(C) 2010 Teresa Bonacci. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Volatile compounds produced by adults of Anchomenus dorsalis under undisturbed and disturbed conditions were investigated with an all-glass aeration apparatus. GC-MS analysis of the crude extracts from undisturbed and disturbed adults highlighted four major volatile compounds, undecane, heneicosane, Z-9 tricosene and tricosane, of which significantly more undecane was released by disturbed adults compared to undisturbed beetles. The pygidial glands of adults of Anchomenus dorsalis were investigated using light and Transmission Electron Microscopy (TEM). Each gland showed dense aggregates of secretory cells organized into visually distinct lobes; a long collecting canal that drains the secretion towards the reservoir, a bean-shaped double lobed muscular reservoir in which secretion is stored and a short duct (efferent duct) through which the secretion is discharged. The function of the pygidial glands and the possible role played by undecane as a defensive allomone and/or chemical signalling molecule are discussed.
undecane, GC/MS, chemical defences, gland morphology, predation avoidance mechanisms
Carabid beetles are known to produce a large variety of
defensive substances, and their chemical nature has been elucidated in
more than 500 species (
Anchomenus dorsalis
(Pontoppidan, 1763) is a gregarious platynine carabid inhabiting muddy
soils and fields across Europe. It is often found in association with
species of Brachinus (
When disturbed, the ground beetles Brachinus dorsalis
releases a strong odour (perceived even by humans) (Bonacci, personal
observation), and quickly retreat (with dispersal movement that produce
a great confusion in the observer) into deeper soil crevices (like the dilution effect). Under laboratory conditions,
Adults of Anchomenus dorsalis were collected by hand from different inter-specific aggregations (each composed by 100–130 individuals) of Brachinus sclopeta and Anchomenus dorsalis found under stones or straw bales in Calabria (Crati Valley, province of Cosenza, 39°35'56"N; 16°15'48"E; elevation: 60 m a.s.l.). Following field collections, monospecific groups were placed in separate plastic cages (30 × 22 × 20 cm) with 4 cm of clay soil in a climatic chamber at 22 °C, photoperiod L/D of 18/6, and fed on veal meat and earthworm pieces (Lumbricus terrestris (Linnaeus, 1758)).
Air collection of adult volatilesThe collection of volatiles from Anchomenus dorsalis
adults was conducted using a horizontal all-glass apparatus 1 l in
volume. Humidified and charcoal filtered air was drawn through the
apparatus at 0.5 l min-1 by a peristaltic pump for 2 h in a conditioned
room at temperature of 22 ± 2 °C. The volatiles produced by experimental
groups of 20 individuals of Anchomenus dorsalis
adults of both sexes were trapped in glass collectors (6 mm ID) loaded
with 600 mg of porapak Q, and held in place by glass wool plugs. Two
experimental individual groups were considered: disturbed and
undisturbed. Adults were considered undisturbed when they were gently
transferred into the glass apparatus and disturbed when, before the
start of the aeration, the glass chamber containing the adults was
vigorously shaken for 10 seconds (
GC–MS analyses were performed using a Hewlett-Packard 5890 GC system interfaced with an HP 5973 quadrupole mass spectrometer detector. As a stationary phase an HP5–MS capillary column (5% diphenyl-95% dimethylpolysiloxane 30 m - 0.2 mm, 0.25 μm film thickness, J&W Scientific, USA) was used. Injector and detector temperatures were 250 °C and 270 °C respectively. Helium was used as the carrier gas. The GC oven temperature program was 60 °C for 5 min, than increased by 10 °C/min to 280 °C. Electron impact ionization spectra were obtained at 70 eV, recording mass spectra from 42 to 550 uma. Compound analysis and identification was carried out using a commercial NIST 2005 mass spectra library search and by comparison with standard analytical grade compounds purchased from Sigma-Aldrich (U.S.A.). Quantitative analysis was carried out for 4 compound identified by GC-MS analysis: undecane, heneicosane, (Z)-9 - tricosene and tricosane.
For this analysis the elutes were diluted in 1 ml of hexane using a volumetric flask. Six point calibration curves, using analytical standards undecane, heneicosane, (Z)-9 - tricosene and tricosane, in the 0.2–100 ng μl-1 range, were used in order to evaluate the chromatographic response. The mean amount ± SE of each of these compounds was calculated dividing the amount of the compound obtained per replicate per the number of individuals used in each replicate.
Gland anatomyFor anatomical study by optical microscopy, adult beetles were killed at -15 °C and their abdomens were treated with 10% potassium hydroxide for 4 days before examination of the chitinous structures. The glands were mounted on clean glass slides and observed by optical microscopy equipped with Nomarsky interference contrast and photographed with a Coolpix 4500 camera (Nikon).
For light and transmission electron microscopy (TEM),
samples were fixed in 3% glutaraldehyde solution in 0.1 M phosphate
buffer (pH. 7.4) for 2 h at 4 °C and post fixed with 3% osmium tetroxide
for 2 h. The specimens were then washed in phosphate buffer,
dehydrated through graded acetone solutions and embedded in Araldite
(Fluka, Buchs, Switzerland). Semithin sections (1 μm) were obtained
with a Leica Ultracut UCT ultramicrotome by using glass knives, mounted
on clean glass slide and stained with 1% toluidine blue. They were then
photographed with the Zeiss Axioskop microscope. For transmission
electron microscopy, ultrathin sections (600–900 Ǻ) were prepared using
a diamond knife and collected on copper grids (G 300 Cu), contrasted
using both lead citrate and uranyl acetate and then examined with a
“Zeiss EM 900” electron microscope (TEM). Gland structure terminology
follows
The quantitative analysis to determine differences in the amount of undecane, heneicosane, (Z)-9 - tricosene and tricosane recovered from Anchomenus dorsalis adults were compared by t-test (
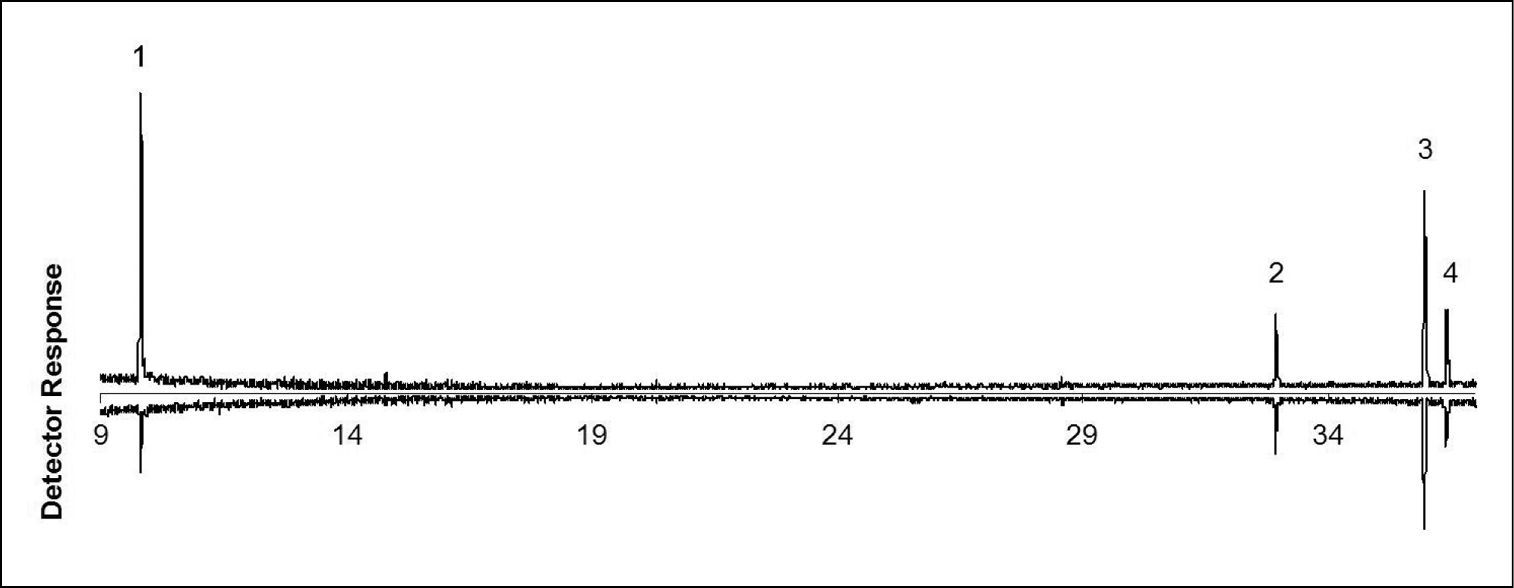
GC-MS analysis of volatile collections showed that disturbed and undisturbed adults of Anchomenus dorsalis released the same four major volatile compounds: undecane, heneicosane, (Z)-9 - tricosene and tricosane (Table 1; Fig. 1). A significant difference between disturbed and undisturbed adults was observed only in the released amount of undecane. An amount of 22.37 ± 8.48 ng (Mean ± SE) were collected from each disturbed adult vs. 0.94 ± 0.29 ng collected from a undisturbed adult (t = 2, 52; df = 8; p = 0.035). Other chemicals (heneicosane, (Z)-9 - tricosene and tricosane) tend to be released more when Anchomenus dorsalis individuals are disturbed but their amount is not statistically significant (Table 1).
Volatile compounds from Anchomenus dorsalis adults obtained from air collections carried out for 2 hours at 0.5 l min-1. RT: retention time at the GC-MS analysis; df: degree of freedom.
| Compound | R.T. (min.) | Amount (ng) /adult (mean ± SE) | t- value | df | p | |
|---|---|---|---|---|---|---|
| Disturbed | Undisturbed | |||||
| Undecane | 9.82 | 22.37 ± 8.48 | 0.94 ± 0.29 | 2.52 | 8 | 0.035 |
| Heneicosane | 32.92 | 1.09 ± 0.96 | 0.44 ± 0.31 | 0.63 | 8 | NS |
| (Z )- 9 - Tricosene | 35.96 | 2.19 ± 2.10 | 0.78 ± 0.70 | 0.63 | 8 | NS |
| Tricosane | 36.98 | 0.93 ± 0.86 | 0.38 ± 0.28 | 0.60 | 8 | NS |
Gas chromatograms of volatile compounds collected from disturbed (up) and undisturbed (down) adults of Anchomenus dorsalis. 1 undecane 2 heneicosane 3 (Z)-9 - tricosene 4 tricosane. On the x axis is reported the retention time (minutes). As a stationary phase an HP5–MS capillary column was used. The GC oven temperature program was 60 °C for 5 min, than increased by 10 °C/min to 280 °C.
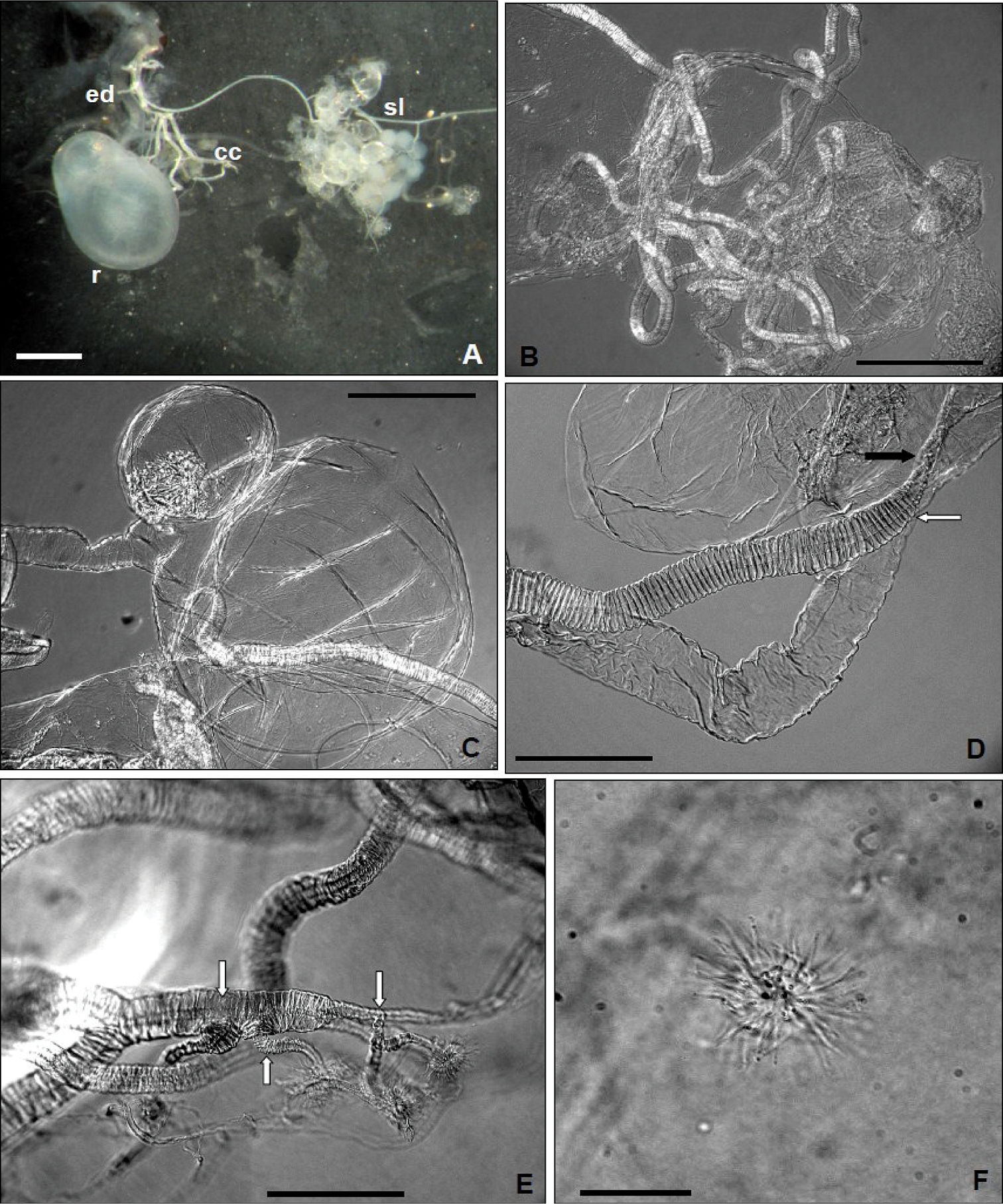
The pygidial glands of Anchomenus dorsalis
are cuticular invaginations of the body wall that open outside
immediately behind the eighth abdominal tergite. Each gland consists of a
aggregate of secretory cells, an collecting canal, a reservoir and an
efferent duct through which the secretion is discharged. The efferent
duct open near the abdominal tip to the sides of anus. Each lobe is
essentially a ball of cells (fig. 2 A) aligned radially around a central collecting lumen (sensu
Light microscope: A Dorsal aspects of pygidial gland; ed, efferent duct; r, reservoir; cc, collecting canal; sl, secretory lobe (Scale bar = 0.5 mm) (not treated with potassium hydroxide) B collecting canal (Scale bar = 0.125 mm) C reservoir with smooth constriction at about one third from its hind end (Scale bar = 0.125 mm) D insertion of collecting canal (black arrow) and efferent duct (white arrow) in the reservoir (Scale bar = 0.05 mm) E collecting canal with apical ramifications (white arrows) (Scale bar = 0.05 mm) F “floret” (sensu
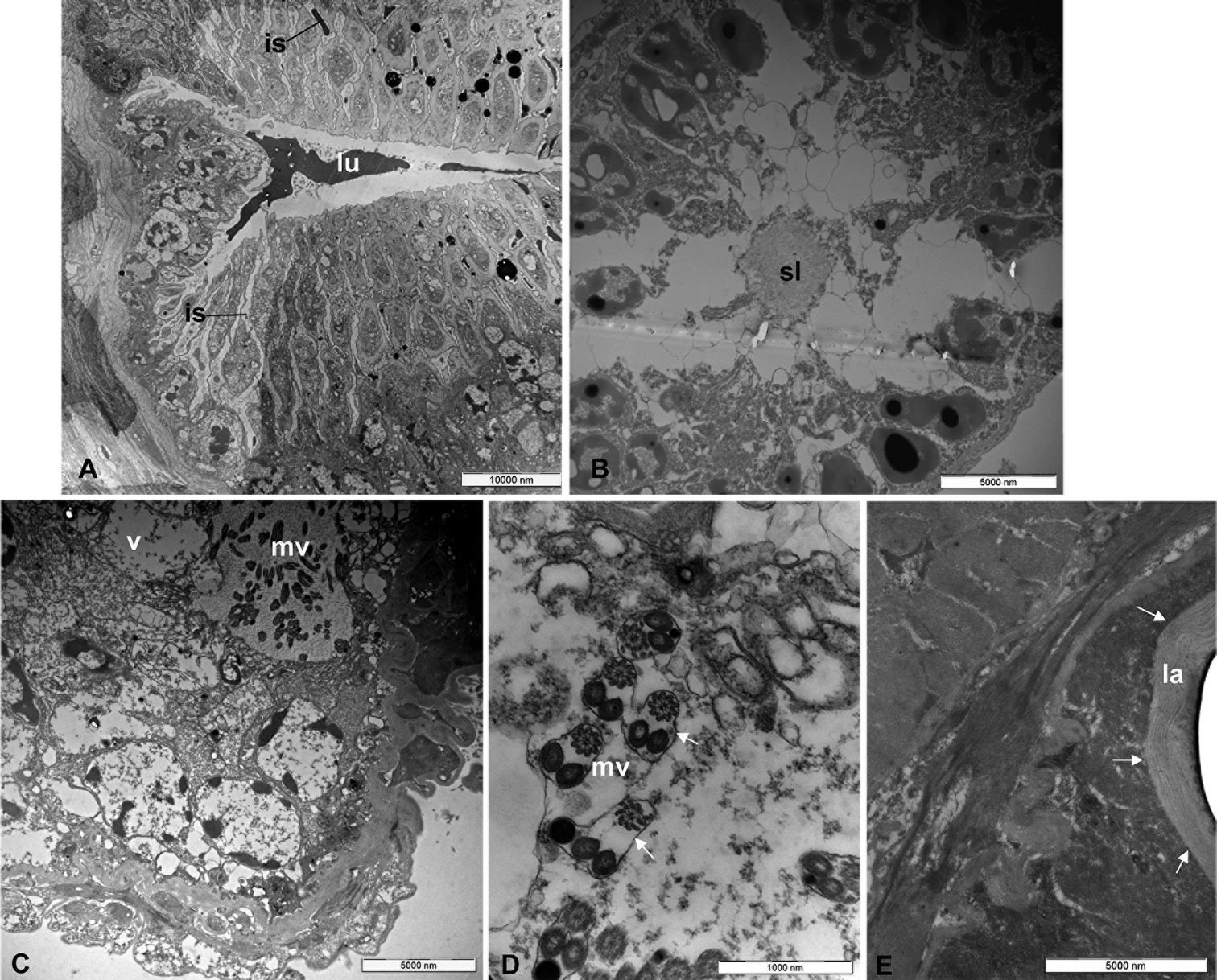
Examined by TEM, the wall of the collecting canal is lined by epidermal part (fig. 3 A) that consist of cells connected to each other by micro-canals projecting into the collecting canal lumen. The lumen of collecting canal contain a heterogeneous secretion (fig. 3 A).
Transmission electron microscope (TEM): A, collecting canal with lumen (lu); the black arrows show interstitial spaces (is) B secretory lobe with secretory lumen (sl) C vesicle (v) with microvilli (mv) D microvilli structure (mv) at highest enlargement (the white arrows show the thin lamina) and E Inner wall of the reservoir with chitinous basal lamina (la) (black arrows) and massive muscle layer around.
Each secretory lobe consist of secretory cells arranged radially around the central lumen. Each secretory cell has an secretory vesicle which is almost as long as the cell itself (fig. 3 B) with a coated membrane and bear many microvilli projecting into the cavity (fig. 3 C). Between the secretory cells are evident (fig. 3 B) the vesicular ducts that carry the secretion in the collecting lumen (fig. 3 B). Each microvillus (fig. 3 D) is formed by three structures: one with a typical spiral shape and other two structures, similar in shape and size. All the structures are enveloped by a thin lamina (fig. 3 D). The inner wall of the reservoir is composed of a thick chitinous layer (basal lamina) (fig. 3 E). A thick muscle layer was found surrounding the reservoir. Likely, the muscles play an important role for the expulsion of the secretory products through the efferent duct. This is composed of muscle bundles that pass spirally around it.
Discussion and conclusionsThe chemical analyses of disturbed and undisturbed Anchomenus dorsalis
adults showed that undecane was produced significantly in larger
amounts in disturbed individuals. This suggests that this compound
(which can be perceived even by humans) could play a prominent role in
the chemical defence of the species. The role of undecane as a defensive
substance has been widely reported in the Insecta: Acanthomyops claviger (Roger, 1862) (
Undecane is an optimal chemical signalling molecule, its
molecular weight and polarity combining moderate olfactory efficiency
with a sufficiently high vapour pressure to broadcast in the centimetre
range when present in microgram quantities or less (
The laboratory observations of the pygidial glands of Anchomenus dorsalis show that they resemble those of other Carabidae in their structure (
As mentioned above, the defense glands in carabid
beetles produce chemical compounds primarily to provide protection
against putative predators (
In summary, undecane and the pygidial glands appear to play a role in the defence mechanism of Anchomenus dorsalis. Further studies will carry on to investigate if undecane emission is able to elicit dispersal and retreating movements both in co-specific and inter-specific groups.