Research Article |
|
Corresponding author: Hongxiang Han ( hanhx@ioz.ac.cn ) Academic editor: Erik J. van Nieukerken
© 2016 Nan Jiang, Shuxian Liu, Dayong Xue, Hongxiang Han.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Jiang N, Liu S, Xue D, Han H (2016) A review of Cyclidiinae from China (Lepidoptera, Drepanidae). ZooKeys 553: 119-148. https://doi.org/10.3897/zookeys.553.6153
|
Abstract
The subfamily Cyclidiinae from China is reviewed: two genera and seven species are reported from China. One new subspecies, Cyclidia fractifasciata indistincta subsp. n., is described. Two new synonyms are established: Cyclidia substigmaria (Hübner, 1831) (= Cyclidia substigmaria brunna Chu & Wang, 1987, syn. n. = Cyclidia tetraspota Chu & Wang, 1987, syn. n.). One misidentification in Chu & Wang (1987) is corrected. Identification keys and diagnoses for all discussed Chinese species are provided. External features and genitalia are depicted. In addition, results of DNA barcoding for five taxa of Cyclidia are briefly discussed.
Keywords
DNA barcodes, morphology, new subspecies, new synonymy, taxonomy
Introduction
The subfamily Cyclidiinae Warren, 1922, is the smallest subfamily within four subfamilies (besides Drepaninae, Oretinae, and Thyatirinae) of Drepanidae. This subfamily was first proposed as Eucherinae by
Recently,
Species of Cyclidiinae are distributed in the Palearctic Asia and Oriental region. Up to the present, two genera (Cyclidia Guenée, 1858 and Mimozethes Warren, 1901) have been recognized in Cyclidiinae. Ten species and eight subspecies are included in Cyclidia, with six species and four subspecies (C. substigmaria substigmaria (Hübner, 1831), C. substigmaria brunna Chu & Wang, 1987, C. substigmaria intermedia Prout, 1918, C. tetraspota Chu & Wang, 1987, C. rectificata rectificata (Walker, 1862a), C. fractifasciata (Leech, 1898), C. sericea Warren, 1922, C. orciferaria Walker, 1860) recorded in China (
The DNA barcoding method using a 658 bp base pair fragment of the cytochrome c oxidase subunit I gene (COI) as a tool for species discrimination was first put forward based on two hundred closely related species of Lepidoptera (
In the present study an overview of the Chinese Cyclidiinae is given with diagnostic characters for each genus and species, one new subspecies is described, two new synonyms are established, and one misidentification in
Materials and methods
Morphology. Studied specimens mainly belong to the Institute of Zoology, Chinese Academy of Sciences, Beijing, China (IZCAS) and the Natural History Museum, London, United Kingdom BMNH. Terminology for wing venation follows the Comstock-Needham System (
DNA-Barcoding. Prior to DNA sequencing, one or two legs were removed from several specimens of each of five examined taxa (C. substigmaria substigmaria, C. rectificata rectificata, C. fractifasciata fractifasciata, C. fractifasciata indistincta, C. orciferaria). DNA extraction was done using Qiagen DNeasy Blood and Tissue Kit (Qiagen, Beijing, China). The primers for the amplification of the 658 bp fragment were LepF1 (5’-ATTCAACCAATCATAAAGATATTGG-3’), LepR1 (5’-TAAACTTCTGGATGTCCAAAAAATCA-3’) (
Cyclidia species included in this study with GenBank accession numbers and BOLD process ID.
| Taxa | Sequence ID | Collecting locality | Collecting date | GenBank accession no. | BOLD process ID |
|---|---|---|---|---|---|
| C. substigmaria substigmaria | DB00162 | West Tianmushan, Zhejiang | Jul. 2003 | KR872896 | CLDC001-15 |
|
DB00173
DB00174 |
Wuzhishan, Hainan Lingshui, Hainan |
May 2007 May 2007 |
KR872897
KR872898 |
CLDC002-15
CLDC003-15 |
|
| DB00181 | Baotianman, Henan | Aug. 2008 | KR872899 | CLDC004-15 | |
| DB00182 | Luoyang, Henan | Aug. 2006 | KR872900 | CLDC005-15 | |
| DB00184 | Baoshan, Yunnan | Aug. 2007 | KR872901 | CLDC006-15 | |
| DB00189 | Yanling, Hunan | Jul. 2008 | KR872902 | CLDC007-15 | |
| IOZ LEP M 01129 | Mengla, Yunnan | Jul. 2013 | KR872903 | CLDC008-15 | |
| IOZ LEP M 01134 | Tengchong, Yunnan | Aug. 2013 | KR872904 | CLDC009-15 | |
| IOZ LEP M 01304 | West Tianmushan, Zhejiang | Jul. 2011 | KR872905 | CLDC010-15 | |
| IOZ LEP M 08961 | Mengla, Yunnan | Jul. 2013 | KR872906 | CLDC011-15 | |
| IOZ LEP M 09195 | Qushi, Yunnan | Aug. 2013 | KR872907 | CLDC012-15 | |
| IOZ LEP M 16605 | Kangxian, Gansu | Aug. 2014 | KR872908 | CLDC013-15 | |
| IOZ LEP M 16606 | Kangxian, Gansu | Aug. 2014 | KR872909 | CLDC014-15 | |
| IOZ LEP M 16607 | Kangxian, Gansu | Aug. 2014 | KR872910 | CLDC015-15 | |
| IOZ LEP M 16608 | Kangxian, Gansu | Aug. 2014 | KR872911 | CLDC016-15 | |
| IOZ LEP M 17993 | Liuku, Yunnan | Sep. 2014 | KR872912 | CLDC017-15 | |
| IOZ LEP M 17994 | Liuku, Yunnan | Sep. 2014 | KR872913 | CLDC018-15 | |
| IOZ LEP M 02790 | Guilin, Guangxi | Apr. 1952 | KR872914 | CLDC019-15 | |
| C. rectificata rectificata | DB00226 | Bomi, Tibet | Aug. 2005 | KR872923 | CLDC020-15 |
| DB00228 | Mêdog, Tibet | Aug. 2006 | KR872924 | CLDC021-15 | |
| DB00229 | Mainling, Tibet | Aug. 2006 | KR872925 | CLDC022-15 | |
| IOZ LEP M 03475 | Zayü, Tibet | Aug. 2014 | KR872926 | CLDC023-15 | |
| IOZ LEP M 03476 | Zayü, Tibet | Aug. 2014 | KR872927 | CLDC024-15 | |
| IOZ LEP M 03477 | Zayü, Tibet | Aug. 2014 | KR872928 | CLDC025-15 | |
| IOZ LEP M 16015 | Zayü, Tibet | Aug. 2014 | KR872929 | CLDC026-15 | |
| C. fractifasciata fractifasciata | IOZ LEP M 00657 | Pianma, Yunnan | May 2011 | KR872930 | CLDC027-15 |
| IOZ LEP M 00683 | Pianma, Yunnan | May 2011 | KR872931 | CLDC028-15 | |
| IOZ LEP M 07012 | Pianma, Yunnan | May 2011 | KR872932 | CLDC029-15 | |
| IOZ LEP M 07013 | Pianma, Yunnan | May 2011 | KR872933 | CLDC030-15 | |
| C. fractifasciata indistincta | IOZ LEP M 16601 | Kangxian, Gansu | Aug. 2014 | KR872934 | CLDC031-15 |
| IOZ LEP M 16602 | Kangxian, Gansu | Aug. 2014 | KR872935 | CLDC032-15 | |
| IOZ LEP M 16603 | Kangxian, Gansu | Aug. 2014 | KR872936 | CLDC033-15 | |
| IOZ LEP M 16604 | Kangxian, Gansu | Aug. 2014 | KR872937 | CLDC034-15 | |
| IOZ LEP M 09387 | Wushan, Chongqing | Jul. 2013 | KT250118 | CLDC035-15 | |
| C. orciferaria | DB00202 | Bawangling, Hainan | May 2007 | KR872915 | CLDC036-15 |
| DB00203 | Wuzhishan, Hainan | Apr. 2008 | KR872916 | CLDC037-15 | |
| DB00210 | Yanling, Hunan | Jul. 2008 | KR872917 | CLDC038-15 | |
| DB00211 | Yanling, Hunan | Jul. 2008 | KR872918 | CLDC039-15 | |
| DB00213 | Shixing, Guangdong | Jun. 2008 | KR872919 | CLDC040-15 | |
| DB00216 | Baoshan, Yunnan | Aug. 2007 | KR872920 | CLDC041-15 | |
| IOZ LEP M 01208 | West Tianmushan, Zhejiang | Jul. 2011 | KR872921 | CLDC042-15 | |
| IOZ LEP M 01324 | West Tianmushan, Zhejiang | Jul. 2011 | KR872922 | CLDC043-15 |
Results
Taxonomy
Cyclidiinae Warren, 1922
Cyclidiinae Warren, 1922: 444
Cyclidia
Cyclidia Guenée, 1858: 62. Type species: Cyclidia substigmaria (Hübner, 1831), by monotypy.
Nelcynda Walker, 1862a: 1142. Type species: Nelcynda rectificata Walker, 1862, by monotypy.
Ciclidia Chou & Xiang, 1984: 159. [Incorrect spelling of Cyclidia Guenée.]
Generic characters
Head. Antennae lamellate, partly unipectinate, rami very short (Fig.
Diagnosis
Cyclidia is quite different from Mimozethes externally and in the genitalia. For example, externally, the rami of the antennae are much shorter; the species of Cyclidia are much larger, and the postmedial lines of forewing are often double, while in Mimozethes, it is single and forms a “>” shaped protrusion near R5; in the male genitalia, the socii are well developed in Cyclidia, but absent in Mimozethes; the sacculus unmodified in Cyclidia but forming a process in Mimozethes; in the female genitalia, the signa are a paired band-like sclerotization in Cyclidia, but absent in Mimozethes.
Distribution
China, Japan, Korean Peninsula, south and southeast Asia.
Key to Chinese Cyclidia species
| 1 | Wings colour white or grey | 2 |
| – | Wings colour blackish brown |
C. orciferaria, Figs |
| 2 | Discal spots on hind wing distinct | 3 |
| – | Discal spots on hind wing indistinct | 4 |
| 3 | Discal spots on hind wing dark grey |
C. substigmaria substigmaria, Figs |
| – | Discal spots on hind wing black |
C. substigmaria intermedia, Fig. |
| 4 | Terminal lines of both wings single |
C. rectificata rectificata, Figs |
| – | Terminal lines of both wings double | 5 |
| 5 | Outer margin of forewing medial line forming an right angle below M3 | 6 |
| – | Outer margin of forewing medial line not forming an right angle below M3 |
C. pitimani, Figs |
| 6 | Outer line of antemedial line and inner line of postmedial line of forewing distinct |
C. fractifasciata fractifasciata, Figs |
| – | Outer line of antemedial line and inner line of postmedial line of forewing invisible |
C. fractifasciata indistincta, Figs |
Adults. 4–9 Cyclidia substigmaria substigmaria 4 male (with dot-like and wavy submarginal line of the forewing, Yunnan) 5 ditto, underside 6 male (with faint, broad and interrupted submarginal line of the forewing, Zhejiang) 7 male (holotype of C. substigmaria brunna, Sichuan) 8 male (holotype of C. tetraspota, Yunnan) 9 C. substigmaria intermedia, male (Tibet) 10–11 C. rectificata 10 male (Tibet) 11 ditto, underside. Scale bar: 1 cm.
Adults. 12–13 Cyclidia pitimani 12 male (Yunnan) 13 ditto, underside 14–15 C. fractifasciata fractifasciata 14 male (Yunnan) 15 ditto, underside 16–17 C. fractifasciata indistincta subsp. n. 16 male (holotype, Gansu) 17 ditto, underside 18–19 C. orciferaria 18 male (Hainan) 19 ditto, underside 20–21 Mimozethes angula 20 male (holotype, Sichuan) 21 ditto, underside 22–23 M. lilacinaria 22 male (holotype, Sichuan) 23 ditto, underside. Scale bar: 1 cm.
Cyclidia substigmaria
Euchera substigmaria Hübner 1831: 29. pl. 90, figs 519, 520. Syntypes, China.
Cyclidia
substigmaria
:
Abraxas capitata Walker, 1862a: 1121. Holotype ♀, China: Hong Kong (BMNH).
Euchera
capitata
:
Cyclidia substigmaria brunna Chu & Wang, 1987: 205. Holotype ♂, China: Sichuan: Emeishan, Qingyinge (IZCAS). Syn. n.
Cyclidia tetraspota Chu & Wang, 1987: 206. Holotype ♂, China: Yunnan: Xishuangbanna, Yunjinghong (IZCAS). Syn. n.
Diagnosis
In external appearance, this species is distinguishable from other congeners by the following characters: the discal spots of hind wing are very distinct on the upper side and the underside; the discal spot of the forewing is covered with white scales on the upper side; two greyish brown markings are present inside the anal angle of the forewing. The male genitalia of the species are close to those of C. rectificata, but the terminal part of the uncus and the socii are narrower; the vesica is much more scobinate. In the female genitalia, the two signa are close to each other posteriorly, while in C. rectificata, they are almost parallel.
Remarks
There are five subspecies of C. substigmaria:
C. s. substigmaria (Hübner, 1831), most parts of China and Vietnam;
C. s. intermedia Prout, 1918 in Tibet;
C. s. nigralbara Warren, 1914 in Japan and Korean Peninsula;
C. s. modesta Bryk, 1943 in Myanmar;
C. s. superstigmaria Prout, 1918 in India and Nepal.
Distribution
China, Japan, Korean Peninsula, India, Nepal, Myanmar, Vietnam.
Biological notes
Cyclidia substigmaria substigmaria
Diagnosis
The subspecies is very similar to C. substigmaria intermedia, but differs externally by the paler discal spot of the hind wing and the two less distinct markings inside the anal angle of the forewing.
Type material examined
CHINA: Sichuan (IZCAS): 1♂ (Holotype of C. substigmaria brunna), Emeishan, Qingyinge, 800–1000 m, 17.V.1957, coll. Wang Zongyuan. Zhejiang (IZCAS): 1♀ (Allotype of C. substigmaria brunna), Hangzhou, 4.V.1975, coll. Zhang Baolin. Fujian (IZCAS): 3♂ (Paratypes of C. substigmaria brunna), Wuyishan, 6–21.V.1983, coll. Wang Linyao. Yunnan (IZCAS): 1♀ (Paratypes of C. substigmaria brunna), Liuku, 2500 m, 23.V.1981, coll. Liao Subai; 1♂ (Holotype of C. tetraspota), Xishuangbanna, Yunjinghong, 650 m, 22.VI.1959, coll. Meng Xuwu; 1♀ (Allotype of C. tetraspota), Yiwubanna, Menglun, 650 m, 23.VII.1959, coll. Zhang Facai; 1♂ (Paratype of C. tetraspota), ibidem, 28.V.1958, coll. Wang Shuyong. Hainan (IZCAS): 1♂ (Paratype of C. tetraspota), Wanning, 10 m, 9.IV.1960, coll. Li Zhenfu. Guangxi (IZCAS): 1♂ (Paratype of C. tetraspota), Guilin, Liangfeng, 20.IV.1952. Hongkong (BMNH): 1 ♀, collector and collecting date unknown (Holotype of C. substigmaria capitata).
Additional material examined
CHINA: Henan (IZCAS): 1♂, Luoyang, Huaguoshan, 4.VIII.2006, coll. Song Hao; 1♀, Baiyunshan, 1400 m, 27.VII.2003, coll. Lu Yanan; 1♂, Jigongshan, 25.VI.1984. Shaanxi (IZCAS): 2♂1♀, Ningshan, Guanghuojie, 1189 m, 28.VII.2014, coll. Liu Shuxian and Ban Xiaoshuang; 1♂, Zhashui, Yingpanzhen, 980 m, 31.VII.2014, Liu Shuxian and Ban Xiaoshuang; 1♂, Xunyang, Bailiuzhen, 386 m, 3.VIII.2014, coll. Liu Shuxian and Ban Xiaoshuang. Gansu (IZCAS): 1♂, Wenxian, Qiujiaba, 2200–2350 m, 29.VI.1998, coll. Yuan Decheng; 1♀, Kangxian, Baiyunshan, 1250–1750 m, 12.VII.1998, coll. Wang Shuyong; 1♂7♀, Kangxian, Yangba, Meiyuangou, 1000 m, 13.VIII.2014, coll. Xue Dayong & Ban Xiaoshuang; 1♀, Wenxian, Lukou, 22.V.1987. Jiangsu (IZCAS): 7♂4♀, Chemo, 22.IV–2.V.1935, coll. O. Piel. Anhui (IZCAS): 1♀, Linzongchang, IX.1970, coll. Mai Weiqiang; 2♀, Yuexi, Linyeju, 11.IX.1982, coll. Zhou Tiying. Zhejiang (IZCAS): 5♂3♀, Lin’an, West Tianmushan, 400–1506 m, 6.IX.1981, 26–30.VII.2003, 27.VII.2011, coll. Xue Dayong et al.; 15♂1♀, Tianmushan, 15–25.VI.1936, 25–30.VIII.1947, 22.VIII.1972, 28–31.VII.1998, coll. O. Piel et al.; 1♂1♀, Hangzhou, 4.V.1975, 1981, coll. Zhang Baolin; 1♂, Qingyuan, Fengyangshan, Datianping, 1290 m, 6–10.VIII.2003, coll. Han Hongxiang. Hubei (IZCAS): 1♂, Shennongjia, Muyu, 22.VII.1998, coll. Zhou Hongzhang; 1♀, Shennongjia, Dalongtan, 2700 m, 27.VII.1998, coll. Zhou Haisheng; 1♂, Xingshan, Longmenhe, 1300 m, 12.IX.1994, coll. Song Shimei; 4♀, Xuan’en, 650 m, 25.V.1989, coll. Li Wei; 1♀, Hefeng, Fenshuiling Linchang, 31.VII.1989, coll. Li Wei. Jiangxi (IZCAS): 1♀, Yifeng, Yuanqian, 8.IX.1959. Hunan (IZCAS): 1♀, Yanling, Taoyuandong, 631 m, 4–8.VII.2008, coll. Chen Fuqiang; 1♀, Fenghuang, 15.IX.1988, coll. Song Shimei; 1♀, Cili, 3.IX.1988, coll. Song Shimei. Fujian (IZCAS): 11♂9♀, Wuyishan, 26.IV–14.VI.1983, coll. Wang Linyao and Zhang Baolin; 1♂, Xinkou, 15.VI.1981, coll. Lin Yibiao; 2♂1♀, Jianyang, Huangkeng, 270–950 m, 23.IV–1.V.1960, coll. Jiang Shengqiao and Zuo Yong; 1♀, Chong’an, Xingcun, Guadun, 840–1210 m, 25.VIII.1960, coll. Ma Chenglin; 1♀, Chong’an, Xingcun, Sangang, 740 m, 17.V.1960, coll. Zhang Yiran. Guangdong (IZCAS): 1♂, Guangzhou, 8.VI.1973, coll. Zhang Baolin; 4♂5♀, Guangzhou, Sanyuanli, 27.IV.1958, coll. Wang Linyao. Hainan (IZCAS): Wanning, 10 m, 14.IV.1960, coll. Li Changqing; 3♂, Xinglong, 24.III.1963, IV.1963, coll. Zhang Baolin; 3♂, Lingshui, Diaoluoshan, 4–5.V.2007, coll. Han Hongxiang; 1♀, Wuzhishan, Shuiman, 600 m, 12.V.2007, coll. Han Hongxiang; 1♀, Baisha, Yinggeling, 434 m, 3–4.XII.2007, coll. Li Jing; 1♀, Jianfengling, Tianchi, 3.III.1982, coll. Long Yongcheng. Guangxi (IZCAS): 1♂1♀, Jinxiu, Luoxiang, 200–400 m, 1–16.V.1999, coll. Huang Fusheng and Han Hongxiang; 1♀, Jinxiu, Yonghe, 500 m, 12.IV.1999, coll. Han Hongxiang; 1♀, Jinxiu, Jinzhong Gonglu, 1100 m, 12.V.1999, coll. Li Wenzhu; 2♂, Guilin, Yanshan, 26.IX.1958, 19.XI.1959; 1♂5♀, Fangcheng, Fulong, 240–260 m, 1.III.1998, 19–20.IV.1998, coll. Li Wenzhu and Wu Chunsheng; 1♂, Napo, Nianjing, 900 m, 11.IV.1998, coll. Wu Chunsheng; 1♀, Napo, Defu, 1350 m, 19.VI.2000, coll. Yao Jian; 1♀, Napo, Nonghua, 990 m, 13.IV.1998, coll. Li Wenzhu; 1♀, Napo, Baihe, 540 m, 8.IV.1998, coll. Qiao Gexia; 1♂, Pingxiang, 230 m, 8.VI.1976, coll. Zhang Baolin; 2♀, Longsheng, 10–11.VI.1980, coll. Zhong Tiesen and Song Shimei; 2♀, Daxin, Xialei, 680 m, 31.III.1998, coll. Li Wenzhu; 2♂, Longzhou, Nonggang, 195 m, 15–17.VII.2013, coll. Liu Shuxian and Li Xinxin. Sichuan (IZCAS): 1♀, Emeishan, Baoguosi, 550–750 m, 8.IV.1957, coll. Wang Zongyuan; 1♂, Emeishan, 580–1100 m, 22.VI.1955, coll. Zi Yunzhen; 36♂34♀, Emeishan, Qingyinge, 800–1000 m, 17.IV–20.V.1957, 19.IX–28.X.1957, coll. Zhu Fuxing et al.; 1♀, Yanyuan, Jinhe, 2.VII.1984, coll. Chen Yixin. Guizhou (IZCAS): 1♀, Sinan, 350 m, 9.V.1983, coll. Liu Yanxian; 1♂, Koei-Yang, 5.IX.1935. Yunnan (IZCAS): 2♂7♀, Xishuangbanna, Mengna, 550 m, 22–30.VI.1959, coll. Zhang Yiran and Li Zhenfu; 1♂3♀, Xiaomengyang, 850–1000 m, 6.V.1957, 12.VII–22.VIII.1957, 10.X.1957, coll. Wang Shuyong et al.; 1♂1♀, Xishuangbanna, Menghun, 160–750 m, 4.VI.1958, coll. Meng Xuwu et al.; 1♀, Xishuangbanna, Yunjinghong, 650 m, 3.VII.1957, coll. Wang Shuyong; 2♂6♀, Xishuangbanna, Mengla, 620–650 m, 2.V–6.VI.1959, coll. Zhang Yiran et al.; 6♂9♀, Mengla, Menglun, 650–665 m, 22–29.X.1958, 3.IV–18.V.1964, 29.VII.2013, coll. Wang Shuyong et al.; 1♂, Xishuangbanna, Menghai, 1200–1600 m, 18.VII.1958, coll. Wang Shuyong; 2♀, Xishuangbanna, Ganlanba, 560 m, 9–10.VII.1958, coll. Li Chuanlong; 1♂, Xishuangbanna, Bubang, 700 m, 14.IX.1993, coll. Yang Longlong; 1♀, Xishuangbanna, Yiwu, 800–1300 m, 13.VII.1959, coll. Pu Fuji; 6♂1♀, Baoshan, Baihualing, 1520 m, V.11–13.VIII.2007, coll. Wu Chunguang and Lang Songyun; 2♂1♀, Baoshan, Bawan, 1040–1100 m, 19–23.1992, 8–10.VIII.2007, 8–10.VIII.2013, coll. Wu Chunguang et al.; 2♂3♀, Baoshan, Xinujiang Hegu, 800–1000 m, 10–11.V.1955, coll. Xue Yufeng; 1♂, Tengchong, Qushi, Dabacun, 1873 m, 4.VIII.2013, coll. Liu Shuxian and Li Xinxin; 7♂1♀, Tengchong, Zhengding, 1833 m, 6–7.VIII.2013, coll. Liu Shuxian and Li Xinxin; 2♀, Tengchong, Heinitang, 1824 m, 26–27.VI.2014, coll. Li Xinxin and Pan Xiaodan; 1♀, Cheli, 620 m, 18.IV.1957, coll. Zang Lingchao; 2♂, Yuanyang, Nansha, 1100 m, 26.V.1979, coll. Luo Kezhong; 1♂1♀, Lushui, Liuku, 860–1220 m, 18–19.IX.2014, coll. Liang Hongbin; 2♂4♀, Lushui, Pianma, 1750–1980 m, 7.V.1981, 8–12.V.2011, 3–4.VII.2014, coll. Zhang Xuezhong et al.; 1♀, Jinping, Mengla, 500 m, 2.V.1956, coll. Huang Keren; 1♀, Jinping, Chang Potou, 1200 m, 23.V.1956, coll. Huang Keren. Vietnam (IZCAS): 1♀, Tonkin, Hoa-Binh, leg. A. de Cooman.
Variation
The submarginal line of the forewing varies from dot-like and wavy to faint, broad and interrupted between veins. In the male genitalia, the terminal half of the costa vary from smooth (Fig.
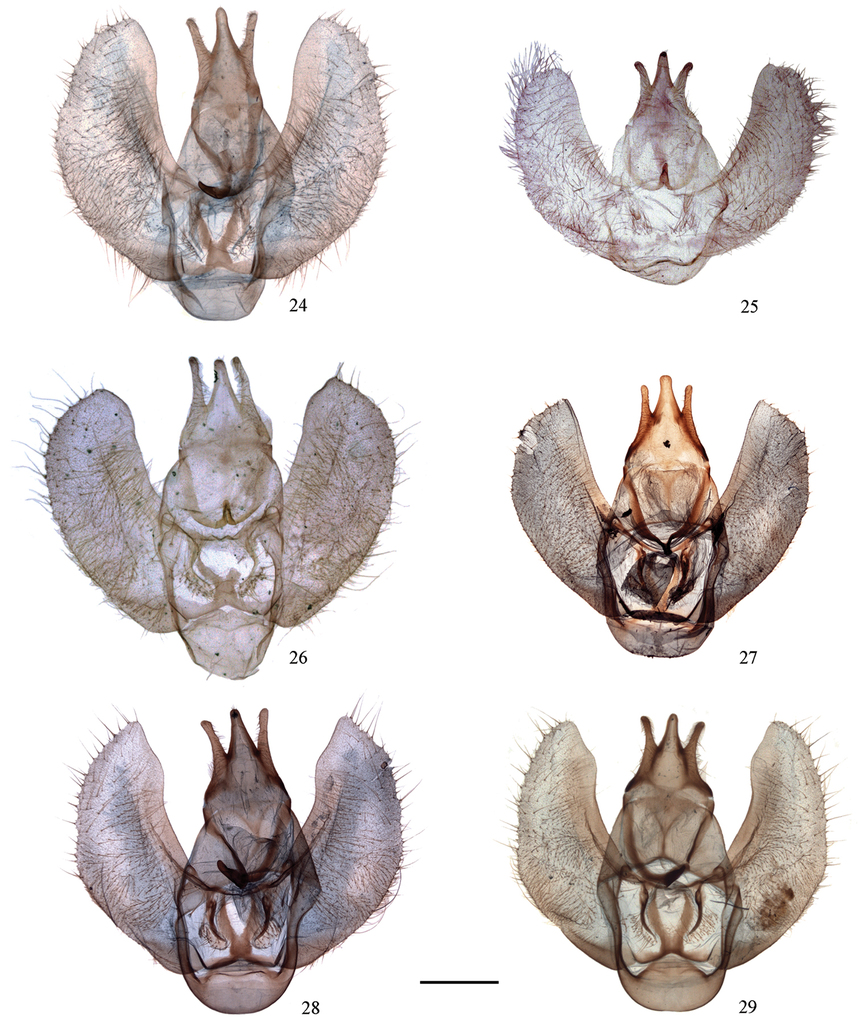
Male genitalia of Cyclidia. 24 C. substigmaria substigmaria (Baoshan, Yunnan, slide no. 41) 25 ditto (holotype of C. substigmaria brunna, Emeishan, Sichuan, slide no. 12) 26 ditto (holotype of C. tetraspota, Xishuangbanna, Yunnan, slide no. 10) 27 ditto (Xishuangbanna, Yunnan, slide no. 681) 28 ditto (Xishuangbanna, Yunnan, slide no. 683) 29 C. substigmaria intermedia (Tibet, slide no. 311). Scale bar: 1 mm.
Genetic data
The distance to the nearest neighbour C. rectificata is 8.92%. The intrasubspecific divergence of the barcode region of C. substigmaria substigmaria ranges from 0%–2.6% (average distance 1%) (n = 19). Some specimens from Yunnan cluster together at some distance from all other specimens (Fig.
Remarks
After examining the types of C. substigmaria brunna, C. tetraspota and a long series of material collected near their type localities, it was found that the external and genital features of C. substigmaria brunna and C. tetraspota are nearly identical to those of C. substigmaria substigmaria. Barcodes of one paratype of C. tetraspota (IOZ LEP M 02790) and two specimens from type locality of C. substigmaria brunna (IOZ LEP M 17993 and 17994) were clustered within C. substigmaria substigmaria in the Neighbour Joining (NJ) tree with the genetic distances from 0.015%–2.6% (see fig. 58). Thus, C. tetraspota and C. substigmaria brunna are considered as junior synonyms of C. substigmaria substigmaria.
Distribution
China (Henan, Shaanxi, Gansu, Jiangsu, Anhui, Zhejiang, Hubei, Jiangxi, Hunan, Fujian, Taiwan, Guangdong, Hainan, Hong Kong, Guangxi, Sichuan, Guizhou, Yunnan), Vietnam.
Cyclidia substigmaria intermedia
Cyclidia substigmaria intermedia Prout, 1918: 416. Holotype ♂, China: Tibet (BMNH).
Diagnosis
See under C. substigmaria substigmaria.
Type material examined
CHINA: Tibet (BMNH): 1♂ (Holotype), Tibet, collector and collecting date unknown, ex. Joicey Collection.
Additional material examined
CHINA: Tibet (IZCAS): 1♂, Mêdog, Yarang, 1091 m, 20–23.VIII.2006, coll. Lang Songyun; 1♀, Mêdog, Beibung, 850 m, 24.VI.1983, coll. Han Yinheng; 2♀, Mêdog, 2750 m, 22.VIII.1982, coll. Han Yinheng; 1♀, Zayü, Dongyan, 1600 m, 17.VII.1973.
Genetic data
No genetic data available.
Distribution
China (Tibet).
Cyclidia rectificata
Nelcynda rectificata Walker, 1862a: 1142. Holotype 1♂, India: Sikkim (BMNH).
Cyclidia muricolaria Walker, 1862b: 1483. Holotype 1♀, India: Darjeeling (BMNH).
Cyclidia patulata Walker, 1866: 1537. Holotype ♀, India: Darjeeling (BMNH).
Chorodna
rectificata
:
Enchera
rectificata
:
Cyclidia rectificata : Warren 1922: 445.
Diagnosis
The species is very similar to C. diehli Lutz & Kobes, 2002 (Sumatra) externally, but can be distinguished by the blackish brown and more distinct forewing submarginal line. The most distinct differences are in the male genitalia: the terminal part of the uncus is much narrower and longer; a rounded process with short setae is absent on the basal part of each socius, while C. diehli has this character; the terminal part of the valva is much broader than that of C. diehli. The male and female genitalia are also similar to those of C. substigmaria, the diagnosis can be seen under C. substigmaria substigmaria.
Remarks
There are two subspecies of C. rectificata. C. rectificata rectificata (Walker, 1862) is distributed in China and India, and C. rectificata malaisei Bryk, 1943 is distributed in Myanmar.
Distribution
China, India, Myanmar.
Cyclidia rectificata rectificata
Diagnosis
See under C. rectificata.
Material examined
CHINA: Yunnan (IZCAS): 1♂, Tengchong, Heinitang, 1930 m, 28–30.V.1992, coll. Xue Dayong. Tibet (IZCAS): 7♀, Nyalam, Zham, 2250 m, 12–20.V.1974, coll. Zhang Xuezhong; 1♂, Cona, 2800 m, 8.VIII.1974, coll. Huang Fusheng; 1♂, Zham, 2200 m, 25.VI.1975, coll. Wang Ziqing; 1♀, Gyirong, 2800 m, 26.VIII.1975, coll. Wang Ziqing; 3♀, Bomi, Yi’ong, 2300 m, 23–29.VIII.1983, coll. Han Yinheng; 2♂5♀, Nyingchi, Bomi, Tangmai, 2100 m, 29–31.VIII.2005, coll. Wang Xuejian; 3♂1♀, Nyingchi, Pêlung, 2115 m, 1–2.IX.2005, coll. Wang Xuejian; 4♂3♀, Zayü, Shang Zayü, 1812–1960 m, 21–23.VIII.2005, 10–11.VIII.2014, coll. Wang Xuejian, Cheng Rui and Cui Le; 1♂1♀, Zayü, Rongcheng Binguan, 2178 m, 8–12.VIII.2014, coll. Cheng Rui and Cui Le; 2♀, Mainling, Pai, 2883 m, 4–6.VIII.2006, coll. Lang Songyun; 8♂11♀, Mêdog, Lage, 3213 m, 7–8.VIII.2006, coll. Lang Songyun; 3♂2♀, Mêdog, Dayandong, 2880 m, 9.VIII.2006, coll. Lang Songyun; 2♂, Mêdog, Hanmi, 2095 m, 10–11.VIII.2006, coll. Lang Songyun; 2♀, Mêdog, Pomo Gonglu 80K, 2118 m, 24–25.VIII.2006, coll. Lang Songyun.
Male genitalia of Cyclidia. 30 C. rectificata (Tibet, slide no. 2) 31 C. pitimani (Yunnan, slide no. 9) 32 C. fractifasciata fractifasciata (Yunnan, slide no. 724) 33 C. fractifasciata indistincta subsp. n. (paratype, Gansu, slide no. 721) 34 C. orciferaria (Hainan, slide no. 728). Scale bar: 1 mm.
35–36 Male genitalia of Mimozethes. 35 M. angula (paratype, Sichuan, slide no. 34) 36 M. lilacinaria (Sichuan, BMNH, slide No. 304) 37–47 Phallus 37 Cyclidia substigmaria substigmaria (Tengchong, Yunnan, slide no. 682) 38 ditto (holotype of C. substigmaria brunna, Emeishan, Sichuan, slide no. 12) 39 ditto (holotype of C. tetraspota, Xishuangbanna, Yunnan, slide no. 10) 40 C. substigmaria intermedia (Tibet, slide no. 311) 41 C. rectificata (Tibet, slide no. 727) 42 C. pitimani (Yunnan, slide no. 9) 43 C. fractifasciata fractifasciata (Yunnan, slide no. 724) 44 C. fractifasciata indistincta subsp. n. (paratype, Gansu, slide no. 721) 45 C. orciferaria (Hainan, slide no. 728) 46 M. angula (holotype, Sichuan, slide no. 19) 47 M. lilacinaria (Sichuan, BMNH, slide No. 304). Sscale bars: 1 mm.
Female genitalia. 48 Cyclidia substigmaria substigmaria (Henan, sldie. no. 726) 49 ditto (Jiangsu, slide. no. 33) 50 ditto (paratype of C. tetraspota, Xishuangbanna, Yunnan, slide no. 36) 51 C. substigmaria intermedia (Tibet, slide no. 685) 52 C. rectificata (Tibet, slide no. 3) 53 C. fractifasciata fractifasciata (Yunnan, slide no. 725) 54 C. fractifasciata indistincta subsp. n. (paratype, Gansu, slide no. 722) 55 C. orciferaria (Hainan, slide no. 729) 56 M. angula (Henan, slide no. 288) 57 M. lilacinaria (Sichuan, slide no. 280). Sscale bars: 1 mm.
Genetic data
The intraspecific divergence of the barcode region of C. rectificata rectificata is 0% (average distance 0%) (n = 7). The distance to the nearest neighbour C. substigmaria is 8.92%.
Distribution
China (Yunnan, Tibet), India.
Cyclidia pitimani
Euchera pitimani Moore, 1886: 99. Syntypes including 1♂, Burma: Tenasserim, Tavoy (BMNH).
Cyclidia pitimani : Warren, 1922: 445.
Cyclidia sericea Warren sensu Chu & Wang, 1987: 206. (Misidentification)
Cyclidia sericea Warren sensu Chu & Wang, 1991: 64, fig. 24, pl. 1: 4. (Misidentification)
Diagnosis
This species is very similar to C. sericea (Borneo, Sumatra), but can be distinguished by the following characters: smaller than C. sericea (the average forewing length of the male is 32 mm, against ca 40 mm in C. sericea); in C. pitimani, the doubled antemedial line form almost right angles anteriorly, especially the inner line, while in C. sericea, the protrusions of the antemedial lines are more rounded; the anterior part of the median band is much narrower in C. pitimani; the terminal spots are less distinct than those of C. sericea. In the male genitalia, the terminal part of the valva is broader and more rounded.
Material examined
CHINA: Yunnan (IZCAS): 2♂, Xishuangbanna, Xiaomengyang, 850 m, 6–7.IX.1957, coll. Zang Lingchao and Zhang Yiran (one male was originally incorrectly recorded as “Qinghai, Gonghe”); 2♂, Xishuangbanna, Bubang, 700 m, 14.IX.1993, coll. Yang Longlong.
Genetic data
No genetic data available.
Remarks
After examining the types of C. pitimani and C. sericea, and studying the descriptions and figures of the two species (
Distribution
China (Yunnan), Myanmar.
Cyclidia fractifasciata
Euchera fractifasciata Leech, 1898: 360. Syntypes 1♂, 1♀, China: Western China (BMNH).
Cyclidia
fractifasciata
:
Diagnosis
The species can be distinguished by the following characters: a black broad subbasal line is present on the forewing; the forewing medial line is broad at anterior half and very narrow and dot-like at posterior half; outer margin of the forewing medial line forms an almost right angle below M3; the phallus of the male genitalia forms a small protrusion posteriorly; the lamella postvaginalis of the female genitalia is rectangle.
Remarks
Distribution
China.
Cyclidia fractifasciata fractifasciata
Diagnosis
See under C. fractifasciata indistincta.
Material examined
CHINA: Yunnan (IZCAS): 1♂, Dulongjiang, 1500 m, 29.V.2006, coll. Xiao Ningnian; 3♂1♀, Lushui, Pianma, 8–12.V.2011, coll. Yang Xiushuai and Wang Ke.
Distribution
China (Yunnan).
Genetic data
The intrasubspecific divergence of the barcode region in C. fractifasciata fractifasciata is 0% (n = 4).
Cyclidia fractifasciata indistincta , subsp. n.
Description
Head. Antennae blackish brown dorsally, flat and unipectinate, basal half without rami, rami very short. Frons blackish grey, not protruding. Labial palpi black with third segment distinct, extending beyond frons. Vertex black scattered with grey scales.
Thorax. Patagia white at basal half and blackish grey at terminal half. Tegula blackish grey. Dorsal side of thorax white with two pairs of blackish grey patches medially. Hind tibia with two pairs of spurs in both sexes. Forewing length: 37–40 mm. Apex of forewing rounded, not falcate; outer margin of both wings smooth. Wings white, transverse lines black. Forewing with a blackish brown patch basally; subbasal line broad; antemedial lines double, outer line indistinct and often invisible; medial line broad band-like at anterior half, very narrow and dot-like at posterior half; outer margin of medial line forming an almost right angle below M3; discal spot white, almost rhombic; postmedial lines double, wavy, inner line very obscure; submarginal line double, broad, and invisible between M3 and CuA1; terminal lines double and discontinuous on each vein, inner line composed of oval markings, outer line appearing as series of short strips, inner markings often fused with outer ones; fringes white mixed with blackish grey. Hind wing with indistinct submarginal line; terminal lines and fringes similar to those of forewing. Underside white, striations indistinct than those of upperside.
Abdomen. Abdominal segments diffused with white scales. Pairs of black quadrate markings on first to seventh abdominal segments. Anterotergal syndeses developed at anterior margin of 2nd tergum. A pair of androconial hair-pencils present on 2nd pleuron of male.
Male genitalia. Uncus triangular. Socii sclerotized, about four-fifths the length of uncus. Gnathos with median process small and triangular. Valva narrow terminally; costa sclerotized and almost straight. Juxta formed a pair of forcipiform processes posteriorly. Saccus semicircular, about two-fifths length of basal width. Phallus slightly curved, with a small triangular lateral process posteriorly; vesica without cornuti.
Female genitalia. Lamella postvaginalis rectangle. Ductus bursae with a colliculum, long and narrow, striate longitudinally. Corpus bursae oval, with a paired slender signa; signa separated and parallel.
Diagnosis
The subspecies is very similar to the nominate subspecies, but differs externally by the following characters: the outer line of the antemedial line and the inner line of the postmedial line on the forewing are invisible, while in the nominate subspecies, they are much more distinct; the forewing discal spot is larger; the inner terminal markings of the forewing are larger and fused with the outer ones partly, while in C. fractifasciata fractifasciata, they are often smaller and separated from the outer ones.
Type material examined
Holotype, ♂, CHINA: Gansu (IZCAS): Kangxian, Yangba, Meiyuangou, 1000 m, 13.VIII.2014, coll. Xue Dayong and Ban Xiaoshuang. Paratypes: 3♂2♀, same data as holotype. Chongqing (IZCAS): 1♀, Wushan, Wulipo, Dangyang, Congping, 1773 m, 25.VII.2013, coll. Cheng Rui.
Genetic data
The intrasubspecific divergence of the barcode region in C. fractifasciata indistincta is 1%. The intraspecific divergence of the barcode region between C. fractifasciata fractifasciata (n = 4) and C. fractifasciata indistincta (n = 5) is 2.3%. The distance between C. fractifasciata with the nearest neighbour species C. substigmaria is 12.5%.
Distribution
China (Gansu, Chongqing).
Etymology
The subspecies is named on the basis of the Latin adjective indistinctus, referring to the transverse lines of the forewing.
Cyclidia orciferaria
Cyclidia orciferaria Walker, 1860: 56. Syntypes, China: North China.
Cyclidia ociferaria Kirby, 1892: 725. [Incorrect spelling of Cyclidia orciferaria Walker.]
Diagnosis
This species is different from other congeners in the following external characters: the apex of the forewing is falcate; the wing colour is blackish brown; two bands covered with greyish blue scales are present on the forewing, and the inner band is narrower and less distinct than the outer band; the discal spot of the forewing is yellowish brown, oblong, with a blackish brown narrow line medially; greyish blue scales are covered on the submarginal lines of both wings, and often absent on the middle part of the hind wing. There are also differences in the male genitalia: the socii are weakly sclerotized and much shorter than the uncus; the valva is short. In the female genitalia, the posterior margin of the lamella postvaginalis is slightly concaved; the two signa are tapered at posterior half and situated very close to each other.
Material examined
CHINA: Zhejiang (IZCAS): 2♂, Tianmushan, 20–23.VII.1973, coll. Zhang Baolin; 1♂1♀, Lin’an, West Tianmushan, 400–1500 m, 26.VII–29.VIII.2003, coll. Xue Dayong et al.; 1♂, West Tianmushan, Zhonglieci, 363 m, 24.VII.2011, coll. Yan Keji; 1♂, West Tianmushan, Xianrending, 1506 m, 27.VII.2011, coll. Yan Keji; 2♂2♀, Taishun, Wuyanling, Shuangkengkou, 680 m, 28–29.VII.2005, coll. Lang Songyun; 1♀, Taishun, Siqianzhen, 250 m, 4.VIII.2005, coll. Lang Songyun; 1♂, Ningbo, V.1981. Jiangxi (IZCAS): 1♀, Huzhi, 28.VII.1990. Hunan (IZCAS): 2♂, Yanling, Taoyuandong, 631 m, 4–8.VII.2008, coll. Chen Fuqiang; 1♂, Tianpingshan, 25.VI.1981. Fujian (IZCAS): 1♂, Jiangle, Longqishan, 800 m, 15.IX.1990, coll. Yang Bin; 8♂, Wuyishan, 24.IV–21.V.1983, coll. Wang Linyao; 1♀, Wuyishan, Sangang, 24.VII.1980; 1♀, Nanping, Shangyang, 9.VI.1963, coll. Zhang Youwei. Guangdong (IZCAS): 1♂, Ruyuan, Nanling, Baohuzhan, 1020 m, 16–20.VII.2008, coll. Chen Fuqiang; 1♀, Shixing, Chebaling, 365–401 m, 22–26.VII.2008, coll. Chen Fuqiang. Hainan (IZCAS): 4♂2♀, Nankai, Nanmaola, 1261 m, 10–14.V.2009, coll. Chen Fuqiang and Yan Keji; 6♂1♀, Jianfengling, Tianchi, 828 m, 1–5.V.2007, 18.V.2009, coll. Chen Fuqiang; 1♂2♀, Bawangling, Dong’er Linchang, 1004–1015 m, 8.V.2007, 7.IV.2008, coll. Chen Fuqiang and Lang Songyun; 11♂, Wuzhishan, Shuiman, 730–900 m, 7–11.V.2007, 1–3.IV.2008, coll. Lang Songyun and Han Hongxiang; 1♂3♀, Lingshui, Diaoluoshan, 190–920 m, 3–7.V.2007, coll. Han Hongxiang and Lang Songyun; 1♀, Qiongzhong, Limuling, 620 m, 15.V.2007, coll. Han Hongxiang; 1♀, Xinglong, 24.IV.1963, coll. Zhang Baolin. Guangxi (IZCAS): 3♂2♀, Fangcheng, Fulong, 200–550 m, 23–26.V.1999, coll. Yuan Decheng et al.; 1♂1♀, Napo, Defu, 1350 m, 19.VI.2000, coll. Zhu Chaodong; 1♂, Jinxiu, Linhai Shanzhuang, 1100 m, 2.VII.2000, coll. Li Wenzhu; 1♂, Jinxiu, Jinzhong Gonglu, 1000 m, 10.V.1999, coll. Han Hongxiang; 1♀, Daxin, Xialei, 680 m, 31.III.1998, coll. Li Wenzhu. Yunnan (IZCAS): 1♂1♀, Hekou, Xiaonanxi, 200 m, 10–11.VI.1956, coll. Huang Keren et al.; 1♀, Pingbian, Daweishan, 1500 m, 20.VI.1956, coll. Huang Keren et al.; 1♂, Xishuangbanna, Mengla, Menglun, 650 m, 1.VI.1964, coll. Zhang Baolin; 1♂1♀, Mengla Linchang, 550 m, 20.IV.1982, coll. Wang Yongxian; 1♂, Mengla, 20.VI.1982, coll. Chen Yixin; 1♀, Mengla, Lengku, 623 m, 10.VI.1980, coll. Guo Zuyun; 1♂, Xishuangbanna, Bubang, 700 m, 14.IX.1993, coll. Yang Longlong; 1♂, Xishuangbanna, Damenglong, 650 m, 1.VIII.1958, coll. Zheng Leyi; 1♂, Xishuangbanna, Dameng’a, 1050–1080m, 15.VIII.1958, coll. Wang Shuyong; 2♂, Cangyuan, 790–1100 m, 19–22.V.1980, coll. Song Shimei and Shang Jinwen; 1♂, Xiaomenglun, 21.IV.1982, coll. Wang Linyao; 1♂, Ruili, Dengga, 6–8.VI.1992, coll. Xue Dayong; 1♂, Baoshan, Baihualing, 1520 m, 11–13.VIII.2007, coll. Wu Chunguang.
Genetic data
The intraspecific divergence of the barcode region of C. orciferaria is ranges from 0%–1.7% (average distance 1.09%) (n = 8). The distance to the nearest neighbour C. substigmaria is 11.5%.
Distribution
China (Jiangsu, Zhejiang, Jiangxi, Hunan, Fujian, Guangdong, Hainan, Guangxi, Sichuan, Yunnan), Myanmar, Vietnam, Indonesia.
Biological notes
The morphology of the larva of C. orciferaria was illustrated in
Mimozethes
Mimozethes Warren, 1901: 190. Type species: Euchera nana Warren, 1897, by original designation.
Generic characters
Head. Antennae lamellate and shortly unipectinate, basal part of antennae without rami (Fig.
Diagnosis
See under Cyclidia.
Remarks
According to
Distribution
China, Japan.
Key to Chinese Mimozethes species
| 1 | Outer margin of forewing weakly protruding; ventral margin of valva forming a small triangular protrusion apically in male genitalia |
M. angula, Figs |
| – | Outer margin of forewing strongly protruding; ventral margin of valva not forming a small triangular protrusion apically in male genitalia |
M. lilacinaria, Figs |
Mimozethes angula
Mimozethes angula Chu & Wang, 1987: 207. Holotype ♂, China: Sichuan: Mt. Emei (IZCAS).
Diagnosis
This species is very similar to M. lilacinaria (Leech, 1897) and M. argentilinearia, but it can be distinguished by the following characters: the outer margin of the forewing is less strongly protruding than that of M. lilacinaria and M. argentilinearia; the black patch inside the anal angle of the forewing is less distinct than that of M. argentilinearia; the yellowish brown patch on the underside of the forewing is much smaller and less distinct than that of M. lilacinaria and M. argentilinearia. In the male genitalia, the uncus is shorter; the ventral margin of the valva forms a small triangular protrusion apically, but M. lilacinaria and M. argentilinearia lack this character; the sacculus process is much longer than that of M. lilacinaria.
Type material examined
CHINA: Sichuan (IZCAS): 1♂ (Holotype), Emeishan, Qingyinge, 800–1000 m, 15.IX.1957, coll. Zhu Fuxing; 1♀ (Allotype), same locality, 22.IX.1957, coll. Zhu Fuxing; 4♂2♀ (Paratype), same locality, 22.VI.1957, 15–19. IX.1957, coll. Zhu Fuxing et al.
Additional material examined
CHINA: Henan (IZCAS): 1♀, Baiyunshan, 13–15.VIII.2008, 1550 m, coll. Jiang Nan. Hubei (IZCAS): 1♂, Shennongjia, Dajiuhu, 1800 m, 1.VIII.1981, coll. Han Yingheng. Sichuan (IZCAS): 9♂2♀, Emeishan, Qingyinge, 800–1000 m, 20.VI.1957, 15–22.IX.1957, coll. Zhu Fuxing et al.; 1♀, Qingchengshan, 1000 m, 4.VI.1979, coll. Shang Jinwen; 1♂, Emeishan, 1288 m, 31.VII.2013, coll. Cheng Rui.
Genetic data
No genetic data available.
Distribution
China (Henan, Hubei, Sichuan).
Mimozethes lilacinaria
Decetia lilacinaria Leech, 1897: 184. Holotype ♂, China: Sichuan: Emeishan (BMNH).
Heteromize lycoraearia Oberthür, 1912: 269. Holotype ♂, China: Sichuan: Mou-pin (BMNH).
Mimozethes
lilacinaria
:
Diagnosis
See under M. angula.
Type material examined
CHINA: Sichuan (BMNH): 1♂ (Holotype), Omei-Shan, 3620 ft., Native coll. July & Aug. 1890, Leech Coll. 1900-64, BMNH (E) 1377104.
Additional material examined
CHINA: Sichuan (BMNH): 1♂, Chasseurs indigènes, de Tà-tsien-lou, Récolle de 1910, Ex Oberthür Coll. Brit. Mus. 1927-3, Drepanidae genitalia slide No. 304; 1♀, Siao-Lou, 1900, Chasseurs indigènes, Ex Oberthür Coll. Brit. Mus. 1927-3. Yunnan (IZCAS): 1♀, Xishuangbanna, Menghai, 21.VII.1958, coll. Wang Shuyong.
Genetic data
No genetic data available.
Remarks
Distribution
China (Sichuan, Yunnan).
DNA barcoding results and discussion
Forty-three DNA barcode sequences of lengths 658bp were obtained for Cyclidia species. The nucleotide composition of Cyclidia species COI genes was 30.60% of A, 38.54 of T, 16.06% of C, 14.80% of G. The interspecific distance within the genus was range from 8.8%–13.9%. The maximum intraspecific distances was 2.6% in C. substigmaria, 1.7% in C. orciferaria, 0.0% in C. rectificata, and 2.3% in C. fractifasciata. The maximum genetic distances observed within species (2.6% at COI) were less than the minimum distances observed between the species (8.8%). There is a clear barcoding gap between intra and interspecific variation; furthermore, NJ tree also provided strong support for the separation of Cyclidia species (Fig.
In recent revisionary work of Drepanidae,
Acknowledgements
We express our sincere thanks to Anthony Galsworthy, and the trustees and staff of the Natural History Museum, London for allowing examination of material under their care. We are grateful to all collectors whose contributions made our work possible. We appreciate the previous work of Dr. Song Wenhui in Chinese Cyclidia species, and the work of Ms. Yang Chao in preparing some specimens and photographs. This work was supported by the National Science Foundation of China (No. 30870320), the National Science Fund for Fostering Talents in Basic Research (NSFC-J1210002), the Ministry of Science and Technology of the People’s Republic of China (MOST Grant No. 2011FY120200) and a grant from the Key Laboratory of the Zoological Systematics and Evolution of the Chinese Academy of Sciences (No. O529YX5105).
References
- Aurivillius CHR (1894) Neue Spinner aus Asien. Entomologisk tidskrift 15: 169–177.
- Beccaloni G, Scoble M, Kitching I, Simonsen T, Robinson G, Pitkin B, Hine A, Lyal C (Eds) (2003) The Global Lepidoptera Names Index (LepIndex). World Wide Web electronic publication. http://www.nhm.ac.uk/entomology/lepindex [accessed 26 Novermber 2015]
- Bryk F (1943) Entomological results from the Swedish expedition 1934 to Burma and British India. Lepidoptera: Drepanidae. Arkiv för Zoologi Band 34A(13): 1–30.
- Chang BS (1989) Illustrated moths of Taiwan 1. Taiwan Provincial Museum, Taipei, 242 pp. [In Chinese]
- Chen YL (2011) Phylogenetic reconstruction of Cyclidiinae (Lepidoptera: Drepanidae). MS thesis, National Sun Yat-sen University, Kaohsiung, Taiwan. http://etd.lib.nsysu.edu.tw/ETD-db/ETD-search-c/getfile?URN=etd-0725111-051148&filename=etd-0725111-051148.pdf [accessed 26 November 2015]
- Chou I, Xiang H (1984) Studo de Drepanedoj el Yunnan Provinco (Lepidoptera: Drepanidae). Entomotaxonomia 6(2–3): 159–169. [Abstract in Esperanto]
- Chu HF, Wang LY (1987) Taxonomy and geographical distribution of Cyclidiidae. Acta Entomologica Sinica 30(2): 203–211. [In Chinese]
- Chu HF, Wang LY (1991) Fauna Sinica. Insecta. Vol. 3. LepidopteraCyclidiidaeDrepanidae. Science Press, Beijing, vii+269 pp. [In Chinese]
- Chu HF (Ed.) (1981) Iconocraphia Heterocerorum Sinicorum. Vol. I. Science Press, Beijing, iv+134 pp.+22 pp. (Index), 38 pls. [In Chinese]
- Comstock JH (1918) The wings of insects. Comstock Publishing Company, Ithaca, New York, 430 pp.
- Cotes EC, Swinhoe C (1888) Geometrites. In: Cotes E, Swinhoe CC (Eds) A catalogue of the moths of India. Part. IV. Trustees of the Indian Museum, Calcutta, 463–590.
- Fletcher DS (1979) Geometroidea. In: Nye WB (Ed.) The Generic Names of Moths of the World. Vol. 3. Trustees of the British Museum (Natural History), London, 243 pp.
- Gaede M (1931) Family: Drepanidae. In: Strand E (Ed.) Lepidopterorum Catalogus. Vol. 49. W. Junk, Berlin, 60 pp.
- Guenée A (1858) Uranides and Phalénites. In: Boisduval JBAD, Guenée A (Eds) Histoire naturelle des insectes: Spécies général des Lépidoptères. IX. Roret, Paris, 1–551. [In French]
- Hajibabaei M, Janzen DH, Burns JM, Hallwachs W, Hebert PDN (2006) DNA barcodes distinguish species of tropical Lepidoptera. Proceedings of the National Academy of Sciences of the United States of America 103: 968–971. doi: 10.1073/pnas.0510466103
- Hampson GF (1893) Illustrations of Typical Specimens of LepidopteraHeterocera in the Collection of the British Museum. Part 9: The MacrolepidopteraHeterocera of Ceylon. Trustees of the British Museum (Natural History), London, v + 182 pp.
- Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Philosophical Transactions of the Royal Society B: Biological Sciences 270: 313–321. doi: 10.1098/rspb.2002.2218
- Hebert PDN, Penton EH, Burns JM (2004a) Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America 101: 14812–14817.
- Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM (2004b) Identification of birds through DNA barcodes. Plos Biology 2: 1657–1663.
- Holloway JD (1998) The Moths of Borneo. Part 8: Family Castniidae. Callidulidae. Drepanidae and Uraniidae. Malayan Nature Journal 52: 7–76.
- Hübner J (1824–1831) Zuträge zur Sammlung exotischer Schmetterlinge, bestehend in Bekundigung einzelner Fliegmuster neuer oder rarer nichteuropäischer Gattungen 3. Im Verlag der Hübner’schen Werke bei C. Geyer, Augsburg, 103 pp.
- Inoue H (1962) Lepidoptera: Cyclidiidae, Drepanidae. In: Inoue H (Ed.) Insecta Japonica. Vol. 2. Hokuryukan Publishing, Tokyo, 1–54, 1–3 pls. [In Japanese]
- Inoue H (1992) Geometridae, Thyatiridae, Cyclidiidae, Drepanidae. In: Heppner JB, Inoue H. Lepidoptera of Taiwan. Volume. 1, part 2: Checklist. Association for Tropical Lepidoptera, Florida, 111–129, 151–153.
- Jiang N, Liu SX, Xue DY, Tang MJ, Xiao Q, Han HX (2014) External morphology and molecular identification of two tea Geometrid moth from southern China. Chinese Journal of Applied Entomology 51: 987–1002. [In Chinese]
- Kadoorie Farm, Botanic Garden (2004) Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001. South China Forest Biodiversity Survey Report Series (Online Simplified Version): No. 37. KFBG, Hong Kong SAR, ii+33 pp.
- Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of molecular evolution 16: 111–120. doi: 10.1007/BF01731581
- Kirby WF (1892) A Synonymic Catalogue of LepidopteraHeterocera. R. Friedlander and Son., Berlin, 951 pp.
- Klots AB (1970) Lepidoptera. In: Tuxen SL (Ed.) Taxonomist’s Glossary of Genitalia in Insects. Munksgaard, Copenhagen, 115–130.
- Kristensen NP (Ed.) (2003) Handbook of Zoology, Vol. IV. Arthropoda: Insecta. Part 36. Walter de Gruyter, Berlin, New York, 564 pp.
- Leech JH (1897) On LepidopteraHeterocera from China, Japan, and Corea. Annals and Magazine of Natural History (6) 19: 180–235. doi: 10.5962/bhl.title.22195
- Leech JH (1898) LepidopteraHeterocera from Northern China, Japan and Corea. Transactions of the Royal Entomological Society of London 46(3): 261–379.
- Lutz W, Kobes R (2002) Cyclidia diehli sp. n. (Lepidoptera: Cyclidiinae) a species new to science. Heterocera Sumatrana 12: 177–183.
- Meier R, Kwong S, Vaidya G, Ng PKL (2006) DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Systematic Biology 55: 715–728. doi: 10.1080/10635150600969864
- Meier R, Zhang GY, Ali1 F (2008) The use of mean instead of smallest interspecific distances exaggerates the size of the “Barcoding Gap” and leads to misidentification. Systematic Biology 57: 809–813. doi: 10.1080/10635150802406343
- Minet J, Scoble MJ (1999) The drepanoid/geometroid Assemblage. In: Kristensen NP (Ed.) Lepidoptera, Moths and Butterflies. Vol. I: Evolution, Systematics, and Biogeography. Handbook of Zoology. Vol. IV, Arthropoda: Insecta, Part 35. Walter de Gruyter, Berlin and New York, 301–320.
- Minet J (1983) Étude morphologique et phylogénétique des organs tympaniques des Pyraloidea. I. généralités et homologies. (Lep. Glossata). Annales de la Société entomologique de France (N.S.) 19: 175–207. [In French]
- Minet J (2002) The Epicopeiidae: Phylogeny and a redefinition, with the description of new taxa (Lepidoptera: Drepanoidea). Annales de la Societe Entomologique de France 38: 463–487. doi: 10.1080/00379271.2002.10697355
- Moore F (1886) List of the Lepidopterous Insects collected in Tavoy and in Siam during 1884 and 1885 by the Indian Museum Collector. Part i. Heterocera. Journal of the Asiatic Society of Bengal 55: 97–101.
- Nichols SW (Ed.) (1989) The Torre-Bueno Glossary of Entomology. New York Entomological Society in cooperation with the American Museum of Natural History, New York, 840 pp.
- Oberthür C (1912) Revision des Phalénites décrites par Guenée dans le species général des Lépidoptéres (Tome IX.). Famille II. Ennomidae, Guenée. Études de Lépidoptérologie Comparée 6: 223–307, 346–355. [In French]
- Park KT, Kim M, Kwon YD, Ji EM (2011) A review of the genus Oreta Walker in Korea, with description of a new species (Lepidoptera: Drepanidae). Journal of Asia-Pacific Entomology 14(3): 311–316. doi: 10.1016/j.aspen.2011.04.001
- Prout LB (1918) New moth species in the Joicey collection. Annals and Magazine of Natural History 9(11): 412–416.
- Ratnasingham S, Hebert PDN (2007) BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes 7: 355–364. doi: 10.1111/j.1471-8286.2007.01678.x
- Saitou N, Nei M (1987) The neighbor–joining method, A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406–425.
- Scoble MJ (1992) The Lepidoptera, Form, Function and Diversity. Oxford University Press, Oxford, xi+404 pp.
- Sihvonen P, Skou P, Flamigni C, Fiumi G, Hausmann A (2014) Revision of the Hylaea fasciaria (Linnaeus, 1758) species group in the western Palaearctic (Lepidoptera: Geometridae, Ennominae). Zootaxa 3768(4): 469–486.
- Song WH, Xue DY, Han HX (2011) A taxonomic revision of Tridrepana Swinhoe, 1895 in China, with descriptions of three new species (Lepidoptera, Drepanidae). Zootaxa 3021: 39–62.
- Song WH, Xue DY, Han HX (2012) Revision of Chinese Oretinae (Lepidoptera, Drepanidae). Zootaxa 3445: 1–36.
- Strand E (1911) Family: Drepanidae. In: Seitz A (Ed.) The Macrolepidoptera of the World. Vol. 2: the Palearctic Bombyces and Sphinges. Alfred Kernen, Stuttgart, 195–206.
- Sugi S (1987) Larvae of Large Moths in Japan. Kodansha, Tokyo, 453 pp, 120 pls. [In Japanese]
- Swinhoe C (1899) New species of Oriental Lepidoptera. The Annals and Magazine of Natural History 7: 102–116. doi: 10.1080/00222939908678084
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using likelihood, distance, and parsimony methods. Molecular Biology and Evolution 28: 2731–2739. doi: 10.1093/molbev/msr121
- Walker F (1860) List of the specimens of Lepidopterous Insects in the collection of the British Museum. Part 20. Edward Newman, London, 1–276.
- Walker F (1862a) List of the specimens of Lepidopterous Insects in the collection of the British Museum. Part 24. Edward Newman, London, 1021–1280.
- Walker F (1862b) List of the specimens of Lepidopterous Insects in the collection of the British Museum. Part 25. Edward Newman, London, 1281–1477.
- Walker F (1866) List of the specimens of Lepidopterous Insects in the collection of the British Museum. Part 35. Edward Newman, London, 1535–2040.
- Warren W (1897) New genera and species of moths from the Old World regions in the Tring Museum. Novitates Zoologicae 4: 12–130.
- Warren W (1901) Drepanulidae, Uraniidae, and Geometridae from the Palaearctic and Indo-Australian Regions. Novitates Zoologicae 8: 190–201.
- Warren W (1901) Drepanulidae, Uraniidae, and Geometridae from the Palaearctic and Indo-Australian Regions. Novitates Zoologicae 8: 190–201.
- Warren W (1914) New species of Drepanulidae, Noctuidae and Geometridae in the Tring Museum. Novitates Zoologicae 21: 401–425.
- Warren W (1922–1928) Family: Drepanidae. In: Seitz A (Ed.) The Macrolepidoptera of the World. Vol. 10: Bombyces and Sphinges of the Indo-Australian Region. Alfred Kernen, Stuttgart, 443–490, pls. 48–50.
- Wu CG, Han HX, Xue DY (2010) A pilot study on the molecular phylogeny of Drepanoidea (Insecta: Lepidoptera) inferred from the nuclear gene EF-1a and the mitochondrial gene COI. Bulletin of Entomological Research 100: 207–216. doi: 10.1017/S0007485309990162
- Yan SH, Chen YL, Wu SW (2009) Biota Taiwanica. Hexapoda: Lepidoptera, Drepanoidea, Drepanidae (Cyclidiinae). National Sun Yat-Sen University, Kaohsiung, 10 pp.
- Yen SH, Robinson GS, Quicke DLJ (2005) The phylogenetic relationships of Chalcosiinae (Lepidoptera, Zygaenoidea, Zygaenidae). Zoological Journal of the Linnean Society 143: 161–341. doi: 10.1111/j.1096-3642.2005.00139.x
- Yang Z, Landry JF, Handfield L, Zhang Y, Alma Solis M, Handfield D, Scholtens BG, Mutanen M, Nuss M, Hebert PDN (2012) DNA barcoding and morphology reveal three cryptic species of Anania (Lepidoptera: Crambidae: Pyraustinae) in North America, all distinct from their European counterpart. Systematic Entomology 37: 686–705. doi: 10.1111/j.1365-3113.2012.00637.x
- Zhou TY, Wang LY (1985) A preliminary study on Cycidia substigmaria. Chinese Bulletin of Entomology 22(3): 113–116. [In Chinese]