Research Article |
|
Corresponding author: Michael W. Hastriter ( michaelhastriter@comcast.net ) Academic editor: Terry Galloway
© 2016 Michael W. Hastriter.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Hastriter MW (2016) Description of two new species of bat fleas of the genus Araeopsylla (Siphonaptera) from Kenya and Madagascar with notes on miscellaneous bat fleas. ZooKeys 572: 7-21. https://doi.org/10.3897/zookeys.572.7823
|
Abstract
The flea genus Araeopsylla Jordan and Rothschild, 1921 contains nine species distributed throughout the Palaearctic, Ethiopian and Oriental Regions primarily on mollosid bats. A new species of bat flea, Araeopsylla goodmani, is described. This new species is represented by three females collected from one male specimen of the mollosid bat Chaerephon jobimena Goodman & Cardiff, 2004 from Fianarantsoa Province, Madagascar. A second new species, Araeopsylla smiti, is described from one male from the Rift Valley, Kenya. It was collected from the molossid bat Chaerephon bivittatus (Heuglin, 1861). This represents the first record of Araeopsylla in Kenya. Previous records of Araeopsylla in the Malagasy region included Araeopsylla martialis (Rothschild, 1903) from Reunion Island and Madagascar. One hundred fifty-eight specimens (64♂, 94♀) of A. martialis were collected from 67 specimens (flea intensity of 2.4 fleas per host) of Mormopterus jugularis (Peters, 1865) across three provinces of Madagascar (Fianarantosa, Toamasina, and Toliara). Mormopterus jugularis is clearly a common host for A. martialis. Dampfia grahami grahami (Waterston, 1915) is also reported from Eptesicus matroka (Thomas & Schwann, 1905) which is the first record from this host species and the first time the genus Dampfia has been documented in Madagascar. Although Lagaropsylla consularis Smit, 1957 and Lagaropsylla idae Smit, 1957 have been reported in Madagascar previously, Mops leucostigma Allen, 1918 is a new host record for L. idae. The flea intensity of L. idae (64♂, 83♀) on 28 specimens of M. leucostigma was extremely high at 5.3 fleas per host. A key to the genus Araeopsylla is provided.
Keywords
Araeopsylla goodmani , Araeopsylla smiti , Dampfia , key, Lagaropsylla
Introduction
There are currently nine species represented in the flea genus Araeopsylla Jordan & Rothschild, 1921 (
During the early 1970’s, the author was associated with a project conducted by the late Hank W. Setzer, Department of Mammalogy, National Museum of Natural History that included the collection of small mammals and their ectoparasites across most of the African countries. The fleas collected were made available to the author and were studied for several years. During those early flea studies, the author recognized a new species of Araeopsylla and has maintained the single male specimen for 40+ years in anticipation that the female might be discovered. To date, no additional specimens of this species have been discovered.
The Field Museum of Natural History, Chicago, IL conducted mammal studies in Madagascar and also collected ectoparasites from those mammals. The fleas were provided to the author and they were subsequently identified and returned to the Field Museum. Among the material examined were three female specimens representing a new species of Araeopsylla and several new country and host records. These two new Araeopsylla taxa from Kenya and Madagascar will be described herein. Additional records of bat fleas from Ghana, Kenya, and Madagascar will also be documented and discussed.
Methods
Details of the genitalia of the whole mounted specimen of A. smiti (described below) were difficult to visualize. Therefore the specimen was photographed, dissolved off the microscope slide with xylene, dissected, and remounted in Canada balsam. Images were prepared using an Olympus BX61 Compound Microscope, Olympus CC12 digital camera accompanied with an Olympus Microsuite™ B3SV program. This system was also used to measure fleas in accordance with anatomical markers annotated in
Madagascar records of bat fleas were extracted from data bases of the
Results
Ischnopsyllidae
Ischnopsyllinae
Araeopsylla goodmani , sp. n.
Diagnosis
Female distinguished from all other Araeopsylla species by the shape of the caudal margin of S-VII. The caudal margin lacks lobes but has a broad concave margin extending to near the ventral margin which terminates in a right angled lobe. The apex of the lobe has a small sinus (Fig.
Description
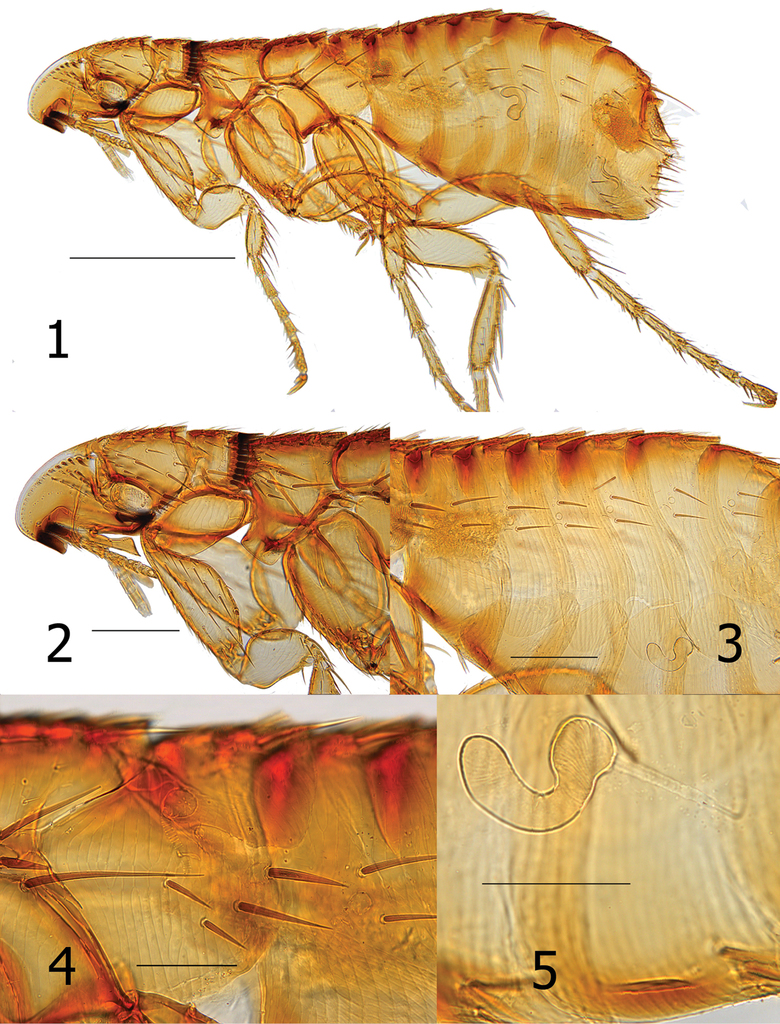
Head. Frons smoothly rounded; frontal row of 19 setae, each successively stouter from oral angle to top of falx; more dorsal setae spiniform. Area between margin of frons and frontal row of setae clear, white, without surface structure except at extreme upper limit with one placoid pit. Eight minute setae post-frontal row and group of 10 mixed setae (spiniform, short and long) dorsal to row of eight setae. Of these, one seta adjacent to eye, very stout and long, extending beyond posterior margin of head. Gena darkly sclerotized, tapering to upturned apex. Eye vestigial, dark pigmented area merging with gena. Two genal teeth; anterior most tooth broader and blunter than posterior tooth. Pre-oral tuber divided into three portions; most posterior strongly hooked downward to pointed apex. Falx well demarcated. Post-antennal area with row of six stout setulae along dorsal margin of antennal fossa. Four rows of setae dorsal to setulae (4, 2, 3, 8); posterior main row without intercalaries and ventral four spiniform and grouped together (characteristic of the genus). Occiput with three dorsal incrassations. Antennal scape with a few minute setae. Margin of pedicel with hyaline extension over first three segme1nts of clavus; along margin of hyaline area are five or six fine long setae extending to seventh or eighth segment of clavus. Maxilla truncate; shaped like a trumpet at apex. Labial palpus of five segments (excluding bulbous palp-bearing segment); penultimate segment longer than other segments (all being quite short). Labial palpus extending about half length of fore coxa. Length of maxillary palpus similar to labial palpus. Galea and lacinia shorter than labial palpus (Figs
Thorax. Pronotum with 18–20 ctenidia; all shorter than length of pronotum. Ctenidia tapered to point, but not sharp at apex. Pronotum with main row of setae minute; more anterior scattered small setae and one long ventral seta. Each thoracic tergum with dorsal incrassations. Twelve stout setae grouped over sclerotic dome of pleural rod. Pleural rod merges with sclerotic dome slightly behind middle of dome. Mesosternum truncate; metasternum diminished but oblique along margin. Metanotum with horizontal row of four setae near interface with dorsal incrassations; one short spinelet at dorsal apex. Lateral metanotal area with one short and one long seta. Metepisternum with one long seta at dorsal margin. Four or five stout (nearly spiniform) setae below level of spiracle on metepimeron. Spiracle on metepimeron large and round (Fig.
Legs. Upper portion of fore coxa very narrow; marginal row of six long setae on upper caudal margin. About 17–18 long lateral setae excluding marginals. All femora lacking lateral or mesal setae. Fore femorotibial joint with one long seta, Meso- and metafemorotibial joints each with two long setae. Lateral surface of fore tibia with six setae; meso- and metatibiae each with eight setae. Dorsal margin of fore tibia with about 10 dorsal notches; meso- (2, 2, 1, 2, 2, 1, 2, 2) and metatibiae (2, 2, 1, 2, 1, 1, 2, 2) each with eight dorsal notches. From proximal to distal, each succeeding tarsus shorter than preceding segment. Each distitarsomere with five lateral plantar bristles; proximal pair placed between second pair on plantar surface (Fig.
Unmodified abdominal segments. Spiracles round on T-II-VII; each segment with one dorsal incrassation and heavily pigmented band extending below incrassation. Terga each with one row of three small setae; single dorsal seta separated from two more ventral setae by large gap. One seta of each row located below spiracle. Heavy sclerotization on ventral surface of S-II–VII. Single row of setae on S-II–VI (1, 2, 2, 3, 3). One antesensilial bristle at margin of T-VII; with internal sclerotized incrassation at base of bristle. Two minute setae on each side of antesensilial bristle (Fig.
Modified abdominal segments. Dorsal portion of T-VIII sclerotized cephalad to trumpet shaped spiracle; all setae below spiracle eight. About 14 setae grouped on apical portion of T-VIII. Caudal margin of S-VII concave to near ventral margin terminating in truncate lobe with small apical sinus. Ventral margin of S-VII with heavy sclerotization; with oblique row of four to six lateral setae (Fig.
Dimensions
Female holotype: 2.2 mm, female average: 2.1 mm (n = 3), range: 2.0–2.2 mm.
Etymology
The new species epithet goodmani is named in honor of its collector, Dr. Steven M. Goodman, Field Museum of Natural History, Chicago, IL for his untiring efforts and excellent contributions to the field of mammalogy, specifically for his work on bats and small mammals in Madagascar from which these specimens were obtained.
Remarks
Although the respective male and female sexes of the two new flea species described in this paper were both collected from the bat genus Chaerephon representing two species [Chaerephon bivittatus (Heuglin, 1861) from Kenya and Chaerephon jobimena Goodman and Cardiff, 2004 from Madagascar], they do not represent the same species of flea. Chaerephon bivittatus and C. jobimena are allopatric in their distributions. Although there exists some sexual dimorphism among fleas, these females differ drastically from the male described below as A. smiti. Characteristics examined included major differences in the nature of the genal teeth, pre-oral tuber, pronotal comb, shape of the gena, variations in chaetotaxy of head and abdomen, and abdominal incrassations.
Araeopsylla lumareti Smit, 1958 (known only from the male sex) could potentially represent the male of this new species for which only females are known; however, this is doubtful based on their differences in hosts, morphology, and geographical remoteness. Araeopsylla lumareti is known only from the type locality in Cambodia from “bat guano” opposed to the occurrence of A. goodmani in Madagascar from C. jobimena, which is endemic to Madagascar. The frontal row of setae of males of A. lumareti are comprised of “small setae” and the occiput is “without marked dorsal incrassations” (
Several species of the bat genus Chaerephon have yielded several bat flea species of the genus Lagaropsylla (
Type material examined
Madagascar, Fianarantsoa Province: Isalo, 3.8 km NW Ranohira, along Namaza River (22°32'24"S, 45°22'48"E), Chaerephon jobimena ♂, 1 XII 2002, SMG, (SGM-13344-1, holotype ♀, SGM-13344-2, paratype ♀,
Araeopsylla smiti , sp. n.
Diagnosis
Distinguished from all other species of Araeopsylla by the details of the telomere and distal arm of S-IX. The telomere is acutely pointed at apex and has a broadly rounded lobe along its ventral margin (Fig.
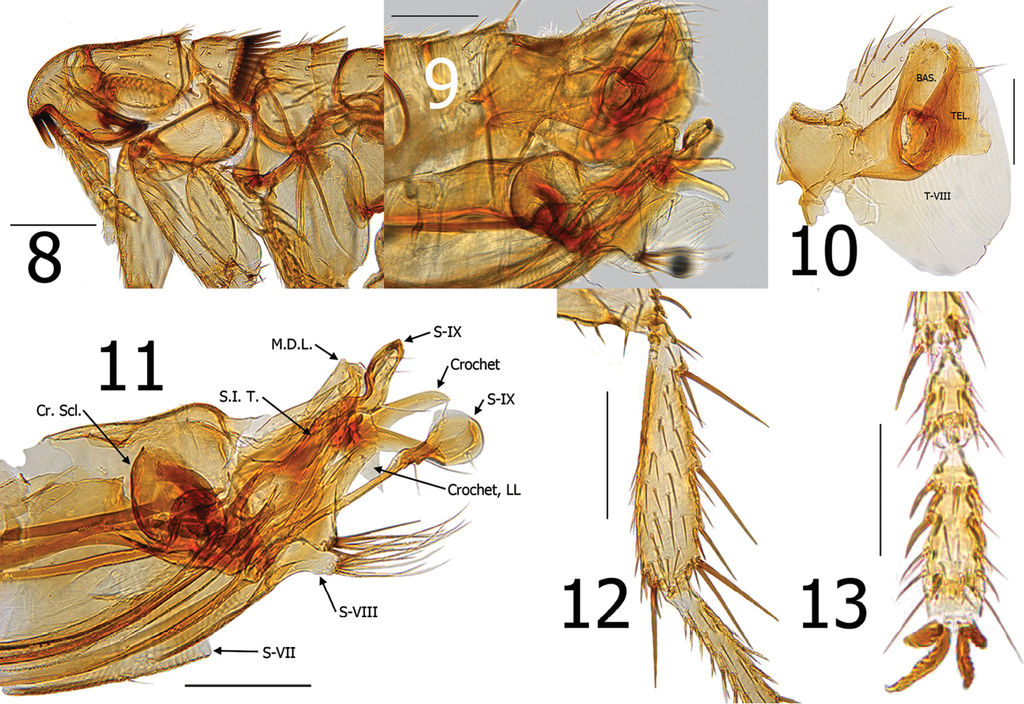
Araeopsylla smiti sp. n., holotype male (BJH-5634). 8 Head and pronotum 9 Terminal segments, before dissection 10 Basimere, telomere, and eighth tergum 11 Aedeagus, eighth sternum, and distal arm of ninth sternum, (Cr. Scl. = Crescent Sclerite, LL = Lower Lobe, M.D.L. = Median Dorsal Lobe, S.I.T. = Sclerotized Inner Tube) 12 Hind tibia 13 Fourth and fifth segments of distitarsomere 2. Scale: 200 µ (8–12), 100 µ (13).
Description
Head. Margin of frons gradually thickened from falx to oral angle. Frontal row of 9–10 minute setae. Area between frons and frontal row of setae with small punctate structures and two placoid pits. Area between frons and frontal row not white or clear, but moderately sclerotized. One placoid pit postad to frontal row of setae near base of genal tooth. Seven to eight minute setae postad to frontal row; group of 6–7 variable sized setae near ventral margin of antennal fossa. Eye fused into darkly sclerotized genal lobe. Genal lobe tapered and broadly rounded at apex; with minute apical tooth. Setae in occipital area rather randomly arranged. Five setulae along dorsal margin of antennal fossa; each as long as other randomly arranged setae in occipital area. Two small setae postad to antennal fossa in position of what is usually 4-5 spiniform setae in other Araeopsylla species. Apex of scape enlarged; three long setae along upper margin and three marginal setae at apex. Pedicel with several fine setae along apical margin; none extending beyond first segment of antenna. Clavus asymmetrical; not extending beyond margin of head. Five segmented labial palpus extended to 1/3 length of fore coxa. Proximal segment of five segmented labial palpus rather bulbous in form (Fig.
Thorax. Pronotum with 26 ctenidia; each acutely pointed and only slightly shorter than length of pronotum. Setae on pronotum randomly arranged. Two dorsal and two ventral pseudosetae under mesonotal collar. Metanotum with three rows of setae; two marginal short, stout spinelets at dorsal apex of sclerite. Pleural rod nearly centrally attached to sclerotic dome. Ventral portion of metasternum lobed downward between coxae. Pleural arch absent. Metepisternum with squamulum and one long seta at dorsal margin. Metepimeron with nine setae; spiracle large and round.
Legs. Fore coxa with ~40 lateral setae. Fore femur with 2 minute setae on mesal surface; none on lateral surface. Mesal surface of mesotibia with single row of five setae; multiple setae on lateral surface. Mesal surface of metatibia with single row of seven setae; multiple setae on lateral surface. Meso- and metatibiae with five well defined notches; two setae at each. Multiple single setae interspersed along margins between defined notches. Each tarsal segment longer than adjacent more distal segments on meso- and metatarsi. Five lateral plantar bristles on all distitarsomeres; first pair displaces between second pair. Two pre-apical plantar bristles on each distitarsomere (Figs
Unmodified abdominal segments. Single spinelet on apex of T-I. Globular sclerotized incrassation at base of each terga (T-I–VII). Pigmented banding extends slightly ventrally from each incrassation. Each terga with single uninterrupted row of setae; one seta below level of each round spiracle. Single long antesensilial bristle. Ventral margin of each sternite is heavily sclerotized. Sternites II–III with one minute seta in row; S-IV–VI with two minute setae in each row.
Modified abdominal segments. Basimere without lobes or sinuses. Telomere with large lobe on lower ventral margin; a few fine setae along ventral margin of telomere above lobe. Tergum VIII encompassing basimere and telomere with all setae restricted to dorsal half of sclerite (Fig.
Etymology
Mr. F.G.A.M. Smit, during his long tenure at the British Museum, London was without doubt, a major contributor to our knowledge of the global flea fauna. It is thus fitting to name this flea smiti in his honor as a noun in apposition.
Remarks
This is the first record of the genus Araeopsylla occurring in Kenya, although the genus has been recorded throughout tropical Africa.
Type material
Holotype ♂, Kenya, Rift Valley, Maji Moto, 4.8 km W of Lake Harrington (00°16'00"S, 03°6'04 “E), Chaerephon bivittatus (
Araeopsylla martialis
Ceratophyllus martialis Rothschild, 1903
Ischnopsyllus martialis Rothschild, 1906: 187–188
Araeopsylla martialis Jordan & Rothschild, 1921: 146;
Remarks
Although Araeopsylla is generally collected only in very small numbers across its range, A. martialis is exceptional. Sixty-seven individual M. jugularis specimens yielded one or more specimens of A. martialis. These were collected in three provinces of Madagascar (Fianarantosa, Toamasina, and Toliara). A total of 158 (64♂, 94♀) specimens were harvested from the 67 specimens of M. jugularis, yielding a flea intensity of 2.4. One male specimen of A. martialis was collected from Rousettus madagascarensis. Other species of bats did not harbor A. martialis. Its occurrence on a Rousettus sp. is likely an accidental association.
Material examined
(BYUC). Madagascar, Fianarantsoa Province: Fianarantsoa église FLM (21°27'32"S, 47°04'36"E), 1190 m, M. jugularis ♂, 18 XI 2004, FHR, 1♂, 1♀.
Dampfia grahami grahami
Ischnopsyllus grahami Waterston, 1915: 115;
Dampfia grahami grahami
Remarks
A total of 15 specimens (8♂, 7♀) was collected from four specimens of the Malagasy endemic Neoromicia matroka (Thomas & Schwann, 1905). This flea is not commonly collected and the only known existing records include the holotype ♂ from Eptesicus capensis = Neoromicia capensis (A. Smith, 1829) from Cape Town, South Africa, 3♀ specimens from Natal, and 2♀ from Orange Free State, South Africa (bat host species undetermined). Neoromicia capensis is widely spread across sub-Saharan Africa. Although not found in Madagascar,
Material examined
(BYUC). Madagascar, Toamasina Province: Andasibe, Ankazinina (18°56'38"S, 48°24'46"E), 970 m, Eptesicus matroka (Thomas & Schwann, 1905) (RHF-58), 18 IX 2004, FHR, 1♀.
Lagaropsylla consularis
Lagaropsylla consularis Smit, 1957: 167,
Remarks
Lagaropsylla consularis has been reported previously in Madagascar and is among the more common species in the genus, primarily parasitizing C. pumilus. Chaerephon pumilus has a broad range from Yemen to Senegal, south to South Africa and Madagascar. Neoromicia somalicus (Thomas, 1901) from which L. consularis was collected in Kenya, is also found in Madagascar. Reported primarily on molossid bats, L. consularis has also been documented on hipposiderid and vespertilionid bats (
Material examined
(BYUC). Kenya: Rift Valley, Maji Moto, Neoromicia somalicus (
Lagaropsylla hoogstraali
Lagaropsylla hoogstraali Smit, 1957: 171–172,
Lagaropsylla traubi Klein, 1967: 127–131;
Lagaropsylla hoogstraali Ribeiro, 1974: 144;
Remarks
Lagaropsylla hoogstraali has been documented in Angola, Rwanda, Sudan, Zaire and Madagascar. Although there are few collections of this flea, most have been collected from Mops midas (Sundevall, 1843), a broadly distributed bat in continental Africa and Madagascar.
Material examined
(BYUC). Madagascar, Mahajanga Province: Ambondromamy, Cite de la Gendarmerie (16°26'03"S, 47°09'26"E), 50 m, Mops midas miarensis = M. midas ♀ (RHF-823), 13 III 2005, FHR, 1♂; same data except M. midas ♂ (RHF-824), 1♂; and M. midas ♂ (RHF-825), 1♀. Toliara Province: Sakaraha, EPP (22°54'26"S, 44°31'48"E). 480 m, M. midas ♂ (RHF-262), 20 X 2004, FHR, 1♀.
Lagaropsylla idae
Lagaropsylla idae Smit, 1957: 165–167;
Lagaropsylla setzeri Segerman, 1970: 3–5;
Lagaropsylla idae Ribiero, 1974: 144;
Remarks
Material examined
(BYUC). Ghana, Eastern Region: Teshi, Accra Plains (05°34"N, 00°00'6"W), M. condylurus (
Key to the species of Araeopsylla
| 1 | Males (A. goodmani sp. n., male unknown) | 2 |
| 1’ | Females (A. lumareti and A. smiti sp. n., females unknown) | 11 |
| 2(1) | Acetabular bristles arranged on prominent long lobe of basimere (lobe longer than wide) | 3 |
| 2’ | Acetabular bristles along margin of basimere, not borne on lobe | 6 |
| 3(2) | Ventral margin of telomere with a sinus and subtending lobe | 4 |
| 3’ | Ventral margin without a sinus or lobe | 5 |
| 4(3) | Caudal margin of T-VIII truncate. Lobe on caudal margin of telomere rounded, not hooked downward (Cambodia) | phnomensis |
| 4’ | Caudal margin of T-VIII narrowing to rounded lobe. Lobe on caudal margin of telomere hooked downward (Cambodia) | immanis |
| 5(3’) | Ventral margin of telomere convex; dorsal margin concave (Cambodia) | lumareti |
| 5’ | Ventral and dorsal margins nearly straight (Thailand) | elbeli |
| 6(2’) | Apex of manubrium spatulate (Rwanda) | faini |
| 6’ | Apex of manubrium not spatulate | 7 |
| 7(6’) | Basal lobe of distal arm of S-IX long and modified. Telomere acutely pointed at apex. Truncate lobe at apex of S-VIII with tuft of long, coarse setae (Kenya) | smiti sp. n. |
| 7’ | Basal lobe of distal arm of S-IX short, without leaf-like apical lobe. Telomere rounded and blunt at apex. Sternum VIII without lobe bearing tuft of long setae | 8 |
| 8(7’) | Apex of manubrium sharp and turned downward. Sinus present above acetabular bristles on basimere (Kenya, Angola, Lesotho, South Africa) | scitula |
| 8’ | Manubrium and basimere otherwise | 9 |
| 9(8’) | Crochet without hook-like lobes (Italy) | gestroi |
| 9’ | Crochet with hook-like lobes | 10 |
| 10(9’) | Basimere quadrate on dorso-apical margin. Telomere broadens towards apex and extends beyond apex of basimere (Réunion Island, Madagascar) | martialis |
| 10’ | Basimere rounded on dorso-apical margin. Telomere somewhat parallel sided, rounded at apex, and sub equal in length to basimere (Egypt) | wassifi |
| 11(1’) | Hilla of spermatheca hardly longer than bulga (Thailand) | elbeli |
| 11’ | Hilla distinctly longer than length of bulga | 12 |
| 12(11’) | Caudal margin of S-VII concave to margin of terminal truncate ventral lobe; ventral lobe with small sinus at apex (Madagascar) | goodmani sp. n. |
| 12’ | Caudal margin of S-VII straight (not concave), or with lobes | 13 |
| 13(12’) | Bursa copulatrix rather straight; without sigmoid-like curves | 14 |
| 13’ | Bursa copulatrix not straight, but with various sigmoid-like curves | 15 |
| 14(12’) | Caudal margin of T-VIII slightly convex with vertical row of three short spiniform setae near apical margin of convexity. Spiracle VIII broadened at apex (Egypt) | scitula |
| 14’ | Caudal margin of T-VIII more straight; without row of three spiniform setae. Spiracle VIII rounded at apex (Egypt) | wassifi |
| 15(13’) | One closely arranged vertical row of six spiniform setae at caudal margin of T-VIII (Réunion Island, Madagascar) | martialis |
| 15’ | Chaetotaxy of caudal margin of T-VIII otherwise | 16 |
| 16(16’) | Caudal margin of S-VII without lobe (Cambodia) | phnomensis |
| 16’ | Caudal margin of S-VII with lobe | 17 |
| 17(16’) | Apex of ventral margin of S-VII extends beyond dorsal lobe (Cambodia) | immanis |
| 17’ | Dorsal lobe projects beyond apex of ventral margin | 18 |
| 18(17’) | Dorsal lobe on caudal margin of S-VII broad, subtended by a broad shallow sinus (Italy) | gestroi |
| 18’ | Broad angular lobe on margin of S-VII without subtending sinus (Rwanda) | fain |
Acknowledgements
The author expresses his sincere gratitude to Carl Dick, Steve Goodman, and James Boone for facilitating this study of a segment of the fleas that were collected in Madagascar that are deposited in the
References
- Beaucournu J-C (2004) Catalogue des puces de la Région Afrotropicale (Insecta-Siphonaptera) (sous-région malgache exclue). Beiträge zur Entomologie 54: 185–239. http://www.worldcat.org/title/beitrage-zur-entomologie/oclc/1519372
- Beaucournu J-C, Fain A (1983) Notes sur les Ischnopsyllinae du continent Africain (Siphonaptera). Revue de Zoologie africaine 97: 453–468.
- Beaucournu J-C, Fontenille D (1993) Contribution a un catalogue des puces de Madagascar (Insecta, Siphonaptera). Archives de l’Institute Pasteur de Madagascar 59: 57–98.
- Beaucournu J-C, Guiguen C (1991) Liste commentée des puces D’Éthiopie (Insecta, Siphonaptera). Annales de Parasitologie Humaine et Comparee (Paris) 66: 126–133. http://www.worldcat.org/title/annales-de-parasitologie-humaine-et-comparee/oclc/1481278
- Beaucournu J-C, Kock D (1994a) Notes sur les Ischnopsyllidae de la Région Orientale, II. Stations inédites et description d’une espèce nouvelle du genre Lagaropsylla Jordan & Rothschild 1921 (Insecta: Siphonaptera). Senckenbergiana biologica 73: 67–75. http://www.senckenberg.de/root/index.php?page_id=946
- Beaucournu J-C, Kock D (1994b) Le genre Lagaropsylla Jordan and Rothschild, 1921 (Siphonaptera: Ischnopsyllidae). Annales de la Société Entomologique de France 30: 193–207. http://www.biodiversitylibrary.org/bibliography/8188
- Beaucournu J-C, Kock D (1996) Notes sur les Ischnopsyllinae du Continent Africain, III. Complements a la repartition des espèce (Insecta: Siphonaptera: Ischnopsyllidae). Senckenbergiana biologica 75: 163–169. http://www.senckenberg.de/root/index.php?page_id=946
- Bedford GAH (1932) A synoptic check-list and host-list of the ectoparasites found on South African Mammalia, Aves, and Reptilia, 2nd ed., 18th Report of the Director of Veterinary Services and Animal Industry, South Africa, 223–522.
- Goodman SM, Taylor PJ, Ratrimomanarivo F, Hoofer SR (2012) The genus Neoromicia (Family Vespertilionidae) in Madagascar, with the description of a new species. Zootaxa 3250: 1–25. http://www.mapress.com/zootaxa
- Hastriter MW, Eckerlin RP (2003) Jellisonia painteri (Siphonaptera: Ceratophyllidae), a new species of flea from Guatemala. Annals of Carnegie Museum 72: 215–221. doi: 10.2992/007.083.0202
- Hopkins GHE, Rothschild M (1956) An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History), Volume II, London, 445 pp., 32 plates.
- Hubbard CA (1969) Tanzania bat fleas, how they were secured. Entomological News 80: 55.
- Jordan K, Rothschild NC (1921) New genera and species of bat-fleas. Ectoparasites 1: 142–161.
- Klein JM, Uilenberg G (1966) Données faunistiques et écologiques sur les puces de Madagascar (Siphonaptera). Cahier O.R.S.T.O., series Entomologie médicale4: 31–60.
- Lewis RE (2006) A catalog of primary types of bat fleas (Siphonaptera: Ischnopsyllidae) of the world. Bat research news 47(3): 43–60. http://www.batresearchnews.org
- Lewis RE, Lewis JH (1990) Catalogue of invalid genus-group and species-group names in Siphonaptera (Insecta).Theses Zoologicae, Volume II, Koeltz Scientific Books, Koenigstein, 263 pp. ISBN 3-87429-302-5
- Lumaret R (1962) Faune de Madagascr XV, Insectes Siponapteres. Publications de L’Institute de Recherche Scientifique Tananarive - Tsimbazaza 107: 12.
- Marcus T (1961) The bat fleas of Southern Africa (Siphonapt.: Ischnopsyllidae). Journal of the Entomological Society of South Africa 24: 173–211. http://content.ajarchive.org/cdm4/index_00128789.php?CISOROOT=/00128789
- Ratrimomanarivo FH, Vivian J, Goodman SM, Lamb JM (2007) Morphological and molecular assessment of the specific status of Mops midas (Chiroptera: Molossidae) from Madagascar and Africa. African Zoology 42: 237–263. doi: 10.3377/1562-7020(2007)42[237:MAMAOT]2.0.CO;2
- Ribiero H (1974) Sifonápteros de Angola (Insecta, Siphonaptera), estudo sistemático e dados bioecológicos interessando á epidemiologia da peste. Dissertation, Instituto de Hygiene e Medicina Tropical, Lisboa, 202 pp.
- Rothschild NC (1903) Further contributions to the knowledge of the Siphonaptera. Novitates Zoologicae 10: 317–325. www.biodiversitylibrary.org/bibliography/3882
- Rothschild NC (1906) Notes on bat fleas. Novitates Zoologicae 13: 186–188. http://www.biodiversitylibrary.org/bibliography/3882
- Smit FGAM (1954) New bat fleas (Siphonaptera: Ischnopsyllidae). Parasitology 44: 144–156. doi: 10.1017/S0031182000018850
- Smit FGAM (1955) A list of African Siphonaptera in the Natal Museum. Annals of the Natal Museum 13: 211–216. http://content.ajarchive.org/cdm4/index_03040798.php?CISOROOT=/03040798
- Smit FGAM (1957) The African species of the bat-flea genus Lagaropsylla. Revue de Zoologie et de Botanique Africaines 55: 163–172.
- Smit FGAM (1958) A new bat-flea from Borneo and Malaya. Entomologische Berichten 18: 236–242.
- Smit FGAM (1964) Siphonaptera. Parc National de la Garamba. – Mission H. de Saeger 44(2): 41–47.
- Smit FGAM (1968) Siphonaptera. Exploration du Parc National Albert, Series 2, 21(1): 3–16.
- Smit FGAM, Wright AM (1978) A list of code numbers of species and subspecies of Siphonaptera. Department of Entomology, British Museum (Natural History), London, 49 pp. [mimeographed]
- Waterston J (1915) Notes on Siphonaptera in the Albany Museum, Grahamstown, South Africa, with descriptions of two new species of the genus Ischnopsyllus. Records in the Albany Museum 3: 107–119.