Research Article |
|
Corresponding author: Carlos Perafán ( caperafanl@gmail.com ) Academic editor: Chris Hamilton
© 2023 Mariana Echeverri, Sebastián Gómez Torres, Nicolás Pinel, Carlos Perafán.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Echeverri M, Gómez Torres S, Pinel N, Perafán C (2023) Four new species of mygalomorph spiders (Araneae, Halonoproctidae and Theraphosidae) from the Colombian Pacific region (Bahía Solano, Chocó). ZooKeys 1166: 49-90. https://doi.org/10.3897/zookeys.1166.101069
|
Abstract
The Colombian Pacific coast is an amazing natural region, immersed in one of the most unknown biodiversity hotspots in the world. An expedition carried out in the north of this area, at the Jardín Botánico del Pacífico (JBP) in Bahía Solano, Chocó, focused on studying the diversity of the mygalomorph spider fauna, allowed us to discover four new species included in the families Halonoproctidae and Theraphosidae. The trapdoor species Ummidia solana sp. nov., and the theraphosids species Euthycaelus cunampia sp. nov. (Schismatothelinae), Melloina pacifica sp. nov. (Glabropelmatinae), and Neischnocolus mecana sp. nov. (Theraphosinae) are illustrated, diagnosed, and described in detail. Photographs of somatic features and copulatory organs and a distribution map are provided. Morphological, taxonomical, and biogeographical aspects are discussed for each species. All these taxonomic novelties represent the first records of these genera for the region, expanding the range of geographic distribution of each of them. This work constitutes the first effort focused on characterizing the community of Mygalomorphae species in the Chocó Biogeographic Region.
Key words
Chocó Biogeographic Region, Euthycaelus, Mecana, Melloina, Neischnocolus, tarantula, trapdoor spider, Tumbes-Chocó-Magdalena hotspot, Ummidia
Introduction
The enigmatic groups of Mygalomorphae spiders, known in a broad sense as tarantulas in America, constitute approximately 6% of the total number of spider species described (
While a few groups of trapdoor spiders can perform short-range ballooning (
The Colombian Pacific region is a fascinating area for its biological characteristics because it’s located in the hearth of the Chocó Biogeographic Region. This area constitutes a global biodiversity hotspot, the ninth most biodiverse in the world and one of the most unknown (
Despite the great potential that Colombia has to become a worldwide reference on Mygalomorphae diversity, due to its geographical location and its enormous variety of ecosystems, only 34 of the 50 species described for the country are known by both sexes, and most of them are distributed in the Andean region (
In this first approach to the knowledge of the Mygalomorphae fauna from the rainforest of the Colombian Pacific, we illustrate, describe, and discuss one species from the Halonoproctidae trapdoor spiders, Ummidia solana sp. nov., and three Theraphosidae tarantulas included in different subfamilies, as follow: Euthycaelus cunampia sp. nov. (Schismatothelinae), Neischnocolus mecana sp. nov. (Theraphosinae), and Melloina pacifica sp. nov (Glabropelmatinae). All these new taxonomic records represent first reports of these genera for the region, which extends the range of geographical distribution of each of them. The results of this work constitute a contribution to the knowledge of the biological diversity of one of the areas with the greatest specific richness of species and endemism in Colombia.
Materials and methods
All specimens herein described were collected under Universidad’s EAFIT General Collection Permit (Resolution 1566 of 24 December 2013; amended via Resolution 02493 of 31 December 2018); and deposited in the Arachnological Collection (Order Araneae) of the Instituto de Ciencias Naturales (ICN), Universidad Nacional de Colombia, Bogotá, Colombia, preserved in 75% ethanol. The specimens were collected during a biological expedition carried out in the Jardín Botánico del Pacífico (JBP), located in Bahía Solano, Chocó, Colombia (Fig.
Primary reproductive structures, palpal bulb and spermathecae, were removed for their description and photographic documentation. All photographs and descriptions of the copulatory bulb correspond to the left palp. Spermathecae were cleaned and cleared with lactic acid (85%) by immersion in a test tube and subjecting them to increased heat for short time intervals. Setae of the male tibia I and palpal tibia were removed in order to illustrate the tibial apophysis and nodules, respectively. Specimens and the structures removed were examined under a LEICA M205C stereo microscope. Photographs were taken with a stereo microscope ZEISS Stereo Discovery V12, then stacked with Helicon Focus 8.2.0 Mac OS (Helicon Soft Ltd. 2019) and processed with Adobe Photoshop CC 2022 (Adobe Inc. 2022).
All measurements are given in millimeters (mm). The total length given does not include the chelicerae or spinnerets. Eye sizes were measured as the maximum diameter in either a dorsal or frontal view and were taken with a digital micrometer. Body measurements were taken with a digital micrometer or a vernier caliper. The length and width of carapace, eye tubercle, labium and sternum are the maximum values obtained. Leg and palp measurements were taken in dorsal view along the central axis of the right-side limbs and were taken with a vernier caliper.
The general descriptive format follows
Abbreviations used in the text and figures are as follows:
A apical keel of palpal bulb;
ALE anterior lateral eyes;
AME anterior median eyes;
ap apical;
d dorsal side;
ITC inferior tarsal claw;
p prolateral side;
PB prolateral branch of tibial apophysis;
PI prolateral inferior keel of palpal bulb;
PLE posterior lateral eyes;
PME posterior median eyes;
PMS posterior median spinnerets;
PLS posterior lateral spinnerets;
PS prolateral superior keel of palpal bulb;
R retrolateral keel of palpal bulb;
RB retrolateral branch of tibial apophysis;
SLS spine like setae;
SR seminal receptacles;
STC superior tarsal claw;
v ventral side.
Taxonomy
Family Halonoproctidae Pocock,1901
Genus Ummidia Thorell, 1875
Ummidia solana sp. nov.
Type material
Holotype ♂: Colombia, Chocó, Bahía Solano, Jardín Botánico del Pacífico, 6.38, -77.40, elevation 60 m a.s.l., 10–25 February 2022, M. Echeverri, S. Gómez Torres and C. Perafán leg. (ICN 12356). Paratype ♀: same data as holotype, except elevation 132 m a.s.l. (ICN 12357).
Etymology
The specific epithet solana is a noun in feminine refers to the municipality of Bahía Solano, one of the most beautiful places in the Colombian Pacific coast, recognized for having large and desolate beaches and landscapes of abundant vegetation. It is immersed in one of the world’s biodiversity hotspots. It is also said that the word “solano” means “wind from where the sun rises”.
Diagnosis
Ummidia solana sp. nov. can be differentiated from all geographically proximate species (see
Distribution
Known only from the type locality (Figs
Description
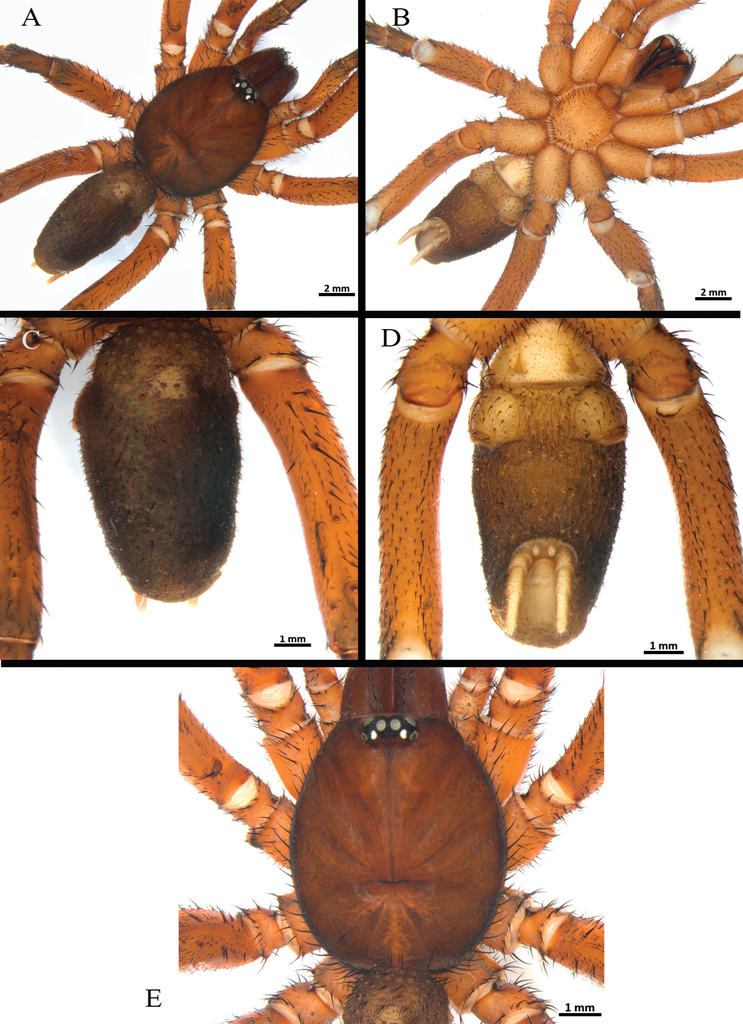
Male (holotype) (Figs
Clypeus: length 0.38, without bristles; protracted onto membranous connection between carapace and chelicerae. Eye group (Fig.
Legs pattern: IV>I>II>III. Lengths of legs and palpal segments on Table
Ummidia solana sp. nov. Male holotype. Lengths of legs and palpal segments.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 6.44 | 4.97 | 4.43 | 5.92 | 4.09 |
| Patella | 3.15 | 2.71 | 2.93 | 2.94 | 2.01 |
| Tibia | 4.13 | 3.11 | 2.28 | 3.65 | 3.13 |
| Metatarsus | 3.24 | 2.65 | 2.63 | 4.18 | - |
| Tarsus | 1.31 | 1.08 | 1.62 | 1.75 | 0.59 |
| Total | 18.27 | 14.52 | 13.89 | 18.44 | 9.82 |
Legs I (Fig.
Palp (Fig.
Coloration. Living spider: carapace black, rugose; ocular area black, PME yellow; chelicerae basal segment, palp, and legs black; tarsi reddish brown; abdomen gray, with cream color spotted pattern. In alcohol: carapace black; sternum brown; labium and maxillae reddish brown; legs dark brown; abdomen gray with spotted pattern; genital area, book lung openings and spinnerets light yellow.
Female (paratype) (Figs
Clypeus: length 0.35, with few bristles; protracted onto membranous connection between carapace and chelicerae. Eye group (Fig.
Legs pattern: IV>I>III>II. Lengths of legs and palpal segments on Table
Ummidia solana sp. nov. Female paratype. Lengths of legs and palpal segments.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 5.25 | 4.77 | 4.59 | 5.96 | 4.70 |
| Patella | 3.57 | 3.36 | 3.07 | 3.46 | 3.24 |
| Tibia | 3.39 | 2.90 | 2.56 | 3.76 | 3.53 |
| Metatarsus | 2.63 | 2.34 | 2.45 | 3.79 | - |
| Tarsus | 1.21 | 1.70 | 2.51 | 2.32 | 2.67 |
| Total | 16.05 | 15.07 | 15.18 | 19.29 | 14.14 |
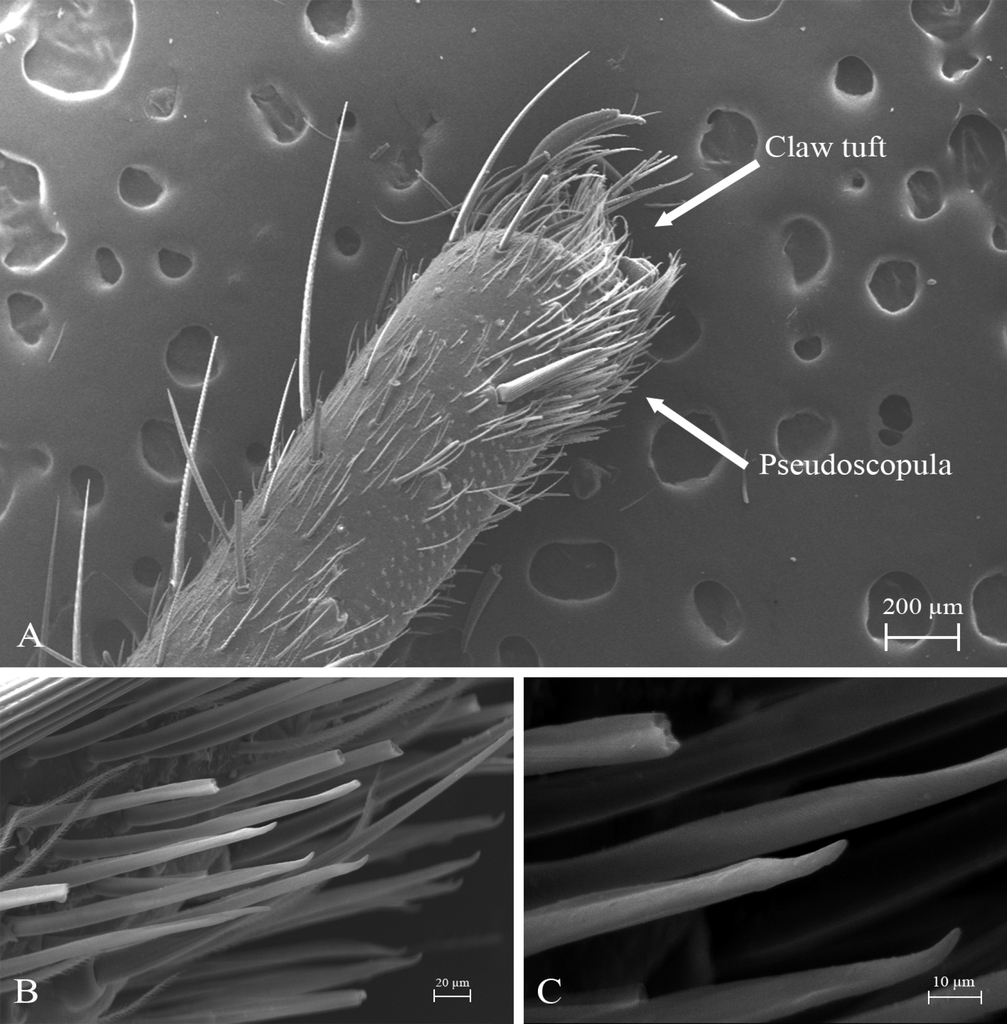
STC with single large and acute proximal tooth, ITC very short and steeply curved in all tarsi. Claw tufts: absent. Pseudoscopulae: absent in all legs. Tarsal trichobothria: Palpal tarsus with ca. 13 clavate trichobothria on medial edge and four filiform trichobothria on distal edge; tarsi I–IV with 1–3 clavate trichobothria and few filiform trichobothria. All femora with wide membranous slits on distal side. All legs and palp with many spiniform setae (Fig.
Legs I (Fig.
Leg III (Fig.
Leg IV. Trochanter and femur: unmodified. Patella: with a wide dorsal central row, prolateral fields of short spines, rise in size toward distal side. Tibia: swollen, dorsal and prolateral with a row of short and fine spines along full length of segment. Metatarsus and tarsus: prodorsal and retrodorsal with SLS along full length of segment, and with ventral long spines covering the totality of the tarsus and 80% of the metatarsus. Retrolateral face of tarsus with defined comb of long spinules over length of the segment (Fig.
Spermathecae (Fig.
Coloration. Living spider: carapace black, smooth, shiny, darker than male; ocular area black, PME yellow; chelicerae basal segment, palp, and legs black; abdomen dark gray, with cream color spotted pattern. In alcohol: carapace dark brown; legs and palp brown with darker overtones, mainly in femora and in the distal segments of all legs; sternum, labium, and maxillae brown; abdomen greyish brown with spotted pattern; genital area, book lung openings, and spinnerets light yellow.
Remarks
Ummidia solana sp. nov. is the third species described from the genus and the family Halonoproctidae for Colombia.
Family Theraphosidae Thorell, 1869
Subfamily Glabropelmatinae
Genus Melloina Brignoli, 1985
Melloina pacifica sp. nov.
Type material
Holotype ♂: Colombia, Chocó, Bahía Solano, Jardín Botánico del Pacífico, 6.38, -77.40, 45 m a.s.l., 10–25 February 2022, M. Echeverri, S. Gómez Torres and C. Perafán leg. (ICN 12358). Paratypes, same data as holotype except elevation, 99–145 m a.s.l.: ♀ (ICN 12359), ♂ (ICN 12360), ♂ (ICN 12361), ♀ (ICN 12362), ♂ (ICN 12363).
Etymology
The specific epithet pacifica is a noun in feminine refers to the Colombian Pacific region, where the species is distributed.
Diagnosis
Male of Melloina pacifica sp. nov. can be distinguished from other Melloina species by the relatively longer embolus (Fig.
Distribution
Known only from the type locality (Figs
Description
Male (holotype) (Figs
Clypeus: absent. Ocular tubercle (Fig.
Legs pattern: IV>I>II>III. Lengths of legs and palpal segments on Table
Melloina pacifica sp. nov. Male holotype. Lengths of legs and palpal segments.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 8.07 | 6.96 | 6.15 | 9.36 | 3.99 |
| Patella | 4.37 | 3.59 | 2.87 | 3.53 | 2.42 |
| Tibia | 7.31 | 5.44 | 4.37 | 7.43 | 3.46 |
| Metatarsus | 6.39 | 5.48 | 5.77 | 9.81 | - |
| Tarsus | 6.80 | 3.63 | 3.35 | 4.26 | 1.37 |
| Total | 32.94 | 25.1 | 22.51 | 34.39 | 11.24 |
Spination (proximal to distal). Femora: palp d 5-8-11, p 0-0-3, v 0-2-0, r 0-2-2; I d 6-4-10, p 0-0-3, v 3-4-10 (8 ap), r 0-0-5; II d 5-5-7, p 0-0-1, v 0-0-6 (5 ap), r 1-1-1; III d 5-5-4, p 3-2-2, v 1-3-10 (8 ap), r 1-2-3; IV d 5-2-4, p 0-0-1 ap, v 0-2-10 (8 ap), r 0-1-1. Patellae: palp d 9-7-8, p 0-1-4 ap, v 1-1-4 ap, r 2-3-2; I d 1-1-3, p 1-1-2, v 0-0-2 ap, r 1-1-2 ap; II d 3-2-5, p 1-2-5 (4 ap), v: 0-1-3 ap, r: 1-1-2; III d: 5-4-5, p: 2-2-2, v: 0-2-2 ap, r: 1-1-3 ap; IV d: 3-1-2, p: 0-1-2 ap, v 0-2-3 (2 ap), r 0-0-1 ap. Tibiae: palp d 2-5-5, p 4-2-4 (2 ap), v 5-6-7 (3 ap), r 2-3-8 (2 ap); I d 1-1-3, p 1-1-2, v 0-0-2 ap, r 1-1-2 ap; II d 2-1-1, p 3-2-3 (2 ap), v 4-4-4 (2 ap), r 2-2-0; III d 3-2-3, p 3-2-3 (1 ap), v 2-2-2 ap, r 3-3-3 ap; IV d 7-5-7, p 5-4-4 (1 ap), v 3-2-3 (2 ap), r 2-2-2 (1 ap). Metatarsi: I d 0, p 2-2-1 ap, v 6-4-6 (2 ap), r 1-1-1; II d 2-2-2, p 4-4-4 (1 ap), v 4-5-6 (3 ap), r 3-2-2 (1 ap); III d 4-4-3, p 2-2-2, v 4-4-5 (2 ap), r 2-3-3 (1 ap); IV d: 3-1-4, p: 2-2-2, v: 3-3-4 (2 ap), r: 3-2-2 (1 ap). Tarsi: cymbium p lobe 4, r lobe 10 (8 ap); I d 0, p 3-1-4 (1 ap), v 0, r 3-3-4 (1 ap); II d 1-1-1, p 3-3-4 (1 ap), v 0, r: 3-3-4 (1 ap); III d 3-2-3, p 3-3-4 (1 ap), v 0, r 3-3-4 (1 ap); IV d 3-4-3, p 2-3-3 (1 ap), v 2-1-2, r 2-3-3 (1 ap).
Palp (Fig.
Coloration. Living spider: carapace, palp, and legs reddish black; femora and tarsi red, distal femora, patellae, tibiae, and metatarsi black; abdomen dark (Fig.
Female (paratype - ICN 12359) (Figs
Clypeus: absent. Ocular tubercle (Fig.
Legs pattern: IV>I>II>III. Lengths of legs and palpal segments on Table
Melloina pacifica sp. nov. Female paratype. Lengths of legs and palpal segments.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 7.12 | 5.96 | 5.4 | 8.01 | 4.32 |
| Patella | 4.41 | 3.63 | 2.82 | 3.56 | 2.7 |
| Tibia | 6.17 | 4.54 | 3.82 | 6.84 | 3.1 |
| Metatarsus | 4.96 | 4.15 | 4.52 | 7.75 | - |
| Tarsus | 2.88 | 2.74 | 2.79 | 3.29 | 3.12 |
| Total | 25.54 | 21.02 | 19.35 | 29.45 | 13.24 |
Spination (proximal to distal). Femora: palp d 2-2-4, p 0-0-1, v 12-9-8, r 0; I d 3-2-3, p 0, v 3-3-5, r 0; II d 1-0-1, p 0, v 4-3-3, r 0; III d 1-2-1, p 1-1-1, v 1-2-3, r 0; IV d 1-1-1, p 0, v 1-2-6, r 0. Patellae: palp d 3-2-3, p 1-1-3 ap, v 0-0-3 ap, r 0-1-2; I d 0, p 0-1-1, v 0-2-2 ap, r 1-1-0; II d 0, p 0, v 0-2-2 ap, r 0; III d 0-1-0, p 0-1-1, v 0-1-2, r 0; IV d 0, p 0, v 0-2-3 (2 ap), r 0. Tibiae: palp d 5-5-3, p 0-1-2, v 6-5-3 ap, r 2-2-2; I d 4-6-4, p 2-1-2, v 3-4-2 ap, r 1-1-1; II d 2-1-1, p 1-1-2, v 4-5-4 (2 ap), r 1-1-1; III d 4-5-5, p 2-2-2, v 3-4-4 (3 ap), r 0-1-1; IV d 2-2-2, p 3-2-1 ap, v 4-5-5 (1 ap), r 1-1-2 (1 ap). Metatarsi: I d 0, p 1-1-0, v 5-5-6 (2 ap), r 0; II d 0, p 1-1-3, v 5-3-5 (2 ap), r 1-2-2 (1 ap); III d 2-3-4, p 2-2-2 (1 ap), v 4-3-3 (1 ap), r 2-2-2; IV d 4-4-4, p 2-2-1, v 4-3-4 ap, r 2-2-2. Tarsi: palp d 0, p 3-3-3, v 1-3-0, r 2-3-2; I d 0, p 3-3-4, v 0-0-2 ap, r 2-3-2; II d 0, p 3-3-3 (1 ap), v 0, r 3-3-3; III d 0, p 3-3-3, v 0, r 3-4-3; IV d 0, p 3-3-4 (1 ap), v 0-3-0, r 4-3-4 (1 ap).
Spermatheca (Fig.
Coloration. In alcohol: as described in the male.
Remarks
Melloina pacifica sp. nov. is the first species of the genus described for Colombia, although it is known that Melloina is distributed in different ecosystems, including cave environments (
Subfamily Schismatothelinae
Genus Euthycaelus Simon, 1889
Euthycaelus cunampia sp. nov.
Type material
Holotype ♂: Colombia, Chocó, Bahía Solano, Jardín Botánico del Pacífico, 6.38, -77.40, elevation 124 m a.s.l., 10–25 February 2022, M. Echeverri, S. Gómez Torres and C. Perafán leg. (ICN 12364).
Etymology
The specific epithet cunampia is a patronym in honor of the family name of Don José and Don Antonio, members of the Emberá indigenous community, from Mecana, Chocó. Mr. José and Mr. Antonio abandoned their hunting traditions for their community to become touristic and academic guides for the JBP. We want to pay tribute to their community and to the JBP with this recognition.
Diagnosis
Males of Euthycaelus cunampia sp. nov. can be distinguished from all other Euthycaelus species by the following combination of morphological features: the shape of the palpal bulb (Fig.
Distribution
Known only from the type locality (Figs
Description
Male holotype (Figs
Euthycaelus cunampia sp. nov., holotype male A–E palpal bulb A ventral view B dorsal view C prolateral view D retrolateral view E detail of prolateral keels F tibial apophysis on leg I, prolateral view G palpal tibia. Arrow indicates prolateral keels. Abbreviations: PB = prolateral branch, RB = retrolateral branch.
Clypeus: absent. Ocular tubercle (Fig.
Legs pattern: IV>I>II>III. Lengths of legs and palpal segments on Table
Euthycaelus cunampia sp. nov. Male holotype. Lengths of legs and palpal segments.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 7.83 | 6.81 | 6.29 | 8.40 | 5.41 |
| Patella | 4.97 | 4.13 | 3.41 | 4.06 | 3.0 |
| Tibia | 6.06 | 4.87 | 3.83 | 6.50 | 4.43 |
| Metatarsus | 6.02 | 5.21 | 5.40 | 8.53 | - |
| Tarsus | 3.96 | 3.31 | 3.05 | 3.79 | 2.34 |
| Total | 28.89 | 24.33 | 21.98 | 31.28 | 15.18 |
Spination (proximal to distal). Cymbium and tarsi without spines. Femora: palp d 0-0-1p; I d 0-0-1p; II d 0-0-1p; III d 0-0-2p-r; IV d 0-0-1r. Patella: I–II, IV and palp 0; III r 0-0-1d. Tibiae: palp p 0-0-1d, r 0-0-7; I v 0-0-1, p 0-0-1; II v 0-0-2 (ap), p 0-0-1; III d 1-0-2, v 1-2-2 (ap); IV d 2-1-2, v 2-2-3 (ap). Metatarsi: I v 1-0-3 (ap); II v 1-0-3 (ap); III d 1-2-2, v 1-2-3 (ap); IV d 1-2-2, v 1-2-4 (3 ap).
Palp (Fig.
Coloration. Living spider: carapace black, covered by brown setae; palp and legs black, femora and patellae darker; tibiae, metatarsi and tarsi covered by very light setae; abdomen brown (Fig.
Female. Unknown.
Remarks
Euthycaelus cunampia sp. nov. represents the first published record of the genus and subfamily Schismatothelinae outside the Andean Region and the Eastern Cordillera for Colombia. This species constitutes the northernmost and westernmost record of the genus and subfamily for the country. Previously, the genus had a characteristic cis-Andean distribution over the Eastern Cordillera of Colombia and the Cordillera de Mérida in Venezuela (
Subfamily Theraphosinae
Genus Neischnocolus Petrunkevitch, 1925
Neischnocolus mecana sp. nov.
Type material
Holotype ♂: Colombia, Chocó, Bahía Solano, Jardín Botánico del Pacífico, 6.38, -77.40, elevation, 28 m a.s.l., 10–25 February 2022, M. Echeverri, S. Gómez Torres and C. Perafán leg. (ICN 12365). Paratype ♀: same data as holotype (ICN 12366).
Etymology
The specific epithet mecana is a noun in apposition related to one of the townships of the municipality of Bahía Solano, where the JBP is located. The name of this small town is due to the fact that it is located on the Mecana riverside, with crystalline waters and abundant biodiversity. The JBP promotes the conservation, research, and recovery of the native biodiversity of this region. We would like to pay tribute to its community and the JBP with this recognition.
Diagnosis
Male of Neischnocolus mecana sp. nov. can be distinguished from the other Neischnocolus species by the following combination of morphological characters: shape of the palpal bulb piriform, with the tip of the embolus continuing the palpal organ axis (not perpendicular), well-developed prolateral (PS and PI) and apical (A) keels with non-serrated edge, PI discontinuous, absence of retrolateral keel (R), and without granulation or microspikes on embolus or tegulum (Fig.
Distribution
Known only from the type locality (Figs
Description
Male holotype (Figs
Neischnocolus mecana sp. nov., holotype male A–D copulatory bulb A ventral view B dorsal view C prolateral view D retrolateral view E palpal tibia F apophysis tibial on leg I, prolateroventral view. Abbreviations: A = apical keel, PB = prolateral branch, PI = prolateral inferior keel, PS = prolateral superior keel, RB = retrolateral branch.
Clypeus: absent. Ocular tubercle: ovoid, length 1.14, width 2.34; elevated, forwardly directed. Anterior eye row slightly procurved, posterior eye row slightly recurved. Eye diameters and interdistances: AME 0.54 (circular), ALE 0.68 (oval), PME 0.40 (oval), PLE 0.55 (oval), AME-AME 0.30, AME-ALE 0.18, PME-PME 1.11, PME-PLE 0.19, PLE-PLE 1.80, ALE-PLE 0.24, AME-PME 0.16. Thoracic fovea: transverse, width 3.25; slightly procurved, deep, 8.75 from the anterior edge of carapace. Chelicerae basal segment: length 4.2, width 3.1; with 11 well-developed teeth on each furrow promargin, and a group of ca. 15 small teeth near last three basal promargin teeth. Maxillae (Fig.
Legs pattern: IV>I>II>III. Lengths of legs and palpal segments on Table
Neischnocolus mecana sp. nov. Male holotype. Lengths of legs and palpal segments.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 14.95 | 14.16 | 13.02 | 16.18 | 9.45 |
| Patella | 7.75 | 7.37 | 6.15 | 6.55 | 5.51 |
| Tibia | 12.31 | 10.90 | 10.20 | 13.56 | 7.43 |
| Metatarsus | 11.02 | 10.91 | 12.01 | 18.56 | - |
| Tarsus | 6.87 | 6.06 | 4.89 | 6.32 | 3.51 |
| Total | 53 | 49.4 | 46.17 | 61.17 | 25.9 |
Urticating setae: types I urticating setae present, subtype Ic (
Spination: All femora, patellae, and tarsi 0. Legs I–II and palp 0. Tibiae: I–II 0; III d 0-0-1, v 0-1-2 ap, p 1-1-0, r 0-1-0; IV d 0-1-0, v 0-1-2 ap, p 0-1-1, r 0-0-1. Metatarsi: I–II 0; III d 0, v 0-2-4 (3 ap), p 1-1-1, r 0-1-1; IV d 0-0-1, v 2-3-4 (3 ap), p 1-1-1, r 0-1-1.
Palp (Fig.
Coloration. Living spiders: body color brown, carapace and femora dark brown, legs brown, and abdomen reddish brown. Ventral abdomen with patterns of dark spots (Fig.
Female paratype (Figs
Clypeus: absent. Ocular tubercle: ovoid, length 1.34, width 2.62; elevated, forwardly directed. Anterior eye row slightly procurved, posterior eye row slightly recurved. Eye sizes and interdistances: AME 0.53 (circular), ALE 0.70 (oval), PME 0.41 (oval), PLE 0.50 (oval), AME-AME 0.34, AME-ALE 0.30, PME-PME 1.27, PME-PLE 0.26, PLE-PLE 2.00, ALE-PLE 0.36, AME-PME 0.21. Thoracic fovea: transverse, width 3.50; slightly procurved, deep, 9.11 from the anterior edge of carapace. Chelicerae basal segment: length 5.1, width 3.6; with 11 well-developed teeth on each furrow promargin, and a group of ca. 12 small teeth near last three basal promargin teeth. Maxillae (Fig.
Legs pattern: IV>I>II>III. Lengths of legs and palpal segments on Table
Neischnocolus mecana sp. nov. Female paratype. Lengths of legs and palpal segments.
| I | II | III | IV | Palp | |
|---|---|---|---|---|---|
| Femur | 11.24 | 10.50 | 8.79 | 12.07 | 9.16 |
| Patella | 7.53 | 6.34 | 5.54 | 6.57 | 5.90 |
| Tibia | 8.04 | 6.54 | 6.30 | 8.80 | 6.14 |
| Metatarsus | 6.20 | 5.83 | 7.69 | 12.07 | - |
| Tarsus | 4.00 | 3.34 | 3.82 | 4.17 | 5.27 |
| Total | 37.01 | 32.55 | 32.14 | 43.68 | 26.47 |
Urticating setae: types I urticating setae present, subtype Ic (
Spination: All femora, patellae, and tarsi 0. Tibiae: palp 0-0-2 ap; I d 0, v 0-0-2 ap, p 0, r 0; II d 0, v 0-0-3 ap, p 0-1-0, r 0; III d 1-0-0, v 0-1-3 ap, p 1-1-0, r 0-1-1; IV d 0, v 0-1-3 ap, p 1-1-0, r 1-1-1. Metatarsi: I 0; II d 0, v 0-1-2 ap, p 0-1-0, r 0; III d 0-1-1, v 0-3-2 ap, p 0-0-1 ap, r 0-0-1 ap; IV d 0-0-1, v 3-4-5 (3 ap), p 1-2-0, r 0-1-1.
Spermathecae (Fig.
Coloration. Living spiders: carapace, abdomen, and legs black, legs with light-colored stripes at the joints. Ventral abdomen with patterns of dark spots (Fig.
Remarks
Neischnocolus mecana sp. nov. it is the fourth species of the genus described for Colombia and it is the first record of Neischnocolus for the Chocó biogeographic region, as well as the first record for the Colombian Pacific. With this description, the known geographic range of the genus is extended. Currently, Neischnocolus is distributed in Colombia in the Andean, Amazonian, and Pacific regions. It is known that Neischnocolus is widely distributed in the Colombian territory, with a very extensive geographical and altitudinal range, and that most of its diversity has not yet been described (
Acknowledgements
We thank the Escuela de Administración, Finanzas e Instituto Tecnológico (EAFIT University) of Medellín, Colombia, for the financial support for the field work and the equipment provided for the field and laboratory work. We thank Luisa Fernanda Puerta, the JBP staff, and the Mecana community for receiving and hosting us, especially José Cunampia and Antonio Cunampia for all the help they gave us during the field work. We would also like to thank Ray Gabriel and Danniella Sherwood for early discussion of Euthycaelus species from Panama and Colombia. The author CP thanks the financial support of the National System of Researchers (SNI), Uruguay. Thanks to D. Ríos Tamayo, L. Montes de Oca, and an anonymous reviewer for their valuable comments and corrections. Thanks to Mateo Giraldo for the photographs of Fig.
Additional information
Conflict of interest
No conflict of interest was declared.
Ethical statement
No ethical statement was reported.
Funding
No funding was reported.
Author contributions
Mariana Echeverri and Sebastián Gómez Torres: participated in the design and execution of the field work, reviewed and analyzed the specimens in the laboratory, described the species, took and edited the photographs, wrote the manuscript. Nicolás Pinel: participated in the design of the field work, wrote the manuscript. Carlos Perafán: participated in the design and execution of the field work, reviewed and analyzed the specimens in the laboratory, performed the taxonomic analysis, wrote the manuscript.
Author ORCIDs
Nicolás Pinel https://orcid.org/0000-0003-1304-3096
Data availability
All of the data that support the findings of this study are available in the main text or Supplementary Information.
References
- Bertani R (2000) Male palpal bulbs and homologous features in Theraphosinae (Araneae, Theraphosidae). The Journal of Arachnology 28(1): 29–42. https://doi.org/10.1636/0161-8202(2000)028[0029:MPBAHF]2.0.CO;2
- Bertani R (2013) A new species of Melloina (Araneae: Paratropididae) from Venezuela. Zoologia 30(1): 101–106. https://doi.org/10.1590/S1984-46702013000100013
- Bond JE, Hendrixson BE, Hamilton CA, Hedin M (2012) A reconsideration of the classification of the spider infraorder Mygalomorphae (Arachnida: Araneae) based on three nuclear genes and morphology. PLoS ONE 7(6): e38753. https://doi.org/10.1371/journal.pone.0038753
- Bristowe WS (1939) The Comity of Spiders. Vol. I. Ray Society, London.
- Cano Á, Bacon CD, Stauffer FW, Antonelli A, Serrano-Serrano ML, Perret M (2018) The roles of dispersal and mass extinction in shaping palm diversity across the Caribbean. Journal of Biogeography 45(6): 1432–1443. https://doi.org/10.1111/jbi.13225
- Cifuentes Y, Estrada-Gomez S, Vargas-Muñoz LJ, Perafán C, Cifuentes Y, Estrada-Gomez S, Vargas-Muñoz LJ, Perafán C (2016) Description and molecular characterization of a new species of tarantula, Pamphobeteus verdolaga, from Colombia (Araneae: Mygalomorphae: Theraphosidae). Zoologia (Curitiba) 33(06): e20160113. [1–6] https://doi.org/10.1590/s1984-4689zool-20160113
- Cooke JAL, Roth VD, Miller FH (1972) The urticating hairs of theraphosid spiders. American Museum Novitates 11(2): 49. http://hdl.handle.net/2246/2705
- Coyle FA (1983) Aerial dispersal by mygalomorph spiderlings (Araneae, Mygalomorphae). The Journal of Arachnology 11(1): 283–286.
- Coyle FA (1985) Ballooning behavior of Ummidia spiderlings (Araneae, Ctenizidae). The Journal of Arachnology 13(1): 137–138.
- Coyle FA (1986) The role of silk in prey capture by non-araneomorph spiders. In: Shear WA (Ed.) Spiders: Webs, Behavior and Evolution. Standfor University Press, California, 269–305.
- Coyle FA, Greenstone MH, Hultsch A, Morgan CE (1985) Ballooning mygalomorphs: estimates of the masses of Sphodros and Ummidia ballooners (Araneae: Atypidae, Ctenizidae). The Journal of Arachnology 13(3): 291–296.
- Decae AE (2010) The genus Ummidia Thorell 1875 in the western Mediterranean, a review (Araneae: Mygalomorphae: Ctenizidae). The Journal of Arachnology 38(2): 328–340. https://doi.org/10.1636/A09-85.1
- Escovar JE, González R, Quiñones ML (2013) Anthropophilic biting behaviour of Anopheles (Kerteszia) neivai Howard, Dyar & Knab associated with Fishermen’s activities in a malaria-endemic area in the Colombian Pacific. Memorias do Instituto Oswaldo Cruz 108(8): 1057–1064. https://doi.org/10.1590/0074-0276130256
- Ferretti N, González A, Pérez-Miles F (2012) Historical biogeography of mygalomorph spiders from the peripampasic orogenic arc based on track analysis and PAE as a panbiogeographical tool. Systematics and Biodiversity 10(2): 179–193. https://doi.org/10.1080/14772000.2012.694375
- Ferretti N, Pompozzi G, Copperi S, Schwerdt L (2013) Aerial dispersal by Actinopus spiderlings (Araneae: Actinopodidae). The Journal of Arachnology 41(3): 407–408. https://doi.org/10.1636/J13-27.1
- Ferretti N, Pérez-Miles F, González A (2014) Historical relationships among Argentinean biogeographic provinces based on mygalomorph spider distribution data (Araneae: Mygalomorphae). Studies on Neotropical Fauna and Environment 49: 1–10. https://doi.org/10.1080/01650521.2014.903616
- Foelix RF (2011) Biology of spiders. 3rd ed. Oxford University Press, Inc., New York, 71–103.
- Gabriel R, Sherwood D (2022) Taxonomy, biogeography, and ecology of some theraphosid spiders of the Darién region with description of five new species (Araneae: Theraphosidae). Revista Iberica de Aracnologia 40: 5–18. http://www.sea-entomologia.org
- Godwin RL, Bond JE (2021) Taxonomic revision of the New World members of the trapdoor spider genus Ummidia Thorell (Araneae, Mygalomorphae, Halonoproctidae). ZooKeys 1027: 1–165. https://doi.org/10.3897/zookeys.1027.54888
- Goloboff-Szumik V, Ríos Tamayo D (2022) Description of the female of Melloina gracilis (Schenkel, 1953) (Mygalomorphae: Theraphosidae) with comments on the familial placement of Melloina. Revista del Museo Argentino de Ciencias Naturales 24(2): 249–255. https://doi.org/10.22179/REVMACN.24.781
- González-Córdoba M, Montoya-Lerma J (2014) Bees (Hymenoptera: Apoidea) of Gorgona Natural National Park, Colombian Pacific. Revista de Biología Tropical 62(1): 297–305. https://doi.org/10.15517/rbt.v62i0.16345
- Guadanucci JPL, Weinmann D (2014) The spider genera Euthycaelus Simon and Schismatothele Karsch (Mygalomorphae, Theraphosidae). Zootaxa 3793(3): 275–288. https://doi.org/10.11646/zootaxa.3795.3.3
- Hedin M, Bond JE (2006) Molecular phylogenetics of the spider infraorder Mygalomorphae using nuclear rRNA genes (18S and 28S): conflict and agreement with the current system of classification. Molecular Phylogenetics and Evolution 41(2): 454–471. https://doi.org/10.1016/j.ympev.2006.05.017
- Hilty SL, Brown WL (2001) Guía de las Aves de Colombia. Asociación Colombiana de Ornitología, 1040 pp.
- Jardín Botánico del Pacífico (2014) Jardín Botánico del Pacífico. http://www.jardinbotanicodelpacifico.org/jardin.html
- Kaderka R, Bulantová J, Heneberg P, Řezáč M (2019) Urticating setae of tarantulas (Araneae: Theraphosidae): Morphology, revision of typology and terminology and implications for taxonomy. PLoS ONE 14(11): e0224384. https://doi.org/10.1371/journal.pone.0224384
- Klinger Braham W, Blandón Mosuera M (2013) Plan estratégico de la macrocuenca del pacífico. Quibdó.
- Lopez L, Valdés-Rodríguez S, Chacón de Ulloa P (2014) Wasps from the arboreal vegetation in Gorgona National Natural Park, Colombian Pacific. Revista de Biología Tropical 62(1): 307–315. https://doi.org/10.15517/rbt.v62i0.16348
- Martínez-Torres SD, Flórez Daza ÁE, Linares-Castillo EL (2011) Meeting between kingdoms: Discovery of a close association between Diplopoda and Bryophyta in a transitional Andean-Pacific forest in Colombia. International Journal of Myriapodology 6: 29–36. https://doi.org/10.3897/ijm.6.2187
- Montes de Oca L, Indicatti RP, Opatova V, Almeida M, Pérez-Miles F, Bond JE (2022) Phylogenomic analysis, reclassification, and evolution of South American nemesioid burrowing mygalomorph spiders. Molecular Phylogenetics and Evolution 168(107377): 1–19. https://doi.org/10.1016/j.ympev.2021.107377
- Mori A, Bertani R (2020) Revision and cladistic analysis of Psalistops Simon, 1889, Trichopelma Simon, 1888 and Cyrtogrammomma Pocock, 1895 (Araneae: Theraphosidae) based on a cladistic analysis of relationships of Theraphosidae, Barychelidae and Paratropididae. Zootaxa 4873(1): 1–132. https://doi.org/10.11646/zootaxa.4873.1.1
- Mosquera RL, Robledo MD, Asprilla PA (2007) Diversidad florística de dos zonas de bosque tropical húmedo en el municipio de Alto Baudó, Chocó-Colombia. Acta Biológica Colombiana 12: 75–90.
- Myers N, Mittermeler RA, Mittermeler CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772): 853–858. https://doi.org/10.1038/35002501
- Narváez-Vásquez A, Gaviria J, Vergara-Navarro EV, Rivera-Pedroza L, Löhr B (2021) Ant (Hymenoptera: Formicidae) species diversity in secondary forest and three agricultural land uses of the Colombian Pacific Coast. Revista Chilena de Entomologia 47(3): 441–458. https://doi.org/10.35249/rche.47.3.21.01
- Opatova V, Hamilton CA, Hedin M, De Oca LM, Král J, Bond JE (2019) Phylogenetic systematics and evolution of the spider infraorder Mygalomorphae using genomic scale data. Systematic Biology 69(4): 671–707. https://doi.org/10.1093/sysbio/syz064
- Padilla-Gil DN (2017) Composition and structure of the heteropterans (Hemiptera) on coastal lotic ecosystems from Colombian Pacific. Actualidades Biologicas 39: 51–57.
- Padilla-Gil DN, Arcos PO (2011) Aquatic Hemiptera associated to estuaries of the Colombian Pacific coast. Revista Colombiana de Entomología 37(107): 350–353. https://doi.org/10.25100/socolen.v37i2.9100
- Paz N (1988) Ecologia y aspectos del comportamiento en Linothele sp. (Araneae, Dipluridae). The Journal of Arachnology 16(1): 5–22. http://www.jstor.org/stable/3705800
- Paz N, Raven RJ (1990) A new species of Linothele from Colombia (Araneae, Mygalomorphae, Dipluridae). The Journal of Arachnology 18(1): 79–86. http://www.jstor.org/stable/3705581
- Perafán C (2017) Distribución actual e histórica del infraorden Mygalomorphae (Araneae) en los Andes del norte. PhD thesis, Universidad de la República, Montevideo, Uruguay.
- Perafán C, Valencia-Cuellar D (2018) Proshapalopus marimbai, a new tarantula species (Mygalomorphae, Theraphosidae) and first genus record from Colombia. Tropical Zoology 31(4): 200–213. https://doi.org/10.1080/03946975.2018.1493181
- Perafán C, Ferretti N, Hendrixson B (2020) Biogeography of New World Theraphosidae. In: Pérez-Miles F (Ed.) New World Tarantulas: Taxonomy, Biogeography and Evolutionary Biology of Theraphosidae. Zoological Monographs, Springer, 6, 153–190. https://doi.org/10.1007/978-3-030-48644-0_6
- Pérez-Escobar OA, Lucas E, Jaramillo C, Monro A, Morris SK, Bogarín D, Greer D, Dodsworth S, Aguilar-Cano J, Sanchez Meseguer A, Antonelli A (2019) The origin and diversification of the hyperdiverse flora in the Chocó Biogeographic Region. Frontiers in Plant Science 10: 1328. https://doi.org/10.3389/fpls.2019.01328
- Pérez-Miles F, Perafán C (2017) Behavior and biology of Mygalomorphae. In: Viera C, Gonzaga MO (Eds) Behaviour and Ecology of Spiders: Contributions from the Neotropical Region. Springer, 29–54. https://doi.org/10.1007/978-3-319-65717-2_2
- Pérez-Miles F, Gabriel R, Miglio L, Bonaldo A, Gallon R, Jimenez JJ, Bertani R (2008) Ami, a new theraphosid genus from Central and South America, with the description of six new species (Araneae: Mygalomorphae). Zootaxa 1915: 54–68. https://doi.org/10.11646/zootaxa.1915.1.3
- Pérez-Miles F, Guadanucci JPL, Jurgilas JP, Becco R, Perafán C (2017) Morphology and evolution of scopula, pseudoscopula and claw tufts in Mygalomorphae (Araneae). Zoomorphology 136: 435–459. https://doi.org/10.1007/s00435-017-0364-9
- Pérez-Miles F, Gabriel R, Sherwood D (2019) Neischnocolus Petrunkevitch, 1925, senior synonym of Ami Pérez-Miles, 2008 and Barropelma Chamberlin, 1940 (Araneae: Theraphosidae). Arachnology 18(2): 150–155. https://doi.org/10.13156/arac.2018.18.2.150
- Petrunkevitch A (1925) Arachnida from Panama. Transactions of the Connecticut Academy of Arts and Sciences 27: 51–248.
- Raven RJ (1980) The evolution and biogeography of the mygalomorph spider family Hexathelidae (Araneae, Chelicerata). Journal of Arachnology 8: 251–266.
- Raven RJ (1985) The spider infraorder Mygalomorphae (Araneae): cladistics and systematics. American Museum of Natural History, New York, 180 pp.
- Raven RJ (1999) Review of the mygalomorph the genus Melloina Brignoli (Paratropididae: Araneae). Memoirs of the Queensland Museum 43(2): 819–825.
- Raven RJ (2010) A review of the Mygalomorphae: biology, morphology and systematics. Book of Abstracts of the 18th International Congress of Arachnology, 2010 Jul 11–17, Siedle, Poland.
- Rossi G de F, Ghirotto VM, Galleti-Lima A, Indicatti RP, Guadanucci JP (2021) “Flying” or digging? The trapdoor spider genus Neocteniza Pocock, 1895: Redescription of three species, new records from Brazil, notes on natural history and first record of ballooning for Idiopidae (Araneae, Mygalomorphae). Zootaxa 5023(4): 451–485. https://doi.org/10.11646/zootaxa.5023.4.1
- Sánchez-Gárcés GC (2017) A review of amphidromous freshwater fishes of the Chocó biogeographical region (Colombia and Ecuador): diversity, ecology, fisheries and conservation. Société Française d’Ichtyologie 41(2): 157–169. https://sfi-cybium.fr/fr/review-amphidromous-freshwater-fishes-chocó-biogeographical-region-colombia-and-ecuador-diversity [March 19, 2022]
- Schenkel E (1953) Bericht über einige Spinnentiere aus Venezuela. Verhandlungen der Naturforschenden Gesellschaft in Basel 64: 1–57.
- SIB (2022) Biodiversidad en cifras. https://cifras.biodiversidad.co/ [March 19, 2022]
- Valencia-Cuéllar D, Perafán C, Guerrero RJ, Leite Guadanucci JP (2019) Schismatothelinae spiders (Araneae, Mygalomorphae, Theraphosidae) from Colombia: Four new species and an approach to their diversity. Zootaxa 4545(4): 548–562. https://doi.org/10.11646/zootaxa.4545.4.6
- WSC [World Spider Catalog] (2023) World Spider Catalog. Natural History Museum Bern. https://doi.org/10.24436/2