Research Article |
|
Corresponding author: Adrian G. Glover ( a.glover@nhm.ac.uk ) Academic editor: Eike Neubert
© 2017 Helena Wiklund, John D. Taylor, Thomas G. Dahlgren, Christiane Todt, Chiho Ikebe, Muriel Rabone, Adrian G. Glover.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Wiklund H, Taylor JD, Dahlgren TG, Todt C, Ikebe C, Rabone M, Glover AG (2017) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Mollusca. ZooKeys 707: 1-46. https://doi.org/10.3897/zookeys.707.13042
|
Abstract
We present the first DNA taxonomy publication on abyssal Mollusca from the Clarion-Clipperton Zone (CCZ), central Pacific ocean, using material collected as part of the Abyssal Baseline (ABYSSLINE) environmental survey cruise ‘AB01’ to the UK Seabed Resources Ltd (UKSRL) polymetallic-nodule exploration area ‘UK-1’ in the eastern CCZ. This is the third paper in a series to provide regional taxonomic data for a region that is undergoing intense deep-sea mineral exploration for high-grade polymetallic nodules.
Taxonomic data are presented for 21 species from 42 records identified by a combination of morphological and genetic data, including molecular phylogenetic analyses. These included 3 heterodont bivalves, 5 protobranch bivalves, 4 pteriomorph bivalves, 1 caudofoveate, 1 monoplacophoran, 1 polyplacophoran, 4 scaphopods and 2 solenogastres. Gastropoda were recovered but will be the subject of a future study. Seven taxa matched published morphological descriptions for species with deep Pacific type localities, and our sequences provide the first genetic data for these taxa. One taxon morphologically matched a known cosmopolitan species but with a type locality in a different ocean basin and was assigned the open nomenclature ‘cf’ as a precautionary approach in taxon assignments to avoid over-estimating species ranges. One taxon is here described as a new species, Ledella knudseni sp. n. For the remaining 12 taxa, we have determined them to be potentially new species, for which we make the raw data, imagery and vouchers available for future taxonomic study. The Clarion-Clipperton Zone is a region undergoing intense exploration for potential deep-sea mineral extraction. We present these data to facilitate future taxonomic and environmental impact study by making both data and voucher materials available through curated and accessible biological collections.
Keywords
New species, Bivalvia , Caudofoveata , Monoplacophora , Polyplacophora , Scaphopoda , Solenogastres , Aplacophora
Introduction
The abyssal zone of the world’s oceans has been defined as that between 3000 m and 6000 m depth, a bathymetric zone that encompasses 54% of the geographic surface of the planet (
The Clarion-Clipperton Zone (hereafter, CCZ) is so called as it lies between the Clarion and Clipperton Fracture Zones, topographical highs that extend longitudinally across almost the entire Pacific Ocean. There is no strict definition of the region, but it has come to be regarded as the area between these fracture zones that lies within international waters and encompasses the main areas of commercial interest for polymetallic nodule mining. Exploration licenses issued by the International Seabed Authority (ISA 2017) extend from 115°W (the easternmost extent of the UK-1 exploration area) to approximately 158°W (the westernmost extent of the COMRA exploration area), as such we use from hereafter a working definition of the CCZ as the box: 13°N158°W; 18°N118°W; 10°N112°W; 2°N155°W. This is an area of almost exactly 5 million sq km, approximately 1.4% of the ocean’s surface.
The Challenger expedition between 1872 and 1876 is said to be the start of modern oceanography, and in total about 4700 new species were described from it. However, in the Pacific Ocean they went from Japan to the Hawaiian Islands and after that fairly straight south down to about 40°S where they turned towards Valparaiso in Chile, and thus they did only touch the western-most part of the CCZ (
Within the entire 5 million sq km CCZ, as defined above, online databased sources prior to this publication list only one benthic mollusc record when specifying depth between 3000-6000 m, and a further four records just south of CCZ (OBIS 2017). This result is due to lack of sampling and/or taxonomic knowledge given that an abundant and diverse mollusc fauna is suspected in the region based on anecdotal reports from past environmental surveys (e.g. ISA 1999;
The UK Seabed Resources Ltd ‘UK-1’ polymetallic nodule exploration contract area ABYSSLINE (AB01) Stratum A, a 30 × 30 km survey box in the northern sector of the 58,000 km2 exploration area. Bathymetric survey and sample localities from the AB01 RV Melville survey cruise, October 2013, data courtesy Craig R. Smith (University of Hawaii), UK Seabed Resources Ltd and Seafloor Investigations, LLC.
This paper aims to provide regional taxonomic information for an area that is undergoing intense deep-sea mineral exploration for high-grade polymetallic nodules regulated by Sponsoring States (here the United Kingdom Government) and the International Seabed Authority (ISA 2017). The study is not a comprehensive faunal guide to the region, but a taxonomic data paper that will be updated with new additions following future collections and analyses. This publication is supported by similar data publications on other taxa from the CCZ. Two have been published (Echinodermata,
Materials and methods
Knowledge of baseline biodiversity and biogeography in the CCZ is severely hampered by a lack of modern DNA-supported taxonomic studies (
Fieldwork
The ABYSSLINE environmental baseline survey consists of a series of 30 × 30 km survey boxes (strata), three within the UK-1 exploration area, and an additional reference site outside the exploration area (
A comprehensive description of our DNA taxonomy pipeline is provided in
Laboratory work
In the laboratory, specimens were re-examined using stereo and compound microscopes, identified and described to best possible taxonomic level with key morphological features photographed with digital cameras and a small tissue-sample taken for DNA extraction.
Extraction of DNA was done with DNeasy Blood and Tissue Kit (Qiagen) using a Hamilton Microlab STAR Robotic Workstation. About 1800 bp of 18S, 450 bp of 16S, and 650 bp of cytochrome c oxidase subunit I (COI) were amplified using primers listed in Table
| Primer | Sequence 5’-3’ | Reference |
|---|---|---|
| 18S | ||
| 18SA | AYCTGGTTGATCCTGCCAGT |
|
| 18SB | ACCTTGTTACGACTTTTACTTCCTC |
|
| 620F | TAAAGYTGYTGCAGTTAAA |
|
| 1324R | CGGCCATGCACCACC |
|
| COI | ||
| LCO1490 | GGTCAACAAATCATAAAGATATTGG |
|
| HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA |
|
| 16S | ||
| ann16SF | GCGGTATCCTGACCGTRCWAAGGTA |
|
| 16SbrH | CCGGTCTGAACTCAGATCACGT | Palumbi et al. 1996 |
Data handling
The field and laboratory work created a series of databases and sample sets that are integrated into a data-management pipeline. This includes the transfer and management of data and samples between a central collections database, a molecular collections database and external repositories (GenBank, WoRMS, OBIS, GBIF, ZooBank) through DarwinCore archive. This provides a robust data framework to support DNA taxonomy, in which openly-available data and voucher material is key to quality data standards. A further elaboration of the data pipeline is published in
Taxonomic assignments
All future studies of biogeographic and bathymetric ranges, gene-flow, extinction risks, natural history, reproductive ecology, functional ecology and geochemical interactions of CCZ species are dependent on accurate identifications faciliated by taxonomy. This taxonomy is dependent on a sound theoretical underpinning – a species concept - coupled with the availability of both raw data and voucher samples. Here we use a phylogenetic species concept sensu
Taxon treatments presented in this paper. Includes Class, DNA Taxonomy ID (a species-level identification based on combined DNA and morphological evidence), GUID (Global Unique Identifier link to data record on http://data.nhm.ac.uk), ABYSSLINE Record number, NHM Accession number, NHM Molecular Collection Facility (MCF) sample ID number (NHMUK_MCF#) and NCBI GenBank accession number (Genbank#) for successfully sequenced genetic markers.
| Class, sub-class | DNA Taxonomy ID | GUID# | ABYSS LINE record# | NHMUK Acc# | NHMUK MCF# | Gen Bank# |
|---|---|---|---|---|---|---|
| Bivalvia, Heterodonta | Myonera sp. (NHM_186) | 45033e06-fb54-49d5-b632-767e63c1cfd3 | NHM_186 | 20170037 | 175138970 |
MF157481
MF157508 |
| Bivalvia, Heterodonta | Thyasira sp. (NHM_180) | 49b2f599-bda4-4177-932f-59effe8a3320 | NHM_051 | 20170038 | 175139015 |
MF157468
MF157501 |
| Bivalvia, Heterodonta | Thyasira sp. (NHM_180) | b84e470d-73bc-413b-88f9-3d702509a37a | NHM_180 | 20170039 | 175139013 | MF157478 |
| Bivalvia, Heterodonta | Vesicomya galatheae | c609ed0c-f881-44c9-a6a0-3e36f0934997 | NHM_143 | 20170040 | 175139017 | MF157474 |
| Bivalvia, Heterodonta | Vesicomya galatheae | 314ef160-7cfa-4705-b091-640c3e69ad1a | NHM_255 | 20170041 | 175138995 |
MF157460
MF157487 MF157509 |
| Bivalvia, Heterodonta | Vesicomya galatheae | 3add2560-71c1-4879-afb8-0a5ed1449c89 | NHM_260 | 20170042 | 175138988 |
MF157488
MF157510 |
| Bivalvia, Protobranchia | Bathyspinula calcar | 3ab74908-1a5d-465f-890c-49373a44906c | NHM_181 | 20170043 | 175138994 |
MF157479
MF157507 |
| Bivalvia, Protobranchia | Bathyspinula calcar | 61f15e3c-f070-48a1-b484-780b37f7feb6 | NHM_146 | 20170044 | 175138993 |
MF157475
MF157505 |
| Bivalvia, Protobranchia | Bathyspinula calcar | c44da298-9b61-4d6d-a1cd-2d6c3bd70859 | NHM_149A | 20170045 | 175138969 | MF157506 |
| Bivalvia, Protobranchia | Bathyspinula calcar | ad2cb87b-1fce-415d-ab45-1619bbc4352b | NHM_284 | 20170046 | 175139011 | MF157514 |
| Bivalvia, Protobranchia | Ledella knudseni sp. n. | 8aec47f4-dcec-4668-8398-9e4b0c28ecb8 | NHM_288A | 20170047 | 175138963 | MF157515 |
| Bivalvia, Protobranchia | Ledella knudseni sp. n. | f1886d78-22bf-403e-bdb2-784b91c0eb12 | NHM_288C | 20170048 | 175139136 |
MF157491
MF157516 |
| Bivalvia, Protobranchia | Ledella sp. (NHM_381) | 8f077dac-baac-4fef-b6a1-7fd02d5f0070 | NHM_381 | 20170049 | 175139009 |
MF157494
MF157521 |
| Bivalvia, Protobranchia | Ledella sp. (NHM_381) | 08d5c39f-b1e4-43d7-a8ea-2fe9abc05752 | NHM_144 | 20170050 | 175139014 |
MF157458
MF157504 |
| Bivalvia, Protobranchia | Nucula profundorum | f2133256-1cad-4255-a5cb-bd5331417127 | NHM_141 | 20170051 | 175139038 |
MF157457
MF157473 MF157503 |
| Bivalvia, Protobranchia | Nucula profundorum | f96a470e-237e-46b4-ba85-4c6196106071 | NHM_274A | 20170052 | 175138964 | MF157512 |
| Bivalvia, Protobranchia | Nucula profundorum | 65f8d1ed-dd6a-4265-90d2-daf07491cd76 | NHM_378 | 20170053 | 175138949 |
MF157464
MF157520 |
| Bivalvia, Protobranchia | Yoldiella sp. (NHM_190) | 621deeed-8f8a-4d2e-9136-4e30794fc68e | NHM_042 | 20170054 | 175139016 | MF157467 |
| Bivalvia, Protobranchia | Yoldiella sp. (NHM_190) | 6dfa8946-aa7a-448d-9f4f-703a3b2a10d9 | NHM_185 | 20170055 | 175138989 | MF157480 |
| Bivalvia, Protobranchia | Yoldiella sp. (NHM_190) | b6e48ff4-2e02-42dc-b9ed-286d297d1459 | NHM_190 | 20170056 | 175138965 | MF157482 |
| Bivalvia, Protobranchia | Yoldiella sp. (NHM_190) | 7a6c76df-989b-4fcd-9e9c-a442d0a02443 | NHM_194 | 20170057 | 175139019 | MF157485 |
| Bivalvia, Protobranchia | Yoldiella sp. (NHM_190) | 37b2493a-a725-4ec4-a720-cc9dd12fb49d | NHM_246 | 20170058 | 175139012 | MF157486 |
| Bivalvia, Protobranchia | Yoldiella sp. (NHM_190) | 17d54bb4-9f38-4073-9bb6-17637773b058 | NHM_289 | 20170059 | 175139034 |
MF157492
MF157517 |
| Bivalvia, Protobranchia | Yoldiella sp. (NHM_190) | 8923576e-4542-4fc7-9a89-016e8fb564cb | NHM_193 | 20170060 | 175139036 | MF157484 |
| Bivalvia, Pteriomorpha | Bentharca cf. asperula | 9d29d7ec-55cd-4b41-929a-2379be221263 | NHM_108 | 20170061 | 175138968 |
MF157470
MF157502 |
| Bivalvia, Pteriomorpha | Bentharca cf. asperula | 96bfe548-f511-49c4-b2a3-0a9a45f9154b | NHM_150 | 20170062 | 175138966 | MF157476 |
| Bivalvia, Pteriomorpha | Bentharca cf. asperula | 8d9beefd-2fbc-4204-9bf8-90551419ac1c | NHM_170 | 20170063 | 175139018 | MF157477 |
| Bivalvia, Pteriomorpha | Bentharca cf. asperula | ccdd114d-c8a8-47da-ba84-8b8ca5125a6a | NHM_282 | 20170064 | 175139035 |
MF157490
MF157513 |
| Bivalvia, Pteriomorpha | Bentharca cf. asperula | 1d462c2a-bb98-4369-afc3-63a7c33a4bdd | NHM_427 | 20170065 | 175139023 | MF157496 |
| Bivalvia, Pteriomorpha | Bentharca cf. asperula | a30eab51-5f52-4fec-89d7-d47152895c92 | NHM_454 | 20170066 | 175138984 | MF157499 |
| Bivalvia, Pteriomorpha | Dacrydium panamensis | 180e485f-f1c2-41e1-b858-f02ba537804b | NHM_117 | 20170067 | 175138967 | MF157471 |
| Bivalvia, Pteriomorpha | Limopsis sp. (NHM_453) | ce9cbed0-82cc-420d-baad-fdfff7cc0986 | NHM_453 | 20170069 | 175138999 |
MF157498
MF157524 |
| Bivalvia, Pteriomorpha | Catillopecten sp. (NHM_105) | 24f5c5bb-e419-48ef-baaa-4a6493f691d9 | NHM_105 | 20170070 | 175138991 | MF157469 |
| Caudofoveata | Prochaetodermatidae sp. (NHM_344) | e68608f9-4b83-4eb9-89f2-0de4f89c21b0 | NHM_344 | 20170071 | 175138997 | MF157462 |
| Monoplaco-phora | Veleropilina oligotropha | bf968b01-1991-43b7-87e4-25da4d5a9dc5 | NHM_405 | 20170072 | 175138950 |
MF157465
MF157495 MF157522 |
| Polyplaco-phora | Leptochiton macleani | d69b581d-8a79-4c4d-8f70-88b2ec07d86e | NHM_446 | 20170073 | 175139008 |
MF157466
MF157497 MF157523 |
| Scaphopoda | Fissidentalium sp. (NHM_261) | 679fa0ca-d647-446d-87c5-e8d33949efe2 | NHM_261 | 20170074 | 175138971 |
MF157461
MF157489 MF157511 |
| Scaphopoda | Gadilida sp. (NHM_192) | fc0e3ae8-9cce-46a0-bb8b-fafe0e2cb46b | NHM_192 | 20170075 | 175138946 |
MF157459
MF157483 |
| Scaphopoda | Gadila sp. (NHM_345) | c301a72f-54cb-435e-8aae-17cf4d37675f | NHM_345 | 20170076 | 175138986 |
MF157463
MF157493 MF157518 |
| Scaphopoda | Gadilida sp. (NHM_132) | 6a1906d9-9ed1-4f6e-a0cf-2d53e2289a01 | NHM_132 | 20170077 | 175138944 |
MF157456
MF157472 |
| Solenogastres | Acanthomeniidae sp. (NHM_367) | c0577fc9-7302-4fec-bc8c-87a17a38bc91 | NHM_367 | 20170078 | 175138973 | MF157519 |
| Solenogastres | Lophomeniinae sp. (NHM_027) | 319fd186-b07f-4be7-986c-b96c20f63723 | NHM_027 | 20170079 | 175139039 | MF157500 |
Systematics
Bivalvia
Heterodonta
Anomalodesmata
Cuspidariidae Dall, 1886
Myonera Dall & E.A Smith, 1886
Myonera
Materials examined
NHM_186 NHMUK 20170037, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/45033e06-fb54-49d5-b632-767e63c1cfd3
Description
Shell thin, translucent, sub-ovate tapering posteriorly. Postero-dorsal margin straight. Rostrum short, demarcated by single, carinate radial rib. Sculpture of a few strong, widely spaced, commarginal lamellae, reduced on rostrum. Shell surface minutely pustulose (Fig.
Remarks
The species resembles the supposedly cosmopolitan form Myonera alleni Poutiers & Bernard, 1995, previously as Myonera atlantica (Allen & Morgan, 1981). However, the type locality for this species is from the deep north Atlantic and no genetic data are available for comparison. No similar species is recorded from deep water of the eastern Pacific. Forms a unique monophyletic clade with two other cuspidariid species distinct from all other AB01 specimens (Fig.
Ecology
Found in polymetallic nodule province.
Lucinida
Thyasiridae Dall, 1900
Thyasira Lamarck, 1818
Thyasira
Material examined
NHM_051 NHMUK 20170038, collected 2013-10-09, 13.8372 -116.55843, 4336 m. http://data.nhm.ac.uk/object/49b2f599-bda4-4177-932f-59effe8a3320
NHM_180 NHMUK 20170039, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/b84e470d-73bc-413b-88f9-3d702509a37a
Description
Minute, thin-shelled, translucent, anteriorly extended, longer than high, umbones posterior of mid-line, posteriorly angulate, antero-dorsal margin long, evenly curved, shell surface smooth. Gill with single demibranch of about 10 widely spaced filaments, ventral edge of the gill does not cover the body pouches. Foot relatively large with distal bulb (Fig.
Remarks
Forms a monophyletic clade with four other thyasirid species (Fig.
Ecology
Found in polymetallic nodule province.
Veneroida
Vesicomyidae Dall & Simpson, 1901
Vesicomya Dall, 1886
Vesicomya galatheae
Material examined
NHM_143 NHMUK 20170040, collected 2013-10-11, 13.75833 -116.69852, 4080 m. http://data.nhm.ac.uk/object/c609ed0c-f881-44c9-a6a0-3e36f0934997
NHM_255 NHMUK 20170041, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/314ef160-7cfa-4705-b091-640c3e69ad1a
NHM_260 NHMUK 20170042.1-2, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/3add2560-71c1-4879-afb8-0a5ed1449c89
Description
Small, inflated sub-spherical. Sculpture of fine closely spaced low commarginal lamellae. Right valve with two cardinal teeth, posterior long, thin, anterior tooth small and short (Fig.
Vesicomya galatheae (Knudsen, 1970) A Live imaged specimens of NHM_260a,b,c habitus B Detail of NHM_143, probable juvenile, oil droplets arrowed C NHM_255 live imaged specimen D–E SEM detail of shell interior and hinge teeth of NHM_260a (right valve). Scale bars: 0.5 mm (B, E). Image attribution Glover, Taylor, Dahlgren & Wiklund, 2017.
Genetic data
Remarks
Vesicomya galatheae was described from off Costa Rica and Panama at 2950- 3570 m. Morphologically similar to Vesicomya pacifica (Smith, 1885) holotype NHMUK 1887.2.9.2710-11 but
Ecology
Found in polymetallic nodule province.
Protobranchia
Nuculanoida
Bathyspinulidae Coan & Scott, 1997
Bathyspinula Allen & Sanders, 1982
Bathyspinula calcar
Material examined
NHM_146 NHMUK 20170044, collected 2013-10-11, 13.75833 -116.69852, 4080 m. http://data.nhm.ac.uk/object/61f15e3c-f070-48a1-b484-780b37f7feb6
NHM_149A NHMUK 20170045, collected 2013-10-11, 13.75833 -116.69852, 4080 m. http://data.nhm.ac.uk/object/c44da298-9b61-4d6d-a1cd-2d6c3bd70859
NHM_181 NHMUK 20170043, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/3ab74908-1a5d-465f-890c-49373a44906c
NHM_284 NHMUK 20170046, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/ad2cb87b-1fce-415d-ab45-1619bbc4352b
Description
Shell sub-ovate, laterally compressed, with long, sharply pointed posterior rostrum. Periostracum shiny, medium brown. Posterior rostrum shorter, less defined in juveniles. Voucher specimen NHM_181 shell length 13.5 mm, width 7.6 mm (Fig.
Bathyspinula calcar (Dall, 1908) A Specimen NHM_181, Image of live specimen after recovery, length 13.5 mm B–D Specimen NHM_149A confirmed juvenile B. calcar using DNA evidence, total length of animal ~2mm. Scale bars: 5 mm (A); 1 mm (B–D). Image attribution Glover, Taylor, Dahlgren & Wiklund, 2017.
Genetic data
Remarks
Widely distributed in the eastern Pacific at depths of 400-5000 m (see
Ecology
Relatively large bivalve recovered from epibenthic sledge tow in polymetallic nodule province.
Nuculanidae H. Adams & A. Adams, 1858
Ledella Verrill & Bush, 1897
Ledella knudseni , sp. n.
Material examined
Paratype NHM_288A NHMUK 20170047.1-2, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/8aec47f4-dcec-4668-8398-9e4b0c28ecb8
Holotype NHM_288C NHMUK 20170048, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/f1886d78-22bf-403e-bdb2-784b91c0eb12
Description
Shell relatively thick, robust. Ovoid with short rostrum, umbones broad, prominent; postero ventral margin sinuous; broad, shallow sulcus extending from umbones to posteroventral margin. Sculpture of low, relatively broad, closely spaced, commarginal lamellae; fine radial striations on rostrum and juvenile shell. Ligament internal, situated on broad resilium beneath umbones. Hinge robust, with 8-9 chevron shaped, blunt teeth to either side of ligament. Inner shell margin smooth. Prodissoconch large, ellipsoidal 0.3 mm long, with sharp rim, surface irregularly pitted. Holotype NHM_288C shell length 2.2 mm, width 1.5 mm; paratype NHM_288A shell length 2.1 mm, height 1.5 mm. (Figure
Ledella knudseni sp. n. A Holotype, specimen NHM_288c B Paratype, specimen NHM_288a C Specimen NHM 288a dissected prior to DNA sequencing and SEM D–G SEM of valve, hinge teeth and protoconch. Scale bars: 1 mm (B–C); 0.5 mm (D–E); 0.1 mm (F–G). Image attribution Glover, Taylor, Dahlgren & Wiklund, 2017.
Remarks
Similar in form to Ledella ultima (Smith, 1885) widespread in the abyssal Atlantic (
Etymology
Named for Jørgen Knudsen (1918-2009), deep-sea bivalve systematist and author of the Galathea Report on abyssal and hadal Bivalvia.
Ecology
Found in polymetallic nodule province.
Ledella
Material examined
NHM_144 NHMUK 20170050, collected 2013-10-11, 13.75833 -116.69852, 4080 m. http://data.nhm.ac.uk/object/08d5c39f-b1e4-43d7-a8ea-2fe9abc05752
NHM_381 NHMUK 20170049, collected 2013-10-19, 13.93307 -116.71628, 4182 m. http://data.nhm.ac.uk/object/8f077dac-baac-4fef-b6a1-7fd02d5f0070
Description
Remarks
This species is morphologically very similar to the new Ledella knudseni, its sister taxon in the molecular phylogenetic analyses (Fig.
Ecology
Found in polymetallic nodule province.
Nuculida
Nuculidae Gray, 1824
Nucula Lamarck, 1799
Nucula profundorum
Material examined
NHM_141 NHMUK 20170051, collected 2013-10-11, 13.75833 -116.69852, 4080 m. http://data.nhm.ac.uk/object/f2133256-1cad-4255-a5cb-bd5331417127
NHM_274A NHMUK 20170052, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/f96a470e-237e-46b4-ba85-4c6196106071
NHM_378 NHMUK 20170053.1-2, collected 2013-10-19, 13.93307 -116.71628, 4182 m. http://data.nhm.ac.uk/object/65f8d1ed-dd6a-4265-90d2-daf07491cd76
Description
Small, trigonal- subovate. Periostracum light brown, shiny. Sculpture of fine radial lirae. Resilifer small. Hinge teeth: 5 anterior, 4 posterior. Inner shell margin finely denticulate. Voucher NHM_274A width 2 mm, height 1.8 mm (Fig.
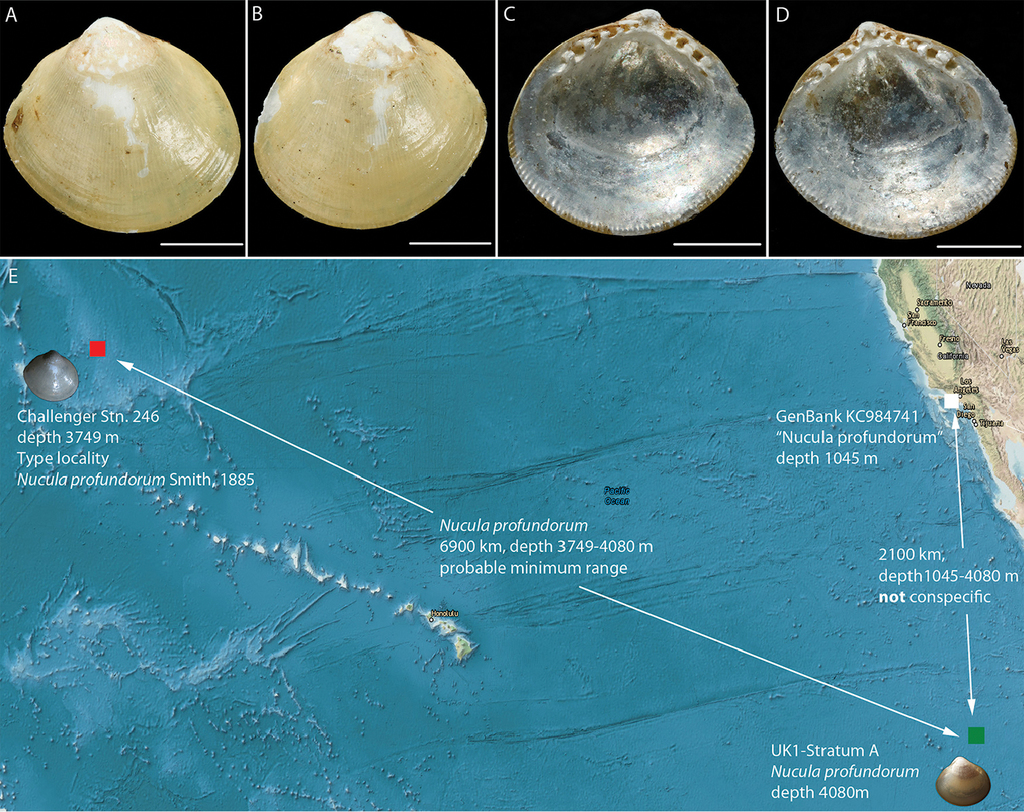
Nucula profundorum Smith, 1885 A Live specimen NHM_141 (for which 18S, CO1 and 16S sequences were obtained) B Live specimens NHM_274 (4 specimens from same sample) C Open shell from single individual NHM_274A with tissue sample taken for DNA sequencing D–E SEM of NHM_378 valve showing hinge teeth. Scale bars: 1.5 mm (B); 0.5 mm (C). Image attribution Glover, Taylor, Dahlgren & Wiklund, 2017.
Genetic data
Remarks
Morphologically matches Nucula profundorum Smith, 1885 based on examination of the syntype specimens [NHMUK 1887.2.9.2919]. In the molecular analysis of nuculoid protobranchs (Fig.
Ecology
The most abundant bivalve mollusc recorded in the ABYSSLINE sampling programme, frequently found in epibenthic sledge and box core samples from region of sediment and polymetallic nodules.
Yoldiidae
Yoldiella A.E Verrill & Bush, 1897
Yoldiella
Material examined
NHM_042 NHMUK 20170054, collected 2013-10-09, 13.8372 -116.55843, 4336 m. http://data.nhm.ac.uk/object/621deeed-8f8a-4d2e-9136-4e30794fc68e
NHM_185 NHMUK 20170055, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/6dfa8946-aa7a-448d-9f4f-703a3b2a10d9
NHM_190 NHMUK 20170056, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/b6e48ff4-2e02-42dc-b9ed-286d297d1459
NHM_193 NHMUK 20170060, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/8923576e-4542-4fc7-9a89-016e8fb564cb
NHM_194 NHMUK 20170057, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/7a6c76df-989b-4fcd-9e9c-a442d0a02443
NHM_246 NHMUK 20170058, collected 2013-10-16, 13.81166 -116.71, 4076 m. http://data.nhm.ac.uk/object/37b2493a-a725-4ec4-a720-cc9dd12fb49d
NHM_289 NHMUK 20170059, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/17d54bb4-9f38-4073-9bb6-17637773b058
Description
Small, sub-ovate, longer than high, umbone at mid-line, dorsal margin horizontal to slightly curved, ventral margin deeply rounded, thin-shelled, shiny, semi-transparent, smooth except for growth increments. Internal features not investigated but 4-5 anterior and posterior chevron teeth. Hindgut visible though the shell forms a simple rounded loop on right side of body. DNA voucher NHM_190 shell length 1.6 mm, height 1 mm. Voucher specimen NHM_185 shell length 1.5 mm, height 1 mm (Fig.
Genetic data
Remarks
Extremely small, semi-transparent bivalves typically about 1 mm in size. Yoldiella species are particularly difficult to identify (see
Ecology
Found in polymetallic nodule province.
Pteriomorphia
Arcoida
Arcidae Lamarck, 1809
Bentharca Verrill & Bush, 1898
Bentharca cf. asperula
Material examined
NHM_108 NHMUK 20170061, collected 2013-10-11, 13.79335 -116.70308, 4081 m. http://data.nhm.ac.uk/object/9d29d7ec-55cd-4b41-929a-2379be221263
NHM_150 NHMUK 20170062.1-2, collected 2013-10-11, 13.75833 -116.69852, 4080 m. http://data.nhm.ac.uk/object/96bfe548-f511-49c4-b2a3-0a9a45f9154b
NHM_170 NHMUK 20170063, collected 2013-10-11, 13.7936 -116.70308, 4078 m. http://data.nhm.ac.uk/object/8d9beefd-2fbc-4204-9bf8-90551419ac1c
NHM_282 NHMUK 20170064, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/ccdd114d-c8a8-47da-ba84-8b8ca5125a6a
NHM_427 NHMUK 20170065, collected 2013-10-20, 13.86367 -116.54432, 4050 m. http://data.nhm.ac.uk/object/1d462c2a-bb98-4369-afc3-63a7c33a4bdd
NHM_454 NHMUK 20170066, collected 2013-10-21, 13.90165 -116.59, 4163 m. http://data.nhm.ac.uk/object/a30eab51-5f52-4fec-89d7-d47152895c92
Description
Shell elongate, trapezoidal, strongly inequilateral, anteriorly attentuated and posteriorly expanded, umbones small, low, dorsal edge straight. Byssal sinus in ventral margin. Sculpture of irregular commarginal lamellae and low radial ribs but covered by a thick, shaggy, brown periostracum with projecting scales. Two pre- and post- umbonal hinge teeth with each tooth crossed by transverse grooves giving a lobate appearance (Fig.
Bentharca cf. asperula (Dall, 1881) A Voucher specimen NHM_150 live after recovery B Specimen NHM_150 after preservation and dissection for DNA sample showing valves C Specimen NHM_108 Live D–F Specimen NHM_150 SEM showing shell ornamentation and hinge teeth. Scale bars: 1 mm (A, E); 0.5 mm (B); 0.2 mm (F). Image attribution Glover, Taylor, Dahlgren & Wiklund, 2017.
Genetic data
Remarks
Bentharca asperula has been regarded as a cosmopolitan deep-water species with a considerable recorded depth range of 430–5005 m (
Ecology
Quite abundant. Found in polymetallic nodule province.
Mytiloida
Mytilidae Rafinesque, 1815
Dacrydium Torell, 1859
Dacrydium panamensis
Material examined
NHM_117 NHMUK 20170067, collected 2013-10-11, 13.79335 -116.70308, 4081 m. http://data.nhm.ac.uk/object/180e485f-f1c2-41e1-b858-f02ba537804b
Description
Shell small, subovate, translucent, anterior-ventral margin slightly produced, highest point near mid-line. Voucher NHM_117 Shell length 1.7 mm, shell height 2.5 mm (Fig.
Genetic data
GenBank NHM_117 18S-MF157471.
Remarks
Identified from figures in
Ecology
Found in polymetallic nodule province.
Limopsidae Dall, 1895
Limopsis Sassi, 1827
Limopsis
Material examined
NHM_453 NHMUK 20170069.1-2, collected 2013-10-21, 13.90165 -116.59, 4163 m. http://data.nhm.ac.uk/object/ce9cbed0-82cc-420d-baad-fdfff7cc0986
Description
Subcircular to slightly oblique with slightly sinuous posterior margin. Periostracum with short, fine, bristles aligned in radial rows. Ligament small, triangular, set in shallow resilifer. Hinge teeth robust, 4 anterior and 5 posterior. Inner shell margin smooth. Voucher NHM_453 shell length 4.6 mm, height 4.3mm (Fig.
Remarks
Dissimilar in shape and periostracal bristle configuration to any recorded Eastern Pacific deep-water species (Coan & Valentich-Scott 2012). However, shape and number of hinge teeth are known to change with age/size in Limopsis species. In molecular analysis (Fig.
Ecology
Found in polymetallic nodule province.
Pectinoida
Propeamussiidae Abbott, 1954
Catillopecten Iredale, 1939
Catillopecten
Material examined
NHM_105 NHMUK 20170070, collected 2013-10-11, 13.79335 -116.70308, 4081 m. http://data.nhm.ac.uk/object/24f5c5bb-e419-48ef-baaa-4a6493f691d9
Description
Small, thin-shelled, subcircular. Right valve flat, left valve slightly convex. Both valves with commarginal undulations that become stronger towards the margin, fine radial striations on both valves. Well defined anterior auricle and byssal notch. Voucher NHM_105 1.8 mm shell length, height 1.5 mm (Fig.
Genetic data
GenBank NHM_105 18S-MF157469.
Remarks
Holotype (ZMUC) from Gulf of Panama, 3270–3670 m Galathea stn 726, figured by
Ecology
Found in polymetallic nodule province.
Caudofoveata
Prochaetodermatidae Salvini-Plawen, 1975
Prochaetodermatidae
Material examined
NHM_344 NHMUK 20170071.1-2, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/e68608f9-4b83-4eb9-89f2-0de4f89c21b0
Description
Voucher NHM_344 (Fig.
Genetic data
GenBank NHM_344 16S-MF157462.
Remarks
The specimen has the typical body shape and sclerite type of Prochaetodermatidae.
Ecology
Found in polymetallic nodule province. Burrows in soft sediment.
Monoplacophora
Neopilinidae Knight & Yochelson, 1958
Veleropilina Starobogatov & Moskalev, 1987
Veleropilina oligotropha
Material examined
NHM_405 NHMUK 20170072, collected 2013-10-20, 13.86328 -116.54885, 4050 m. http://data.nhm.ac.uk/object/bf968b01-1991-43b7-87e4-25da4d5a9dc5
Description
Shell transparent, sculpture is reticulate, reticulation not covering the smooth apical area. Voucher specimen NHM_405, specimen length 2.2 mm, specimen width 1 mm (Fig.
Veleropilina oligotropha (Rokop, 1972) Specimen NHM_405. A Dorsal view of living specimen B Ventral view of living specimen. C Lateral view of ethanol-preserved specimen D Dorsal shell sculpture detail, just below apex E Ventral view of mouth and shell margin. Scale bars: 1mm. Image attribution Glover, Dahlgren & Wiklund, 2017.
Remarks
Morphologically agrees with description of Veleropilina oligotropha (Rokop, 1972) described from ~6000 m water depth in the central North Pacific.
Forms a unique monophyletic clade distinct from all other AB01 specimens. No genetic matches on GenBank. In the molecular analyses based on the 16S gene, the Monoplacophora clade is strongly supported, but internal branches are unresolved or, when clades are present, they have low support (Fig.
Ecology
Specimen collected from an epibenthic sledge tow across region of sediment and polymetallic nodules.
Polyplacophora
Leptochitonidae Dall, 1899
Leptochiton Gray, 1847
Leptochiton macleani
Material examined
NHM_446 NHMUK 20170073.1-2, collected 2013-10-20, 13.86367 -116.54432, 4050 m. http://data.nhm.ac.uk/object/d69b581d-8a79-4c4d-8f70-88b2ec07d86e
Description
The form and pattern of tegmental granules together with the three aesthete pores are most similar to the images of Leptochiton macleani (Sirenko, 2015: figs 34–36). Voucher NHM_446 length approx 10 mm, width 3.2 mm (Fig.
Leptochiton macleani Sirenko, 2015. NHM_446 voucher specimen. A Live specimen (lateral view) after recovery from the ROV scoop sample B Preserved specimen (ventro-lateral view) following DNA extraction C Dorsal view D surface detail E SEM of tegmentum surface and pores. Scale bars: 4 mm (A); 0.5 mm (D); 0.3 mm (E). Image attribution Glover, Taylor, Ikebe, Dahlgren & Wiklund, 2017.
Remarks
Ecology
Specimen collected from an ROV scoop in region of sediment and polymetallic nodules, presumed living associated or on the nodule surface, but not directly observed doing so.
Scaphopoda
Dentaliida Starobogatov, 1974
Dentaliidae Children, 1834
Fissidentalium Fischer, 1885
Fissidentalium
Material examined
NHM_261 NHMUK 20170074, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/679fa0ca-d647-446d-87c5-e8d33949efe2
Description
A damaged shell with rib features and curvature similar to Fissidentalium species (see Scarabino, 1995). Voucher NHM_261, poor preservation, length 21 mm, maximum width 3.1 mm (Fig.
Remarks
Forms a unique monophyletic clade distinct from other AB01 specimens. In the molecular analysis it groups with other Fissidentalium species, but with very low support. No genetic matches on GenBank. Phylogenetic tree supports placement in order Dentaliida, family Dentaliidae (Fig.
Ecology
Specimen collected from an epibenthic sledge tow across region of sediment and polymetallic nodules.
Gadilida Starobogatov, 1974
Gadilida
Material examined
NHM_192 NHMUK 20170075, collected 2013-10-13, 13.93482 -116.55018, 4082 m. http://data.nhm.ac.uk/object/fc0e3ae8-9cce-46a0-bb8b-fafe0e2cb46b
Description
Slender, smooth, transparent, annular growth increments, maximum diameter at mouth. Voucher NHM_192, length 4 mm, maximum width 0.5 mm (Fig.
Remarks
Forms a unique monophyletic clade distinct from other AB01 specimens. No genetic matches on GenBank. Phylogenetic tree (Fig.
Ecology
Specimen collected from an epibenthic sledge tow across region of sediment and polymetallic nodules.
Gadilidae Stoliczka, 1868
Gadila Gray, 1847
Gadila
Material examined
NHM_345 NHMUK 20170076, collected 2013-10-17, 13.75583 -116.48667, 4076 m. http://data.nhm.ac.uk/object/c301a72f-54cb-435e-8aae-17cf4d37675f
Description
Short, glossy, transparent, maximum diameter near centre, ventral side curved, dorsal side near straight. Mouth simple, oblique. NHM_345 voucher specimen length 6 mm, width 1.4 mm (Fig.
Remarks
Forms a unique monophyletic clade distinct from other AB01 specimens. No genetic matches on GenBank. Phylogenetic tree supports placement in order Gadilida (Figure
Ecology
Specimen collected from an epibenthic sledge tow across region of sediment and polymetallic nodules.
Gadilida
Material examined
NHM_132 NHMUK 20170077, collected 2013-10-11, 13.75833 -116.69852, 4080 m. http://data.nhm.ac.uk/object/6a1906d9-9ed1-4f6e-a0cf-2d53e2289a01
Description
Shell slender, smooth, fairly transparent, increasing in diameter to a maximum about 2.5 mm from the anterior aperture, then decreasing towards the mouth. NHM_132 voucher specimen length 16.6 mm, max width 3 mm (Fig.
Remarks
Forms a unique monophyletic clade distinct from other AB01 specimens. No genetic matches on GenBank. Phylogenetic tree (Fig.
Ecology
Specimen collected from an epibenthic sledge tow across region of sediment and polymetallic nodules.
Solenogastres
Acanthomeniidae Salvini-Plawen, 1978
Acanthomeniidae
Material examined
NHM_367 NHMUK 20170078.1-2, collected 2013-10-19, 13.93307 -116.71628, 4182 m. http://data.nhm.ac.uk/object/c0577fc9-7302-4fec-bc8c-87a17a38bc91
Description
Voucher specimen NHM_367, small solenogaster specimen, anterior end lacking; fragment ca. 2.5 mm long and 0.5 mm in maximum diameter (Fig.
Genetic data
GenBank NHM_367 COI-MF157519.
Remarks
The combination of scales and hollow spicules as main epidermal sclerites is diagnostic for the family Acanthomeniidae. Forms a unique monophyletic clade distinct from other AB01 specimens (Fig.
Ecology
Specimen collected from an epibenthic sledge tow across region of sediment and polymetallic nodules.
Pruvotinidae Heath, 1911
Lophomeniinae Salvini-Plawen, 1978
Lophomeniinae
Material examined
NHM_027 NHMUK 20170079.1-2, collected 2013-10-09, 13.8372 -116.55843, 4336 m. http://data.nhm.ac.uk/object/319fd186-b07f-4be7-986c-b96c20f63723
Description
Voucher specimen NHM_027, small, probably juvenile, solenogaster specimen (Fig.
Genetic data
GenBank NHM_027 COI-MF157500.
Remarks
Forms a unique monophyletic clade distinct from other AB01 specimens (Fig.
Ecology
Specimen collected from an epibenthic sledge tow across region of sediment and polymetallic nodules.
Discussion
Only one record of benthic mollusc taxa in the CCZ is hitherto reported on OBIS (OBIS 1017; iobis.org), with a further four just south of CCZ. In this study we report 42 records for 21 taxa, of which one is described as a new species. All our data and material from this study are made publicly available through this publication, and through depositing DNA extractions and tissue for further molecular analyses in the Molecular Collections Facility as well as morphological vouchers at the Natural History Museum in London, UK.
Mollusca is a diverse group with its members having very differing life histories, and in this study there are representatives of both sediment-dwelling species and nodule fauna. Not much is known about the mollusc species distribution and connectivity within the CCZ, an information deficit that makes it impossible to assess impact from anthropogenic activities. Genetic data is crucial for distribution analyses as some taxa look very similar and can be difficult to separate to species level based on morphology only, e.g. the new species Ledella knudseni and its sister taxon Ledella sp. (NHM_381). In our study we have used a precautionary approach when reporting taxa that are preliminary identified as described species with type locality far from CCZ, e.g. our Bentharca cf. asperula which is very similar to Bentharca asperula with type locality in Gulf of Mexico. Without genetic information from specimens collected at the type locality, we can not rule out that ours is a different species despite the similarity in morphology.
The protobranch bivalve Nucula profundorum is the most abundant bivalve mollusc in our samples, and population connectivity analyses are underway (Dahlgren et al. in prep). Morphologically it is identical to type material of the original Nucula profundorum, which was described from collections of HMS Challenger in the mid-North Pacific (36°N, 178°E) at about 3750 m depth (Fig.
There are very few DNA sequences from a few faunal groups from the CCZ available on GenBank, e.g. echinoderms (
Acknowledgements
The ABYSSLINE (ABYSSal baseLINE) environmental survey of the UK-1 exploration area was supported by a collaborative partnership between six non-profit global academic research institutes (University of Hawaii (UH) at Manoa, Natural History Museum (NHM), Uni Research, National Oceanography Centre, Senckenberg Institute, IRIS Norway) and through an arrangement with UK Seabed Resources Ltd. We gratefully acknowledge the leadership of Prof Craig R Smith (UH) on this project and during the research cruises. Additional support for the project was provided by the Swedish research council FORMAS (TGD). We would like to acknowledge the support of Maggie Georgieva (NHM) and Iris Altamira (UH) in sorting on board ship, and Henk H. Dijkstra from Naturalis Biodiversity Center in Leiden, Netherlands for taxonomic advice on Catillopecten. We would also like to acknowledge the expert support from the Senckenberg Institute team in the deployment and recovery of successful Brenke Epibenthic Sledge samples on the first ABYSSLINE cruise (Nils Brenke, Pedro Martinez Arbizu and Inga Mohrbeck). This study was made possible only by the dedicated help from the entire scientific party and the Masters and Crew of the Research Vessel Melville during the first cruise of the ABYSSLINE project in October 2013. Thanks also to Jackie Mackenzie-Dodds at the NHM Molecular Collection Facility, and Ben Scott of the NHM Diversity and Informatics group for work on the NHM Data Portal.
References
- Abbott RT (1954) American seashells. Van Nostrand Company, New York, 541 pp.
- Adams H, Adams A (1858) The genera of recent Mollusca arranged according to their organisation. J. van Voorst, London.
- Allen JA (2008) Bivalvia of the deep Atlantic. Malacologia 50: 57–103. https://doi.org/10.4002/0076-2997-50.1.57
- Allen JA, Hannah FJ (1989) Studies on the deep sea Protobranchia: the subfamily Ledellinae (Nuculanidae). The Natural History Museum (London), Bulletin (Zoology) 55: 123–171.
- Allen JA, Morgan RE (1981) The functional morphology of Atlantic deep water species of the families Cuspidariidae and Poromyidae (Bivalvia): an analysis of the evolution of the septibranch condition. Philosophical Transactions of the Royal Society of London 294: 413–546. https://doi.org/10.1098/rstb.1981.0117
- Allen JA, Sanders HL (1982) Studies on the deep sea Protobranchia: the subfamily Spinulinae (family Nuculanidae). Bulletin of the Museum of Comparative Zoology 150: 1–30.
- Bruun AF, Greve S, Mielche H, Spärck R (Eds) (1956) The Galathea deep sea expedition 1950–1952. Described by members of the expedition. George Allen and Unwin Ltd, London, 296 pp.
- Children JG (1834) Synopsis of the contents of the British Museum (28th edition). British Museum, London, 240 pp.
- Coan EV, Scott PH (1997) Checklist of marine bivalves of the northeastern Pacific Ocean. Santa Barbara Museum of Natural History, Contributions in Science 1: 1–28.
- Coan EV, Valentich-Scott PH (2012) Bivalve seashells of tropical west America: Marine bivalves from Baja California to northern Peru. Santa Barbara Museum of Natural History Monographs 6: 1–1258.
- Cohen BL, Gawthrop A, Cavalier-Smith T (1998) Molecular phylogeny of brachiopods and phoronids based on nuclear-encoded small subunit ribosomal RNA gene sequences. Philosophical Transactions of the Royal Society B Biological Sciences 353: 2039–2061. https://doi.org/10.1098/rstb.1998.0351
- Dahlgren TG, Wiklund H, Rabone M, Amon DJ, Ikebe C, Watling L, Smith CR, Glover AG (2016) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Cnidaria. Biodiversity Data Journal 4: e9277. https://doi.org/10.3897/BDJ.4.e9277
- Dall WH (1908) The Mollusca and the Brachiopoda. Reports on the dredging operations off the west coast of Central America to the Galapagos, to the west coast of Mexico, and in the Gulf of California, in charge of Alexander Agassiz. Bulletin of the Museum of Comparative Zoology 43: 203–487.
- Dall WH (1881) Reports on the results of dredging, under the supervision of Alexander Agassiz, in the Gulf of Mexico and in the Caribbean Sea (1877-78), by the United States Coast Survey Steamer “Blake”, Lieutenant-Commander C.D. Sigsbee, U.S.N., and Commander J.R. Bart. Bulletin of the Museum of Comparative Zoology at Harvard College 9: 33–144.
- Dall WH (1900) Contributions to the Tertiary fauna of Florida, with especial reference to the Miocene silex-beds of Tampa and the Pliocene beds of the Calooshatchie River. Transactions of the Wagner Free Institute of Science of Philadelphia 3: 949–1189.
- Dall WH (1878) Descriptions of new forms of mollusks from Alaska contained in the collection of the National Museum. Proceedings of the United States National Museum 1: 1–3. https://doi.org/10.5479/si.00963801.1
- Dall WH (1895) Science results of explorations by the US Fish Commission Steamer Albatross. No. 34. Report on Mollusca and Brachiopoda dredged in deep water, chiefly near the Hawaiian Islands, with illustrations of the hitherto unfigured species from northwest America. In: US Nat. Mus., Proc., 675–733.
- Dall WH (1886) Reports on the results of dredging, under the supervision of Alexander Agassiz, in the Gulf of Mexico (1877–78) and in the Carribean Sea (1879–80), by the U.S. Coast Survey steamer “Blake”, Lieut.-Commander C.D. Sigsbee, U.S.N. and Commander J.R. Bartlett. Bulletin of the Museum of Comparative Zoology at Harvard College 12: 171–318.
- Dall WH (1916) Diagnoses of new species of marine bivalve mollusks from the northwest coast of America in the collection of the United States National Museum. Proceedings of the United States National Museum 52: 393–417. https://doi.org/10.5479/si.00963801.52-2183.393
- Dall WH, Simpson CT (1901) The Mollusca of Puerto Rico. Bulletin of the US Fish Commission 20: 351–524.
- Donoghue MJ (1985) A Critique of the Biological Species Concept and Recommendations for a Phylogenetic. The Bryologist 88: 172–181. https://doi.org/10.2307/3243026
- Ebbe B, Billett DSM, Brandt A, Ellingsen K, Glover A, Keller S, Malyutina M, Martínez Arbizu P, Molodtsova T, Rex M, Smith C, Tselepides A (2010) Diversity of Abyssal Marine Life. In: Life in the World’s Oceans. Wiley-Blackwell, 139–160. https://doi.org/10.1002/9781444325508.ch8
- Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. https://doi.org/10.1093/nar/gkh340
- Etter RJ, Rex MA (1990) Population differentiation decreases with depth in deep-sea gastropods. Deep Sea Research Part A, Oceanographic Research Papers 37: 1251–1261. https://doi.org/10.1016/0198-0149(90)90041-S
- Fischer P (1885) 1880-1887. Manuel de conchyliologie et de paleontologie conchyliologique ou histoire naturelle des mollusques vivants et fossiles. Libraire F. Savy, Paris, 1369 pp.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299. https://doi.org/10.1371/journal.pone.0013102
- Forbes E (1844) On the Mollusca and Radiata of the Aegean Sea and on their distribution, considered as bearing on geology. Report of the British Association for the Advancement of Science 1843: 130–193.
- Glover AG, Dahlgren TG, Wiklund H, Mohrbeck I, Smith CR (2016a) An End-to-End DNA Taxonomy Methodology for Benthic Biodiversity Survey in the Clarion-Clipperton Zone, Central Pacific Abyss. Journal of Marine Science and Engineering 4: 2. https://doi.org/10.3390/jmse4010002
- Glover AG, Wiklund H, Rabone M, Amon DJ, Smith CR, O’Hara T, Mah CL, Dahlgren TG (2016b) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Echinodermata. Biodiversity Data Journal 4: e7251. https://doi.org/10.3897/BDJ.4.e7251
- Gray JE (1824) Shells. In: Parry WE (Ed.) Supplement to Appendix, Parry’s Voyage for the Discovery of a north-west passage in the years 1819-1820, containing an account of the subjects of Natural History London, John Murray.Appendix 10, Zool, 240–246.
- Gray JE (1847) A list of the genera of recent Mollusca, their synonymy and types. Proceedings of the Zoological Society London 15: 129–206.
- Heath H (1911) Reports on the scientific results of the expedition to the Tropical Pacific, in charge of Alexander Agassiz, by the U. S. Fish Commission Steamer Albatross, from August 1899 to June 1900, Commander Jefferson F. Moser. XVI. The Solenogastres. Memoirs of the Museum of Comparative Zoology at Harvard College 45: 1–182.
- Hedley C (1907) The results of deep-sea investigation in the Tasman Sea. II. The expedition of the ‘Woy Woy. ’ Records of the Australian Museum 6: 356–364. https://doi.org/10.3853/j.0067-1975.6.1907.1020
- Hendrickx M, Valentich-Scott P, Suárez-Mozo N (2016) Deep-water bivalve mollusks collected during the TALUD XV cruise off the west coast of the southern Baja California Peninsula, Mexico. Biodiversity Data Journal 4: e8661. https://doi.org/10.3897/BDJ.4.e8661
- International Seabed Authority (1999) Deep-Seabed Polymetallic Nodule Exploration: Development of Environmental Guidelines. Kingston, Jamaica
- Iredale T (1924) Results from Roy Bell’s molluscan collections. Proceedings of the Linnean Society of New South Wales 49: 179–278.
- Janssen A, Kaiser S, Meißner K, Brenke N, Menot L, Arbizu PM (2015) A reverse taxonomic approach to assess macrofaunal distribution patterns in abyssal pacific polymetallic nodule fields. PLoS ONE. https://doi.org/10.1371/journal.pone.0117790
- Jennings RM, Etter RJ (2014) Phylogeographic estimates of colonization of the deep Atlantic by the protobranch bivalve Nucula atacellana. Polish Polar Research 35: 261–278. https://doi.org/10.2478/popore-2014-0017
- Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic acids research 30: 3059–3066. https://doi.org/10.1093/nar/gkf436
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
- Killeen IJ, Turner JA (2009) Yoldiella and Portlandia (Bivalvia) from the Faroe-Shetland Channel and Rockall Trough, Northeast Atlantic. Journal of Conchology 39: 733–778.
- Knight JB, Yochelson EL (1958) A reconsideration of the Monoplacophora and the primitive Gastropoda. Proceedings of the Malacological Society of London 33: 37–48.
- Knudsen J (1967) The deep-sea Bivalvia. John Murray Expedition Scientific Reports 11: 237–343.
- Knudsen J (1970) The systematics and biology of abyssal and hadal Bivalvia. Galathea Reports 11: 1–241.
- Krylova EM, Kamenev GM, Vladychenskaya IP, Petrov NB (2015) Vesicomyinae (Bivalvia: Vesicomyidae) of the Kuril-Kamchatka Trench and adjacent abyssal regions. Deep-Sea Research Part II: Topical Studies in Oceanography 111: 198–209. https://doi.org/10.1016/j.dsr2.2014.10.004
- Lamarck J-B (1818) Histoire naturelle des animaux sans vertèbres ... précédée d’une introduction offrant la détermination des caractères essentiels de l’animal, sa distinction du végétal et des autres corps naturels, enfin, l’exposition des principes fondamentaux de la zool. Verdière, Paris, 1–636. http://www.biodiversitylibrary.org/item/46337 [March 15, 2017]
- Lamarck J-B (1799) Prodrome d’une nouvelle classification des coquilles, comprenant une rédaction appropriée des caractères génériques, et l’établissement d’un grand nombre de genres nouveaux. Mémoires de la Société d’Histoire Naturelle de Paris: 63–91.
- Medlin L, Elwood HJ, Stickel S, Sogin ML (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71: 491–499. https://doi.org/10.1016/0378-1119(88)90066-2
- Nygren A, Sundberg P (2003) Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Molecular Phylogenetics and Evolution 29: 235–249. https://doi.org/10.1016/S1055-7903(03)00095-2
- Payne CM, Allen JA (1991) The Morphology of Deep-Sea Thyasiridae (Mollusca: Bivalvia) from the Atlantic Ocean. Philosophical Transactions of the Royal Society B: Biological Sciences 334: 481–562. https://doi.org/10.1098/rstb.1991.0128
- Poutiers J, Bernard F (1995) Carnivorous bivalve molluscs (Anomalodesmata) from the tropical western Pacific Ocean, with a proposed classification and a catalogue of Recent species. Mémoires du Muséum National d’Histoire Naturelle 167: 107–187.
- Rafinesque CS (1815) Analyse de la nature ou tableau de l’univers et des corps organisés. Published by the author, Palerme, 214 pp. https://doi.org/10.5962/bhl.title.106607
- Rokop FJ (1972) A new species of monoplacophoran from the abyssal north Pacific. Veliger 15: 91–95.
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Salvini-Plawen L (1978) Antarktische und subantarktische Solenogastres (eine Monographie: 1898-1974). Zoologica 128: 1–305.
- Salvini-Plawen L (1975) MolluscaCaudofoveata. Marine invertebrates of Scandinavia, 4. Universitetsforlaget, Oslo, 54 pp.
- Sassi A (1827) Saggio geologico sopra il Bacino terziario di Albenga. Giornale ligustico di scienze: 467–484.
- Scarabino V (1995) Scaphopoda of the tropical Pacific and Indian Oceans, with description of 3 new genera and 42 new species. Mémoires du Muséum National d’Histoire Naturelle 167: 189–379.
- Schenck G (1939) Revised nomenclature for some nuculid pelecypods. Journal of Paleontology 13: 21–41.
- Sharma PP, Zardus JD, Boyle EE, González VL, Jennings RM, McIntyre E, Wheeler WC, Etter RJ, Giribet G (2013) Into the deep: A phylogenetic approach to the bivalve subclass Protobranchia. Molecular Phylogenetics and Evolution 69: 188–204. https://doi.org/10.1016/j.ympev.2013.05.018
- Sirenko B (2015) Shallow and deep-sea chitons of the genus Leptochiton Gray, 1847 (Mollusca: Polyplacophora: Lepidopleurida) from Peruvian and Chilean waters. Zootaxa 4033: 151–202. https://doi.org/10.11646/zootaxa.4033.2.1
- Sjölin E, Erséus C, Källersjö M (2005) Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Molecular Phylogenetics and Evolution 35: 431–441. https://doi.org/10.1016/j.ympev.2004.12.018
- Smith CR, Dahlgren TG, Drazen J, Gooday A, Glover AG, Kurras G, Martinez-Arbizu P, Shulse C, Spickermann R, Sweetman AK, Vetter E (2013) Abyssal Baseline Study (ABYSSLINE) Cruise Report. Seafloor Investigations Report 2013-1304-051J-SRDL-AB01.
- Smith CR, De Leo FC, Bernardino AF, Sweetman AK, Arbizu PM (2008) Abyssal food limitation, ecosystem structure and climate change. Trends in Ecology and Evolution 23: 518–528. https://doi.org/10.1016/j.tree.2008.05.002
- Smith EA (1885) Report on the Lamellibranchiata collected by HMS Challenger during the years 1873-1876. Reports of the scientific results of the voyage of H.M.S. “Challenger”, Zoology 13: 1–341.
- Starobogatov YI (1974) Xenoconchias and their bearing on the phylogeny and systematics of some molluscan classes. Paleontologicheskii Zhurnal 1: 3–18.
- Starobogatov YI, Moskalev LI (1987) Systematics of the Monoplacophora. In: Starobogatov YI, Golikov AN, Likarev IM (Eds) Molluscs, results and perspectives of investigation. USSR Academy of Sciences, Zoological Institute, Leningrad.
- Tizard TH, Moseley HN, Buchanan JY, Murray J (1885) Narrative of the cruise of HMS Challenger with a general account of the scientific results of the expedition. HMSO, London.
- Torell O (1859) Bidrag till Spitsbergens Mollusk-fauna. Föreningens Boktryckeri, Stockholm, 154 pp.
- Verrill AE, Bush KJ (1897) Revision of the genera of Ledidae and Nuculidae of the Atlantic coast of the United States. American Journal of Science 3: 51–63. https://doi.org/10.2475/ajs.s4-3.13.51