Research Article |
|
Corresponding author: Mårten J. Klinth ( mjeriksson90@gmail.com ) Academic editor: Samuel James
© 2017 Mårten J. Klinth, Emilia Rota, Christer Erséus.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Klinth MJ, Rota E, Erséus C (2017) Taxonomy of North European Lumbricillus (Clitellata, Enchytraeidae). ZooKeys 703: 15-96. https://doi.org/10.3897/zookeys.703.13385
|
Abstract
Lumbricillus is a genus of clitellate worms with about 80 described species that inhabit marine and limnic habitats. This study follows a recent analysis of the phylogeny of the genus based on 24 species of Lumbricillus collected mainly in Norway and Sweden. We provide the illustrated taxonomic descriptions of all these species and describe two of them as new; Lumbricillus latithecatus sp. n. and L. scandicus sp. n. Using the recent phylogeny, we informally divide Lumbricillus into five distinct morphological groups, into which we also tentatively place the Lumbricillus species not included in this study. Furthermore, we establish Claparedrilus gen. n., with the type species C. semifuscoides sp. n., and transfer Pachydrilus semifuscus Claparède, 1861 (previously referred to Lumbricillus) into said genus.
Keywords
Annelida, Oligochaeta, Lumbricillus latithecatus sp. n., Lumbricillus scandicus sp. n., Claparedrilus gen. n., Claparedrilus semifuscoides sp. n., Claparedrilus semifuscus (Claparède, 1861) comb. nov.
Introduction
Enchytraeids (Annelida, Clitellata, Enchytraeidae) are small clitellate worms that mainly inhabit terrestrial soils but the family is well represented in the aquatic environment. One of the about 30 genera, Lumbricillus Ørsted, 1844, is primarily found in marine and freshwater habitats, but also in humid soils (
Returning to the 19th Century, Claparède described his Pachydrilus Claparède, 1861 as an assemblage of marine littoral species lacking “hair bristles”, but having a single pair of spermathecae in segment V, clitellum covering segments XI–XIII, male pores in XII, and simple vascular and nervous systems. Pachydrilus (and its five representatives) shared these basic traits with the terrestrial species then classified in Enchytraeus Henle, 1837, but was distinguished by the lack of dorsal pores and by generally possessing red-colored blood. Pachydrilus was later redefined by Vejdovský (1879) to include only species with sigmoid chaetae and small nephridial anteseptals and then further restricted by
The name Pachydrilus soon became a competitor for Lumbricillus in the taxonomic literature. Some scientists favored Pachydrilus (
Phylogeny of North European Lumbricillus, modified from
In 1885, Saint-Loup described the species Pachydrilus enchytraeoides Saint-Loup, 1885 from the Marseille harbor. The rather brief description mentioned pouch-like spermathecae, a simple circulatory system and irregularly lobed testes (
Among the new species described by
The molecular phylogenetic study by
The aim of this study is to increase the knowledge of the taxonomy of North European Lumbricillus, based on the most recent phylogenetic reconstruction and molecular delimitation of species (
Material and methods
Worms were collected in marine, brackish and limnic habitats, mainly in Norway and Sweden (Appendix
Results
General notes
All descriptions are based on fixed worms mounted on slides. This has some disadvantages for discerning the shape of certain internal organs such as the nephridia, but is not an unusual method for marine worms, and it improves the description of other characters such as chaetae. Nevertheless, morphology can differ from descriptions in the literature based on living specimens.
All specimens in this study are amputated of their posterior segments (used for DNA extraction). Therefore, comparisons of total length and segment number with original descriptions have not been possible. When available, the length of the fifteen first segments as well as the width at the clitellum of the worms has been used to compare the general body size of the species.
In fixed Lumbricillus worms the origin of the dorsal vessel can be difficult to establish since vessel expansions are more or less conspicuous according to the peristaltic movement of the blood at the time of fixation. Thus, due to the varying conditions when animals were killed and fixed, the dorsal vessel may appear to originate in different segments.
Abbreviations in the figures
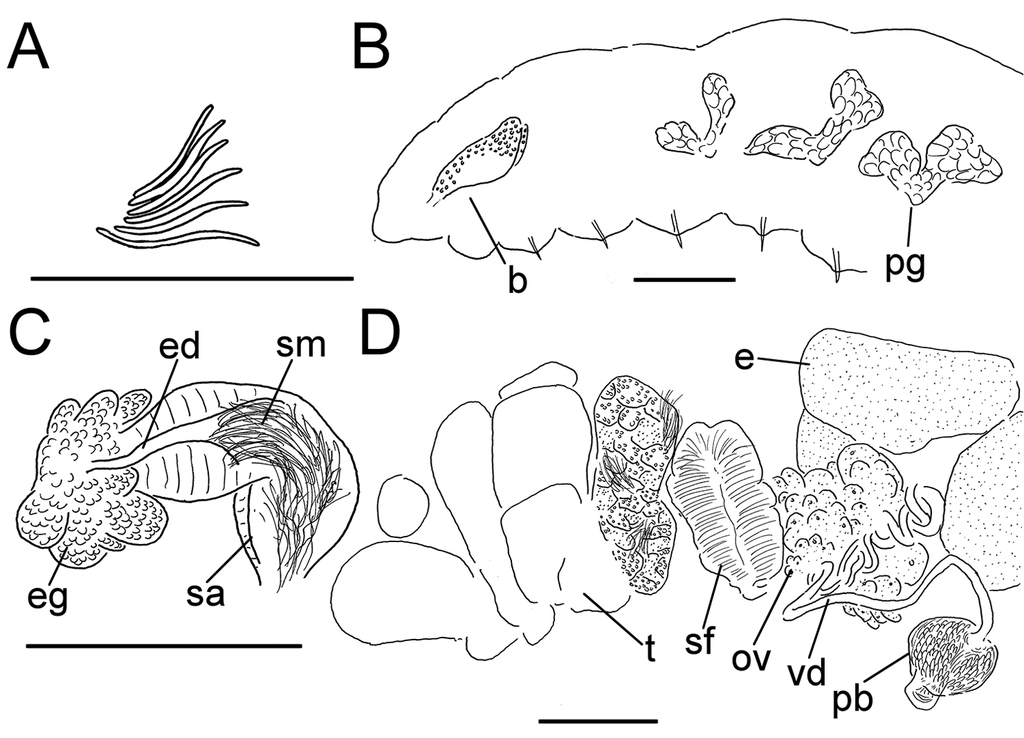
as=anteseptale, b=brain, cl=clitellum, dg=duct glands, e=egg, ed=ectal duct, eg=ectal gland, mu=musculature, nd=nephridial duct, oe=ooesophagus, ov=ovaries, pb=penial bulb, pg=pharyngeal glands, ps=postseptale, s=spermatheca, sa=spermathecal ampulla, sf=sperm funnel, sm=sperm mass, sp=spermathecal pore, t=testis, ts=testis sac, vd=vas deferens.
Taxonomy
Lumbricillus
Genus description/diagnosis
Mainly red, pink, orange, yellow or white when alive, sometimes green or black. Living worms ranging from about 5 to 20 (35 in extremes) mm, fixed from 3 to 14 mm (35 mm in L. maximus (Michaelsen, 1888) even after fixation;
Type species
Lumbricus lineatus Müller, 1774.
Other species
See Table
Remarks
Based on the recent phylogenetic analysis of North European Lumbricillus, a number of monophyletic groups within the genus were recognized (
The informal division of Lumbricillus into five morphological groups based on the phylogenies by
| Monophyletic (Based solely on Klinth et al. 2017) | Testis sacs | Spermathecal shape | Spermathecal duct glands | Penial bulb | Sperm funnels | Chaetae per bundle | ||
| lineatus group | Yes | Regularly lobed into bunch-shape | Spindle-shaped, indistinct ampulla | No | Round | 1-5 times longer than wide | 3-6 or more | |
| pagenstecheri group | Yes | Regularly lobed into bunch-shape | Club-shaped, distinct ampulla | Yes | Round | About twice longer than wide | 3-6 or more | |
| “tuba” group | No | Regularly lobed into bunch-shape | Club-shaped, distinct ampulla | No | Round | About as long as wide | 3-6 | |
| buelowi group | Yes | Irregularly lobed without bunch-shape | Club-shaped, distinct ampulla | No | Round | About as long as wide | 2-3 | |
| arenarius group | Yes | Irregularly lobed without bunch-shape | Pouch-shaped, indistinct ampulla | No | Round or bilobed | 3-10 times longer than wide | 2-3 | |
| Species included in the present study | Species not included in the present study, placed on the basis of their descriptions | |||||||
| lineatus group |
L. fennicus Nurminen, 1964 L. kaloensis Nielsen & Christensen, 1959 L. latithecatus sp. n. * L. lineatus (Müller, 1774) L. pumilio Stephenson, 1932a L. rivalis Levinsen, 1884 L. rubidus Finogenova & Streltsov, 1978 L. rutilus Welch, 1914 L. verrucosus (Claparède, 1861) L. sp F L. sp G |
L. aestuum (Stephenson, 1932b) L. alaricus Shurova, 1974 L. antarcticus Stephenson, 1932b L. americanus (Ude, 1896) L. benhami Stephenson, 1932b L. enteromorphae von Bülow, 1957 L. griseus (Stephenson, 1932b) L. healyae Rodriguez & Rico, 2008 L. incisus Wang & Liang, 1997 L. insularis (Ude, 1896) L. immoderatus Finogenova, 1988 L. macqueriensis Benham, 1905 L. maximus (Michaelsen, 1888) L. minimus (Černosvitov, 1929) |
L. minutus (Müller, 1776) sensu Michaelsen, 1911 L. murmanicus Finogenova & Streltsov, 1978 L. parabolus Shurova, 1978 L. parvus (Ude, 1896) L. pseudominutus Timm, 1988 L. pygmaeus (Michaelsen, 1935) L. rupertensis Coates, 1981 L. sadovskyi Marcus, 1965 L. santaeclarae Eisen, 1904 L. scoticus Elmhirst & Stephenson, 1926 L. werthi (Michaelsen, 1905) |
|||||
| pagenstecheri group |
L. pagenstecheri A-D (Ratzel, 1868) L. viridis Stephenson, 1911 |
L. annulatus Eisen, 1904 L. belli Tynen, 1969 L. corallinae Shurova, 1977 L. curtus Coates, 1981 L. franciscanus Eisen, 1904 L. ignotus Shurova, 1977 L. kalatdlitus Nurminen, 1970 L. kamtschatkanus (Michaelsen, 1929) L. kurilensis Shurova, 1974 L. maritimus (Ude, 1896) L. merriami Eisen, 1904 L. mirabilis Tynen, 1969 |
L. nipponicus (Yamaguchi, 1937) L. orientalis Shurova, 1974 L. pinquis Shurova, 1977 L. qualicumensis Tynen, 1969 L. reynoldsoni Backlund, 1948 L. rufulus Shurova, 1974 L. taisiae Shurova, 1978 L. tenuis (Ude, 1896) L. tsimpseanis Coates, 1981 L. sapitus Shurova, 1979 L. similis Shurova, 1977 |
|||||
| Species included in the present study | Species not included in the present study, placed on the basis of their descriptions | |||||||
| “tuba” group |
L. scandicus sp. n. ** L. tuba Stephenson, 1911 L. helgolandicus (Michaelsen, 1927) |
L. balticus von Bülow, 1957 L. charae (Tynen, 1970) L. imakus Nurminen, 1970 L. lentus Shurova, 1978 |
L. macrothecatus Erséus, 1976 L. niger Southern, 1909 L. ochotensis Shurova, 1979 |
|||||
| buelowi group |
L. buelowi Nielsen & Christensen, 1959 L. knoellneri Nielsen & Christensen, 1959 |
L. cervisiae Kossmagk-Stephan, 1983 L. eltoni (Stephenson, 1924) syn. L. knoellneri? L. mangeri (Michaelsen, 1914) |
L. muscicolus (Stephenson, 1924) syn. L. knoellneri? L. nielseni Nurminen, 1965 |
|||||
| arenarius group |
L. arenarius (Michaelsen, 1889) L. dubius (Stephenson, 1911) L. sp. H |
L. christenseni Tynen, 1966 L. crymodes (Stephenson, 1922) syn. L. arenarius? L. eudioptus (von Bülow, 1955) L. westheidei Kossmagk-Stephan, 1983 |
||||||
| Species with uncertain placement |
L. algensis Erséus, 1977 (seminal vesicles irregularly lobed without bunch-shape but otherwise lineatus-like) L. brunoi Martinez-Ansemil, 1982 (seminal vesicles irregularly lobed without bunch-shape but otherwise lineatus-like) L. colpites (Stephenson, 1932b) (seminal vesicles irregularly lobed without bunch-shape, penial bulb with several lobe-shaped glands) L. horridus Finogenova, 1988 (intricate penial apparatus unlike that of other Lumbricillus) L. intricatus Finogenova, 1977 (spermathecae with ventral openings, nodulate chaetae, but otherwise lineatus-like) |
|||||||
Preliminary key to species groups (based on species included in study only)
| 1 | Chaetae 3–6 (or higher) per bundle, upper bundles located dorsolaterally; testis sacs with lobes in bunch-like arrangement | 2 |
| – | Chaetae 2–3 (occasionally higher) per bundle, upper bundles located midlaterally, just above the lateral line; testis sacs irregularly lobed, not bunch-like | 4 |
| 2 | Spermathecae with short, indistinct ducts, and spindle-shaped ampullae; sperm funnels about 1–5 times longer than wide | lineatus group |
| – | Spermathecae club-shaped, with rather long ducts and clearly set-off ampullae; sperm funnels about 1–2 times longer than wide | 3 |
| 3 | Sperm funnels about as long as wide; no glands along each spermathecal duct inside compact gland around duct at spermathecal pore | ”tuba” group |
| – | Sperm funnels about 2 times longer than wide; numerous glands along each spermathecal duct (inside compact gland around duct at spermathecal pore) | pagenstecheri group |
| 4 | Sperm funnels about as long as wide; spermathecae club-shaped, with rather long ducts and clearly set-off ampullae | buelowi group |
| – | Sperm funnels about 3–10 times longer than wide; spermathecae club-shaped, but ducts gradually widening into ampullae | arenarius group |
The lineatus group
Characteristics: Testis sacs regularly lobed in bunch-like arrangement. Spermathecae spindle-shaped with short duct which is difficult to distinguish from ampulla, and glands surrounding the ectal pore. Chaetae sigmoid and usually 3–6 or more per bundle; upper bundles located dorsolaterally. Penial bulbs round. Sperm funnel from as long as wide to about 5 times longer than wide.
Lumbricillus lineatus
Lumbricus lineatus Müller, 1774: p. 29.
Lumbricillus lineatus;
Pachydrilus claparedeanus Ditlevsen, 1904: pp. 431–435, figs 28 a–b.
Lumbricillus agilis Moore, 1905: pp. 395–397, pl. XXXIII, figs 23–28.
Lumbricillus lineatus
partim;
“Lumbricillus lineatus L2”; BOLD (unpublished records)
Non Pachydrilus lineatus; sensu Backlund 1947: pp. 3–5, figs 1–2 (see Lumbricillus latithecatus sp. n. below).
Type material (neotype)
Lumbricillus lineatus was described long before reference to types had become common practice and there is no remaining original material (“Typus amissus” in Nomenclatura Oligochaetologica). We designate
Other material examined
Description
Orange, red or pink worms. Length (fixed worms) more than 2.2–5.5 mm (amputated specimens), first 15 segments 2.1–2.8 mm long, width at clitellum 0.45–0.75 mm. More than 14–38 segments. Chaetae sigmoid (Fig.
Coelomocytes, in some specimens numerous, 10–20 µm long, round, oval or spindle-shaped, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Figs
Details of neotype
Length 3.3 mm (amputated specimen), first 15 segments 2.4 mm long, width at clitellum 0.45 mm. 20 segments. Dorsal bundles with 4–6 chaetae anterior to clitellum, 3–4 chaetae in postclitellar segments. Ventral bundles with 6–8 chaetae anterior to clitellum, 3–5 chaetae posteriorly. Longest chaetae 50 µm, about 3 µm wide. Clitellum extending over XII–XIII.
Coelomocytes 15 µm long. Dorsal vessel originating in XIII. Nephridia observed in VIII–X and XIII–XX, about 110 µm long.
Testes originating in XI, extending forwards into X. Sperm funnels folded, length and width unclear. Vasa deferentia 15 µm wide. Penial bulbs 115 µm in diameter. No mature eggs observed.
Spermathecae (Fig.
Geographical distribution including BOLD data
Remarks
Lumbricillus lineatus, possibly the first ever described enchytraeid, has an interesting history and was for a long time poorly defined as a species. It was given the name Lumbricus lineatus by
The specimens of L. lineatus in this study were smaller than the ones in the re-description by
Lumbricillus lineatus is morphologically most similar to L. verrucosus and L. latithecatus sp. n. (compare spermathecae in Fig.
Lumbricillus rutilus
Lumbricillus rutilus
Welch, 1914: 143–151, pl. VIII, fig. 13, pl. IX, figs 14–24;
“Lumbricillus rivalis”; BOLD (published records;
Type material
USNM 25507, 26318, 30863–4 (Nomenclatura Oligochaetologica). Type locality: Chicago Sewage Testing Station, United States (
Material examined
Description
Orange-reddish worms. Length (fixed worms) more than 2.7–7.2 mm (amputated specimens), first 15 segments 2.4–4.8 mm long, width at clitellum 0.39–0.65 mm. More than 14–30 segments. Chaetae (Fig.
Coelomocytes in some specimens numerous, 10–25 µm long, round, oval or spindle-shaped, granulated. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Originally described from USA, now genetically identified from Norway, Sweden and United Kingdom; also recognized from Canada and Switzerland (BIN-number: BOLD:ACV8942).
Remarks
Our newly sampled material matches
Lumbricillus rutilus is genetically most closely related to L. lineatus and L. latithecatus sp. n. (Fig.
Lumbricillus latithecatus sp. n.
“Lumbricillus lineatus L1”; BOLD (unpublished records)
Lumbricillus
sp. E;
? Pachydrilus lineatus; sensu Backlund 1947: pp. 3–5, figs 1–2.
Holotype
Type locality
Norway, Rogaland, Sola, Ölbörhamna, intertidal in decomposing algae, 58.8697N, 5.5654E, collected 15 June 2012 by C. Erséus. Norway.
Paratype
Other material examined
Etymology
Named from the Latin latus meaning wide and theca for spermatheca.
Diagnosis
This species can be distinguished from other Lumbricillus species by the shape of the spermathecae, which do not gradually widen from the ectal pore but instead originates from a very wide pore followed by an ectal duct and ampulla of even width throughout. This makes the duct and ampulla of the spermathecae virtually indistinguishable. There is at least a superficial similarity to the spermathecae of Lumbricillus lineatus and L. verrucosus with a midway constriction or bend and sperm aggregated in the ental part of the ampulla (Fig.
Description of all material
Length (fixed worms) more than 2.5–7.8 mm (amputated specimens), first 15 segments 2.5–4.7 mm long, width at clitellum 0.42–0.85 mm. More than 15–27 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 10–25 µm long, round, oval or spindle-shaped, granulated. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Figs
Details of holotype
The largest specimen of the lot. Length 7.8 mm (amputated specimen), first 15 segments 4.7 mm long, width at clitellum 0.85 mm. 27 segments. Dorsal bundles with 5–8 chaetae anterior to clitellum, 4–7 chaetae in postclitellar segments. Ventral bundles with 5–10 chaetae anterior to clitellum, 5–9 chaetae posteriorly. Longest measured chaetae 75 µm long, about 5 µm wide. Clitellum extending over XII–1/2XIII.
Coelomocytes 10–25 µm long. Dorsal vessel originating in XIII. Nephridia observed in IX and XXIII–XXV, about 125–135 µm long.
Testis sacs extending forwards into IX. Sperm funnels in XI, 1100 µm long, 210 µm wide, making them about 5 times longer than wide. Vasa deferentia 30 µm wide. Penial bulbs 285 µm in diameter. No mature eggs present.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from Norway and Sweden; also recognized from Denmark (BIN-number BOLD :AAU0294).
Remarks
The measured lengths of the sperm funnels are probably underestimated due to the difficulty of tracing them through the worms and due to folding. The two Swedish specimens were somewhat smaller than the Norwegian ones, and their funnels folded and only measurable for about 360 µm, but the length:width ratio was close to 4–6:1, as noted for the Norwegian specimens.
The description of Pachydrilus lineatus by Backlund (1947), from a drainpipe in Southern Sweden, in some ways reminds of our new species. Backlund was uncertain if his species belonged to P. lineatus because of the very wide spermathecal duct (which seemed as wide at the pore as in its medial part), the lack of a distinct ampulla and the possession of a large gland around the ectal pore. Furthermore, he described the penial bulbs as bilobed with a larger dorsal and a smaller ventral lobe. The description of the spermathecae sounds like the one of those of L. latithecatus, and after having examined the penial bulbs in the whole-mounted specimens of the latter species, it seems as if there could be a small lobe hidden behind the large spherical lobe (when viewed laterally). However, we would need transverse sections to truly compare this character to that which Backlund described. Lastly, Backlund reported small pharyngeal glands without dorsal development, which does not match with what we have observed for our species. Therefore, we are not certain as to the identity of Backlund’s P. lineatus.
Lumbricillus latithecatus is genetically most closely related to L. lineatus and L. rutilus (Fig.
Lumbricillus verrucosus
Pachydrilus verrucosus Claparède, 1861: pp. 82–85, pl. I, figs 1–6;
Lumbricillus verrucosus;
Pachydrilus lineatus forma verrucosus;
Lumbricillus lineatus
partim;
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Sound of Sleat, Isle of Skye, Hebrides, United Kingdom (Claparède, 1861). We did not designate a neotype as we do not have material from the type locality.
Material examined
Description
White to yellow worms. Length (fixed worms) more than 2.3–5.7 mm (amputated specimens), first 15 segments 2.3–3.4 mm long, width at clitellum 0.42–0.60 mm. More than 18–33 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes in some specimens numerous, 10–25 µm long, round, oval or spindle-shaped, granulated. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Figs
Geographical distribution
Originally described from the United Kingdom, now genetically identified from Norway and Sweden. The full extent of this species’ distribution is difficult to ascertain since it was previously synonymized with L. lineatus, a species distributed worldwide. BIN-number BOLD:ACV7714.
Remarks
Lumbricillus verrucosus was originally described by
Interestingly, Lumbricillus verrucosus is genetically most closely related to L. rivalis (Levinsen, 1883) and not to L. lineatus (Fig.
Lumbricillus rivalis
Pachydrilus rivalis
Levinsen, 1883: p. 231;
Lumbricillus rivalis;
Pachydrilus subterraneus Vejdovsky, 1889: pp. 1–3.
Pachydrilus germanicus Michaelsen, 1886: pp. 43–44.
Lumbricillus evansi Southern, 1909: pp. 151–152, pl. X, figs 10a–f.
Non Lumbricillus enteromorphae von Bülow, 1957: pp. 82–84, pl. XXVI, figs 6–10, pl. XXVII, fig. 1, pl. XXX, fig. 16.
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Langelinie, Denmark (Levinsen, 1883). We did not designate a neotype as we do not have material from the type locality.
Material examined
Description
Orange-red worms. Length (fixed worms) more than 3.4–7.6 mm (amputated specimens), first 15 segments 2.6–3.8 mm long, width at clitellum 0.60–0.85 mm. More than 17–44 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 15–20 µm long, spindle-shaped, oval, granulated. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution
Genetically identified from Greenland, Norway and Sweden; also recognized from Canada (BOLD:AAF9076). This species was originally described from Denmark and is considered well distributed throughout Europe, and North America.
Remarks
Pachydrilus rivalis was originally defined by Levinsen (1884) as a species with up to 9 chaetae per bundle, spermatheca formed by a large, red, pear-shaped container (Danish “beholder”), ending ectally with a glandular rosette, but bearing no glands on duct. The nephridial efferent duct originated at the posterior end of postseptale; the postseptals had red spots; the body color was red.
Our new specimens fit well with the more detailed re-descriptions, particularly in the number of chaetae and the shape of the spermathecae. The only differences concern the body size, where our worms are much smaller than those reported before, and the length/width ratio of the sperm funnels, which Nielsen and Christensen described as up to 10 times longer than wide, compared to our observed 4.5 times. These differences could be explained by our examination of fixed instead of live material.
Lumbricillus rivalis is genetically most closely related to L. verrucosus (Fig.
Lumbricillus
Lumbricillus
sp. G;
Material examined
Specimen
Description
Colour of worms unknown. Length (fixed worms) more than 3.3–4.1 mm (amputated specimens), first 15 segments 2–2.8 mm long, width at clitellum 0.42–0.49 mm. More than 17–33 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 15–30 µm long, round, oval or spindle-shaped, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution
Only known from Norway and the United Kingdom.
Remarks
Unfortunately only one mature specimen was available for this study making it difficult to assign it to a known species. On the other hand, the description of a new species on such limited material would be premature. Lumbricillus sp. G is genetically placed within the lineatus group (Fig.
Lumbricillus kaloensis
Lumbricillus kaloensis
Nielsen & Christensen, 1959: p. 100, figs 113–114;
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Kalø Vig, Denmark. We did not designate a neotype as we do not have material from the type locality.
Material examined
Description
Orange or whitish worms. Length (fixed worms) more than 2.8–3.6 mm (amputated specimens), first 15 segments 2.5–3.1 mm long, width at clitellum 0.32–0.37 mm. More than 18 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 15 µm long, round, oval, granulated. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Originally described from Denmark, now genetically identified from Norway and Sweden. BIN-number: BOLD:AAU0152.
Remarks
The specimens examined in this study match the original description by
Lumbricillus kaloensis is genetically placed within the lineatus group. Its possible sister-group relationship with Lumbricillus sp. F (suggested by the tree in Fig.
Lumbricillus
Lumbricillus
sp. F;
Material examined
Description
Colour of worms unknown. Length (fixed worm) more than 3.1 mm (amputated specimen), first 15 segments 2.2 mm long, width at clitellum 0.35 mm. More than 21 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 15 µm long, round, oval or spindle-shaped, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution. Only known from the United Kingdom and Norway (unpublished results).
Remarks. Unfortunately only one mature specimen was available for this study, making it difficult to assign it to a known species. On the other hand, the description of a new species on such limited material is not recommended. Lumbricillus sp. F is genetically placed within the lineatus group; it is possibly phylogenetically close to L. kaloensis, but this is not strongly supported (Fig.
Lumbricillus pumilio
Lumbricillus pumilio
Stephenson, 1932a: pp. 902–904, figs 1–3;
Lumbricillus pumillio
(sic);
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Wembury Bay, Plymouth, United Kingdom (
Material examined
Description
Short and stout worms. Colour unknown. Length (fixed worms) more than 1.7–3.2 mm (amputated specimens), first 15 segments 1.3–2.3 mm long, width at clitellum 0.24–0.38 mm. More than 15–23 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes difficult to identify as such in this species, but small round, oval or spindle-shaped granulated cells about 5–7 µm long were noted in the coelomic cavity. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution
Genetically identified from the United Kingdom. Also reported and briefly described from Iceland (
Remarks
Our measurements of the coelomic corpuscles (5–7 µm long) contradict the original description where they are described as being 20–28 µm. This could either be due to a high degree of variation in this trait or that we are comparing non-homologous cell types. The smaller subneural gland in XV described by Stephenson could not be distinguished, either because of its small size or because it was absent. Despite a few discrepancies from the original description the small body size of the worms together with the shape of the spermathecae and other reproductive organs supports that the sampled specimens belong to L. pumilio.
Lumbricillus pumilio is genetically closely related to L. rubidus Finogenova & Streltsov, 1978 (Fig.
Lumbricillus rubidus
Lumbricillus rubidus
Finogenova & Streltsov, 1978: pp. 17–23, fig. 1;
Lumbricillus enteromorphae; sensu
Type material
ZIAS 1/42509 (Nomenclatura Oligochaetologica). Type locality: Dal’nii Plyazh in Dal’nie Zelentsy Bay, Murmansk, Russia (
Material examined
Description
Pale to pinkish worms. Length (fixed worms) more than 2.2–3.9 mm (amputated specimens), first 15 segments 2.0–3.1 mm long, width at clitellum 0.31–0.68 mm. More than 17–23 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 10–20 µm long, round, oval or spindle-shaped. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution
Described from Russia and Germany, now genetically identified from Sweden.
Remarks
The specimens in this study match the original description of L. rubidus by
In 1959, Nielsen and Christensen classified the species L. enteromorphae von Bülow, 1957 as a hunger form of L. rivalis.
Lumbricillus rubidus is genetically closely related to L. pumilio (Fig.
Lumbricillus fennicus
Lumbricillus fennicus
Nurminen, 1964: pp. 48–51, fig. 2;
Type material
HUZM (Nomenclatura Oligochaetologica). Type locality: Tvärminne, Finland (
Material examined
Description
Colour of worms unknown. Length (fixed worms) more than 1.8–3.5 mm (amputated specimens), first 15 segments 2.0–2.3 mm long, width at clitellum 0.4–0.48 mm. More than 12–23 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 15–25 µm long, round, oval or spindle-shaped. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution
Originally described from Finland, but also reported from Denmark, France (
Remarks
The original description of L. fennicus matches the specimens of this study in most characters, but there are a few differences. Our specimens measured 2–3.5 mm in length after fixation, but considering that some had been cut directly posterior to the clitellum the length of the complete worms probably was 3–5 mm. This is smaller than the 8 mm reported by
The lobed, as opposed to cylindrical, sperm funnels are so far (in European species) only reported for L. fennicus, and this, together with the matching shape of the spermathecae, allowed confident allocation of the specimens to this species despite some incongruence among the characters mentioned above. The interpretation of the lobes of the sperm funnels probably also differs between living and fixed specimens. Our Swedish specimens were collected in freshwater habitats, but the sites are possibly subjected to brackish water at times, making the range of salinity similar to the original records from the Gulf of Finland. Most other records in Europe are from coastal oligohaline or inland freshwater habitats.
Lumbricillus fennicus is both genetically (Fig.
The pagenstecheri group
Characteristics: Testes with testis sacs regularly lobed in bunch-like arrangement. Spermathecae with distinct ampulla and glands both surrounding the ectal pore and distributed along the duct. Chaetae 3–6 per bundle, or more; upper bundles dorsolateral. Penial bulbs round. Sperm funnels about twice as long as wide.
Lumbricillus pagenstecheri
Enchytraeus pagenstecheri Ratzel, 1869: pp. 587–588, pl. XLII, figs 2, 13, 20b & 21.
Pachydrilus pagenstecheri; Vejdovsky 1877: p. 298;
Lumbricillus pagenstecheri;
Lumbricillus henkingi
Ude, 1901: pp. 9–10, pl. II, figs 15–18;
Lumbricillus ritteri
Eisen, 1904: pp. 84–86, figs 53–54, pl. XIII, figs 5–9;
Lumbricillus aegialites
Stephenson, 1922: pp. 1126–1130, figs 2–3;
Lumbricillus necrophagus Stephenson, 1922: pp. 1130–1133, figs 4–5.
Lumbricillus georgiensis Tynen, 1969: pp. 390–391, figs 1–3.
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: The original material was collected in Rhine River near Karlsruhe, and in ponds around Heidelberg, Germany (
Remarks
The molecular studies by
Lumbricillus pagenstecheri was originally described by
For this study, we chose to present the morphological measurements only for our cryptic species A, which is the only one with a sufficient sample size, and provide a comparison of some characters with the other three cryptic species in Table
Lumbricillus pagenstecheri
Material examined
Description
White to yellow worms. Length (fixed worms) more than 2.8–9.3 mm (amputated specimens), first 15 segments 2.4–4.2 mm long, width at clitellum 0.59–0.75 mm. More than 17–40 segments. Chaetae sigmoid (Fig.
Comparison of selected measured traits from the four possibly cryptic species of L. pagenstecheri, as well as their known geographical distribution. As our specimens were amputated for the extraction of DNA we could only compare the size of the first 15 segments.
| L. pagenstecheri cryptic species: distribution | Length I–XV (mm) /Width at clitellum (mm) | Chaetae | Penial bulbs diameter (µm) | Spermathecae | ||||||
| Dorsal | Ventral | Length (µm) | ||||||||
| Preclit. | Postclit. | Preclit. | Postclit. | Length (µm) | Width of ampulla (µm) | Ectal gland diameter (µm) | ||||
| A: Canada, Denmark, Sweden | 2.4–4.2 /0.6–0.8 | 2–5 | 2–5(6) | (2)4–7 | (2)3–6(8) | 70–95 | 135–185 | 140–215 | 75–110 | 80–195 |
| B: Norway | 4.3–5.3 /0.9–1.8 | 5–6(7) | 3–5 | (6)7–8 | 4–6(7) | 125–135 | 365–390 | 170–265 | 125–225 | 260–340 |
| C: Canada, Norway, Spain | 2.5–2.7 /0.3–0.7 | 3–5 | 2–4 | 4–6 | 2–7 | 65–75 | 130 | 110 | 80 | 105 |
| D: Canada, Norway | 2.5–5.0 /0.6–1.0 | 3–5 | 2–3(4) | 4–6(7) | 2–4 | 95–110 | 115–245 | 180–205 | 145–170 | 190–250 |
Coelomocytes numerous, 15–25 µm long, spindle-shaped, oval, round, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI; two first pairs connected dorsally, third pair with uncertain connection (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from Sweden; also recognized from Canada and Denmark (BIN-number: BOLD:AAF9627).
Lumbricillus viridis
Lumbricillus viridis
Stephenson, 1911: pp. 46–50, figs 6a–b & 7a–c;
Pachydrilus orthochaetus Delphy, 1921: pp. 64–82, figs 29–41.
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Firth of Clyde, Wemyss Bay, United Kingdom (Stephenson, 1911). We did not designate a neotype as we do not have material from the type locality.
Material examined
Description
Green worms (sometimes yellowish-green). Length (fixed worms) more than 7.9–10.6 mm (amputated specimens), first 15 segments 3.8–6.2 mm long, width at clitellum 0.74–1.05 mm. More than 23–41 segments. Chaetae straight or slightly sigmoid (Fig.
Coelomocytes numerous, 20–35 µm long, spindle-shaped, oval, round, granulated. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from France and Norway. Previously described from Denmark, Norway (
Remarks
Specimens in this study are smaller and possess somewhat fewer chaetae, than the ones from the original description by Stephenson and the later re-description by Nielsen and Christensen. Furthermore, the observed proportions of the sperm funnels (twice longer than wide) differ greatly from those (7–10:1, or 6–8:1) described by Stephenson and Nielsen and Christensen, respectively. However, folding of these organs may have caused us to underestimate their true length. Nevertheless, the distinct greenish colour of the sampled specimens and the resemblance between their spermathecae and particularly the one described by Nielsen and Christensen confirm these specimens as Lumbricillus viridis.
According to our knowledge, the presence of gland cells along the spermathecal ectal duct has not been reported for L. viridis before, possibly because of the difficulty of distinguishing these gland cells from the large ones surrounding the ectal pore. In this study, similar duct glands have only been observed in L. pagenstecheri sensu lato.
Lumbricillus viridis is genetically most closely related to the L. pagenstecheri species complex (Fig.
The “tuba” group
Characteristics: Testes with testis sacs regularly lobed in bunch-shaped arrangement. Spermathecae with ampulla distinctly set off from the duct and glands surrounding the ectal pore. Chaetae usually 3–6 per bundle; upper bundles dorsolateral. Penial bulbs round. Sperm funnels about as long as wide.
Note that this group, containing also L. scandicus sp. n., is not monophyletic (Fig.
Lumbricillus tuba
Lumbricillus tuba
Stephenson, 1911: pp. 42–46, figs 5a–b, pl. I, figs 6–8;
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Firth of Clyde, Millport, Island of Cumbrae, United Kingdom (
Material examined
Description
White to grey worm. Length (fixed worm) more than 4.8 mm (amputated specimen), first 15 segments 2.0 mm long, width at clitellum 0.39 mm. More than 39 segments. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 20 µm long, spindle-shaped, oval, round, granulated. Paired pharyngeal glands present in IV, V and VI (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from Norway and Sweden. Also described from Denmark and the United Kingdom. BIN-number: BOLD:ACQ1913.
Remarks
Our specimen matches the original description by
Lumbricillus tuba is genetically most closely related to the L. pagenstecheri group (including L. viridis), but it is morphologically most similar to L. scandicus sp. n. described below.
Lumbricillus scandicus sp. n.
Lumbricillus cf. helgolandicus
Lumbricillus helgolandicus
sensu
Non Pachydrilus helgolandicus Michaelsen, 1927: p. 12, fig. 11;
Holotype
Type locality
Sweden, Öland, Borgholm, Neptuni Åkrar, beach with mixed shelly sand, pebbles and organic material, 57.3346 N, 17.0102 E, collected 11 June 2006 by L. Matamoros.
Paratype
Other material examined
Etymology
Named after Scandinavia where the species has been found.
Diagnosis
This species is morphologically most similar to L. helgolandicus and L. tuba. It is distinguished from L. helgolandicus in having shorter sperm funnels, sperm arranged circularly in the spermathecae and generally possessing more chaetae per bundle. Lumbricillus scandicus can be distinguished from L. tuba in having spermathecal ectal glands that are larger than the ampulla and generally possessing more chaetae per bundle.
Description of all material
Pale, white to pinkish or orange worms. Length (fixed worms) more than 2.6–3.9 mm (amputated specimens), first 15 segments 2.0–2.9 mm long, width at clitellum 0.3–0.7 mm. More than 18–24 segments. Prostomium hemispherical, sometimes triangular. Chaetae slightly sigmoid (Fig.
Coelomocytes numerous, 15–20 µm long, round or oval. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally, with large ventral lobes (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Details of holotype
Length 3.5 mm (amputated specimen), first 15 segments 2.7 mm long, width at clitellum 0.5 mm. More than 20 segments. Dorsal bundles with 3–5, chaetae anterior to clitellum, 3–4 chaetae in postclitellar segments. Ventral bundles with 4–7 chaetae anterior to clitellum, 3–5 chaetae posteriorly. Longest chaetae about 60 µm long and about 2.5 µm wide.
Coelomocytes about 20 µm long.
Sperm funnels about 155 µm long and 160 µm. Vasa deferentia about 7 µm wide. Penial bulbs 145 µm in diameter. Four mature eggs present.
Spermathecae (Fig.
Geographical distribution
Genetically identified from Norway and Sweden. Also reported from Denmark and Russia (White Sea).
Remarks
The new species corresponds well to the description of Lumbricillus cf. helgolandicus (Michaelsen, 1927) by
These circumstances prompted re-examination of the last remaining syntype of Pachydrilus helgolandicus from Michaelsen’s collection in the Zoological Museum in Hamburg (see description of that material below). We found that the sperm funnels were more than 4 times longer than wide, compared to his reported 12 times. This difference could be explained by Michaelsen having examined live material, whereas the syntype that we studied had been fixed (contracted) in formalin or alcohol, shortening the sperm funnels. Furthermore, we might have underestimated the true length of the sperm funnels due to the difficulties with measuring folded organs in mounted material. Regardless, compared to our material of “L. cf. helgolandicus”, here described as L. scandicus sp. n., the sperm funnels of L. helgolandicus sensu stricto clearly have a higher length/width ratio.
The spermathecae of L. helgolandicus are similar to those of L. scandicus in having a distinct ampulla and a very large ectal gland. However, in L. helgolandicus, the spermatheca contains sperm that are arranged in an irregular mass, and it has a very distinct musculature covering the ectal duct (possibly made more apparent by the aging of the material), whereas the spermatheca of L. scandicus has sperm arranged in a more circular manner and only weakly defined musculature covering the ectal duct.
Lumbricillus helgolandicus is larger than L. scandicus and has generally larger internal organs. It also has fewer chaetae per bundle, no more than 5 in preclitellar, and 2–3 in postclitellar bundles, whereas L. scandicus has up to 7 chaetae in preclitellar, and up to 6 in postclitellar bundles.
Based on our assessment of the syntype from Helgoland, we conclude that our Scandinavian material is not conspecific with L. helgolandicus (Michaelsen, 1927), and instead deserves to be treated as a new species (L. scandicus). Furthermore, we conclude that L. helgolandicus sensu
Lumbricillus scandicus was genetically found as sister to the L. lineatus group (Fig.
Lumbricillus helgolandicus
Pachydrilus helgolandicus
Michaelsen, 1927: p. 12, fig. 11;
Type material
Description of lectotype
White worm. Length (fixed) 13 mm, 41 segments; first 15 segments 4.9 mm long, width at clitellum 1.1 mm. Prostomium hemispherical. Chaetae straight to slightly sigmoid (Fig.
Coelomocytes numerous, about 25 µm long, round or oval. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally, with large ventral lobes. Nephridia observed in XX, XXVI–XXVII, and possibly VIII–X, about 120–130 µm long, anteseptale consisting of funnel only, duct originating posteroventrally.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution
Originally described from Germany.
Remarks
We have mounted and re-examined the only remaining syntype of L. helgolandicus from the Zoological Museum in Hamburg and found the worm to correspond well to the descriptions of L. helgolandicus by
Based on morphology, L. helgolandicus is similar to L. scandicus and is probably closely related to this species. In the DNA-based phylogeny, L. scandicus is placed close to the lineatus group (Fig.
The buelowi group
Characteristics: Testes with testis sacs irregularly lobed and compact. Spermathecae with long duct distinctly set off from ampulla, and glands surrounding the ectal pore. Chaetae usually 2–3 per bundle; upper bundles midlateral, just above the lateral line. Penial bulbs round. Sperm funnels about as long as wide.
Lumbricillus buelowi
Lumbricillus buelowi
Nielsen & Christensen, 1959: pp. 106, figs 121–124 & 129;
Fridericia bulbosa; sensu
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality not precisely defined; the species was originally described from four different sites (Kalø, Femmøller, Ebeltoft and Avedøre) in Denmark (
Material examined
Geographical distribution including BOLD data
Genetically identified from Norway and Sweden. Also known from Denmark and Germany. BIN-number: BOLD:ACQ3084.
Description
White to slightly pink or yellow worms. Length (fixed worms) more than 2.4–5.2 mm (amputated specimens), first 15 segments 1.7–2.4 mm long, width at clitellum 0.28–0.49 mm. More than 21–32 segments. Chaetae straight or slightly sigmoid (Fig.
Coelomocytes numerous, 10–25 µm long, spindle-shaped, oval, round, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI; each pair converging dorsally (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from Norway and Sweden. Also known from Denmark and Germany. BIN-number: BOLD:ACQ3084.
Remarks
It is clear that the two species here identified as L. buelowi and L. knoellneri Nielsen & Christensen, 1959 are closely related (
There are a number of species with descriptions similar to the ones of L. buelowi, and therefore also of L. knoellneri, such as L. eltoni (Stephenson, 1924), L. muscicolus (Stephenson, 1924) and L. nielseni Nurminen, 1965. All these latter three were described from Svalbard where we found specimens of L. knoellneri but not L. buelowi. Unfortunately, the two species described by
Lumbricillus knoellneri
Lumbricillus knoellneri
Nielsen & Christensen, 1959: pp. 106–107, figs 125–126, 130;
Fridericia bulbosa; sensu
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Ebeltoft Vig, Denmark (
Material examined
Description
White to yellow worms. Length (fixed worms) more than 2.1–3.6 mm (amputated specimens), first 15 segments 1.6–1.9 mm long, width at clitellum 0.20–0.32 mm. More than 16–32 segments. Chaetae straight or slightly sigmoid (Fig.
Coelomocytes numerous, 10–25 µm long, spindle-shaped, oval, round, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from Norway (mainland and Svalbard) and Sweden. Also known from Denmark and Germany. BIN-number: BOLD:ACM5261.
Remarks
Lumbricillus knoellneri is described as having only 2 chaetae throughout the body but the newly studied material suggests that the preclitellar ventral bundles possess 2–3 chaetae. In fact, all of the eight studied specimens had 3 chaetae in at least 2 of the preclitellar ventral bundles, some in as many as 9. We found no pattern of this distribution and for each preclitellar ventral segment bearing chaetae (II–XI), we found representatives with either 2 or 3 chaetae. This shows how variable this trait is and could explain the difference to the description by Nielsen and Christensen. However, it could also mean that our “L. knoellneri” is in fact another species. Many of the internal organs of L. knoellneri were as long as or even slightly longer than the ones in L. buelowi. This in combination with a generally smaller size caused the segments of L. knoellneri to appear more contracted. For a further discussion see the Remarks for L. buelowi above.
In 1985, Kossmagk-Stephan synonymized L. cervisiae (which he himself had described as a new species two years earlier) and L. christenseni Tynen, 1966 with L. knoellneri. All three species are small, have only two chaetae per bundle (at least according to the original descriptions) and similarly shaped spermathecae. However, L. christenseni has a sperm funnel that is 7–8 times longer than wide which is significantly longer than the 1.5 times measured in L. knoellneri. The sperm funnel of L. cervisiae is 3–4 times longer than wide which is also more than that of L. knoellneri. Furthermore, the testis sacs of L. cervisiae cover several small scattered lobes and the vasa deferentia extends backwards into XIII. Finally, L. cervisiae appears to be more slender than L. knoellneri and has significantly smaller internal organs, which we were able to discern by examining the mounted original material of Kossmagk-Stephan. Therefore, we reject the idea of L. cervisiae and L. christenseni being synonyms of L. knoellneri and treat them as separate species.
The arenarius group
Characteristics: Testes with testis sacs irregularly lobed. Spermathecae with short gradually widening duct, which is difficult to distinguish from ampulla, and glands surrounding the ectal pore. Chaetae usually 2–3 or more per bundle; upper bundles midlateral, just above the lateral line. Penial bulbs round or bilobed. Sperm funnels three to ten times longer than wide.
Lumbricillus arenarius
Enchytraeus arenarius Michaelsen, 1889: pp. 12–14, figs 5a–d.
Marionina arenaria;
Enchytraeoides arenarius;
Lumbricillus arenarius;
Lumbricillus magdalenae Nurminen, 1965: pp. 6–7, figs 2e–g.
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Elbe River, Hamburg, Germany (
Material examined
Description
White to yellow worms. Length (fixed worms) more than 5.0–8.6 mm (amputated specimens), first 15 segments 3.5–4.0 mm long, width at clitellum 0.31–0.51 mm. More than 19–35 segments. Chaetae straight or slightly sigmoid (Fig.
Coelomocytes numerous, 20–50 µm long, spindle-shaped, oval, round, granulated with distinct nucleus, some with distally hooked ends. Paired pharyngeal glands present in IV, V and VI (Fig.
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from Norway (mainland and Svalbard) and Sweden. Also reported from Denmark, Canada, Germany, Greenland, Iceland, Ireland, Wales and North–Western Australia (
Remarks
The original description by
Lumbricillus arenarius is genetically closely related to L. sp. H and L. dubius (Fig.
Lumbricillus
Lumbricillus
sp. H;
Material examined
Description
White to orange worms. Length (fixed worms) more than 3.8–5.4 mm (amputated specimens), first 15 segments 2.0–2.8 mm long, width at clitellum 0.40–0.42 mm. More than 31–33 segments. Chaetae straight or slightly sigmoid (Fig.
Coelomocytes numerous, 15–20 µm long, spindle-shaped, oval, round, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI, sometimes extending into VII; each pair converging dorsally (Fig.
Male genitalia paired. Testes (Fig.
Spermathecae (Fig.
Geographical distribution
Genetically identified from Norway.
Remarks
Initial comparisons found similarities between this species and Lumbricillus westheidei Kossmagk-Stephan, 1983, such as similar shape of spermathecae and slightly bilobed penial bulbs. However, having re-examined Kossmagk-Stephan’s type material we found some important differences compared to our specimens. First, L. westheidei has only two chaetae per bundle, whereas our specimens have up to three chaetae in the preclitellar segments (the position of the upper bundles is identical in the two species). Second, the three pairs of pharyngeal glands are clearly separated in L. westheidei but in our specimens at least the first two pairs appear to have a dorsal connection. Third, the testis sacs of L. westheidei are much smaller than the ones we observed in our specimens. Fourth, the vasa deferentia appear to be much longer and form many more coils in segment XII in L. westheidei compared to our L. sp. H. Finally, the sperm funnels of L. westheidei are about 10 times longer than wide, against the 4:1 length:width ratio observed in our specimens. Unfortunately, none of our examined specimens appeared to be fully mature, as sperm were not observed either at the sperm funnels or in the spermathecae, and there were no mature eggs present. This suggests that the sperm funnels and spermathecae were not fully developed and could at maturity resemble those of L. westheidei more. Due to this uncertainty we cannot completely rule out that our specimens are of the same species as L. westheidei; however, for now we will continue to treat it as an unknown species, simply referred to as L. sp. H.
Since L. westheidei resembles, in its general morphology, our L. sp. H, it is important to add some notes on its generic allocation.
Lumbricillus sp. H is genetically closely related to L. arenarius and L. dubius (Stephenson, 1911) (Fig.
Lumbricillus dubius
Enchytraeus dubius Stephenson, 1911: pp. 54–58, figs 10–12 & pl. II, figs 12–14;
Lumbricillus dubius;
Type material
Typus amissus (Nomenclatura Oligochaetologica). Type locality: Firth of Clyde, Wemyss Bay, United Kingdom (
Material examined
Description
White to yellow worms. Length (fixed worms) more than 2.1–6.1 mm (amputated specimens), first 15 segments 1.5–2.5 mm long, width at clitellum 0.32–0.55 mm. More than 20–44 segments. Chaetae straight or slightly sigmoid (Fig.
Coelomocytes numerous, 15–30 µm long, spindle-shaped, oval, round, granulated with distinct nucleus. Paired pharyngeal glands present in IV, V and VI. Each pair converges dorsally, connection, if present at all, indistinct (Fig.
Male genitalia paired. Testes (Fig.
Spermathecae (Fig.
Geographical distribution including BOLD data
Genetically identified from Norway, Russia (White Sea), Sweden and the United Kingdom (
Remarks
The specimens examined match the description of Lumbricillus dubius by
Lumbricillus dubius has irregularly lobed testis sacs and spermathecae that are at least superficially similar to those of L. arenarius. The chaetae are straight to slightly sigmoid and few in number, which further supports the close relationship with L. arenarius and L. sp. H.
Lumbricillus dubius is genetically closely related to L. sp. H and L. arenarius (Fig.
Claparedrilus gen. n.
Genus description/diagnosis
Prostomium hemispherical. Head pore at 0/1. Epidermis with transverse rows of gland cells. Chaetae straight to sigmoid, without nodulus, grouped into two dorsolateral and two ventrolateral bundles per segment. Oesophageal appendages absent. Pharyngeal glands in four pairs, in IV–VII, converging but not connected dorsally, some with ventral lobes, but without secondary glands. Only nucleated coelomocytes present. Dorsal vessel originating intra or in segments posterior to clitellum. Nephridia with anteseptale made up of funnel on a short stalk. Clitellum more or less covering segments XII–XIII. Testes paired, surrounded by testis sacs; the latter forming compact mass with shallow lobes irregularly arranged. Penial bulbs round and compact. Midventral subneural glands present in XIV–XV. Spermathecae in V, attached to and usually communicating with oesophagus lumen, and with crown of glands surrounding ectal part of ectal duct. Spermathecae club-shaped with ampulla distinctly set off from duct. Spermathecal diverticula absent. Marine, living in the littoral zone.
Type species
Claparedrilus semifuscoides sp. n.
Other species
Claparedrilus semifuscus (Claparède, 1961) comb. nov.
Etymology
Clapare- from Claparède, the original author of the species C. semifuscus, a poorly defined species with which the type species for this new genus (C. semifuscoides) has been misidentified, and -drilus (latinized Greek) for worm.
Remarks
The need for this new genus arose from the difficulty of placing the type species C. semifuscoides (which we previously referred to as L. semifuscus) in the phylogeny of the Enchytraeidae. Molecular data had previously supported that this species was not a member of Lumbricillus and instead closer to, but not a member of, Globulidrilus and Bryodrilus (
A comparison of characters distinguishing Claparedrilus gen. n. from other marine enchytraeid taxa. Traits of particular importance highlighted in boldface. *Coded according to
| Genus | Chaetal shape Upper bundles |
Brain posterior | Pharyngeal glands | Coelomocytes | Gut appendages | Nephridial anteseptal | Blood vessel End/Origin |
Testes | Subneural glands | Penial bulb | Spermathecal ampulla |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Claparedrilus | Slightly sigmoid dorsolateral |
Indented | Dorsally free; With ventral lobes; 4 pairs |
Nucleated | No. | Funnel on a short thin stalk | Peristomial/ XIII | Compact With seminal vesicles |
Yes |
Compact | No diverticula |
|
Christensenidrilus blocki (Dózsa-Farkas & Convey, 1997) |
Sigmoid dorsolateral |
Slightly indented | Dorsally free; With secondary lobes; 3 pairs |
Anucleate | No | Funnel and some coils | ?/ XIII | Compact With seminal vesicles |
No |
Compact | No diverticula |
|
Lumbricillus Ørsted, 1844 |
Straight to sigmoid Dorso- or Midlateral |
Indented | Dorsally free or fused; With ventral lobes; 3 pairs |
Nucleated | No | Funnel only | Peristomial/ XIII-XV |
Reg. or irreg. lobed With testis sacs |
Yes or No | Compact, rarely bilobed | No diverticula |
|
Marionina georgiana (Michaelsen, 1888) |
Sigmoid dorsolateral |
Indented | Dorsally free; No ventral lobes; 3 pairs |
Nucleated | No | Funnel only | Peristomial/ XIII | Compact With seminal vesicles |
No* | Small* | No diverticula |
|
Randidrilus Coates & Erséus, 1985 |
Slightly curved Absent |
Deeply indented | Dorsally fused With ventral lobes; 3–4 pairs** |
Nucleated | No | Funnel only | Peristomial/ XX-XXIII | Compact With sperm sacs |
No | Bilobed | No diverticula |
| Stephensoniella Černosvitov, 1934 | Str. or slightly sigmoid Midlateral |
Slightly indented | Dorsally fused With ventral lobes; 3 pairs |
Nucleated | No | Funnel only | Peristomial/ XII-XXIII | Compact With seminal vesicles |
No | Compact | Diverticulate |
Claparedrilus semifuscoides sp. n.
Marionina semifusca; sensu
Lumbricillus semifuscus; sensu
? Marionina semifusca; sensu
? Lumbricillus semifuscus;
Non Pachydrilus semifuscus Claparède, 1861: pp. 76–79, pl. II, figs 1–5.
Non Marionia semifusca;
Non Marionina semifusca;
Non Enchytraeoides semifuscus;
Holotype
Type locality
United Kingdom, Wales, Anglesey, Beaumaris, intertidal zone of beach with sand and algae, 53.2623 N, 4.0914 W, collected 15 Febuary 2007 by M. Strand and P. Sundberg.
Paratypes
Other material examined
Etymology
Named after its similarity to Claparedrilus semifuscus, which it has previously been confused with and misidentified as.
Diagnosis
This species can be distinguished from C. semifuscus by the size of the penial bulbs. In C. semifuscus, the bulbs are much larger than the sperm funnels, whereas in C. semifuscoides they are of about the same size as the funnels or smaller.
Description
White, grey to pinkish worms. Length (fixed worms) more than 4.0–7.3 mm (amputated specimens), first 15 segments 2.1–3.4 mm long, width at clitellum 0.54–0.69 mm. More than 22–45 segments. Chaetae sigmoid or straight (Fig.
Coelomocytes numerous, 10–20 µm long, spindle-shaped, oval, round, granulated with distinct nucleus. Paired pharyngeal glands 4 pairs, in IV, V, VI and VII, respectively; each pair converging but not connected dorsally (Figs
Male genitalia paired (Fig.
Spermathecae (Fig.
Geographical distribution
Genetically identified from Norway and the United Kingdom. Also known (by morphology) from Iceland (
Remarks
In 1861, Claparède described Pachydrilus semifuscus from the Hebrides in Scotland. Due to its unusual and confusing morphology, this species has been moved around among some enchytraeid genera. It was transferred to Marionina (
Claparedrilus semifuscoides can be separated from C. semifuscus by the size of the penial bulbs, where the former species have bulbs about the same size as the sperm funnels (about 150 µm in diameter) whereas the latter have bulbs larger than the funnels; they are 400–500 µm long. The nephridium illustrated by Claparède is reminiscent of what we observed (Fig.
Our specimens of C. semifuscoides are smaller than the ones described by Stephenson as M. semifusca, and they possess fewer chaetae, but we still believe that they belong to the same species. Stephenson remarks that (1) the nephridia can be found from V, (2) the anteseptale is made up of funnel only, and (3) the efferent duct extends backwards towards the pore, not forwards as illustrated by Claparède for C. semifuscus. We found nephridia from VI (possibly not finding any in V because they were obscured by the pharyngeal glands) and observed that the anteseptale consists of a funnel on a thin stalk. As this character was difficult to see and because there is no true nephridial tissue anterior to the septa this could still have been interpreted by Stephenson as a funnel only. We found that the efferent duct extended forward towards the pore which is more in agreement with Claparède’s illustration of C. semifuscus than what Stephenson noted, but the interpretation of this character may differ as the animal extends or contracts. Finally, Stephenson stated that the efferent duct originates well in front of the middle of the postseptale, whereas we observed it originating behind the middle or even from the posterior end. However, Stephenson also noted that this was not apparent in living specimens and only became clear from sections, which we have not studied.
Our specimens of C. semifuscoides also largely agree with Southern’s account of M. semifusca except for his description of the cylindrical penial bulbs. It is possible that the bulbs he studied were everted (as illustrated for L. pagenstecheri A in the present study; Fig.
The species reported as L. semifuscus from Iceland (
Compared to the species of Lumbricillus, i.e., the genus in which we previously placed this species (and erroneously referred to it as L. semifuscus), C. semifuscoides can be distinguished mainly by its four pairs of pharyngeal glands, the stalked nephridial funnel, and the irregularly lobed testes.
Discussion
General comments on Lumbricillus taxonomy
Diagnosing and delimiting Lumbricillus are problematic, as one of its most striking features, the bunch-like arrangement of the lobed testis sacs, is not shared by all species in the genus. This character appears to be a synapomorphy of the lineatus, pagenstecheri and “tuba” groups, which together make up a monophyletic clade, but excludes the buelowi and arenarius groups, which have unlobed or irregularly lobed testis sacs (Fig.
Comments on Enchytraeoides
Before we came to the conclusion that the arenarius group should remain within Lumbricillus, we explored the possibility that it could be treated as a separate genus. Searching the taxonomic literature for possible candidate genera we found Enchytraeoides, in which L. arenarius had previously been placed by some authors (
It is difficult to establish whether Pachydrilus enchytraeoides Saint-Loup, 1885 is the same species as Enchytraeoides marioni Roule, 1888, but through personal communications with Roule,
Roule described the penial bulbs as situated in segment XI, a trait neither seen in any Lumbricillus nor in any other typical enchytraeid in which the bulbs are in XII. Furthermore, there were some discrepancies in Roule’s description as to the location of the spermathecae, which were described as located in segment VI, but were illustrated as in segment V. This could be explained by Roule counting the prostomium as a separate segment, but this still does not explain the placement of the penial bulbs which are also illustrated as part of segment XI. If the material was studied live without proper magnification, and the description and illustration were produced later, this might have caused a misinterpretation of the position of the penial bulbs. However, given the extensive descriptions and illustrations based on such large amounts of material this seems unlikely. Therefore, the true phylogenetic placement of Enchytraeoides remains uncertain until newly sampled species have been examined and sequenced and for now it should remain as a junior synonym of Lumbricillus.
Geographical distribution and habitat
It is difficult to make any conclusions of the full geographical distribution of the species in this study, as most of our samples are from Norway and Sweden, with some also from the United Kingdom and other parts of Northern Europe. When taking into account the BOLD data and the reports by other authors it seems that some species are very common, such as L. lineatus, L. rivalis and L. pagenstecheri sensu lato (e.g.
As evident from Appendix I and the taxonomic literature on Lumbricillus, this genus is mostly associated with seashores and brackish waters, but many species (such as L. arenarius, L. fennicus, L. knoellneri, L. rutilus and L. scandicus) are commonly encountered also in freshwater habitats.
Future research
Having studied only about one fourth of the 80 or so described species of Lumbricillus with a primarily molecular approach, it is clear that a lot of taxonomic and genetic work remains to be done on this genus. Ideally, we would like to be able to link each of the described species to one or more molecular barcodes of COI, and to clearly delimit the species from each other using also nuclear genetic data (as in
Conclusions
Having studied the morphology of the Lumbricillus species included in the molecular study by
Acknowledgements
We would like to thank Robert Almstrand, Anna Ansebo, Nicholas Bekkouche, Mike Dempsey, Torbjørn Ekrem, Diego Fontaneto, Ton van Haaren, Karstein Hårsaker, Magnus Johansson, Sebastian Kvist, Svante Martinsson, Lisa Matamoros, Erica Mejlon (=E. Sjölin), Urban Olsson, Belén Reboreda Rivera, Malin Strand, Per Sundberg, David Templeman, Hong-Zhou Wang, Pierre De Wit and Endre Willassen, for assistance in the field and/or collecting or otherwise providing material; to Anna Ansebo, Daniel Gustafsson, Per Hjelmstedt, Magnus Johansson, Emelie Lindqvist, Maria Lindström, Svante Martinsson, Lisa Matamoros, Urban Olsson and Marcus Svensson for lab assistance; and to the Norwegian Barcode of Life (NorBOL), and to The Canadian Center of DNA Barcoding, for COI-barcoding some of our specimens. Financial support was given by The Swedish Research Council (VR), Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), Swedish Taxonomy Initiative (ArtDatabanken, Uppsala), Norwegian Taxonomy Initiative (Artsdatabanken, Trondheim), Royal Society of Arts and Sciences in Gothenburg and Adlerbert Foundation (also Gothenburg).
References
- Backlund HO (1948) Lumbricillus reynoldsoni n. sp., an enchytraeid from the beaches of North Wales. Journal of the marine biological Association of the United Kingdom 27: 710–717. https://doi.org/10.1017/S0025315400056083
- Benham WB (1905) On the Oligochaeta from the Southern Islands of the New Zealand Region. Transactions and Proceedings of the New Zealand Institute 37: 285–297.
- BOLD – Barcoding of Life Database. http://boldsystems.org/ (accessed 24 October 2016)
- Brinkhurst RO, Jamieson BGM (1971) Aquatic Oligochaeta of the World, Edinburgh: Oliver & Boyd.
- von Bülow T (1955) Oligochaeten aus den Endgebieten der Schlei. Kieler Meeresforschungen 11: 253–264.
- von Bülow T (1957) Systematisch-autökologische Studien an eulitoralen Oligochaeten der Kimbrischen Halbinsel. Kieler Meeresforschungen 13: 69–116.
- Černosvitov L (1929) Communication préliminaire sur les Oligochètes récoltés par MP Remy pendant la croisière arctique effectuée par le” Pourquoi-Pas?” en 1926 sous la direction du Dr. J.-B. Charcot. Bulletin du Muséum National d’Histoire Naturelle. Paris, 2(1): 144–149.
- Černosvitov L (1934) Zur Kenntnis der Enchytraeiden. I. Zoologischer Anzeiger 105: 233–247.
- Černosvitov L (1937) System der Enchytraeiden. Bulletin de l’Association Russe pour les Recherches Scientifiques à Prague 5: 263–295.
- Christensen B (1960) A comparative cytological investigation of the reproductive cycle of an amphimictic diploid and a parthenogenetic triploid form of Lumbricillus lineatus (O.F.M.) (Oligochaeta, Enchytraeidae). Chromosoma 11(1): 365–379. https://doi.org/10.1007/BF00328661
- Christensen B (1961) Studies on cyto-taxonomy and reproduction in the Enchytraeidae. Hereditas 47(3‐4): 387–450. https://doi.org/10.1111/j.1601-5223.1961.tb01782.x
- Christensen B, Dózsa-Farkas K (2012) A new genus Globulidrilus and three new enchytraeid species (Oligochaeta: Enchytraeidae) from Seoraksan National Park (Korea). Journal of Natural History 46(45–46): 2769–2785. https://doi.org/10.1080/00222933.2012.737038
- Christensen B, Jelnes J, Berg U (1978) Long-term isozyme variation in parthenogenetic polyploid forms of Lumbricillus lineatus (Enchytraeidae, Oligochaeta) in recently established environments. Hereditas 88(1): 65–73. https://doi.org/10.1111/j.1601-5223.1978.tb01604.x
- Christensen B, O’Connor FB (1958) Pseudofertlization in the genus Lumbricillus (Enchytraeidae). Nature 181: 1085–1086.
- Claparède E (1861) Études anatomiques sur les Annélides, Turbellariés, Opalines et Grégarines observés dans les Hébrides. Mémoires de la Société de Physique et d’Histoire Naturelle de Genève 16: 71–164.
- Coates KA (1989) Phylogeny and origins of Enchytraeidae. In Aquatic Oligochaete Biology, Springer, Netherlands, 17–33. https://doi.org/10.1007/BF00027534
- Coates K, Ellis DV (1981) Taxonomy and distribution of marine Enchytraeidae (Oligochaeta) in British Columbia. Canadian Journal of Zoology 59(11): 2129–2150. https://doi.org/10.1139/z81-290
- Coates KA, Erséus C (1985) Marine enchytraeids (Oligochaeta) of the coastal northwest Atlantic (northern and mid USA). Zoologica Scripta 14(2): 103–116. https://doi.org/10.1111/j.1463-6409.1985.tb00181.x
- Delphy J (1920) Recherches sur les lombriciens (Oligochètes) limicoles. V—Quelques questions de nomenclature. Bulletin de la Société Zoologique de France 45: 242–245.
- Delphy J (1921) Études sur l’organisation et le développement des Lombriciens limicoles thalassophiles. Travaux du Laboratoire de Zoologie Comparative de l’École Pratique des Hautes-Études. Pillu-Roland, Valognes: 1–137.
- Ditlevsen A (1904) Studien an Oligochäten. Zeitschrift für wissenschaftliche Zoologie 77: 398–480.
- Dózsa-Farkas K, Convey P (1997) Christensenia, a new terrestrial enchytraeid genus from Antarctica. Polar Biology, 17(6): 482–486. https://doi.org/10.1007/s003000050146
- Eisen G (1904) Enchytraeidae of the west coast of North America. In Harriman Alaska Expedition series. Vol. XII. Annelids. Smithsonian Institution, New York, 1–166.
- Elmhirst R, Stephenson J (1926) On Lumbricillus scoticus n. sp. Journal of the marine biological Association of the United Kingdom 14: 469–473. https://doi.org/10.1017/S0025315400007943
- Erséus C (1976) Littoral Oligochaeta (Annelida) from Eyjafjörôur, North Coast of Iceland. Zoologica Scripta 5: 5–11. https://doi.org/10.1111/j.1463-6409.1976.tb00677.x
- Erséus C (1977) Marine Oligochaeta from the Koster Area, west coast of Sweden, with descriptions of two new enchytraeid species. Zoologica Scripta 6: 293–298. https://doi.org/10.1111/j.1463-6409.1978.tb00781.x
- Erséus C (1994) The Oligochaeta. In: Blake J.A. & Hilbig B. (Eds.), Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel, Volume 4, Oligochaeta and Polychaeta: Phyllodocida (Phyllodocidae to Paralacydoniidae). Santa Barbara Museum of Natural History, Santa Barbara California, 5–38.
- Erséus C, Grimm R, Healy B, Lundberg S, Rota E, Timm T (1999) Clitellate diversity in Nationalstadsparken, an urban national park in Stockholm, Sweden. Hydrobiologia 406: 101–110. https://doi.org/10.1007/978-94-011-4207-6_10
- Erséus C, Rota E, Matamoros L, De Wit P (2010) Molecular phylogeny of Enchytraeidae (Annelida, Clitellata). Molecular Phylogenetics and Evolution 57(2): 849–858. https://doi.org/10.1016/j.ympev.2010.07.005
- Finogenova NP (1977) The oligochaetes of Tyup River and Tyup Bay on Lake Issyk-kul’ Hydrobiological studies of the Tyup River and of Tyup Bay on Lake Issyk-Kul’. Collection of scientific papers; USSR Academy of Sciences, Zoological Institute 115–123.
- Finogenova NP, Streltsov VE (1978) Two new species of oligochaetes of the genus Lumbricillus from the east Murman littoral. Biologiya Morya 1: 17–23.
- Finogenova NP, Timm T (1988) Enchytraeids (Oligochaeta, Enchytraeidae) of the littoral of the White Sea. Benthic ecosystems of the south-eastern part of the Kandalaksha Bay and adjacent waters of the White Sea. Explorations of the fauna of the seas 38(46): 91–108.
- Giere O (1976) Zur Kenntnis der litoralen Oligochaetenfauna Südfinnlands. Annales Zoologici Fennici 13: 156–160.
- GIMP (2016) 2.8.10. GNU Image Manipulation Program. http://www.gimp.org/ (accessed 24 October 2016)
- Graefe U, Schmelz RM (1999) Indicator values, strategy types and life forms of terrestrial Enchytraeidae and other microannelids. Newsletter on Enchytraeidae 6: 59–67.
- Healy B (2007) Ecological studies of Enchytraeidae in Irish brackish habitats. FOLIA Facultatis scientiarum naturalium Universitatis Masarykianae Brunensis. Biologia, 110.
- Henle FGJ (1837) Ueber Enchytraeus, eine neue Anneliden-Gattung. Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin, Berlin 1837: 74–90.
- Klinth MJ, Martinsson S, Erséus C (2017) Phylogeny and species delimitation of North European Lumbricillus (Clitellata, Enchytraeidae). Zoologica Scripta 46: 96–110. https://doi.org/10.1111/zsc.12187
- Knöllner FH (1935) Ökologische und systematische Untersuchungen über litorale und marine Oligochäten der Kieler Bucht. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tier 66: 425–563.
- Kossmagk-Stephan KJ (1983) Marine Oligochaeta from a sandy beach of the island of Sylt (North Sea) with description of four new enchytraeid species. Mikrofauna des Meeresbodens 89: 1–28.
- Kossmagk-Stephan KJ (1985) Systematik, Faunistik und Lebenszyklus mariner Oligochaeta der Nord- und Ostseeküste. Dr.rer.nat. Dissertation, University Göttingen: 306 pp.
- Lafont M, Vivier A (2006) Oligochaete assemblages in the hyporheic zone and coarse surface sediments: their importance for understanding of ecological functioning of watercourses. Hydrobiologia 564: 171–181. https://doi.org/10.1007/s10750-005-1717-9
- Lasserre P (1971) The marine Enchytraeidae (Annelida, Oligochaeta) of the eastern coast of North America with notes on their geographical distribution and habitat. The Biological Bulletin 140(3): 440–460. https://doi.org/10.2307/1540280
- Levinsen GMR (1883) Systematisk-geografisk Oversigt over de nordiske Annulata, Gephyrea, Chaetognathi og Balanoglossi. II. Videnskabelige Meddelelser fra den Naturhistoriske Forening i Kjøbenhavn for Aaret 1883: 92–350.
- Mackie ASY, Erséus C (1997) Annelida. In: Howson, C.M. & Picton, B.E. (Eds.), The species directory of the marine fauna and flora of the British Isles and surrounding seas (No. 276). Ulster Museum, 102–136.
- Marcus E (1965) Naidomorpha aus brasilianischem Brackwasser. Beiträge zur neotropischen Fauna 4: 61–83. https://doi.org/10.1080/01650526509360380
- Martínez-Ansemil E (1982) Les Oligochètes aquatiques de la peninsule Iberique (2e note) avec la description de Lumbricillus brunoi n. sp. (Enchytraeidae). Bulletin de la Société d’Histoire Naturelle,Toulouse 118: 145–151.
- Martinsson S, Dózsa-Farkas K, Rota E, Erséus C (2017) Placing the forgotten: on the positions of Euenchytraeus and Chamaedrilus in an updated enchytraeid phylogeny (Clitellata: Enchytraeidae). Invertebrate Systematics 31(1): 85–90. https://doi.org/10.1071/IS16042
- Michaelsen W (1886) Untersuchungen über Enchytraeus Möbii Mich. und andere Enchytraeiden. Inaugural-Dissertation. Kiel. Verlag von Lipsius & Tischer.
- Michaelsen W (1888) Die Oligochaeten von Süd-Georgien nach der Ausbeute der Deutschen Station von 1882–1883. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten 5: 53–73.
- Michaelsen W (1889) Oligochaeten des Naturhistorischen Museums in Hamburg. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten 6: 1–17.
- Michaelsen W (1900) Das Tierreich Vol, 10, Oligochaeta. Friedländer & Sohn, Berlin, pp. XXIX: 1–575. https://doi.org/10.5962/bhl. title.1108
- Michaelsen W (1905) Die Oligochaeten der Deutschen Südpolar‐Expedition 1901-1903 nebst Erörterung der Hypothese über einen früheren groβen die Südspitzen der Kontinente verbindenden antarktischen Kontinent. Deutsche Südpolar-Expedition IX, Zoologie 1: 1–58.
- Michaelsen W (1911) Littorale Oligochäten von der Nordküste Russlands. Travaux de la Société Impériale des Naturalistes de St. Pétersbourg 42: 106–110.
- Michaelsen W (1914) Oligochaeta. In: Beiträge zur Kenntnis der Land- und Süβwasserfauna Deutsch- Südwestafrikas. Ergebnisse der Hamburger deutsch-südwestafrikanischen Studienreise 1911: 137–182.
- Michaelsen W (1925) Zur Kenntnis einheimischer und ausländischer Oligochäten. Zoologische Jahrbucher. Abteilung für Systematik, Geographie und Biologie der Tiere 51: 255–328.
- Michaelsen W (1927) Oligochaeta. In: Grimpe G, Wagler E (Eds.) , Die Tierwelt der Nord- und Ostsee VIc. Leipzig, 1–44.
- Michaelsen W (1929) Oligochaeten der Kamtschatka-Expedition. Yearbook of the AS USSR Zoological Museum 30(2): 315–329.
- Michaelsen W (1934) Ein neuer Strand-Enchyträide von Helgoland. Zoologischer Anzeiger 108: 135–141.
- Michaelsen W (1935) Meeresstrand-Enchyträiden des südlichen Atlantischen Ozeans. Scientific Results of the Norwegian Antarctic Expeditions 1927-1928 et sqq. , instituted and financed by consul Lars Christensen 14: 2–7.
- Moore JP (1905) Some marine Oligochaeta of New England. Proceedings of the Academy of Natural Sciences of Philadelphia 57(2): 373–399.
- Müller OF (1771) Von Würmern des süssen und salzigen Wassers, mit Kupfern. Verlegts Heineck und Faber: 1–200.
- Müller OF (1774) Vermium Terrestrium et Fluviatilum, seu Animalium Infusoriorum, Helminthicorum, et Testaceorum, non Marinorum, Succincta Historia. Volume 1, Part 2, 72 pp., Heineck et Faber, Havniae et Lipsiae.
- Nielsen CO, Christensen B (1959) The Enchytraeidae: Critical revision and taxonomy of European species. Natura Jutlandica 8–9: 1–160.
- Nomenclatura Oligochaetologica (2016) http://wwx.inhs.illinois.edu/people/mjwetzel/nomenoligo/ [accessed 24 October 2016]
- Nurminen M (1964) Lumbricillus fennicus sp.n. and some other Enchytraeids (Oligochaeta) from Finland. Annales Zoologici Fennici 1: 48–51.
- Nurminen M (1965a) Enchytraeid and lumbricid records (Oligochaeta) from Spitsbergen. Annales Zoologici Fennici 2(1): 1–10.
- Nurminen M (1965b) Enchytraeids (Oligochaeta) from northern Norway and western Lapland. Annales Zoologici Fennici 2(1): 11–15.
- Nurminen M (1970) Records of Enchytraeidae (Oligochaeta) from the west coast of Greenland. Annales Zoologici Fennici 7: 199–209.
- Ratzel F (1869) Beiträge zur anatomischen und systematischen Kenntniss der Oligochaeten. Zeitschrift für wissenschaftliche Zoologie 18(4): 563–591.
- Rodriguez P, Rico E (2008) A new freshwater oligochaete species (Clitellata: Enchytraeidae) from Livingston Island, Antarctica. Polar Biology, 31(10): 1267–1279. https://doi.org/10.1007/s00300-008-0465-5
- Rosa D (1887) II Neoenchytraeus bulbosus n. sp. Bollettino dei Musei di Zoologia e Anatomia Comparata della R. Universita di Torino 2: 1–3.
- Rota E (2001) Oversized enchytraeids (Annelida, Clitellata): a comparative study, with a revised description of Lumbricillus maximus (Michaelsen). Organisms Diversity & Evolution 1(3): 225–238. https://doi.org/10.1078/1439-6092-00019
- Rota E, Healy B (1999) A taxonomic study of some Swedish Enchytraeidae (Oligochaeta), with descriptions of four new species and notes on the genus Fridericia. Journal of Natural History 33(1): 29–64. https://doi.org/10.1080/002229399300461
- Rota E, Matamoros L, Erséus C (2008) In search of Marionina (Clitellata, Enchytraeidae): A taxonomic history of the genus and re‐description of the type species Pachydrilus georgianus Michaelsen, 1888. Italian Journal of Zoology 75(4): 417–436. https://doi.org/10.1080/11250000801930433
- Roule L (1888) Sur la structure histologique d’un oligochaete marin appartenant à un genre nouveau. Comptes rendus hebdomadaires des Séances de l’Académie des Sciences 116: 308–310.
- Roule L (1889) Etudes sur le développement des Annélides et en particulier d’un oligochaete limicole marin (Enchytraeoides Marioni nov. sp.). Annales des Sciences Naturelles 7: 107–442.
- Saint-Loup R (1885) Sur l’organisation du Pachydrilus enchytraeoides. Comptes rendus hebdomadaires des Séances de l’Académie des Sciences 101: 482–485.
- Schmelz RM, Collado R (2012) An updated checklist of currently accepted species of Enchytraeidae (Oligochaeta, Annelida). Newsletter on Enchytraeidae 12: 67–87.
- Shurova NM (1974) Enchytraeidae of the genus Lumbricillus (Oligochaeta) from the intertidal zone of the Kurile Islands. In: The plant and animal world of the littoral of the Kurile Islands. Far-Eastern Scientific Centre, Novosibirsk, 128–136.
- Shurova NM (1977) New littoral species of the genus Lumbricillus (Oligochaeta). Biologiya Morya 1: 57–62.
- Shurova NM (1978) The intertidal oligochaetes from the eastern coast of Kamchatka. In: Kusakin O.G. (Ed.), Litoral Beringova morya i yugo-vostochnoj Kamchatki (Littoral of the Bering Sea and the southeastern Kamchatka). Nauka, Moscow, 98–105.
- Shurova NM (1979) Enchytraeids (Oligochaeta) of the far-east seas of the USSR. In: Studies on the pelagic and ground inhabiting organisms of the Far East seas. Vladivostok, 75–89.
- Southern R (1907) Irish Naturalist 16: 68–82.
- Southern R (1909) Contributions towards a monograph of the British and Irish Oligochaeta. Proceedings of the Royal Irish Academy 27: Section B, 119–182.
- Southern R (1913) Clare Island survey part 48: Oligochaeta. Proceedings of the Royal Irish Academy 31: 1–48. https://doi.org/10.5962/bhl.title.64352
- Stephenson J (1911) On some littoral Oligochaeta of the Clyde. Transactions of the Royal Society of Edinburgh 48: 31–65. https://doi.org/10.1017/s0080456800018755
- Stephenson J (1922) The Oligochaeta of the Oxford University Spitsbergen Expedition. Proceedings of the Zoological Society of London 92(4): 1109–1138. https://doi.org/10.1111/j.1469-7998.1922.tb07096.x
- Stephenson J (1924) On some Oligochaete worms from Spitsbergen. Results of the Merton College expedition to Spitsbergen. Annals and Magazine of Natural History. London 13: 210–216.
- Stephenson J (1925) The Oligochæta of Spitsbergen and Bear Island; some additions and a summary. Proceedings of the Zoological Society of London 95(4): 1293–1322. https://doi.org/10.1111/j.1469-7998.1925.tb07438.x
- Stephenson J (1930) The Oligochaeta. Oxford: Clarendon Press.
- Stephenson J (1932a) Oligochaeta from Australia, North Carolina, and other parts of the world. Proceedings of the Zoological Society of London 1932: 899–941. https://doi.org/10.1111/j.1096-3642.1932.tb01571.x
- Stephenson J (1932b) Oligochaeta. Part. I. Microdrili. Discovery Reports 4: 233–264.
- Timm T (2005) On the distribution and taxonomic limits of Lumbricillus pagenstecheri (Oligochaeta, Enchytraeidae). Proceedings of the Estonian Academy of Sciences: Biology, Ecology 54(4): 292–301.
- Tynen MJ (1966) A new species of Lumbricillus, with a revised check-list of the British Enchytraeidae [Oligochaeta]. Journal of the Marine Biological Association of the United Kingdom, 46(1): 89–96. https://doi.org/10.1017/S0025315400017562
- Tynen MJ (1969) New Enchytraeidae (Oligochaeta) from the east coast of Vancouver Island. Canadian Journal of Zoology 47(3): 387–393. https://doi.org/10.1139/z69-072
- Tynen MJ (1970) Marionina charae, a new species of enchytraeid (Oligochaeta) from the Canadian arctic. Canadian Journal of Zoology 48: 1359–1361. https://doi.org/10.1139/z70-232
- Tynen MJ, Coates KA, Smith CAS, Tomlin AD (1991) Henlea yukonensis (Oligochaeta: Enchytraeidae), a new species from the Yukon Territory, with comments on Henlea Michaelsen, 1889 and Punahenlea Nurminen, 1980. Canadian Journal of Zoology 69: 1375–1388.
- Tynen MJ, Nurminen M (1969) A key to the European littoral Enchytraeidae (Oligochaeta). Annales Zoologici Fennici 6: 150–155.
- Ude H (1892) Ein neues Enchytraeiden–Genus. Zoologischer Anzeiger 401: 344–345.
- Ude H (1896) Enchytraeiden. Hamburger Magalhaensische Sammelreise III (5). Hamburg: L. Friedrichsen & Co., 1–43.
- Ude H (1901) Jena 2: 1–34.
- Ude H (1929) Oligochaeta. Die Tierwelt Deutschlands 15: 1–132.
- Vejdovský F (1877) Zur Anatomie und Systematik der Enchytraeiden. Sitzungsberichte der Königlichen Böhmischen Gesellschaft der Wissenschaften in Prag: 294–304.
- Vejdovský F (1879) Beiträge zur vergleichenden Morphologie der Anneliden. I. Monographie der Enchytraeiden. Friedrich Tempsky, Prag: 62 pp.
- Vejdovský F (1884) System und Morphologie der Oligochaeten. Prag: Verlag Franz Rivnac: 172 pp.
- Vejdovský F (1889) Note sur Pachydrilus subterraneus n. sp. Revue biologique du nord de la France 1(4): 1–3.
- Vivien R, Wyler S, Lafont M, Pawlowski J (2015) Molecular barcoding of aquatic oligochaetes: implications for biomonitoring. PloS one 10(4): e0125485. https://doi.org/10.1371/journal.pone.0125485
- Wang H, Liang Y (1997) Two new species of Oligochaeta (Annelida) from King George Island, Antarctica. Oceanologia et Limnologia Sinica, 28: 177–184.
- Welch PS (1914) Studies on the Enchytraeidae of North America. Bulletin of the Illinois State Laboratory of Natural History, Urbana 10: 123–211.
- Welch PS (1917) The Enchytraeidae (Oligochaeta) of the Woods Hole Region, Mass. Transactions of the American Microscopical Society 36(3): 119–138. https://doi.org/10.2307/3221697
- Yamaguchi H (1937) The fauna of Akkeshi Bay III. Oligochaeta. Journal of the Faculty of Sciences, Hokkaido Imperial University, Series VI, Zoology 5: 137–142.
- Ørsted AS (1842) Conspectus generum specierumque Naidum ad faunam Danicam pertinentium. Naturhistorisk Tidsskrift 4: 128–140.
- Ørsted AS (1844) De regionibus marinis. København: J.C. Scharling: 1–89.
Appendix 1
List of specimens used in this study, with specimen identification number, collection data, GPS coordinates (in decimal degrees), GenBank accession numbers for the COI barcode, and voucher numbers. Letters for Lumbricillus pagenstecheri refer to barcoding clusters. Country codes: ES=Spain, GL=Greenland, NO=Norway, SE=Sweden and UK=United Kingdom.
| Species | ID | Collection locality | Coordinates | Date | Leg | Habitat | Barcode Acc. No. | Voucher No. |
|---|---|---|---|---|---|---|---|---|
| Lat. / Long. | ||||||||
| Claparedrilus semifuscoides sp n. | CE2247 | UK, Wales, Anglesey, Beaumaris | 53.2623, -4.0914 | 15-Feb-2007 | M. Strand & P. Sundberg | Intertidal, sand, algae | KU893993 |
|
| Claparedrilus semifuscoides sp n. | CE2248 | UK, Wales, Anglesey, Beaumaris | 53.2623, -4.0914 | 15-Feb-2007 | M. Strand & P. Sundberg | Intertidal, sand, algae | KU893994 |
|
| Claparedrilus semifuscoides sp n. | CE2249 | UK, Wales, Anglesey, Beaumaris | 53.2623, -4.0914 | 15-Feb-2007 | M. Strand & P. Sundberg | Intertidal, sand, algae | KU893995 |
|
| Claparedrilus semifuscoides sp n. | CE2252 | UK, Wales, Anglesey, Beaumaris | 53.2623, -4.0914 | 15-Feb-2007 | M. Strand & P. Sundberg | Intertidal, sand, algae | KU893996 |
|
| Claparedrilus semifuscoides sp n. | CE23750 | NO, Nordland, Bognäs, Tysfjorden | 68.2248, 16.0771 | 17-Aug-2014 | C. Erséus | Upper intertidal, seaweeds, stones | KU894097 |
|
| Claparedrilus semifuscoides sp n. | CE24657 | NO, Nordland, Lofoten, Grimsöystraumen Strait | 68.2607, 14.2679 | 10-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, coarse shelly sand | KU893989 |
|
| Lumbricillus arenarius | CE1001 | SE, Bohuslän, Lysekil, Fiskebäckskil, Kristineberg | 58.248, 11.4458 | 16 May 2005 | A. Ansebo | Intertidal shelly sand and gravel | KU893917 |
|
| L. arenarius | CE8474 | NO, Svalbard, Ny-Ålesund, Kongsfjorden | 78.973, 11.4443 | 21-Jul-2010 | D. Fontaneto | Land-locked lagoon | KU894140 |
|
| L. arenarius | CE20748 | NO, Svalbard, Spitsbergen, Nordfjorden, Tschermak | 78.4903, 15.3289 | 27-Jul-2013 | K. Hårsaker | Freshwater stream, sand | KU893909 |
|
| L. arenarius | CE20749 | NO, Svalbard, Spitsbergen, Nordfjorden, Tschermak | 78.4903, 15.3289 | 27-Jul-2013 | K. Hårsaker | Freshwater stream, sand | KU893910 |
|
| L. arenarius | CE20750 | NO, Svalbard, Spitsbergen, Nordfjorden, Tschermak | 78.4903, 15.3289 | 27-Jul-2013 | K. Hårsaker | Freshwater stream, sand | KU893911 |
|
| L. buelowi | CE5224 | SE, Bohuslän, Strömstad, Tjärnö | 58.8754, 11.1458 | 8-Oct-2008 | C. Erséus | Upper intertidal, sand | KU893945 |
|
| L. buelowi | CE22293 | NO, Sogn og Fjordane, Neröyfjorden, Bakka | 60.9202, 6.8674 | 15-May-2014 | C. Erséus & M. Klinth | Lower intertidal, gravel, sand, algae | KU893941 |
|
| L. buelowi | CE23273 | NO, Troms, Tromsö, Tromsö, Lanes | 69.629, 18.9207 | 14-Aug-2014 | C. Erséus | Intertidal, sand, gravel | KU894284 |
|
| L. buelowi | CE23375 | NO, Troms, Tromsö, Sommaröya, Gurahaugen | 69.6321, 18.0278 | 15-Aug-2014 | C. Erséus | Lower intertidal, mixed mineral and shell sand | KU893942 |
|
| L. buelowi | CE23376 | NO, Troms, Tromsö, Sommaröya, Gurahaugen | 69.6321, 18.0278 | 15-Aug-2014 | C. Erséus | Lower intertidal, mixed mineral and shell sand | KU893943 |
|
| L. buelowi | CE24678 | NO, Nordland, Lofoten, Grimsöystraumen Strait | 68.2607, 14.2679 | 10-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, coarse shelly sand | KU893946 |
|
| L. buelowi | CE24688 | NO, Nordland, Lofoten, Grimsöystraumen Strait | 68.2607, 14.2679 | 10-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, coarse shelly sand | KU894208 |
|
| L. buelowi | CE24690 | NO, Nordland, Lofoten, Grimsöystraumen Strait | 68.2607, 14.2679 | 10-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, coarse shelly sand | KU893947 |
|
| L. dubius | CE5221 | SE, Bohuslän, Strömstad, Tjärnö | 58.8754, 11.1458 | 8-Oct-2008 | C. Erséus | Upper intertidal, coarse sand | KU893930 |
|
| L. dubius | CE5223 | SE, Bohuslän, Strömstad, Tjärnö | 58.8754, 11.1458 | 8-Oct-2008 | C. Erséus | Upper intertidal, coarse sand | KU893926 |
|
| L. dubius | CE22767 | NO, Finnmark, Mageröya, Skipsfjorden | 71.0093, 25.8933 | 12-Aug-2014 | C. Erséus | Grass at high water mark, roots and sand | KU893931 |
|
| L. dubius | CE23370 | NO, Troms, Tromsö, Sommaröya, Gurahaugen | 69.6321, 18.0278 | 15-Aug-2014 | C. Erséus | Lower intertidal, mixed mineral and shell sand | KU893934 |
|
| L. dubius | CE23371 | NO, Troms, Tromsö, Sommaröya, Gurahaugen | 69.6321, 18.0278 | 15-Aug-2014 | C. Erséus | Lower intertidal, mixed mineral and shell sand | KU893932 |
|
| L. dubius | CE24700 | NO, Nordland, Lofoten, Grimsöystraumen Strait | 68.2607, 14.2679 | 10-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, coarse shelly sand | KU893928 |
|
| L. dubius | CE24711 | NO, Nordland, Lofoten, Grimsöystraumen Strait | 68.2607, 14.2679 | 10-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, coarse shelly sand | KU893929 |
|
| L. dubius | CE24726 | NO, Nordland, Lofoten, Grimsöystraumen Strait | 68.2608, 14.2678 | 10-Sep-2014 | C. Erséus & E. Willassen | Mid-intertidal, coarse shelly sand, algae | KU893927 |
|
| L. fennicus | CE2767 | SE, Öland, Borgholm, S of Föra | 56.9855, 16.8764 | 12-Jun-2007 | A. Ansebo, L. Matamoros & C. Erséus | Stream, muddy clay | KU894132 |
|
| L. fennicus | CE2768 | SE, Öland, Borgholm, S of Föra | 56.9855, 16.8764 | 12-Jun-2007 | A. Ansebo, L. Matamoros & C. Erséus | Stream, muddy clay | KU894003 |
|
| L. fennicus | CE2988 | SE, Öland, Borgholm, S of Föra | 56.9855, 16.8764 | 12-Jun-2007 | A. Ansebo, L. Matamoros & C. Erséus | Stream, muddy clay | KU894125 |
|
| L. fennicus | CE6092 | SE, Bohuslän, Lysekil, Färlev, Färlevsfjorden | 58.4785, 11.5722 | 27-May-2009 | C. Erséus, A. Ansebo & M. Johansson | Sand, fine mud | KU894002 |
|
| L. kaloensis | CE978 | SE, Torslanda, Lilleby, Sillvik | 57.7467, 11.755 | 10-Apr-2005 | A. Ansebo | Intertidal sand | KU894128 |
|
| L. kaloensis | CE5412 | NO, Hordaland, Bergen, Blomsterdalen | 60.2689, 5.2212 | 4-Nov-2011 | P. De Wit | Intertidal, sand, rocks | KU894149 |
|
| L. knoellneri | CE980 | SE, Västergötland, Göteborg, Fiskebäck | 57.6567, 11.8483 | 15-May-2005 | A. Ansebo | Intertidal sand | KU894121 |
|
| L. knoellneri | CE982 | SE, Västergötland, Göteborg, Fiskebäck | 57.6567, 11.8483 | 15-May-2005 | A. Ansebo | Intertidal sand | KU894122 |
|
| L. knoellneri | CE19369 | NO, Sogn og Fjordane, Luster Nes, Lustrafjorden | 61.3864, 7.369 | 12-Aug-2013 | C. Erséus | Upper intertidal, sand | KU893940 |
|
| L. knoellneri | CE20761 | NO, Svalbard, Spitsbergen, Nordfjorden, Tschermak | 78.4903, 15.3289 | 27-Jul-2013 | K. Hårsaker | Freshwater stream, sand | KU893938 |
|
| L. knoellneri | CE20762 | NO, Svalbard, Spitsbergen, Nordfjorden, Tschermak | 78.4903, 15.3289 | 27-Jul-2013 | K. Hårsaker | Freshwater stream, sand | KU893939 |
|
| L. knoellneri | CE22615 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU893937 |
|
| L. knoellneri | CE23252 | NO, Troms, Tromsö, Tromsö, Lanes | 69.6291, 18.9205 | 14-Aug-2014 | C. Erséus | Supralittoral, seaweed, herbs growing on top | KU894131 |
|
| L. knoellneri | CE23253 | NO, Troms, Tromsö, Tromsö, Lanes | 69.6291, 18.9205 | 14-Aug-2014 | C. Erséus | Supralittoral, seaweed, herbs growing on top | KU894106 |
|
| L. latithecatus sp. n. | CE1976 | SE, Skåne, Höganäs, Mölle | 56.2576, 12.5192 | 29-Jul-2006 | C. Erséus | Decaying algae, sand | KU894053 |
|
| L. latithecatus sp. n. | CE1979 | SE, Skåne, Höganäs, Mölle | 56.2576, 12.5192 | 29-Jul-2006 | C. Erséus | Decaying algae, sand | KU894055 |
|
| L. latithecatus sp. n. | CE12041 | NO, Rogaland, Sola, Ölbörhamna | 58.8697, 5.5654 | 15-Jun-2012 | C. Erséus | Intertidal, algae | KU894054 |
|
| L. latithecatus sp. n. | CE12042 | NO, Rogaland, Sola, Ölbörhamna | 58.8697, 5.5654 | 15-Jun-2012 | C. Erséus | Intertidal, algae | KU894127 |
|
| L. lineatus | CE1640 | SE, Bohuslän, Strömstad, Tjärnö | 58.8766, 11.1456 | 1-Sep-2004 | C. Erséus | Intertidal, sand, gravel | KU894030 |
|
| L. lineatus | CE1694 | ES, Galicia, Ponevedra, Illa de Arousa | 42.56, -8.87 | 1-Apr-2006 | B. Reboreda Rivera | Ulva compost culture | KU894118 |
|
| L. lineatus | CE1894 | SE, Öland, Mörbylånga, Skärlöv | 56.4241, 16.5815 | 10-Jun-2006 | L. Matamoros | Beach, shelley sand, organic material | KU894040 |
|
| L. lineatus | CE12043 | NO, Rogaland, Sola, Ölbörhamna | 58.8697, 5.5654 | 15-Jun-2012 | C. Erséus | Intertidal, algae | KU894033 |
|
| L. lineatus | CE21688 | NO, Telemark, Kragerö, Gunnarsholmen Island | 58.8649, 9.4117 | 12-May-2014 | C. Erséus & M. Klinth | Anaerobic, sand, gravel, 0.3-0.5 m depth. | KU894181 |
|
| L. lineatus | CE21986 | NO, Hordaland, Maurangerfjorden, Nedrehus | 60.1295, 6.3146 | 14-May-2014 | C. Erséus & M. Klinth | High-water mark, grass roots with sand | KU894177 |
|
| L. lineatus | CE22604 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU894196 |
|
| L. lineatus | CE22605 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU894029 |
|
| L. pagenstecheri A | CE1896 | SE, Öland, Mörbylånga, Skärlöv | 56.4241, 16.5815 | 10-Jun-2006 | L. Matamoros | Beach, shelley sand, organic material | KU893986 |
|
| L. pagenstecheri A | CE1897 | SE, Öland, Mörbylånga, Skärlöv | 56.4241, 16.5815 | 10-Jun-2006 | L. Matamoros | Beach, shelley sand, organic material | KU893987 |
|
| L. pagenstecheri A | CE1899 | SE, Öland, Mörbylånga, Skärlöv | 56.4241, 16.5815 | 10-Jun-2006 | L. Matamoros | Beach, shelley sand, organic material | KU893988 |
|
| L. pagenstecheri A | CE2497 | SE, St Förö, Västergötland, Göteborg, Stora Förö Island | 57.6252, 11.8469 | 2-Jun-2007 | C. Erséus | Supralittoral, algae, gravel and pebbles | - |
|
| L. pagenstecheri A | CE2498 | SE, St Förö, Västergötland, Göteborg, Stora Förö Island | 57.6252, 11.8469 | 2-Jun-2007 | C. Erséus | Supralittoral, algae, gravel and pebbles | KU894141 |
|
| L. pagenstecheri A | CE2500 | SE, St Förö, Västergötland, Göteborg, Stora Förö Island | 57.62512, 11.8469 | 2-Jun-2007 | C. Erséus | Supralittoral, algae, gravel and pebbles | KU893985 |
|
| L. pagenstecheri B | CE22586 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU893983 |
|
| L. pagenstecheri B | CE22727 | NO, Finnmark, Porsanger, Olderfjorden | 70.4799, 25.209 | 12-Aug-2014 | C. Erséus | Driftline of dead algae | KU893984 |
|
| L. pagenstecheri C | CE1699 | ES, Galicia, Ponevedra, Illa de Arousa | 42.56, -8.87 | 1-Apr-2006 | B. Reboreda Rivera | Ulva compost culture | KU893981 |
|
| L. pagenstecheri C | CE20718 | NO, Svalbard, Spitsbergen, Wijdefjorden, Gunvorvatn | 79.8186, 15.6646 | 21-Jul-2013 | K. Hårsaker & T. Ekrem | Decaying seaweed | KU893982 |
|
| L. pagenstecheri D | CE22728 | NO, Finnmark, Porsanger, Olderfjorden | 70.4799, 25.209 | 12-Aug-2014 | C. Erséus | Driftline of dead algae | KU894110 |
|
| L. pagenstecheri D | CE22729 | NO, Finnmark, Porsanger, Olderfjorden | 70.4799, 25.209 | 12-Aug-2014 | C. Erséus | Driftline of dead algae | KU894210 |
|
| L. pagenstecheri D | CE23482 | NO, Nordland, Bjerkvik | 68.5481, 17.5422 | 15-Aug-2014 | C. Erséus | Highwater line, sand | KU893980 |
|
| L. pagenstecheri D | SM191 | GL, Disko Island, Qeqertarsuaq | 69.2489, -53.5437 | 27-Jul-2013 | S. Martinsson | Beach, sand, algae | KU893978 |
|
| L. pumilio | CE3346 | UK, Wales, Cardiff, Newport, St. Brides | 51.517, -3.000 | 13-Nov-2007 | S. Kvist | Supralittoral peat | KU894205 |
|
| L. pumilio | CE3347 | UK, Wales, Cardiff, Newport, St. Brides | 51.517, -3.000 | 13-Nov-2007 | S. Kvist | Supralittoral peat | KU894195 |
|
| L. pumilio | CE3427 | UK, Wales, Cardiff, Newport, St. Brides | 51.517, -3.000 | 13-Nov-2007 | S. Kvist | Supralittoral peat | KU894084 |
|
| L. pumilio | CE3428 | UK, Wales, Cardiff, Newport, St. Brides | 51.517, -3.000 | 13-Nov-2007 | S. Kvist | Supralittoral peat | KU894085 |
|
| L. pumilio | CE3430 | UK, Wales, Cardiff, Newport, St. Brides | 51.517, -3.000 | 13-Nov-2007 | S. Kvist | Supralittoral peat | KU894086 |
|
| L. pumilio | CE3436 | UK, Wales, Cardiff, Newport, St. Brides | 51.517, -3.000 | 13-Nov-2007 | S. Kvist | Supralittoral peat | KU894087 |
|
| L. pumilio | CE3437 | UK, Wales, Cardiff, Newport, St. Brides | 51.517, -3.000 | 13-Nov-2007 | S. Kvist | Supralittoral peat | KU894088 |
|
| L. rivalis | CE1873 | SE, Blekinge, Sölvesborg, Norje | 56.1413, 14.6742 | 7-Jun-2006 | L. Matamoros | Beach, sand, clay | KU894064 |
|
| L. rivalis | CE1874 | SE, Blekinge, Karlskrona, Torhamn | 56.0726, 15.8402 | 8-Jun-2006 | L. Matamoros | Beach, stones, organic material | KU894056 |
|
| L. rivalis | CE2503 | SE, St Förö, Västergötland, Göteborg, Stora Förö Island | 57.6252, 11.8469 | 2-Jun-2007 | C. Erséus | Supralittoral, algae, gravel and pebbles | KU894146 |
|
| L. rivalis | CE22596 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU894103 |
|
| L. rivalis | CE22600 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU894112 |
|
| L. rivalis | CE22602 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU894102 |
|
| L. rubidus | CE2549 | SE, Saltholmen, Västergötland, Göteborg, Saltholmen | 57.6631, 11.8516 | 7-Jun-2007 | C. Erséus | Supralittoral, roots and brown soil | KU894094 |
|
| L. rubidus | CE2551 | SE, Saltholmen, Västergötland, Göteborg, Saltholmen | 57.6631, 11.8516 | 7-Jun-2007 | C. Erséus | Supralittoral, roots and brown soil | KU894153 |
|
| L. rubidus | CE2553 | SE, Saltholmen, Västergötland, Göteborg, Saltholmen | 57.6631, 11.8516 | 7-Jun-2007 | C. Erséus | Supralittoral, roots and brown soil | KU894152 |
|
| L. rubidus | CE6105 | SE, Bohuslän, Lysekil, Färlev, Färlevsfjorden | 58.4765, 11.576 | 27-May-2009 | C. Erséus, A. Ansebo & M. Johansson | Sand and clay | KU894091 |
|
| L. rubidus | CE6106 | SE, Bohuslän, Lysekil, Färlev, Färlevsfjorden | 58.4765, 11.576 | 27-May-2009 | C. Erséus, A. Ansebo & M. Johansson | Sand and clay | KU894092 |
|
| L. rubidus | CE6107 | SE, Bohuslän, Lysekil, Färlev, Färlevsfjorden | 58.4765, 11.576 | 27-May-2009 | C. Erséus, A. Ansebo & M. Johansson | Sand and clay | KU894089 |
|
| L. rubidus | CE6108 | SE, Bohuslän, Lysekil, Färlev, Färlevsfjorden | 58.4765, 11.576 | 27-May-2009 | C. Erséus, A. Ansebo & M. Johansson | Sand and clay | KU894150 |
|
| L. rutilus | CE1887 | SE, Blekinge, Karlskrona, Torhamn | 56.0723, 15.8402 | 8-Jun-2006 | L. Matamoros | Organic sediment | KU894115 |
|
| L. rutilus | CE1903 | SE, Öland, Borgholm, Neptuni Åkrar | 57.3346, 17.0102 | 11-Jun-2006 | L. Matamoros | Sand, shells, org. material | KU894013 |
|
| L. rutilus | CE2510 | SE, St Förö, Västergötland, Göteborg, Stora Förö Island | 57.6252, 11.8469 | 2-Jun-2007 | C. Erséus | Supralittoral, algae, gravel and pebbles | KU894004 |
|
| L. rutilus | CE2937 | SE, Öland, Mörbylånga, Sandbyborg | 56.5536, 16.6394 | 13-Jun-2007 | A. Ansebo, L. Matamoros & C. Erséus | Seashore, sand, organic material, 0.1 m deep in sand | KU894203 |
|
| L. rutilus | CE2939 | SE, Öland, Mörbylånga, Sandbyborg | 56.5536, 16.6394 | 13-Jun-2007 | A. Ansebo, L. Matamoros & C. Erséus | Seashore, sand, organic material, 0.1 m deep in sand | KU894011 |
|
| L. rutilus | CE3060 | SE, Västergötland, Göteborg, Ryaverket | 57.6974, 11.8928 | 15-Aug-2007 | R. Almstrand | Sewage treatment plant | KU894006 |
|
| L. rutilus | CE3061 | SE, Västergötland, Göteborg, Ryaverket | 57.6974, 11.8928 | 15-Aug-2007 | R. Almstrand | Sewage treatment plant | KU894007 |
|
| L. rutilus | CE3502 | UK, Manchester, Urmston, United Utilities, Davyhulme Wastewater Treatment Works | 53.4639, -2.3741 | 10-Feb-2008 | M. Dempsey | Biofilter (indoors) | KU894008 |
|
| L. rutilus | CE3506 | UK, Manchester, Urmston, United Utilities, Davyhulme Wastewater Treatment Works | 53.4639, -2.3741 | 10-Feb-2008 | M. Dempsey | Biofilter (indoors) | KU894126 |
|
| L. rutilus | CE9267 | SE, Gotland, Visby, Palissaderna Park | 57.6346, 18.284 | 7-Aug-2010 | C. Erséus | Freshwater | KU894142 |
|
| L. scandicus sp. n. | CE975 | SE, Torslanda, Lilleby, Sillvik | 57.7467, 11.755 | 10-Apr-2005 | A. Ansebo | Intertidal sand | KU893954 |
|
| L. scandicus sp. n. | CE1905 | SE, Öland, Borgholm, Neptuni Åkrar | 57.3346, 17.0102 | 11-Jun-2006 | L. Matamoros | Sand, shells, org. material | KU893950 |
|
| L. scandicus sp. n. | CE1907 | SE, Öland, Borgholm, Neptuni Åkrar | 57.3346, 17.0102 | 11-Jun-2006 | L. Matamoros | Sand, shells, org. material | KU893951 |
|
| L. scandicus sp. n. | CE1915 | SE, Öland, Borgholm, Ölands Norra Udde | 57.3681, 17.0918 | 11-Jun-2006 | L. Matamoros | Beach, sand, stones | KU893952 |
|
| L. scandicus sp. n. | CE2548 | SE, Saltholmen, Västergötland, Göteborg, Saltholmen | 57.6631, 11.8516 | 7-Jun-2007 | C. Erséus | Supralittoral, roots and brown soil | KU893953 |
|
| L. scandicus sp. n. | CE2552 | SE, Saltholmen, Västergötland, Göteborg, Saltholmen | 57.6631, 11.8516 | 7-Jun-2007 | C. Erséus | Supralittoral, roots and brown soil | KU893955 |
|
| L. tuba | CE22614 | NO, Finnmark, Porsanger, Roddinessjöen | 70.0927, 25.0739 | 11-Aug-2014 | C. Erséus | Intertidal, algae, rubble | KU894207 |
|
| L. verrucosus | CE968 | SE, Torslanda, Lilleby, Sillvik | 57.7467, 11.755 | 10-Apr-2005 | A. Ansebo | Intertidal sand | KU894068 |
|
| L. verrucosus | CE21479 | NO, Vestfold, Larvik, Jordfallen | 59.0427, 10.0174 | 12-May-2014 | C. Erséus & M. Klinth | Subtidal, sand, gravel | KU894193 |
|
| L. verrucosus | CE21486 | NO, Vestfold, Larvik, Jordfallen | 59.0427, 10.0174 | 12-May-2014 | C. Erséus & M. Klinth | Subtidal, sand, gravel | KU894186 |
|
| L. verrucosus | CE21490 | NO, Vestfold, Larvik, Jordfallen | 59.0427, 10.0174 | 12-May-2014 | C. Erséus & M. Klinth | Subtidal, sand, gravel | KU894184 |
|
| L. verrucosus | CE21494 | NO, Vestfold, Larvik, Jordfallen | 59.0427, 10.0174 | 12-May-2014 | C. Erséus & M. Klinth | Subtidal, sand, gravel | KU894067 |
|
| L. verrucosus | CE21811 | NO, Vest-Agder, Lyngdal, Lene, Lenefjorden | 58.1353, 7.1817 | 13-May-2014 | C. Erséus & M. Klinth | Subtidal, sulfidic gravel | KU894071 |
|
| L. verrucosus | CE21816 | NO, Vest-Agder, Lyngdal, Lene, Lenefjorden | 58.1353, 7.1817 | 13-May-2014 | C. Erséus & M. Klinth | Subtidal, sulfidic gravel | KU894114 |
|
| L. verrucosus | CE21821 | NO, Vest-Agder, Lyngdal, Lene, Lenefjorden | 58.1353, 7.1817 | 13-May-2014 | C. Erséus & M. Klinth | Subtidal, sulfidic gravel | KU894073 |
|
| L. viridis | CE12037 | NO, Rogaland, Sola, Ölbörhamna | 58.8697, 5.5654 | 15-Jun-2012 | C. Erséus | Intertidal, decomp. algae | KU893969 |
|
| L. viridis | CE12038 | NO, Rogaland, Sola, Ölbörhamna | 58.8697, 5.5654 | 15-Jun-2012 | C. Erséus | Intertidal, decomp. algae | KU893970 |
|
| L. viridis | CE12039 | NO, Rogaland, Sola, Ölbörhamna | 58.8697, 5.5654 | 15-Jun-2012 | C. Erséus | Intertidal, decomp. algae | KU893971 |
|
| L. viridis | CE23255 | NO, Troms, Tromsö, Tromsö, Lanes | 69.629, 18.9207 | 14-Aug-2014 | C. Erséus | Intertidal, sand, gravel | KU893967 |
|
| L. sp. F | CE2659 | UK, Devon, Plymouth, River Plym | 50.3757, -4.1082 | 20-Jul-2007 | L. Matamoros | Iintertidal, stones, clay | KU893997 |
|
| L. sp. G | CE2246 | UK, Wales, Anglesey, Beaumaris | 53.2623, -4.0914 | 15-Feb-2007 | M. Strand & P. Sundberg | Intertidal, sand, algae | KU894001 |
|
| L. sp. G | CE2661 | UK, Devon, Plymouth, River Plym | 50.3757, -4.1082 | 20-Jul-2007 | L. Matamoros | Iintertidal, stones, clay | KU893999 |
|
| L. sp. G | CE23373 | NO, Troms, Tromsö, Sommaröya, Gurahaugen | 69.6321, 18.0278 | 15-Aug-2014 | C. Erséus | Lower intertidal, mixed mineral and shell sand | KU894000 |
|
| L. sp. H | CE23136 | NO, Troms, Rotsundselv | 69.8001, 20.7158 | 14-Aug-2014 | C. Erséus | Upper intertidal, sand | KU893920 |
|
| L. sp. H | CE24967 | NO, Nordland, Gildeskål, Kjöpstad, Holmsundsfjorden Bay | 67.0536, 14.2729 | 11-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, rock pool, gravel, sand | KU893921 |
|
| L. sp. H | CE24968 | NO, Nordland, Gildeskål, Kjöpstad, Holmsundsfjorden Bay | 67.0536, 14.2729 | 11-Sep-2014 | C. Erséus & E. Willassen | Upper intertidal, rock pool, gravel, sand | KU893919 |
|