Citation: Gonzalez-Cueto J, Quiroga S, Norenburg J (2014) A shore-based preliminary survey of marine ribbon worms (Nemertea) from the Caribbean coast of Colombia. ZooKeys 439: 83–108. doi: 10.3897/zookeys.439.5965
A checklist of benthic ribbon worm species from the Caribbean coast of Colombia is presented, including synonyms, distributions, a photographic record, and the main morphologic characters of each species for a rapid identification. This is the first research focused broadly on nemerteans in Colombia. 54 specimens of nemerteans were hand-collected from the rocky littoral of two different localities, and identified according to personal experience and specialist literature. 13 species were found; of which 11 represent new records for the country. These species belong to eight different traditionally used families: Tubulanidae, Valenciniidae, Lineidae, Amphiporidae, Cratenemertidae, Emplectonematidae, Drepanophoridae and Ototyphlonemertidae. The most common and abundant species was Dushia atra. The biodiversity of nemerteans in Colombia seems to overlap with the nemertean fauna from Florida and Brazil, explained by the convergence of the North Brazil Current, Guiana Current, Caribbean Currents and the Panama-Colombia Contracurrent in the sampled region. The results of this work suggest that the Caribbean coast of Colombia is a region with a high diversity of nemerteans, and provide important taxonomic data for environmental assessments and future biological research.
Nemertini, Rhynchocoela, Caribbean biodiversity, benthic species
Nemerteans, phylum Nemertea, also known as Nemertini, Rhynchocoela, or ribbon worms, comprise a group of bilateral, coelomate and non-segmented worms (
About 36 species have been recorded for the Caribbean Sea (
Detailed taxonomic understanding of animals also may require them to be properly fixed for histological examination and, increasingly, for genetic studies. There are few species-level identification keys to nemerteans, and those that do exist cannot be applied reliably beyond their region of origin. This reflects the paucity of experts available to make regional keys and also the low level of explicit morphological variation available in nemerteans, which results in extensive superficial similarity among species. Taxonomy, and therefore phylogeny, within the phylum also is poorly resolved, because many (perhaps most) nemertean species descriptions are inadequate and diagnoses for genera and families often are conflicting or insufficiently diagnostic (
A recent higher-level phylogeny of the nemerteans was proposed on the basis of DNA sequence data (
Recent phylogenetic studies also re-enforce views by a number of nemertean systematists that several of the most species-rich genera (especially Cerebratulus, Lineus, Micrura, Amphiporus, and Tetrastemma) are non-monophyletic (
Although in the last decades research in biodiversity of Colombia has increased, the phylum Nemertea remains one of its most neglected taxa (
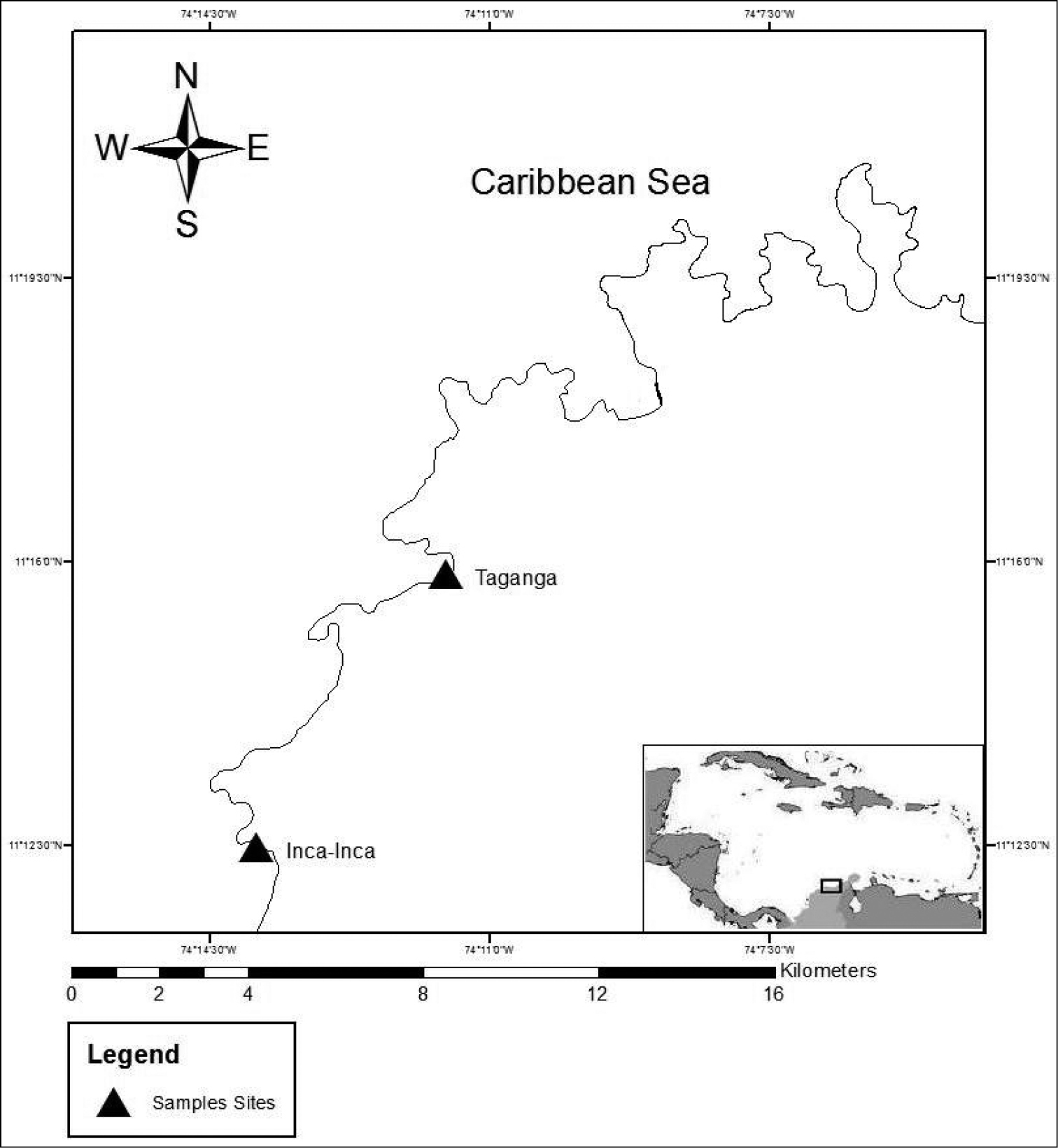
The material was collected from two different localities in the Santa Marta region on the Caribbean coast of Colombia: Inca-Inca and Taganga (Fig. 1).
Map of the region of Santa Marta, Colombia, South America. The triangles show the sampled sites.
The sites are in the bays formed by the extensions into the sea of the foothills of the Sierra Nevada de Santa Marta (SNSM), which is considered the highest coastal mountain range in the world. The common characteristic of these sites is the presence of a rocky littoral zone formed by metamorphic rocks interspersed with sandy beaches. There are two main climatic seasons – dry from December to April and rainy from May to November – influenced mainly by the Alisios winds (
Specimens were collected from the rocky littoral zone. Three methods were used to look for the worms: inspecting the surface of rocks, breaking apart rocks when they had crevices, and leaving a portion of coarse substrate in a bowl of sea water so that worms were obligated to go up to the surface as the seawater de-oxygenated. Each specimen was relaxed in 7.5% magnesium chloride, measured and photographed in vivo, fixed in 10% buffered formalin and finally transferred to 70% ethanol after at least 24 hours of fixation. When necessary, histological sections were done at the level of the head and in the middle region of the body. For this, cross sections of paraplast embedded tissue were made and stained with hematoxylin and eosin. Taxonomic identification was based on
A total of 54 specimens was collected, of which 46 were identified to 13 species, and 2 only to family; 4 individuals could not be identified and they are recorded as 4-eyed monostiliferans (Table 1). Two specimens self-destructed; one was a heteronemertean and the other a hoplonemertean (their tissues were preserved in absolute alcohol for future molecular study). All the named species found in this work have been recorded from other localities, including continental and insular Caribbean, Brazil, and Gulf of Mexico (
Nemerteans from Caribbean Coast of Colombia. The systematics is based on
| Taxon | Locality | Habitat | Voucher | Synonyms |
|---|---|---|---|---|
| PALAEONEMERTEA: TUBULANIDAE | ||||
| Tubulanus rhabdotus Corrêa, 1954 | II | 1 | CBUMAG:NEM00042 | |
| HETERONEMERTEA: VALENCINIIDAE | ||||
| Baseodiscus delineatus (Delle Chiaje, 1825) | II, TA | 2 | CBUMAG:NEM:00002, CBUMAG:NEM:00008, CBUMAG:NEM:00012, CBUMAG:NEM00046, CBUMAG:NEM00051, CBUMAG:NEM00052 | (a) |
| HETERONEMERTEA: LINEIDAE (sensu lato) | ||||
| Dushia atra (Girard, 1851) | II, TA | 2, 3 | CBUMAG:NEM:0003, CBUMAG:NEM:00006, CBUMAG:NEM00020, CBUMAG:NEM00027, CBUMAG:NEM00028, CBUMAG:NEM00029, CBUMAG:NEM00030, CBUMAG:NEM00031, CBUMAG:NEM00032, CBUMAG:NEM00033, CBUMAG:NEM00034, CBUMAG:NEM00035, CBUMAG:NEM00036, CBUMAG:NEM00037, CBUMAG:NEM00038 | (b) |
| Lineus stigmatus Coe, 1951 | TA | 2 | CBUMAG:NEM00050 | |
| Micrura ignea Schwartz & Norenburg, 2005 | II | 1, 2 | CBUMAG:NEM00001, CBUMAG:NEM00041 | |
| HOPLONEMERTEA: MONOSTILIFERA | ||||
| Amphiporus cruentatus Verrill, 1879 | II | 2, 4 | CBUMAG:NEM:00015, CBUMAG:NEM:00016 | |
| Amphiporus cf. ochraceus (Verrill, 1873) | II | 2, 4 | CBUMAG:NEM:0011, CBUMAG:NEM00025, CBUMAG:NEM00026, CBUMAG:NEM00048 | (c) |
| Amphiporus texanus Coe, 1951 | II, TA | 2, 4 | CBUMAG:NEM00004 CBUMAG:NEM00017 CBUMAG:NEM00018 CBUMAG:NEM00019 | |
| Nemertopsis bivittata (Delle Chiaje, 1841) | II | 4 | CBUMAG:NEM00048 | (d) |
| Ototyphlonemertes erneba (Corrêa, 1950) | GA | |||
| Ototyphlonemertes lactea (Corrêa, 1954) | TA, GA | 3 | CBUMAG:NEM00054, CBUMAG:NEM00055 | (e) |
| Zygonemertes fragariae Corrêa, 1954 | II | 2 | CBUMAG:NEM00040 | |
| Zygonemertes virescens (Verrill, 1879) | II | 2, 4 | CBUMAG:NEM:00007, CBUMAG:NEM:00009, CBUMAG:NEM:00014, CBUMAG:NEM:00021 CBUMAG:NEM:00022, CBUMAG:NEM:00023 | (f) |
| 4-eyed monostiliferan sp.1 | TA | 2 | CBUMAG:NEM:00010, CBUMAG:NEM00024 | |
| 4-eyed monostiliferan sp.2 | II | 2 | CBUMAG:NEM:0005 | |
| 4-eyed monostiliferan sp.3 | TA | 2 | CBUMAG:NEM00044 | |
| Cratenemertidae sp. | TA | 2, 5 | CBUMAG:NEM00043, CBUMAG:NEM00049 | |
| HOPLONEMERTEA: POLYSTILIFERA: REPTANTIA | ||||
| Punnettia cf. natans (Kirsteuer, 1973) | TA | 5 | CBUMAG:NEM00045 | (g) |
The following taxonomic key (see below) and the descriptions of the main characteristics, together with photographs of the different species, provide a tool for rapid visual identification of the nemerteans found in this survey of the Santa Marta region of Colombia. That for Ototyphlonemertes erneba is based on published descriptions.
| 1a | Mouth ventral and posterior to brain; proboscis without modified middle region bearing armature | “Class” Anopla 2 |
| 1b | Mouth antero-terminal or subterminal and usually sharing a common opening with the rhynchopore but rarely the two open separately; proboscis with modified middle region bearing armature of one or more stylets | “Class” Enopla 6 |
| 2a | Cephalic lobe (head) demarcated posteriorly with pair lateral vertical furrows (may be indistinct) | 3 |
| 2b | Cephalic lobe lateral margins each a distinct longitudinal furrow | Lineidae 4 |
| 3a | Ground color pale ochre; numerous irregularly spaced blackish-brown rings encircle body – most anterior interrupted by mouth, fourth bears pale, often indistinct, oval lateral sense organ | Tubulanus rhabdotus |
| 3b | Ground color pale, milky tan; numerous reddish brown short but irregular length longitudinal lines cover dorsal surface and may extend ventrally; cephalic furrows with orthogonal secondary furrows | Baseodiscus delineatus |
| 4a | Caudal cirrus present in adults but may be very small | 5 |
| 4b | Caudal cirrus lacking in adults; blackish-brown ground color with numerous pairs of white markings dorsally along length of body; anterior 2/3 of cephalic lobe white with symmetrical brown patterning | Lineus stigmatus |
| 5a | Body uniformly blackish except for white anterior margin of cephalic lobe and white caudal cirrus; mouth longer than relaxed width of worm | Dushia atra |
| 5b | Body uniform bright orange anteriorly, grading to yellowish posteriorly | Micrura ignea |
| 6a | Proboscis armature a fig with numerous tiny tack-like stylets; also numerous sacs of accessory stylets; long and wide oblique cephalic furrows with orthogonal secondary furrows; numerous conspicuous ocelli set in approximately 4 rows | Punnettia cf. natans |
| 6b | Proboscis armature a distinct single stylet resting on a basis, usually with a pair of sacs containing accessory stylets | 7 |
| 7a | Ocelli present (not always evident) | 9 |
| 7b | Ocelli absent; statocyst present | Ototyphlonemertes 8 |
| 8a | Statocyst with usually 3 statolith granules; stylet smooth; papillae at anterior of anterior proboscis chamber each with rod-shaped inclusion | Ototyphlonemertes erneba |
| 8b | Statocyst with about 12 statolith granules; proboscis extremely short (about length of head); stylet helically sculpted; cerebral organs and cephalic cirri absent | Ototyphlonemertes lactea |
| 9a | Four ocelli set as rectangle | Unidentified monostiliferan spp 1–3 (see text) |
| 9b | More than four ocelli (not always evident) | 10 |
| 10a | Blood vessels conspicuously filled with red corpuscles | Amphiporus cruentatus |
| 10b | Blood vessels “colorless” | 11 |
| 11a | Body uniformly dark brown and opaque, cephalic lobe rimmed by white “halo”; about 6 ocelli along each antero-lateral margin visible only in specimens squeezed under coverslip | Amphiporus texanus |
| 11b | Ocelli visible without squeezing specimen | 12 |
| 12a | Ocelli extend laterally next to and posterior to cerebral ganglia; armature basis concave or flat posterior margin; epidermis contains small, intracellular, crescent-shaped hooks (requires squeezing specimen under coverslip and compound microscopy at 200–400×) | Zygonemertes 13 |
| 12b | Ocelli all precerebral; armature basis convex posterior margin | 14 |
| 13a | Body pale to dark green as adult, may be grayish-white as juvenile | Zygonemertes virescens |
| 13b | Body uniformly rosy pink | Zygonemertes fragariae |
| 14a | Ocelli small, 6-10 in a pair of rows parallel to and near lateral margins of head; body uniformly translucent milky gray to yellowish, and cerebral ganglia with orange hue | Amphiporus cf. ochraceus |
| 14b | Ocelli large, about 26 set in four groups but may appear to form one large group along each lateral margin of the head; body relatively opaque rosy red; armature basis very rounded and short relative to stylet; good swimmer when irritated | Cratenemertidae sp. 1 |
The mouth is ventral and posterior to the cerebral ganglia in Palaeonemertea and Heteronemertea (e.g., Fig. 2D, K). In all the Hoplonemertea Monostilifera encountered here the mouth and proboscis share a common pore, the rhynchopore, located at or subterminal to the tip of the head (NB: they open separately in most polystiliferans and in a few monostiliferans, but both openings are at the tip of the head). In lineid Heteronemertea (generally and in all specimens encountered here) a more or less deep furrow extends longitudinally along each side of the head and often is referred to as a cephalic slit, groove or furrow; the cerebral organ pore opens at its posterior. Cephalic furrows (when present) of tubulanid palaeonemerteans, baseodiscid heteronemerteans and hoplonemerteans are shallow and vertical or oblique, at the sides of the head near the cerebral ganglia, and they may be inconspicuous; the cerebral organ pore opens into the middle of each furrow. These furrows might best be referred to as cerebral organ furrows to distinguish them from a circumferential cephalic groove; a shallow epidermal groove that encircles the body and demarcates the “head” from the foregut region and found in most benthic Hoplonemertea and a very few Palaeonemertea and Pilidiophora. It commonly is post-cerebral and in Hoplonemertea usually takes the form of a dorsal posteriorly directed “V” and a ventral anteriorly directed “V”. The hoplonemertean proboscis when everted reveals a characteristic cylindrically uniform coating of more or less conspicuous epidermal papillae, whereas the anoplan proboscis generally lacks conspicuous papillae and often is bilaterally differentiated. The mid-region of the monostiliferan hoplonemertean proboscis is conspicuously differentiated into a bulb-like structure posteriorly and an anterior diaphragm bearing a basis with stylet and two or more sacs containing accessory stylets (e.g., Fig. 3G, M, P; 4C, G, J, M; 5H, K), whereas the mid-proboscis of polystiliferan Hoplonemertea is inconspicuously differentiated and bears an ovoid basis with multiple tiny stylets that may be very difficult to observe even with the 40× objective of a compound microscope. Measurements given below are from animals collected in this study.
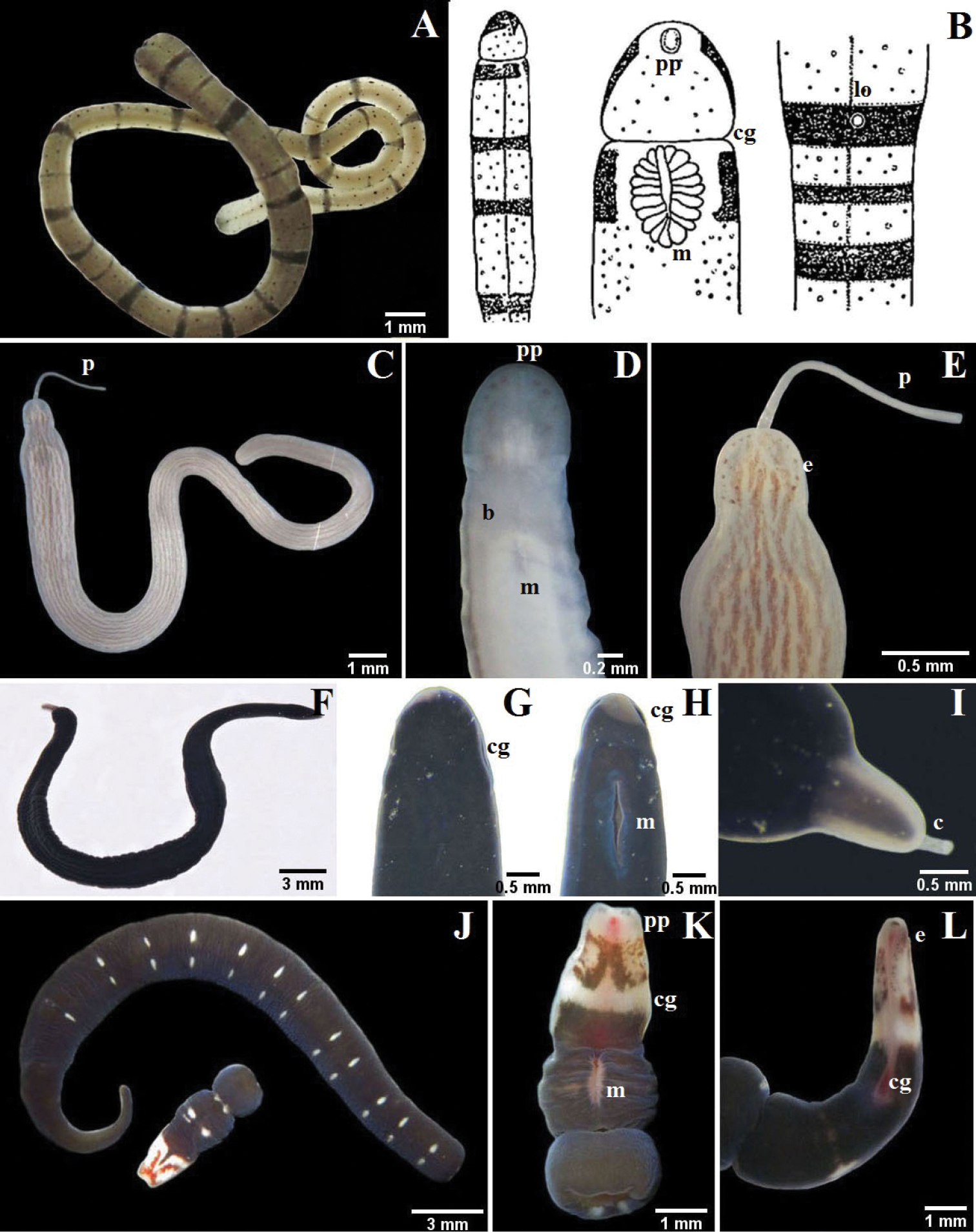
A–B Tubulanus rhabdotus: B detail of the head, mouth and lateral organ (modified from
A–D Micrura ignea: A entire specimen, the worm has expulsed the proboscis B dorsal detail of the head C ventral detail of the head D detail of the tail E–G Amphiporus cruentatus: F dorsal detail of the head G detail of the stylets H–J Amphiporus cf. ochraceus: I dorsal detail of the head J drawing of the stylet K–M Amphiporus texanus: K entire worm L dorsal detail of the head M detail of the stylets. ac accessory stylet, b base of the stylet, br brain, c cirrus, cg cephalic grooves, e eyes, m mouth, p proboscis, pp proboscis pore, s sylet, v blood vessel.
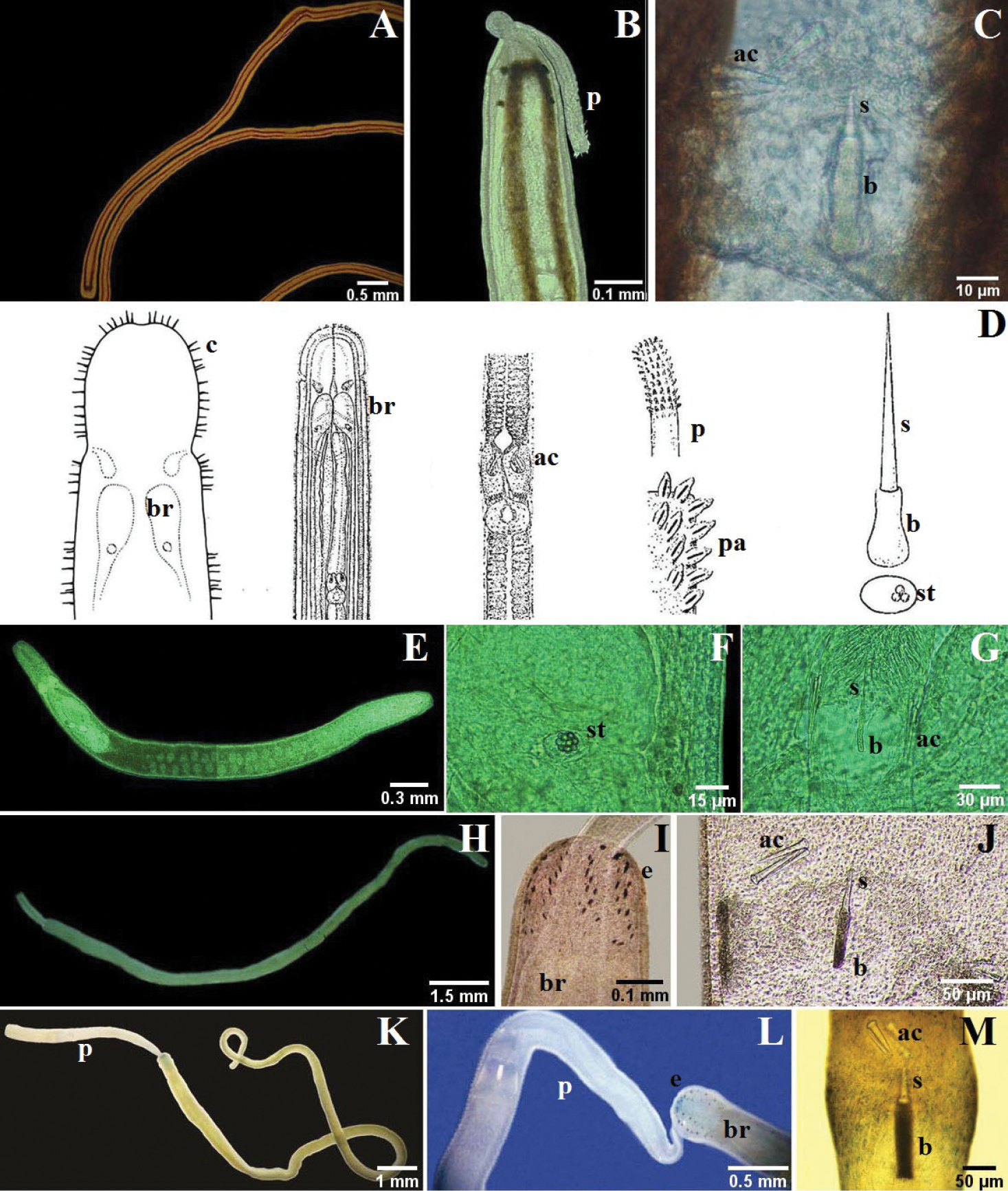
A–C Nemertopsis bivittata: B dorsal detail of the head and proboscis, C detail of the stylets D Ototyphlonemertes erneba (modified from
A–C 4-eyed monostiliferan sp. 1: B dorsal detail of the head C drawing of the stylet D–E 4-eyed monostiliferan sp. 2: E dorsal detail of the head and proboscis F–H 4-eyed monostiliferan sp. 3: G dorsal detail of the head H detail of the stylets I–K Cratenemertidae sp.: J dorsal detail of the head and proboscis K detail of the stylets L–M Punnettia cf. natans: M dorsal detail of the head. ac accessory stylet, b base of the stylet, br brain, e eyes, ec epithelial crests, p proboscis, s sylet.
(a) Baseodiscus delineatus (Delle Chiaje, 1825): Baseodiscus curtus, Baseodiscus delineatus var. curta, Baseodiscus delineatus var. curtus, Baseodiscus insignis, Borlasia carmelina, Eupolia amboinensis, Eupolia ascophora, Eupolia curta, Nemertes delineatus, Nemertes delineatus, Polia delineata Delle Chiaje, 1825 (
(b) Dushia atra (Girard, 1851): Cerebratulus ater, Lineus ater, Meckelia atra Girard, 1851 (
(c) Amphiporus cf. ochraceus (Verrill, 1873): Cosmocephala ochracea Verrill, 1873 (
(d) Nemertopsis bivittata (Delle Chiaje, 1841): Eunemertes peronea, Nemerteopsis peronea, Nemertes peronea, Nemertopsis peronea, Omatoplea peronea, Ommatoplea peronea, Polia bivittata Delle Chiaje, 1841; Prosorhochmus bistriatus, Prosorochmus bistriatus (
(e) Ototyphlonemertes lactea Corrêa, 1954: Norenburgia lactea Chernyshev, 1993 (
(f) Zygonemertes virescens (Verrill, 1879): Amphiporus agilis, Amphiporus virescens Verrill, 1879; Nemertes verrilli, Ophionemertes agilis (
(g) Curranemertes natans Kirsteuer, 1973.
One specimen up to about 30 mm long and 1 mm wide; dorsoventrally flattened, cephalic lobe broadly rounded anteriorly; caudal terminus blunt. Ochre with numerous small dark dots arranged in longitudinal lines, dark brown rings of differing thickness spaced irregularly along body, first four rings thickest. Prominent but short, vertical cerebral organ furrows demarcate head from rest of body. Eyespots lacking. Mouth ventral, just posterior to cephalic grooves. Proboscis pore subterminal. Lateral sensory organ present in fourth ring. Worm secretes and lives in soft tube of honey color.
Curaçao (
Six specimens up to about 50 mm long, 2 mm wide; dorsoventrally flattened; cephalic lobe broadly rounded. Milky ground color; dorsally with abundant short interrupted reddish to brown longitudinal lines, paler or completely absent on ventral surface. Shallow, cerebral organ furrows post-cerebral, vertical and slightly oblique, with inconspicuous, orthogonally oriented secondary furrows. Numerous ocelli arranged irregularly along antero-lateral margin of head. Mouth ventral, posterior to cerebral organ furrows. Proboscis pore at anterior of head; proboscis short and thin.
This species seems to be circumglobal in tropical and subtropical seas (
Fifteen specimens up to about 160 mm long, 2.5 mm wide; dorsoventrally flattened; head elongate, can be pointy; short, slender caudal cirrus present. Black body, lips of cephalic furrows, tip of head and tail grayish or milky white. Deep cephalic furrows form lateral margins of head. Ocelli lacking. Mouth ventral, a large longitudinal slit posterior to cephalic furrows. Proboscis long, yellow, with smooth surface when everted.
Curaçao (
This was the most frequently found species, though not necessarily the most abundant; we did not do quantitative sampling in this study. Given the wide regional distributions of other nemerteans in this and other recent studies (e.g.,
One specimen up to about 30 mm long, 2 mm wide; dorsoventrally flattened, anterior margin somewhat squared, posterior end tapered, caudal cirrus absent. Body dark violet or dark olive green; paired, widely spaced, transversely elongate white markings dorsally, each pair part of barely perceptible thin white ring encircling body; anterior two-thirds of head white with prominent brown pigment patterning, including a V-shaped marking dorsally and ventrally, each pointed anteriorly. Deep cephalic furrows form lateral margins of head. About 20 to 30 small ocelli disposed in a single irregular line on each side of head along anterior third of cephalic furrow. Mouth ventral, a large longitudinal slit posterior to cephalic furrows. Cephalic ganglion visible through body wall as a pink mass.
Florida (
Two specimens up to about 100 mm long, 3 mm wide; dorsoventrally flattened; head “triangular”, pointed anteriorly and widening posteriorly; posterior end blunt with short and slender caudal cirrus. Orange grading posteriorly to yellowish. Deep cephalic furrows form lateral margins of head. Mouth slit-shaped, posterior to cephalic grooves. Eyespots lacking. Proboscis long, thick and pink; when everted it possesses ruffles.
Belize (
Two specimens up to about 20 mm long, < 1 mm wide; dorsoventrally flattened, bluntly rounded at both ends. Pale yellow, with three thin, longitudinal blood vessels made bright red by corpuscles that can be observed flowing through the vessels. Cerebral organ furrows, vertical, not prominent, precerebral. About 6–10 conspicuous, blackish, precerebral ocelli, in single row along each. Rhynchopore subterminal at tip of head; rhynchocoel extends to about middle of body length; proboscis long and thick; armature approximately at center of proboscis; stylet slender (length: 30 µm), supported on cylindrical basis (33 × 8 µm); 2 pouches with 3 accessory stylets each. Mature females with dark or bright gray eggs visible through body wall.
Gulf of Mexico, New England (USA) and Washington (
Four specimens up to about 10 mm long, 0.5 mm wide; dorsoventrally flattened, bluntly rounded at both ends. Variable color, yellowish to milky gray, sometimes with light orange pigmentation in cephalic region corresponding to cerebral ganglia. Cerebral organ furrows vertical, precerebral. About 6-10 conspicuous, blackish, precerebral ocelli, arranged in single regular row, along each lateral margin of head. Rhynchopore subterminal; rhynchocoel about three quarters of body length; proboscis long; medially constricted basis same length as slender stylet (length: 29 µm); 2 pouches with 2 accessory stylets each.
Gulf of Mexico and New England (USA) (
Four specimens up to about 15 mm long, 0.5 mm wide; dorsoventrally flattened, bluntly rounded at both ends; cephalic lobe narrower than foregut region. Dark brown body; with magnification, thick unpigmented margin (i.e., epidermis) appears white. Cerebral organ furrows vertical, precerebral. Row of about 6 ocelli present along each lateral margin of head; visible in squeeze preparation. Rhynchopore subterminal; proboscis large and thick, conspicuous papillae; central stylet slender (length: 42 µm), supported on wide cylindrical basis (34 × 10 µm) at middle of proboscis; two pouches with 2-4 accessory stylets each.
Gulf of Mexico and Southern Florida (
One specimen up to about 20 mm long; 1 mm wide; rounded at both ends. Yellow milky base color, dorsally with 2 brown to reddish longitudinal lines joined at anterior and posterior ends. Cerebral organ furrows precerebral, difficult to see. Head almost undifferentiated from body. Cephalic grooves not visible. Two pre-cerebral eyes on each lateral margin of head. Rhynchopore subterminal; proboscis small, slender provided with papillae; short central stylet (length: 11 µm), supported on a massive base (27 × 7 µm). Two pouches containing three accessory stylet each.
USA East Coast – Florida (
Up to about 12 mm long; < 1 mm wide; according to (
Brazil (
Two specimens up to about 3.5 mm long; < 0.3 mm wide; truncated at both ends. Milky white color. Cerebral organs and associated furrows absent. Without ocelli. Two ovoid statocysts present, statolith formed by more than 10 spherical granules. Rhynchocoel short, about one-third of body length; proboscis very short; posterior proboscis vesicular (translucent) with transmitted light; armature at middle of proboscis; stylet slender, helically sculpted (length: 15 µm); basis thin, cylindrical (14 × 2 µm); 2 pouches with 3 accessory stylets each.
Brazil (
One specimen up to about 12 mm long, 1 mm wide; dorsoventrally flattened, bluntly rounded at both ends. Body vivid pinkish strawberry-red. Cerebral organ furrows shallow, precerebral. Pair irregular rows of 8-13 ocelli each on each side of head; row of about 10 ocelli between brain and each lateral margin of body and reaching post-cerebrally into foregut region. Rhynchopore subterminal; rhynchocoel to about middle of body length; proboscis long and thick with thin papillae; central stylet thin (length: 45 µm); basis smooth, cylindrical (54 × 14 µm); 2 pouches with 2 or 3 accessory stylets each. Epidermis with minute crescent-shaped intracellular spicules.
São Sebastião (Brazil) (
Six specimens up to about 30 mm long, < 2 mm wide; dorsoventrally flattened, bluntly rounded at both ends. Variable color, from white to yellow or greenish. Cerebral organ furrows shallow, precerebral. Numerous pre-cerebral small ocelli arranged in two pair irregular rows of about 15–20 ocelli each; row of post-cerebral ocelli each side between brain and lateral margin of body, extending far posterior to brain along lateral nerve cord. Rhynchopore subterminal; rhynchocoel wide and almost full body length; proboscis long with small papillae; stylet slender (length: 60 µm) supported on massive and medially constricted basis (112 × 28 µm); two pouches, each bearing 3 accessory stylets. Epidermis with minute crescent-sphaped intracellular spicules.
Gulf of Mexico and New England (
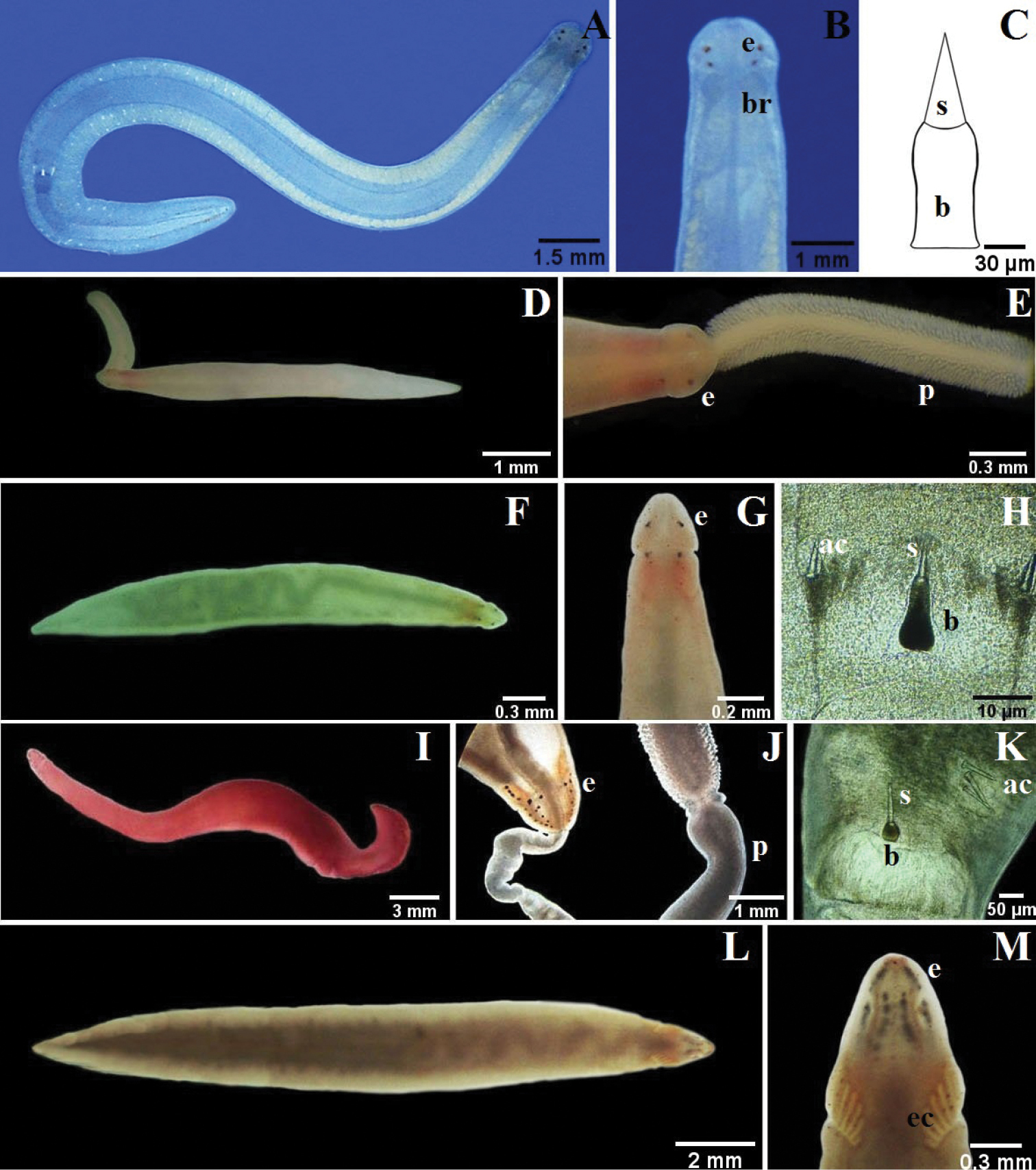
Fig. 5A–C
Description. Two specimens about 12 mm long, 1 mm wide; dorsoventrally flattened. White to yellow-brownish color. Cerebral organ furrows shallow, precerebral. Four precerebral ocelli set as corners of a wide rectangle. Rhynchocoel voluminous, extending almost full body length. Rhynchopore subterminal. Proboscis stout; armature far posterior; Stylet (length 45 µm) supported on massive medially constricted basis (110 × 50 µm); two pouches with 2 accessory stylets each.
Distribution. Santa Marta, Colombia.
Fig. 5D–E
Description. One specimen about 10 mm long, < 1 mm wide; dorsoventrally flattened; head arrow-shaped, tail tapered. Milky white color. Brain appears as a pink orange spot in head. Cerebral organ furrows shallow, precerebral. Postcerebral groove inconspicuous, forms dorsal “V”. Two pair ocelli, anterior and posterior separated by cerebral organ furrow. Rhynchopore subterminal. Proboscis stout, densely papillated.
Distribution. Santa Marta, Colombia.
Fig. 5F–H
Description. One specimen about 6 mm long, < 1 mm wide; dorsoventrally flattened; head arrow-shaped; tail tapered. Cream to greenish color. Brain region pink. Cerebral organ furrows precerebral, deep. Postcerebral groove forms a “V” dorsally. Two pair ocelli, set as square, anterior and posterior separated by cerebral organ furrows. Rhynchopore subterminal; rhynchocoel extends three fourths of body length. Stylet (length: 70 µm) supported on massive pear-shaped basis (130 × 60 µm).
Distribution. Santa Marta, Colombia.
Fig. 5I–K
Description. Two specimens up to about 22 mm long, 2 mm wide; tapered at both ends. Uniform bright red color. Conspicuous mid-dorsal cephalic crest. Cerebral organ furrows precerebral, inconspicuous, with few faint ridges orthoganol to furrow axis. About 26 ocelli scattered in four elongate, irregular groups, anterior and posterior separated by cerebral organ furrows. Rhynchopore subterminal. Proboscis long and stout, with dense, large papillae; stylet (length: 120 µm) on short, wide and rounded basis (50 × 48 µm); two pouches containing three accessory stylets each. Worms capable of swimming with strong undulating movements.
Distribution. Santa Marta, Colombia.
One specimen about 17 mm long, < 1 mm wide; dorsoventrally flattened, tapered at both ends. Gray to brown color, darker on head and along mid-dorsal line of body; ventral surface milky gray. Head narrow with respect to body. Cerebral organ furrows wide, postcerebral, subdivided by about 5 longitudinal epithelial ridges (secondary furrows) orthoganol to furrow axis. Numerous large ocelli, precerebral, arranged in four irregular longitudinal rows, outer rows possibly divided into anterior and posterior clusters. Armature normally several stylets supported on a single basis, but not documented by us. Individuals capable of swimming by undulatory movements, leaving mucus behind it.
Bahía Mochima (Venezuela) (
Two named polystiliferan species are known from the region. Polyschista curacaoensis Stiasny-Wijnhoff, 1925 is known only from three pieces – two heads and a tail – already preserved and strongly contracted when first examined by Stiasny-Wijnhoff; therefore, of dubious value for anatomical study. The heads are described as having a “a well defined brown longitudinal marking on their back” while “the margins are a milky, transparent white and two to three times as broad as the thick [brown] middle part”. The tail is described as being transparent and white. This does not fit well the present species. Curranemertes natans Kirsteuer, 1973 is known only from Venezuela and described as “orange to brownish” dorsally, with the thicker median region of the head being a “darker brownish shade”. Though
Direct observations of the nemerteans “in vivo” facilitates collection of information about nemertean species that is more reliable than possible with preserved specimens, and permits photographic records useful for their identification (
Although, the purpose of this research was taxonomical, it is worth mentioning that the most frequent species was Dushia atra, representing 30% of the total of collected specimens. It was followed by Baseodiscus delineatus and Zygonemertes virescens at 12% each. The major diversity, in terms of number of species, was observed at Inca-Inca, with 7 species. However, the sampling effort was not the same in all collection sites, so it is not possible to make a reliable comparison of the biodiversity among the stations. Most studies of nemerteans in the Caribbean, and elsewhere, have focused on taxonomy (
Frequently, aggregations of 3–5 con-specific specimens of Amphiporus cruentatus, Amphiporus texanus, Dushia atra and Zygonemertes virescens, were found under rocks or in rock crevices. This behavior has been observed before in several species of nemerteans and in some cases may be related with reproduction events (
This study represents the first taxonomic work focused on nemerteans of Colombia, and specifically the Caribbean coast. Except for Ototyphlonemertes lactea, all species are new records for Colombia. Among the 36 species reported from the Caribbean region (
We thank professors Marcela Bolaños, Sandra Vilardy, Lina Saavedra, María Negritto, Joseph Dunn, and Juan Manuel Renjifo; the biologists Ana Lagos, Anisbeth Daza, Darlim Botto, Rosana Londoño, Cristina Cedeño and Sebastian Hernandez, and the biology students, Mariela Ramos, Maria León, Pedro Prado and Julio Pernett for their contributions and help. This work was supported by the “Programa de Semilleros de Investigación” of the Universidad del Magdalena and The Fundación Alejandro Ángel Escobar. We are grateful to three anonymous reviewers of an earlier version of this manuscript for their detailed and helpful comments.