(C) 2011 Fred Kraus. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
I describe two new species in the miniaturized microhylid frog genus Paedophryne from forests in southeastern Papua New Guinea. The first species is described on the basis of two specimens and exhibits female snout-vent length of 8.5–9.0 mm (no males known), whereas that of the second species, described on the basis of 12 specimens, is 8.8–9.3 mm, with males 8.1–8.9 mm. These frogs are smaller than the other two diminutive species described when the genus was recently erected, and they represent what are currently the smallest known species of tetrapods. The two species replace each other elevationally on the same mountain massif and occur in relative geographic proximity to the other named species of the genus. Females of both species contain only two enlarged ova, suggesting that they also possess clutch sizes at the extreme lower end of variation in frogs. All species of Paedophryne inhabit leaf litter, as seen for most other miniaturized anurans.
clutch size, diminutive, ecomorph, Milne Bay Province, Mt. Dayman, Mt. Suckling
Asterophryine frogs represent a large subfamily of the Microhylidae that contains 22 named genera, more than 240 named species, and scores of unnamed forms. The subfamily is monophyletic (
Among those asterophryines of the “terrestrial” ecomorph, which solely inhabit the forest floor, I recently described from southeastern New Guinea a new genus, Paedophryne, that included two new species comprising some of the smallest frogs in the world (
On a recent survey of an isolated mountain massif in southeastern Papua New Guinea I discovered two more species of Paedophryne. Each is of a smaller size than the two species already known in the genus and stands at the lower size limit currently known for anurans (
All measurements were made with an optical micrometer to the nearest 0.1 mm, except for toe disc width, measured to the nearest 0.03 mm; measurements, terminology, and abbreviations follow
I recorded calls in the field using a Sennheiser K6 microphone and a Marantz 660 digital audio recorder; I analyzed call structure using the computer program Avisoft-SASLab Pro(v4.34) available from Avisoft Bioacoustics (http://www.avisoft.com/).
Type specimens are deposited in the Bernice P. Bishop Museum, Honolulu (BPBM) and the Papua New Guinea National Museum and Art Gallery, Port Moresby (PNGNM). All latitude and longitude coordinates use the World Geodetic System, 1984 (WGS 84) and were taken from a hand-held GPS unit.
urn:lsid:zoobank.org:act:3A6D9296-93A9-45C6-96F4-92FBAE0D428D
http://species-id.net/wiki/Paedophryne_dekot
Figs 1, 2A, BBPBM 37753 (field tag FK 15615), alcohol specimen, adult female, collected by F. Kraus and local villagers at Binigun, W slope Mt. Dayman, 9.7071°S, 149.2498°E, 900 m, Milne Bay Province, Papua New Guinea, 1 April 2011.
(n = 1). BPBM 37754, same data as holotype, except collected 4 April 2011.
A minute microhylid (female SV = 8.5–9.0 mm) with smooth dorsal skin; a relatively long leg (TL/SV = 0.45–0.46); short and broad snout (EN/SV = 0.067–0.071, IN/SV = 0.106–0.111, EN/IN = 0.60–0.67); relatively large discs on third and fourth toes (4thT/SV = 0.044–0.052, 3rdF/4thT = 0.43–0.58, Fig. 1E); a uniform brown or red-brown dorsum with two large dorsolateral, downward-pointing, black triangular blotches on each side; and a pale gray venter with brown flecks.
Paedophryne dekot differs from Paedophryne kathismaphlox and Paedophryne oyatabu, the only two other species currently described in this genus, in its smaller size (female SV = 8.5–9.0 in Paedophryne dekot, 10.4–10.9 mm in Paedophryne kathismaphlox, 11.3 mm in Paedophryne oyatabu), longer leg (TL/SV = 0.45–0.46 in Paedophryne dekot, 0.35–0.39 in Paedophryne kathismaphlox, 0.40 in Paedophryne oyatabu), shorter snout (IN/SV = 0.106–0.111, EN/IN = 0.60–0.67, EN/SV = 0.067–0.071 in Paedophryne dekot; IN/SV = 0.087–0.099, EN/IN = 0.78–0.80 in Paedophryne kathismaphlox; EN/SV = 0.062, IN/SV = 0.097 in Paedophryne oyatabu), larger discs on third and fourth toes (4thT/SV = 0.044–0.052 in Paedophryne dekot, 0.032–0.037 in P. kathismaphlox, 0.031 in Paedophryne oyatabu; 3rdF/4thT = 0.43–0.58 in Paedophryne dekot, 0.66–0.86 in Paedophryne kathismaphlox, 0.80 in Paedophryne oyatabu), dorsum with two large dorsolateral triangular blotches on each side (dorsum brown vaguely mottled with black or dark brown in Paedophryne kathismaphlox, dorsum brown with two dark mid-dorsal chevrons in Paedophryne oyatabu), venter pale gray with brown flecks (venter dark brown with scattered light straw-brown or gray flecks in Paedophryne kathismaphlox and Paedophryne oyatabu), and no brightly colored patch below anus (a burnt-orange patch below anus in Paedophryne kathismaphlox).
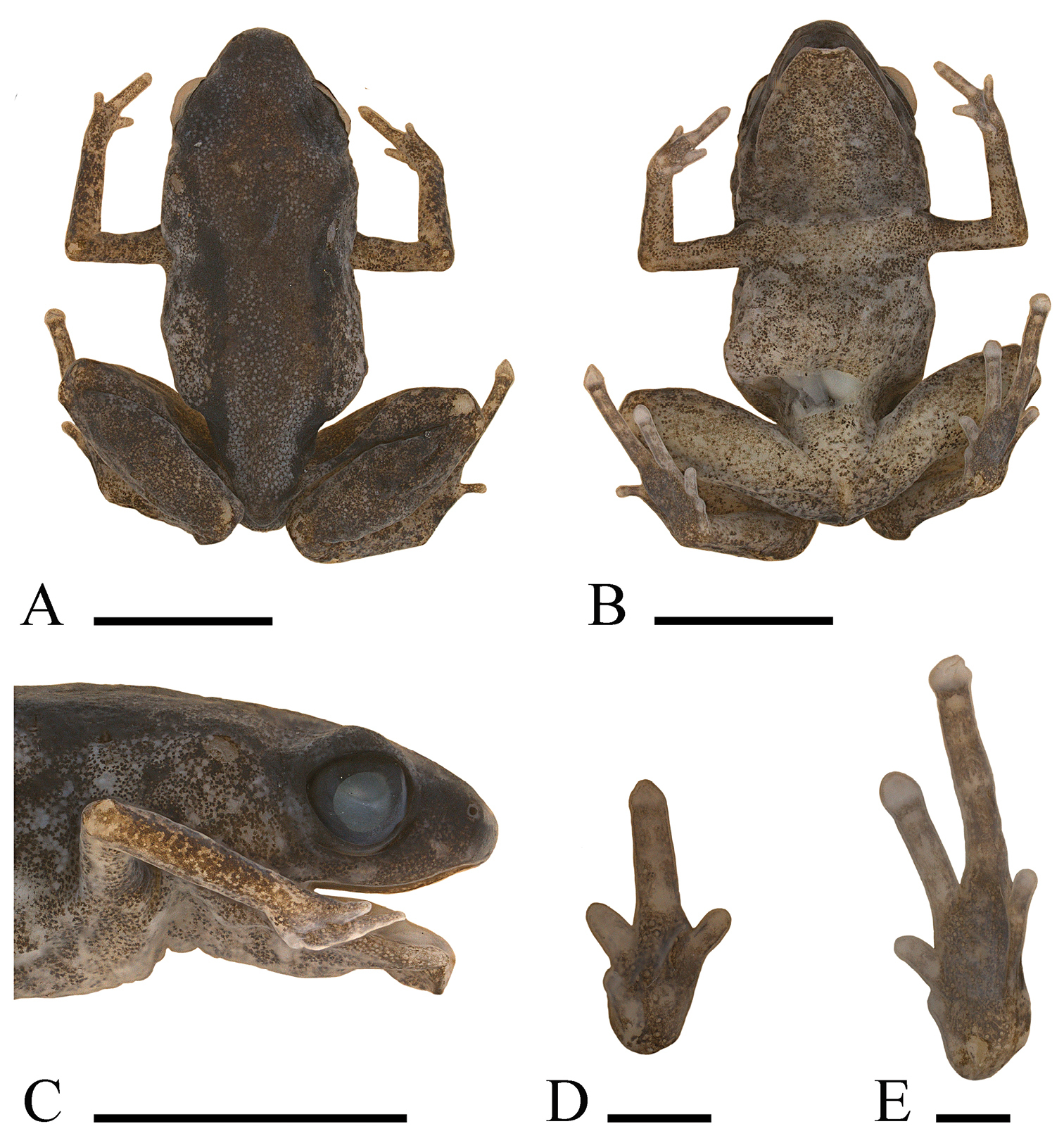
An adult female with an incision on right side and across rear of abdomen; liver removed and stored separately for DNA analysis. Head moderately wide (HW/SV = 0.37, Fig. 1A), with steeply oblique loreal region; canthus rostralis rounded, slightly convex when viewed from above; nostrils directed anterolaterally, closer to tip of snout than to eyes; internarial distance much larger than distance from naris to eye (EN/IN = 0.60, IN/SV = 0.111, EN/SV = 0.067); snout rounded when viewed from the side or from above (Fig. 1A, C); eyes moderately large (EY/SV = 0.13; EY/SN = 1.0, Fig. 1C), pupil horizontal; eyelid approximately two-thirds width of interorbital distance; tympanum indistinct and small (TY/SV = 0.044), visible only when skin dries slightly, hidden posterodorsally. Skin smooth; supratympanic fold absent. Fingers unwebbed, flattened; F1 reduced to a vestigial nub; relative lengths 3>2=4>1 (Fig. 1D); discs absent. Subarticular and metacarpal tubercles absent. Toes unwebbed; T3 and T4 with flattened discs and terminal grooves; disc of T4 not wider than penultimate phalanx. Second and fifth toes with round tips and no discs; T1 a vestigial nub; relative lengths of toes 4>3>2=5>1 (Fig. 1E). Subarticular and metatarsal tubercles absent. Plantar and palmar surfaces smooth. Hind legs rather long (TL/SV = 0.46, Fig. 1A). Tongue elongate, straplike, anterior third attached to floor of mouth.
In preservative, dorsum brown with a more-or-less continuous dorsolateral row of black blotchs and flecks; sides and front and rear of thighs pale gray heavily flecked with dark brown. Face dark brown. Ventral surfaces pale gray flecked with dark brown. Iris black.
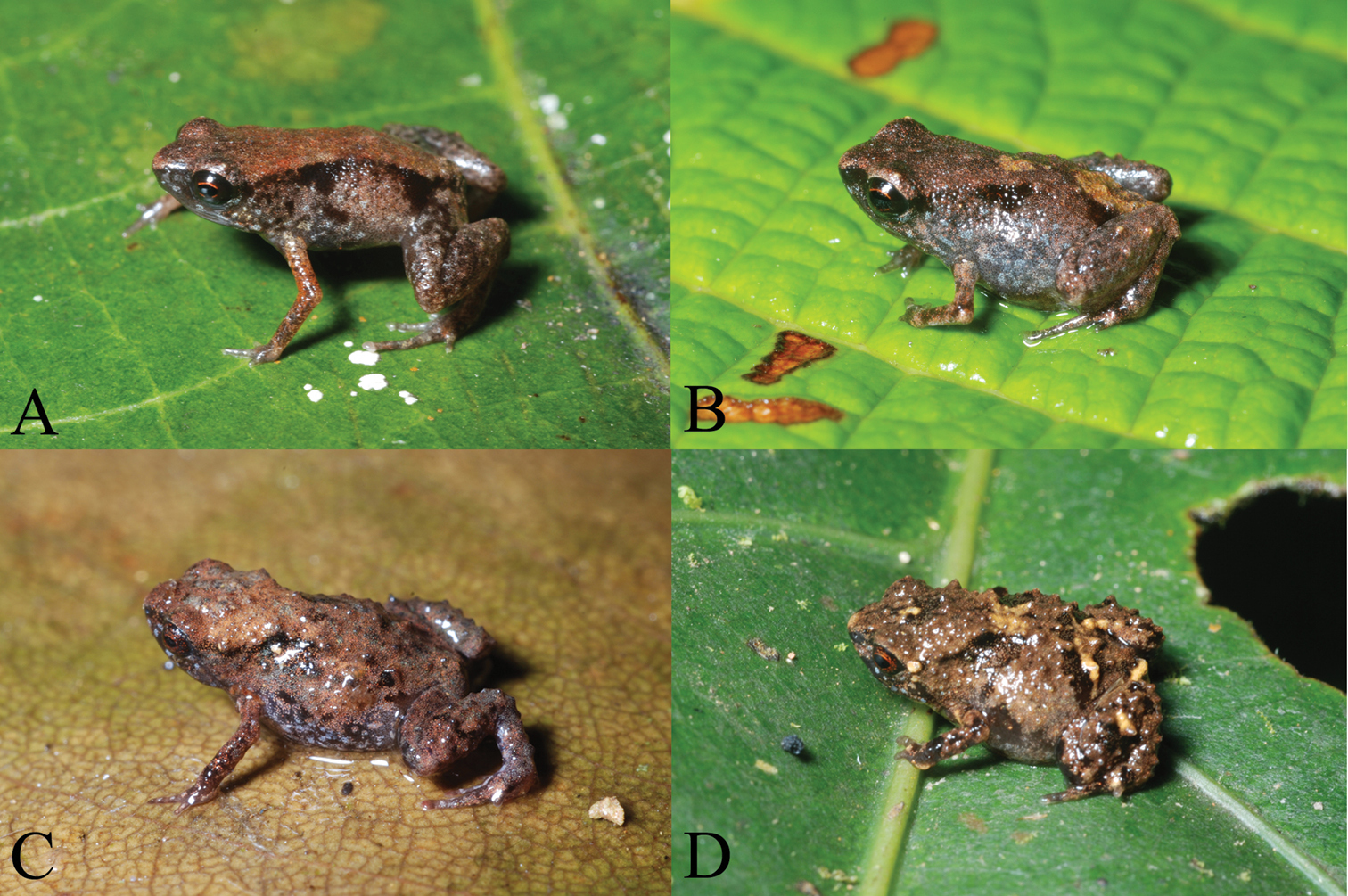
In life, the holotype was noted as: “Dorsum red brown, sides gray, dorsolateral series of black flecks. Rear of thighs light red brown with black punctations. Face black, posterior to eye spotted with light gray. Venter dark gray with small pale-gray flecks.” The iris was black with a red rim around the pupil, and scattered pale blue-gray flecks are apparent on the lower sides and limbs (Fig. 2A).
Measurements (in mm).—SV = 9.0, TL = 4.1, HW = 3.3, HL = 2.9, IN = 1.0, EN = 0.6, SN = 1.2, EY = 1.2, TY = 0.4, 4th T = 0.40.
There is little mensural difference between the paratype and holotype, except that the former has a somewhat larger tympanum (TY/SV = 0.059) and a slightly longer snout (EN/IN = 0.67). In coloration, the paratype is similar to the holotype but the black dorsal blotching is not concentrated into dorsolateral lines; there is a pale tan blotch over the rump; and the dark brown on the sides, limbs, and ventral surfaces is reduced to even stippling instead of flecking. Liver was also removed from the paratype for DNA analysis. Measurements for the paratype are: SV = 8.5, TL = 3.8, HW = 3.2, HL = 2.7, IN = 0.9, EN = 0.6, SN = 1.1, EY = 1.3, TY = 0.5, 4th T = 0.44.
The species name “dekot” is the word for “very small” in Daga, the language spoken in the area from which this species was collected.
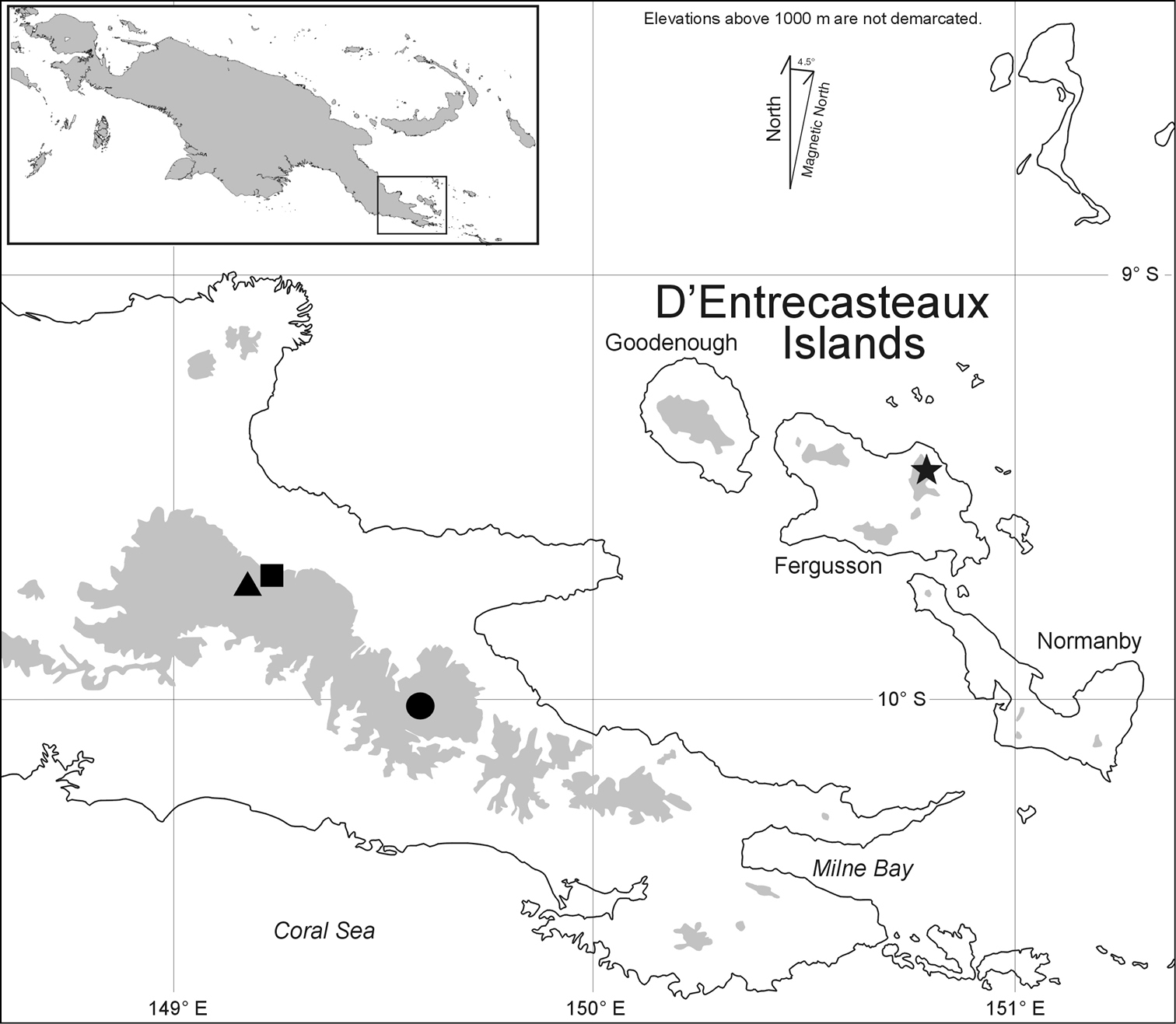
Known only from the western slope of Mt. Dayman in the saddle where it joins Mt. Suckling to the northwest, Milne Bay Province, Papua New Guinea (Fig. 3, square).
Paedophryne dekot inhabits leaf litter on the floor of steeply sloping primary foothill rainforest. Canopy at the type locality was approximately 35 m high; understory was dense, with some moss on trees and ground. This forest type terminates at approximately 1200 m elevation in this area, so Paedophryne dekot seems unlikely to occur higher than that.
This species was heard calling from the forest floor in mid- to late afternoon and at dusk but could not be recorded by me.
Both females contained two enlarged, well-yolked, cream-colored eggs and approximately a dozen small white oocytes.
A Dorsum, B ventrum, C side of head, D palmar view of left hand, and E plantar view of left foot of holotype of Paedophryne dekot (BPBM 37753). Scale bars = 5 mm A–C and 1 mm D, E.
Portraits in life of A holotype of Paedophryne dekot (BPBM 37753), B paratype of Paedophryne dekot (BPBM 37754), C paratype of Paedophryne verrucosa (BPBM 37743), and D paratype of Paedophryne verrucosa (BPBM 37745).
urn:lsid:zoobank.org:act:8DEA8ED4-D3D2-49DC-AFF2-8B8A21CF0044
http://species-id.net/wiki/Paedophryne_verrucosa
Figs 2C, D, 4BPBM 37747 (field tag FK 15516), alcohol specimen, adult male, collected by F. Kraus and local villagers at Sota, SE slope Mt. Dayman, 9.7580°S, 149.1822°E, 1860 m, Milne Bay Province, Papua New Guinea, 27 March 2011.
(n = 11). BPBM 37745–46, same data as holotype; BPBM 37743, same data as holotype, except female collected 23 March 2011; BPBM 37744, same data as holotype, except collected 26 March 2011; BPBM 37748–50, PNGNM 24121–22, same data as holotype, except collected 28 March 2011; BPBM 37751–52, same data as holotype, except females collected 29 March 2011.
A minute microhylid (male SV = 8.1–8.9 mm, female SV = 8.8–9.3 mm) with highly pustulose dorsal skin and plantar surfaces; a relatively short leg (TL/SV = 0.37–0.42); short and broad snout (EN/SV = 0.067–0.080, IN/SV = 0.108–0.123, EN/IN = 0.60–0.70); wide head (HW/SV = 0.38–0.44), fifth toe distinctly shorter than second; relatively large discs on third and fourth toes (4thT/SV = 0.044–0.055); and a light-brown dorsum and sides flecked with black.
Paedophryne verrucosa differs from all other members of the genus in its warty plantar surfaces and in having the fifth toe distinctly shorter than the second; it further differs from Paedophryne kathismaphlox in its smaller size (male SV = 8.1–8.9 mm in Paedophryne verrucosa, 10.1 mm in Paedophryne kathismaphlox; female SV = 8.8–9.3 mm in Paedophryne verrucosa, 10.4–10.9 mm in Paedophryne kathismaphlox), more heavily warty dorsal skin, shorter snout (IN/SV = 0.108–0.123 in Paedophryne verrucosa, 0.087–0.099 in Paedophryne kathismaphlox; EN/SV = 0.067–0.080 in Paedophryne verrucosa, 0.78–0.80 in Paedophryne kathismaphlox), larger discs on third and fourth toes (4thT/SV = 0.044–0.055 in Paedophryne verrucosa, 0.032–0.037 in Paedophryne kathismaphlox), in having the lateral surfaces the same color as the dorsum (lateral surfaces sharply darker than dorsum and punctated with pale gray in Paedophryne kathismaphlox), and in generally lacking a colored patch below anus (tan in one specimen of Paedophryne verrucosa, burnt-orange patch below anus in all Paedophryne kathismaphlox). The new species further differs from Paedophryne oyatabu in its smaller size (female SV = 8.8–9.3 mm in Paedophryne verrucosa, 11.3 mm in Paedophryne oyatabu), heavily warty dorsal skin (smooth in Paedophryne oyatabu), shorter snout (EN/SV = 0.067–0.080 in Paedophryne verrucosa, 0.062 in Paedophryne oyatabu, IN/SV = 0.108–0.123 in Paedophryne verrucosa, 0.097 in Paedophryne oyatabu), larger discs on third and fourth toes (4thT/SV = 0.044–0.055 in Paedophryne verrucosa, 0.031 in Paedophryne oyatabu), dorsum brown with black flecks (brown with two darker scapular chevrons in Paedophryne oyatabu); it further differs from Paedophryne dekot in its heavily warty dorsal skin (smooth in Paedophryne dekot), shorter leg (TL/SV = 0.37–0.42 in Paedophryne verrucosa, 0.45–0.46 in Paedophryne dekot), wider head (HW/SV = 0.38–0.44 in Paedophryne verrucosa, 0.37–0.38 in Paedophryne dekot), and light-brown dorsum flecked with black (dorsum brown or red-brown with dorsolateral black triangular blotches in Paedophryne dekot).
An adult male with vocal slits. Head wide (HW/SV = 0.43, Fig. 3A), with steeply oblique loreal region; canthus rostralis rounded, straight when viewed from above; nostrils directed anterolaterally, closer to tip of snout than to eyes; internarial distance much larger than distance from naris to eye (EN/IN = 0.60, IN/SV = 0.112, EN/SV = 0.067); snout somewhat pointed, sharply rounded when viewed from the side or from above (Fig. 4A, C); eyes moderately large (EY/SV = 0.13; EY/SN = 1.0, Fig. 4C), pupil horizontal; eyelid more than half width of interorbital distance; tympanum indistinct and small (TY/SV = 0.056), visible only when skin dries slightly, hidden posterodorsally. Skin granular and highly pustulose dorsally, granular to slightly pustulose ventrally; supratympanic fold absent. Fingers unwebbed, flattened; F1 very reduced in size; relative lengths 3>2=4>1 (Fig. 4D); discs absent. Subarticular and metacarpal tubercles absent; plantar surfaces granular to slightly pustulose. Toes unwebbed; T3 and T4 with flattened discs and terminal grooves; disc of T4 not wider than penultimate phalanx. Second and fifth toes reduced in size, with round tip and no disc; T1 a vestigial nub; relative lengths of toes 4>3>2>5>1 (Fig. 4E). Subarticular and metatarsal tubercles absent, but plantar surfaces heavily pustulose. Hind legs rather short (TL/SV = 0.38, Fig. 4A). Tongue elongate, straplike, anterior one-third attached to floor of mouth.
In preservative, uniform dark brown above, lighter on sides, where many granules are light brown. Face dark brown with few pale-brown spots. Ventral surfaces and front and rear of thighs pale brown heavily flecked with dark brown, the latter most heavily concentrated on chin and throat. Iris black.
Measurements (in mm).—SV = 8.9, TL = 3.4, HW = 3.8, HL = 2.9, IN = 1.0, EN = 0.6, SN = 1.2, EY = 1.2, TY = 0.5, 4th T = 0.49.
Females attain larger size and have smaller tympana than males (Table 1). Mensural variation in the sample is slight, and important differences are not obvious between the sexes.
In preservative, most specimens are somewhat lighter than the holotype, varying to medium brown dorsally, in which case a few small black blotches are evident. Three specimens directly fixed in alcohol each exhibit a pale gray-brown dorsum with a pair of black scapular blotches and a pair of black lumbar blotches, with a smaller mid-dorsal black blotch between the lumbar blotches. This pattern is evident in life in some specimens (Fig. 2D). Ventral coloration can be lighter in overall tone than seen in the holotype due to presence of fewer dark-brown flecks. Both tan and black spots may also occur sparsely in the ventral pattern. Three specimens have a pale-gray patch below the anus.
Field notes for paratype BPBM 37743 (Fig. 2C): “Brown with black flecks; warty. Face and venter black with light-gray flecks, posterior of abdomen brown. Rear of thighs brown, each with one large black spot.” For paratype BPBM 37745 (Fig. 2D): “Dark brown with black markings, some tan flecks posteriorly and on legs, tan patch around anus. Venter charcoal gray and light gray.” BPBM 37746 was light brown above with two black scapular triangles and no light anal patch. Its chin, throat, and chest were charcoal gray and its abdomen brown with light-gray punctations and dark-gray flecks. BPBM 37747 was dark brown above and also lacked a light-colored anal patch. Its venter was charcoal gray with light-gray flecks. BPBM 37748 was dorsally as for BPBM 37745 and ventrally as for BPBM 37746 and also lacked an anal patch. PNGNM 24121 was brown and black above and gray flecked with light brown below; PNGNM 24122 tan with black flecks above and gray with light-brown flecks below; BPBM 37749 also brown and black above and light and dark gray below.
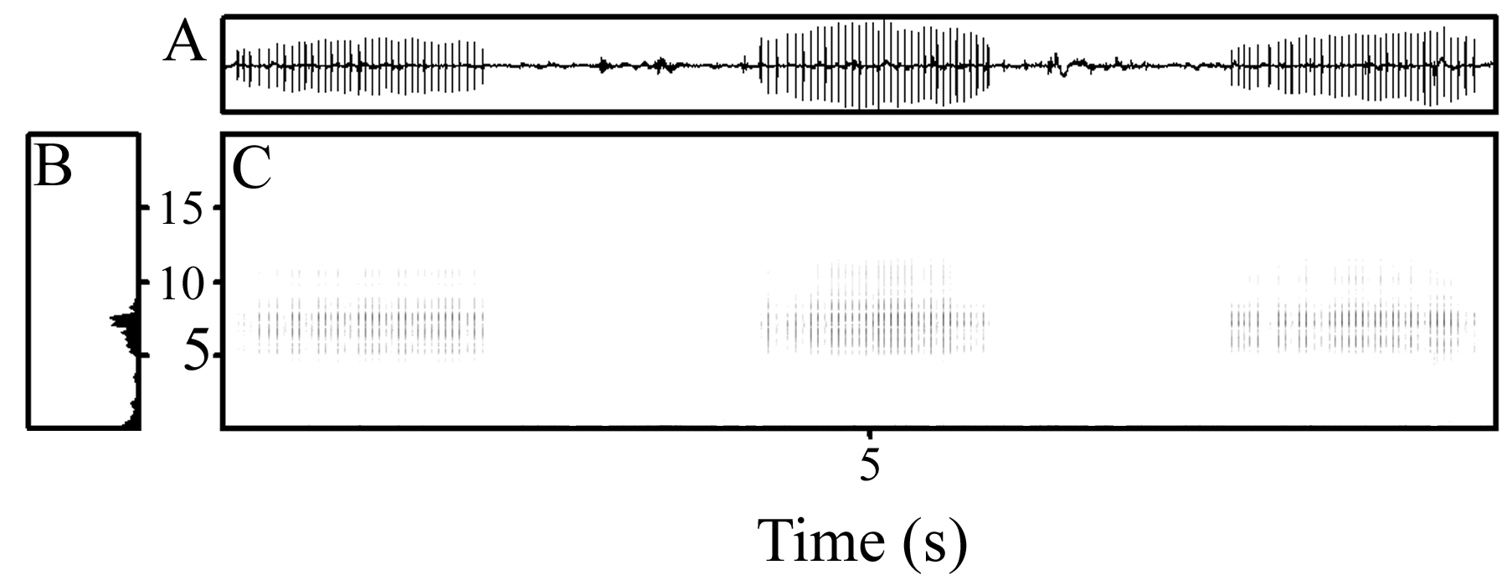
The advertisement call of the holotype was recorded. Each call is a single drawn-out pulsed note given in a long train, with call trains varying from 67–102 s in the two series recorded by me. To the human ear, each call sounds like a quick drag of a finger over a comb. Calls are brief, with average duration varying from 1.210–1.652 s between animals and ranging from 0.712–1.942 s overall (Table 2, Fig. 5). Calls are given at a rate of 0.19–0.46 notes/s, with faster rates at higher temperatures (Table 2). Intervals between calls were longer than the calls themselves and were shorter at higher temperatures, averaging 1.093 s (range 0.897–3.876 s) at 14.9°C, and longer at colder temperatures, averaging 3.694 s (range 1.824–11.655 s) at 19.4°C (Table 2). Calls are highly pulsed, with 15–36 pulses/call, each pulse lasting 0.032–0.066 s (Table 2), and they increase and decrease in maximum amplitude gradually, with maximum amplitude sustained over most of the call duration (Fig. 5A). The power spectrum was rather broad, with a dominant frequency varying from 6510–7890 Hz (Fig. 5B).
The species name “verrucosa” is a Latin adjective meaning “full of warts”.
Known only from the southeastern slope of Mt. Suckling near the saddle where it joins Mt. Dayman to the southeast, Milne Bay Province, Papua New Guinea.
Paedophryne verrucosa inhabits leaf litter on the floor of primary mid-montane rainforest, seeming to prefer the lower slopes of steep hillsides. Canopy at the type locality was approximately 35 m high; understory was dense, with fallen trees, Nastus, and melastomes common. This mid-montane forest begins at approximately 1200 m elevation, so Paedophryne verrucosa seems likely to inhabit forest down to that elevation.
This species typically called at dusk, even continuing through the deafening period of cicada calling at approximately 1800–1830 h, but calling ceased soon after dark. It also frequently called before dawn, and occasional individuals were heard to call briefly in mid-morning. It was not heard by me to call on days lacking rain.
All three females contained two enlarged, well-yolked, cream-colored eggs and approximately a dozen small white oocytes.
Map of southeastern Papua New Guinea, showing type localities for Paedophryne dekot (square) and Paedophryne verrucosa (triangle). Only known localities for the related and geographically proximate Paedophryne kathismaphlox (filled circle) and Paedophryne oyatabu (star) are shown for comparison.
A Dorsum, B ventrum, C side of head, D palmar view of left hand, and E plantar view of left foot of holotype of Paedophryne verrucosa (BPBM 37747). Scale bars = 5 mm A–C and 1 mm D, E.
Mensural data for type series of Paedophryne verrucosa.
| Character | Males (n = 9) | Females (n = 3) | ||
| mean | range | mean | range | |
| SV (mm) | 8.5 | 8.1–8.9 | 9.0 | 8.8–9.3 |
| TL/SV | 0.40 | 0.37–0.42 | 0.39 | 0.39–0.40 |
| EN/SV | 0.070 | 0.067–0.074 | 0.074 | 0.068–0.080 |
| IN/SV | 0.12 | 0.11–0.12 | 0.11 | 0.11–0.11 |
| SN/SV | 0.13 | 0.12–0.14 | 0.13 | 0.13–0.14 |
| TY/SV | 0.057 | 0.048–0.062 | 0.044 | 0.043–0.045 |
| EY/SV | 0.14 | 0.12–0.14 | 0.14 | 0.13–0.15 |
| HW/SV | 0.41 | 0.38–0.44 | 0.39 | 0.38–0.40 |
| HL/SV | 0.33 | 0.32–0.35 | 0.32 | 0.30–0.33 |
| 4thT/SV | 0.049 | 0.045–0.055 | 0.044 | 0.044–0.044 |
| EN/IN | 0.61 | 0.60–0.67 | 0.67 | 0.60–0.70 |
| HL/HW | 0.79 | 0.76–0.85 | 0.81 | 0.80–0.83 |
Call data for two specimens of Paedophryne verrucosa from Mt. Suckling. Numbers for call parameters are mean (range).
| Specimen | Temperature (˚C) | Number of calls | Calling duration (s) | Call rate (calls/s) | Call duration (s) | Interval between calls (s) | Number of pulses/ call | Number of pulses/ call | Pulse length (s) | Dominant frequency (kHz) |
| BPBM 37747 | 14.9 | 13 | 66.7 | 0.19 | 1.652(0.985–1.942) | 3.694(1.824–11.655) | 30.3(15–36) | 30.3(15–36) | 0.055(0.051–0.066) | 7.27(6.51–7.60) |
| uncap-tured | 19.4 | 47 | 102.1 | 0.46 | 1.210(0.712–1.315) | 1.093(0.897–3.876) | 29.8(22–32) | 29.8(22–32) | 0.040(0.032–0.043) | 7.65(7.35–7.89) |
These two new frog species are at the lower size limit known for tetrapods and appear to marginally extend that limit. The smallest known tetrapods are all frogs (
As discussed earlier (
It is uncertain whether the presence of so many minute frogs in the Papuan region represents a biological oddity of that region or whether similar frogs have simply been overlooked or underappreciated elsewhere. Given the difficulty of locating miniaturized frogs in the field and the rate at which they’ve been discovered during the past 15 years, additional miniaturized species no doubt await discovery or description in other poorly surveyed areas of the tropics. For example, further species are known in the diminutive Madagascan Stumpffia but have been awaiting description for years (
Given these observations, I tentatively suggest that the remarkable diversity of miniaturized frogs may represent a biological oddity of the Papuan region. Not only are a surprisingly large number of species involved, but the taxonomic diversity is also large: extremely diminutized frogs occur in seven genera of Papuan asterophryines (one genus omitted from Table 3 because its representative species is not yet described), whereas only 12 genera are involved across the remainder of the globe (
The connection between anuran miniaturization and exploitation of leaf-litter and moss habitats has been briefly discussed previously (
Also consistent with other diminutive frogs, the new species of Paedophryne exhibit clutch sizes at the lower end of those known for anurans. All five females of the two species (2 Paedophryne dekot, 3 Paedophryne verrucosa) each contained two enlarged ova and a complement of approximately one dozen tiny oocytes. This strongly suggests that clutch size in these species is one or two, although it remains to be determined how frequently females deposit clutches. Other species of minute frogs (e.g., Brachycephalus didactylus, Eleutherodactylus iberia Estrada and Hedges, Eleutherodactylus limbatus Cope, Eleutherodactylus orientalis Barbour and Shreve) have even smaller clutches, with females producing only a single egg at a time (
A Waveform, B power spectrum, and C spectrogram (frequency axis in kHz) of three calls of holotype of Paedophryne verrucosa (BPBM 37747) recorded on southeastern slope of Mt. Suckling, 27 March 2011, air temperature 14.9°C.
Body sizes of minute frog species omitted from the survey of
| Species | Max SV male | Max SV female | Mean SV males | Mean SV females | Mean SV males and females | Range males | Range females | Sample size males | Sample size females | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Paedophryne dekot | – | 9.0 | – | 8.8 | – | – | 8.5–9.0 | – | 2 | this study |
| Paedophryne verrucosa | 8.9 | 9.3 | 8.5 | 9.0 | 8.7 | 8.1–8.9 | 8.8–9.3 | 9 | 3 | this study |
| Paedophryne kathismaphlox | 10.1 | 10.9 | – | 10.6 | 10.5 | – | 10.4–10.9 | 1 | 3 |
|
| Paedophryne oyatabu | – | 11.3 | – | – | – | – | – | – | 1 |
|
| Microhyla perparva | 10.5 | 12.4 | – | – | – | 10.1–10.5 | 11.4–12.4 | – | – |
|
| Oreophryne minuta | 11.5 | – | – | – | – | 9.2–11.5 | – | 4 | – |
|
| Choerophryne allisoni | 11.6 | – | 11.6 | – | – | 11.5–11.6 | – | 2 | – |
|
| Aphantophryne minuta | – | 11.8 | – | – | – | – | – | – | 1 |
|
| Choerophryne burtoni | 12.4 | – | 12.3 | – | – | 12.1–12.4 | – | 3 | – |
|
| Microhyla borneensis | 12.8 | 18.8 | 11.7 | 18.4 | – | 10.6–12.8 | 17.9–18.8 | 8 | 2 |
|
| Cophixalus kethuk | 13.5 | 15.0 | 12.9 | 13.9 | 13.4 | 12.4–13.5 | 13.2–15.0 | 6 | 5 |
|
| Cophixalus sisyphus | 14.1 | 13.6 | 13.3 | 13.6 | 13.4 | 12.0–14.1 | 13.5–13.6 | 24 | 2 |
|
| Cophixalus linnaeus | 14.7 | 16.7 | 14.1 | 15.6 | 14.7 | 13.4–14.7 | 14.9–16.7 | 4 | 3 |
|
| Choerophryne arndtorum | 14.8 | – | 13.8 | – | – | 11.2–14.8 | – | 13 | – |
|
| Choerophryne amomani | 15.1 | – | 13.8 | – | – | 11.8–15.1 | – | 9 | – |
|
| Austrochaperina minutissima | 15.8 | 16.6 | 15.5 | – | 15.7 | 15.0–15.8 | – | 4 | 1 |
|
| Cophixalus iovaorum | 16.0 | 17.2 | 14.5 | 16.9 | 14.6 | 13.2–16.0 | 16.6–17.2 | 29 | 2 |
|
| Cophixalus tomaiodactylus | 16.1 | 16.6 | 14.2 | 15.7 | 14.7 | 13.2–16.1 | 14.2–16.6 | 11 | 5 |
|
| Cophixalus desticans | 16.2 | 19.1 | 14.6 | 18.4 | 14.8 | 13.1–16.2 | 17.6–19.1 | 32 | 2 |
|
I thank Elliot Gagomin for his kind permission to work on his land and for his tireless logistical coordination of the expedition to Mt. Suckling; the many inhabitants of Bonenau and Yamsiai who joined in or otherwise assisted this expedition; Shep Myers for preparing Figures 1 and 4; and Pumehana Imada for specimen processing and documentation. I thank the PNG National Museum and Art Gallery for providing in-country collaborative assistance and the Department of Environment and Conservation, National Research Institute, and Milne Bay Provincial Government for permission to conduct this research. This research was supported by National Science Foundation grants DEB-0103794 and DEB-0743890. This is contribution 2011-020 from the Pacific Biological Survey at the Bishop Museum.