Research Article |
|
Corresponding author: Adrienne Jochum ( adrienne.jochum@gmail.com ) Academic editor: Thomas A. Neubauer
© 2023 Adrienne Jochum, Estée Bochud, David Haberthür, Harry G. Lee, Ruslan Hlushchuk, Roger W. Portell.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Jochum A, Bochud E, Haberthür D, Lee HG, Hlushchuk R, Portell RW (2023) Fossil Carychiidae (Eupulmonata, Ellobioidea) from the Lower Pleistocene Nashua Formation of Florida, with the description of a new species. ZooKeys 1167: 89-107. https://doi.org/10.3897/zookeys.1167.102840
|
Abstract
Recent fossil shell mining for a new rail line in the Orlando area of Orange County, Florida has uncovered two species of the ellobioid genus Carychium O. F. Müller, 1773 in a bed of freshwater marl from the Lower Pleistocene Nashua Formation. To taxonomically interpret these finds, the well-preserved shells were imaged via high-resolution X-ray tomography (micro-CT) to view significant internal diagnostic characters such as the columellar configuration and the degree of lamellar sinuosity and their relationship in context to the entire shell. The image data are compared to that of type material and extant and fossil Carychium species inhabiting the SE USA, Mexico, Central America, and Jamaica. Based on these results, the species Carychium floridanum G. H. Clapp, 1918 and Carychium nashuaense sp. nov. are identified from fossil shells dating from the Early Pleistocene. This work documents the first fossil members of C. floridanum and the first fossil Carychium from the SE USA.
Key words
Central Florida, computer tomography, freshwater marl, mollusc assemblage, Orlando, Pleistocene
Introduction
Records of nonmarine fossil molluscs are rare in Florida, and little is known about their distributions in the state. Studies of Pleistocene mollusc faunas in Florida largely focus on marine taxa while fossil nonmarine mollusc beds remain poorly investigated (
Though the shells of this malacofaunule will be taxonomically treated and analyzed later in stratigraphic, paleobiogeographic, and ecological contexts, the focus of this present work is on the morphological assessment and taxonomic treatment of the Carychium shells. So far, records of fossil Carychium are not yet known in Florida. North American Late Cenozoic congeners include fossils of the extant species, Carychium exiguum (Say, 1822) from the Late Pliocene of southwestern Kansas and northern Oklahoma (
By using high-resolution X-ray tomography (micro-CT), we compare the internal shell morphology with that of type material of extant congeners found in the SE USA, Mexico, Central America, and Jamaica (
Material and methods
Sampling, imaging, and measurements
Two 7.5 l bulk samples from the middle of the freshwater layer were sorted by hand, washed through a graded series of sieves, and allowed to dry for further processing. Thirty-two Carychium shells were culled from a mixture of other molluscs and debris under a stereomicroscope.
All eight shells analyzed in this study are in a good state of preservation and are deposited in the Natural History Museum Bern, Bern, Switzerland (
Several qualitative aspects of shell morphology are addressed including peristome shape; whorl profile (whorl convexity); teleoconch sculpture; development of apertural barriers visible in frontal view, including the presence of a deeply immersed denticle/lamella on the parieto-columellar region of the aperture; development of the columellar lamella as discernable in the micro-CT scans of the ventral, dorsal, side-left, side-right and umbilical perspectives of the adult shell.
Light microscopy
Different perspectives of the shells were imaged using a Leica MC190 HD digital camera attached to a Leica M205 stereo microscope (Leica Microsystems GmbH, Wetzlar, Germany). The multifocal images were processed using the software Leica Application Suite X (LAS X) version 5.1.0.25593 (Leica Microsystems). All measurements are in millimeters (mm). Shell measurements are expressed as SH (shell height), SW (shell width), PH (peristome height) and PW (peristome width). All measurements were made using the measuring tool in the Leica LASX application. Shell whorl number was counted (to the closest 0.25 whorl) according to
X-ray tomographic microscopy (micro-CT)
Aside from the extant Carychium floridanum shell [
The Recent C. floridanum shell [
For the fossils in this study, shells were packaged in radiotransparent Basotect melamine resin foam and individually imaged on a Bruker SkyScan 2214 multiscale X-ray micro-computed tomography system at the Institute of Anatomy of the University of Bern in Switzerland (with the control software version 1.8, Bruker micro-CT, Kontich, Belgium). The system is equipped with both a flat-panel detector for scanning large samples and a high-resolution CCD camera, which was used for scanning the Carychium shells. The Hamamatsu L10711 X-ray source was set to a tube voltage of 60 kV and a tube current of 130 µA. Individual shells were scanned at a voxel size of 2.0 µm with one shell [C. floridanum (
The scans with a resulting voxel size of 2.0 µm were acquired with a set of 1913 projections of 4032 × 2688 pixels at every 0.1° over a sample rotation of 180°. Every single projection was exposed for 2187 min, three projections were averaged to reduce image noise. This resulted in a scan time of approximately 5 h and 15 min.
The scan of C. floridanum (
The projection images of each scan were reconstructed into a 3D stack of images with NRecon (Version 2.1.0.1, Bruker micro-CT, Kontich Belgium). The entire process resulted in datasets with the isometric voxel size of 2.0 and 0.5 µm, respectively. Scanning parameters are given in Suppl. material
Repositories
AJC Adrienne Jochum Collection: now housed at the
Results
Taxonomy
Family Carychiidae Jeffreys, 1830
Genus Carychium O. F. Müller, 1773
Carychium floridanum
Carychium exiguum floridanum G. H. Clapp, 1918: 73–75, pl. 8.
Carychium floridanum
G. H. Clapp, 1918:
Carychium floridanum
G. H. Clapp, 1918:
Material examined
USA, Florida • Orange County, Orlando; 28.4489, −81.0375 (WGS84) encompassing 500 m radius; Oct. 2021; R. Portell and H. Means leg.;
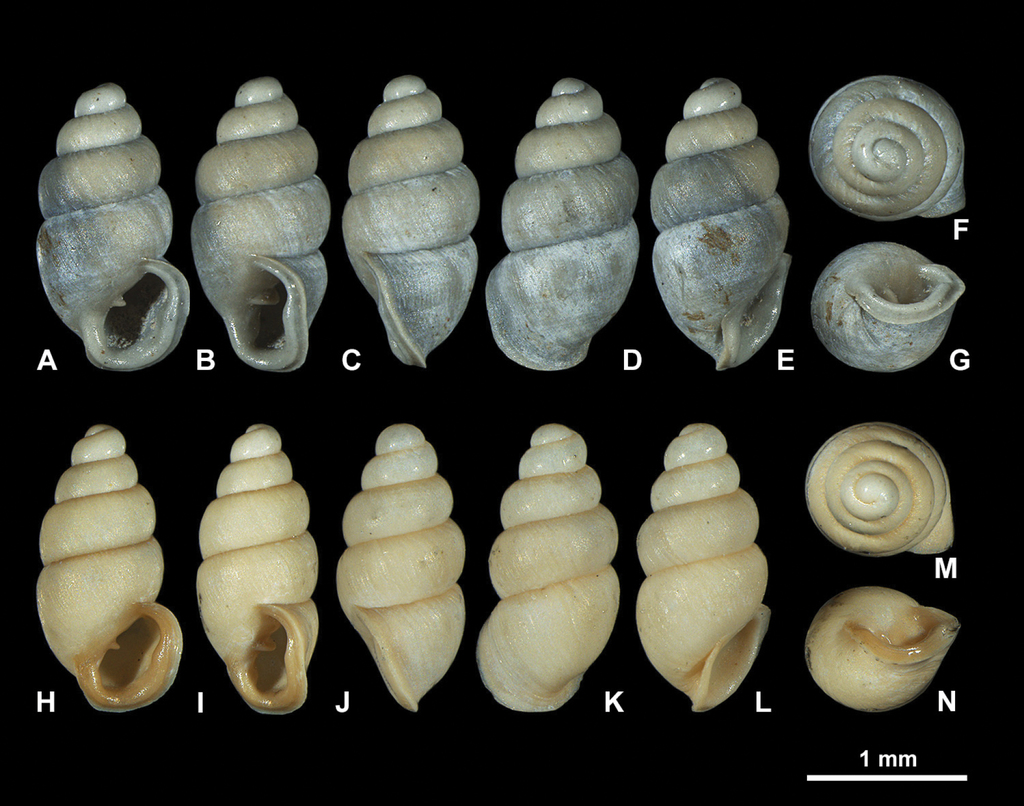
Fossil Carychium floridanum G. H. Clapp, 1918 (
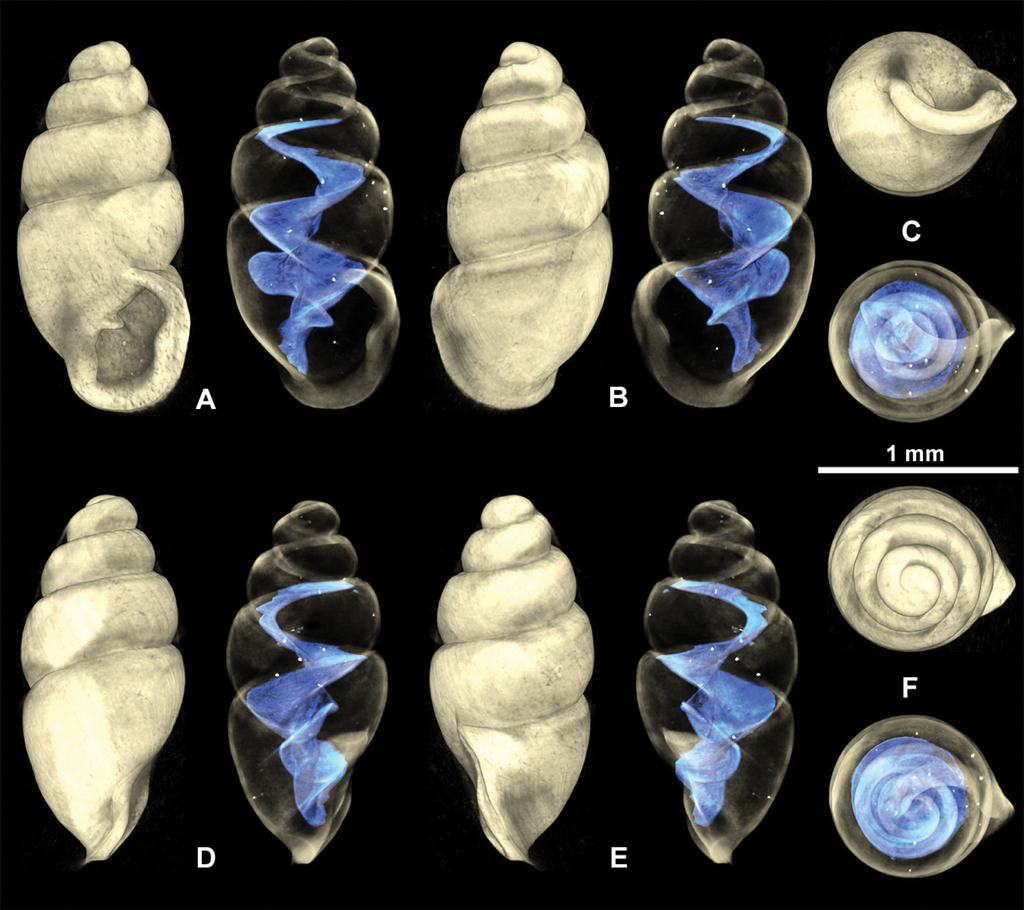
3D visualizations of X-ray micro-CT data of fossil Carychium floridanum G. H. Clapp, 1918 (
The two shells were compared to the syntype shell from Snapper Creek Hammock, Miami, FL (
Recent Carychium floridanum G. H. Clapp, 1918 (
3D visualizations of X-ray micro-CT data of extant Carychium floridanum G. H. Clapp, 1918 (
Stratigraphic occurrence
Freshwater Marl Bed (middle nonmarine layer) Florida Museum of Natural History Invertebrate Paleontology (
Description
Shell medium-sized for genus (SH: 1.6–1.8 mm), pupiform in shape, glossy; teleoconch with occasional, fine threadlike ribs, with 4.5–5 tumid whorls. Suture deep. Protoconch smooth, bulbous, shiny, and distinct from teleoconch. Whorl profile strongly convex, aperture higher than wide, elliptical, with 2 barriers, parietal robust with the columellaris constituting a low swollen hump deep in the shell. Peristome doubled and heavily callused, expanded in palatal, basal and columellar regions; largely reflected at the base. Palatal lip weakly sigmoid and buttressed with heavy callus inside and marked with a concavity or line at mid-section behind the apertural rim, extending 2/3 the body whorl in aperture facing left profile view (clearly visible on the grey shell and not detectable on the yellowish one) (Fig.
Measurements
SH = 1.6–1.8 mm; SW = 0.81–0.92 mm; PH = 0.60–0.7 mm; PW = 0.61–0.67 mm (N = 3).
Occurrence
A frequent find in the bulk samples of the Freshwater Marl Bed (middle nonmarine layer).
Living C. floridanum are known today from central Florida up to Wakulla Springs (
Recent material for comparison
Carychium floridanum G. H. Clapp, 1918, Wakulla Springs, Wakulla County, FL; 30.2355, −84.3031; BARCA032-10; [
Description and comparison
Shell pupiform, translucent with fine threadlike ribbing on the teleoconch. 4.5 tumid whorls. Aperture elliptical with 2 barriers; parietalis points downwards and columellaris is stronger than in the fossil shells. The fossil shells show greater whorl convexity and a bulbous protoconch. Peristome unevenly shaped and thickly callused, parietal shield more extensive than in fossil shells. Measurements (
Remarks
Considering that C. floridanum is clearly genetically and morphologically distinct from its SE North American congeners [EL C5 in
Carychium nashuaense , sp. nov.
Type material
Holotype
: USA, Florida • SH = 1.58 mm, SW = 0.72 mm, PH = 0.56 mm, PW = 0.53 mm; Orange County, Orlando; 28.4489, −81.0375 (WGS84) encompassing 500 m radius; Oct. 2021; R. Portell and H. Means leg.;
Measurements
SH = 1.43–1.63 mm, SW = 0.68–0.69 mm, PH = 0.53–0.57 mm, PW = 0.51–0.54 mm.
Diagnosis
Shell 1.54 mm (mean) in height, elongate-pupiform with elliptical-ovate shaped aperture, thickly callused double peristome with a deeply set columellar-basal apertural barrier and a pronounced parietal denticle. Internally, C. nashuaense sp. nov. has a highly sinuate, tightly coiled, double structured lamellar configuration.
Description
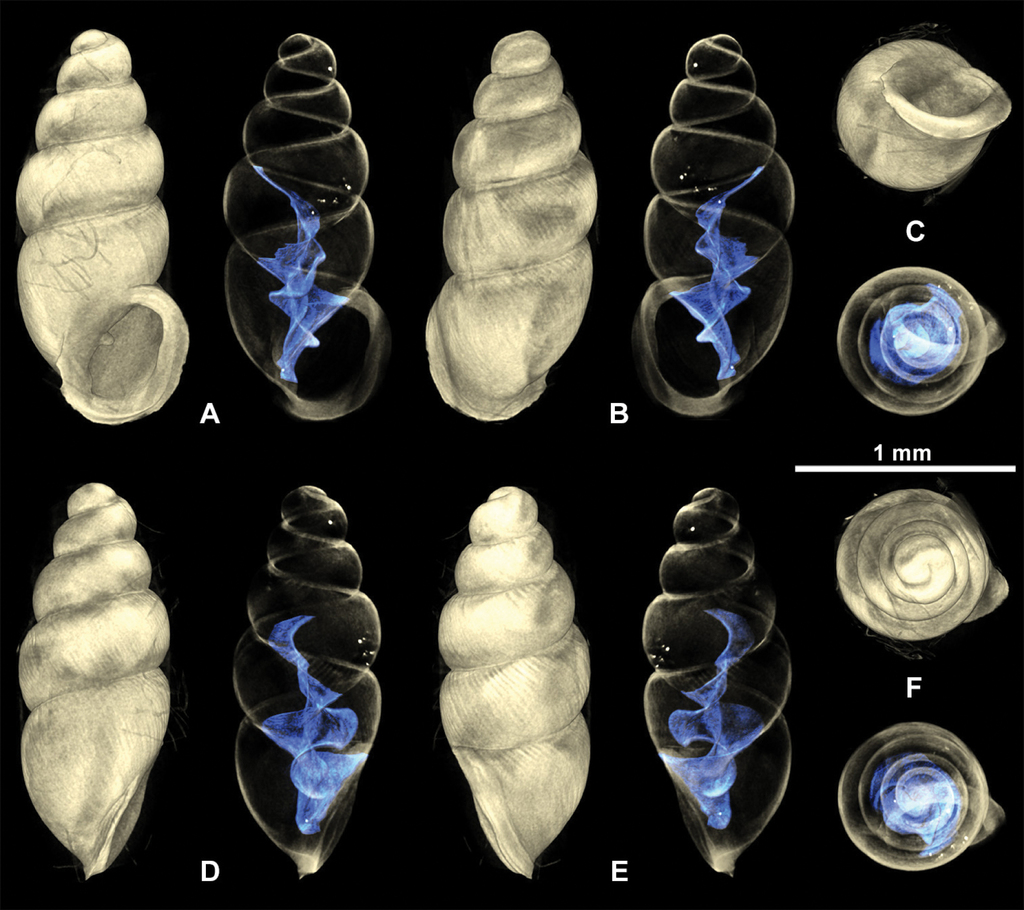
Shell medium-sized for the genus, elongate-pupiform, robust, with 5 tumid whorls and a large aperture. PH is 36% of SH, elliptical-ovate, the inner callused, upper palatal side is somewhat angular. Protoconch bulbous, smooth, and shiny; teleoconch ornamented by equidistantly aligned, broad ribs. Suture deeply impressed, not descending towards the aperture. Whorl profile strongly convex, especially on middle whorls, less so on body whorl. Peristome thickly callused and doubled with a thinner, sharp rim at the margins. Columellar portion broadly expanded, heavily callused, palatal lip thicker at mid-section. Outer, basal and columellar margins reflected, parietal callus thick. Two apertural barriers, visible in apertural view; large prominent deeply set parietalis situated almost medially with a slight downward tendency, not reaching margin of peristome; small columellaris near the base of the columella. The moderately thick parietalis is set deep and advances a short distance beyond the thickly callused inner margin of the peristome in umbilical view. Umbilicus chink-like. From apical view, the spire is dominated by the tumid third and fourth whorls causing the body whorl to completely disappear under their convexity. The reflected, wing-like apertural lip projects away from the spire in apical and umbilical view (Figs
3D visualizations of X-ray micro-CT data of Carychium nashuaense sp. nov. holotype (
3D visualizations of X-ray micro-CT data of Carychium nashuaense sp. nov. paratype (
3D visualizations of X-ray micro-CT data of Carychium nashuaense sp. nov. Paratype (
Differential diagnosis
Carychium nashuaense sp. nov. appears morphologically to be a cross between C. belizeense Jochum & Weigand, 2017 and C. hardiei Jochum & Weigand, 2017. These two species are much larger than C. nashuaense sp. nov. and have smoother shells. Even though C. nashuaense sp. nov. was buried under marl for about 2 million years, the ribbing structure on the teleoconch is remarkably well preserved. The shell shape with the highly convex third and fourth whorls extending over the body whorl, preventing it from being observed in apical view, is most similar to that of C. belizeense. On the other hand, the prominent parietalis proceeding far above the peristome edge (umbilical view) and the elliptical apertural form and reduced peristome thickness in that species differs significantly from that of the highly callused C. nashuaense sp. nov. Internally, the lamella of C. belizeense is highly sinuate and complexly structured on a very compact and short columella.
Etymology
The specific epithet refers to this species being found in the Freshwater Marl Bed of the Nashua Formation.
Stratigraphic occurrence
Freshwater Marl Bed (middle nonmarine layer) (
Discussion
The two Carychium species of this study show on the one hand, the relative constancy of internal character expression in members of northern and central Floridian C. floridanum since the Early Pleistocene and on the other, potential affinity of C. nashuaense sp. nov. with a Central American congener for c. 2 million years ago. Though clarified by
Considering the remarkable similarity of C. nashuaense sp. nov. with that of C. hardiei in Georgia and more so that of C. belizeense from Belize in Central America, some parallels can be drawn in conjunction with avian community data from the Plio-Pleistocene in Florida (
Birds are considered to have facilitated long distance dispersal in snails and as a result, their distributions. They are known vectors of expanded snail distributions via snail transport in the gut and in their plumage (
Given the fossil site’s close proximity (less than 50 km) to the coast of the Atlantic Ocean and its surrounding low-lying topography (< 21 m at the surface), the area has been subject to transgressive (landward migration of the shoreline) and regressive (oceanward migration of the shoreline) episodes due to sea-level oscillations during the Pleistocene (
We strongly encourage continued exploration and investigations in other regions of Florida to glean more knowledge about Carychium distribution, ecology, and community structure in the state.
Acknowledgements
We thank Jesse McKinnon for allowing access to exposures at his mining operation, Guy H. Means for assistance in bulk matrix sampling, Sean Roberts for drafting Figs
Additional information
Conflict of interest
No conflict of interest was declared.
Ethical statement
No ethical statement was reported.
Funding
No funding was reported.
Author contributions
All authors contributed to this work.
Author ORCIDs
Adrienne Jochum https://orcid.org/0000-0002-6624-6412
David Haberthür https://orcid.org/0000-0003-3388-9187
Data availability
All of the data that support the findings of this study are available in the main text or Supplementary Information.
References
- Auffenberg K, Quitmyer IR, Williams JD, Jones DS (2006) Non-marine Mollusca. In: Webb SD (Ed.) First Floridians and Last Mastodons: The Page-Ladson Site in the Aucilla River. Springer, Dordrecht, 247–261. https://doi.org/10.1007/978-1-4020-4694-0
- Chitty E (1853) Descriptions of thirty supposed new species and varieties of land and fluviatile shells of Jamaica, with observations on some shells already described. Contributions to Conchology 1(13): 1–19.
- Clapp GH (1918) New southern forms of Carychium and Thysanophora. The Nautilus 31(3): 73–74.
- Emslie SD (1998) Avian community, climate, and sea-level changes in the Plio-Pleistocene of the Florida Peninsula. Ornithological Monographs 50(50): 1–113. https://doi.org/10.2307/40166707
- Green AJ, Figuerola J (2005) Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Diversity & Distributions 11(2): 149–156. https://doi.org/10.1111/j.1366-9516.2005.00147.x
- Greger DK (1933) The Pleistocene Mollusca of Missouri. American Midland Naturalist 14(1): 58–61. https://doi.org/10.2307/2420053
- Hibbard CW, Taylor DW (1960) Two late Pleistocene faunas from southwestern Kansas. Contributions from the Museum of Paleontology. The University of Michigan, Ann Arbor 16(1): 1–222.
- Hine AC (2013) Geologic History of Florida. University Press of Florida, 229 pp.
- Hubricht L (1964) Some Pleistocene land snail records from Missouri and Illinois. Sterkiana 13: 7–17.
- Hubricht L (1985) The distributions of the native land mollusks of the eastern United States. Fieldiana: Zoology New Series 24, Field Museum of Natural History, Chicago, Illinois, 191 pp. https://doi.org/10.5962/bhl.title.3329
- Huddlestun PF (1988) A revision of the lithostratigraphic units of the coastal plain of Georgia; The Miocene through Holocene. Georgia Geologic Survey Bulletin 104: 1–166.
- Jochum A, Weigand AM, Bochud E, Inäbnit T, Dörge D, Ruthensteiner B, Favre A, Martels G, Kampschulte M (2017) Three new species of Carychium O. F. Müller, 1773 from the southeastern USA, Belize, and Panama are described using computer tomography (CT) (Eupulmonata, Ellobioidea, Carychiidae). ZooKeys 675: 97–127. https://doi.org/10.3897/zookeys.675.12453
- Kappes H, Haase P (2012) Slow, but steady: Dispersal of freshwater molluscs. Aquatic Sciences 74(1): 1–14. https://doi.org/10.1007/s00027-011-0187-6
- Karrow PF, Morgan GS, Portell RW, Simmons E, Auffenberg K (1996) Middle Pleistocene (early Rancholabrean) vertebrates and associated marine and non-marine invertebrates from Oldsmar, Pinellas County. In: Stewart KM, Seymour L (Eds) Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals: Tributes to the Career of CS (Rufus) Churcher. University of Toronto Press, 97–133. https://doi.org/10.3138/9781487574154-009
- Kerney MP, Cameron RAD (1979) A Field Guide to the Land Snails of Britain and North-west Europe. Collins, London, 299 pp.
- Lea HC (1842) Description of eight new species of shells, native to the United States. The American Journal of Science and Arts 42(1): 106–112.
- Müller OF (1773) Vermivm terrestrium et fluviatilium, seu animalium infusoriorum, helminthicorum, et testaceorum, non marinorum, succincta historia. Volumen Imi pars Ima [1–33]. Havniæ & Lipsiæ. (Heineck & Faber), 315 pp. https://doi.org/10.5962/bhl.title.46299
- Paul CRC, Donovan SK (2005) Quaternary and Recent land snails (Mollusca: Gastropoda) from Red Hills Road Cave, Jamaica. Bulletin of the Mizunami Fossil Museum 32: 109–144.
- Pierce HG (1975) Diversity of Late Cenozoic gastropods of the southern High Plains [DPhil thesis]. Lubbock, Texas, USA: Texas Tech University, 267 pp.
- Pilsbry HA (1891) Land and fresh-water molluscs collected in Yucatan and Mexico. Proceedings. Academy of Natural Sciences of Philadelphia 43: 318–334.
- Pilsbry HA (1948) Land Mollusca of North America (north of Mexico). Volume 2, Part 2. The Academy of Natural Sciences of Philadelphia, Monographs 3: i–xlvii + 521–1113.
- Puri HS, Vanstrum VV (1969) Geologic History of the Miocene and younger sediments in south Florida. In: DuBar JR, DuBar SS (Eds) Late Cenozoic stratigraphy of southwestern Florida. Gulf Coast Association of Geological Societies, Society of Economic Mineralogists and Paleontologists, Miami Beach, Guidebook Field Trip 4: 70–86.
- Say T (1822) Description of univalve terrestrial and fluviatile shells of the United States. Journal of the Academy of Natural Sciences of Philadelphia 2(2): 370–381.
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: An open-source platform for biological-image analysis. Nature Methods 9(7): 676–682. https://doi.org/10.1038/nmeth.2019
- Schmid B, Tripal P, Fraaß T, Kersten C, Ruder B, Grüneboom A, Huisken J, Palmisano R (2019) 3Dscript: Animating 3D/4D microscopy data using a natural-language-based syntax. Nature Methods 16(4): 278–280. https://doi.org/10.1038/s41592-019-0359-1
- van Leeuwen CHA, van der Velde G (2012) Prerequisites for flying snails: External transport potential of aquatic snails by waterbirds. Freshwater Science 31(3): 963–972. https://doi.org/10.1899/12-023.1
- van Leeuwen CHA, van der Velde G, van Lith B, Klaassen M (2012) Experimental quantification of long distance dispersal potential of aquatic snails in the gut of migratory birds. PLoS ONE 7(3): e32292. https://doi.org/10.1371/journal.pone.0032292
- van Leeuwen CHA, Huig N, van der Velde G, van Alen TA, Wagemaker CAM, Sherman CDH, Klaassen M, Figuerola J (2013) How did this snail get here? Several dispersal vectors inferred for an aquatic invasive species. Freshwater Biology 58(1): 88–99. https://doi.org/10.1111/fwb.12041
- Weigand AM, Jochum A, Slapnik R, Schnitzler J, Zarza E, Klussmann-Kolb A (2013) Evolution of microgastropods (Ellobioidea, Carychiidae): Integrating taxonomic, phylogenetic and evolutionary hypotheses. BMC Evolutionary Biology 13(1): 18. https://doi.org/10.1186/1471-2148-13-18
Supplementary material
X-ray micro-CT parameters
Data type: micro-CT data (.xlsx file)