Citation: Santos MDS, Porto TJ, Lira-da-Silva RM, Brazil TK (2014) Description of the male of Tityus kuryi Lourenço, 1997 and notes about males of Tityus stigmurus (Thorell, 1877) and Tityus serrulatus Lutz & Mello, 1922 (Scorpiones, Buthidae). ZooKeys 435: 49–61. doi: 10.3897/zookeys.435.6694
The male of Tityus kuryi Lourenço, 1997 is described for the first time. Despite being very similar to the female, the male presents more robust metasomal segments. Additionally, the distribution of the sexual populations of another two species of the T. stigmurus complex is reported herein: T. serrulatus Lutz & Mello, 1922 and T. stigmurus (Thorell, 1877). Males of T. serrulatus were, until now, restricted to the Minas Gerais State (Southwestern region of Brazil), and with new records reported here, its known distribution now encompasses the Northeastern region of Brazil. Males of T. stigmurus were previously recorded only for two municipalities in the State of Bahia, and here we present eight new records for Bahia State and one for Pernambuco State. We present a key to related species of the T. stigmurus complex based on morphology and coloration pattern.
Sexual population, scorpions, Tityus, Brazil
The description of males in population of scorpions is an important contribution, not only as regards taxonomic knowledge of the species, but also to enable understanding of its reproductive strategy. Tityus kuryi Lourenço, 1997 was described based on a single adult female collected in Palmeiras Municipality, in the Chapada Diamantina region, Bahia State, Brazil (
The two more widely distributed species from the Tityus stigmurus complex are parthenogenetic: Tityus serrulatus and Tityus stigmurus (
In this paper, we describe the male of Tityus kuryi and report new records of Tityus serrulatus and Tityus stigmurus males, widening the known distribution of their sexual populations.
Specimens of Tityus kuryi (n = 9), Tityus serrulatus (n = 1595) and Tityus stigmurus (n = 380) present in the scientific collection of the “Museu de Zoologia da Universidade Federal da Bahia (MZUFBA)” were analyzed. Along with these, another 280 specimens of Tityus stigmurus present in the reference collection of the “Centro de Informações Anti-veneno do Estado da Bahia” (CIAVE), Health Department of Bahia State, were also studied. Of the nine Tityus kuryi examined, four of them were kept in captivity for a year and a half (August 2009 to February 2011), a fact that allowed us to identify the spermatophore and to confirm the presence of two males.
The measurements were obtained following the methodology of
The males of Tityus serrulatus and Tityus stigmurus were identified based on observation of the external morphological characteristics, as diagnosed by
Brazil, Bahia State, Palmeiras Municipality, Vale do Capão, 12°37'04"S, 41°29'20"W, 850 m, 24/XII/2006 (T.J. Porto leg.), adult male (MZUFBA 2569); Brazil, Bahia State, Palmeiras Municipality, Vale do Capão, 12°37'11"S, 41°29'23"W, 850 m, 24/XII/2006 (T.J. Porto leg.), adult male (MZUFBA 2570); Brazil, Bahia State, Palmeiras Municipality, Vale do Capão and Cachoeira da Fumaça, 12°31'44"S, 41°33'32"W, 04/VI/1999, 23/II/2007 and 17/VII/2009 (C. M. Pinto-Leite & G. Carvalho leg.), five adult females (MZUFBA 1000, 1602, 2166, 2505, 2529); Brazil, Bahia State, Ibicoara Municipality, 13°24'4"S, 41°16'5"W, I/2005 and VII/ 2011, two adult females (MZUFBA 2451, 2572).
Scorpion of medium to large size, ranging from 55 to 78mm in total length. General coloration dark reddish with blackish spots on the pedipalps, legs, lateral surfaces of mesosomal tergites and ventral submedian carinae of all metasomal segments, as well as transversal blackish spots on the posterior margin of sternites. Carinae moderately to strongly marked; granulations moderately to weakly marked. Fixed and movable fingers with 16–17 oblique rows of granules. Pectines with 24–25 teeth in males, 23–26 in female. Secondary sexual dimorphism evident.
Tityus kuryi Lourenço, 1997, belongs to the “Tityus stigmurus” species complex. The male of Tityus kuryi can be distinguished from the other males of the species complex, particular from Tityus aba, Tityus stigmurus and Tityus martinpaechi, by the absence of three longitudinal dark brown stripes on mesosomal tergites. Furthermore, in Tityus aba and Tityus martinpaechi, the pedipalp of the males is much thinner than of females, which also occurs in Tityus melici although there is no metasomal dimorfism in it (
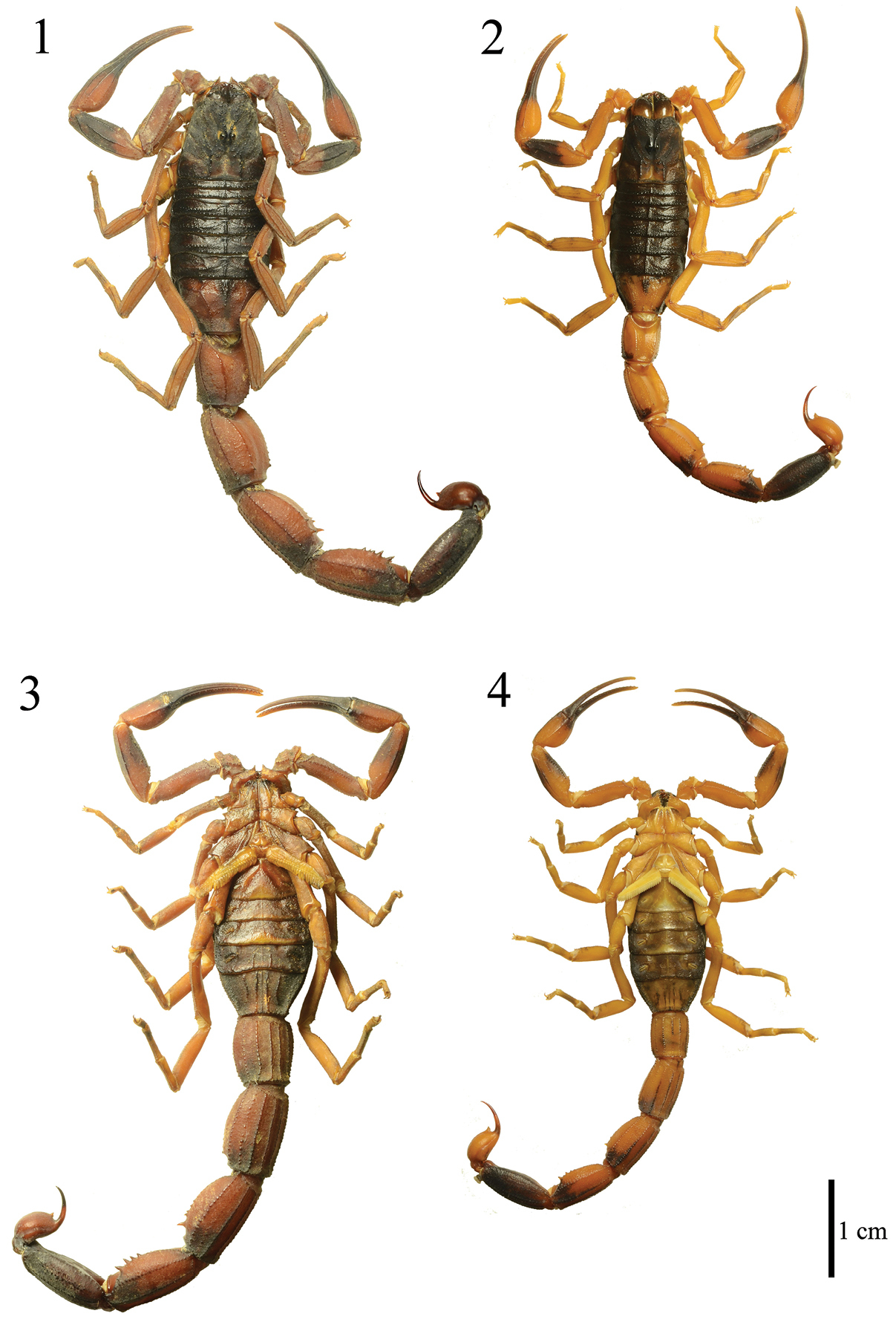
Tityus kuryi. Male (MZUFBA 2569 - Palmeiras, Bahia, Brasil): dorsal (1) and ventral (3) views. Mature female (MZUFBA 2451 - Ibicoara, Bahia, Brazil): dorsal (2) and ventral (4) views.
| 1 | Metasomal segments III and IV without or with 1 to 3 granules modified as spines | 2 |
| − | Metasomal segments III and IV with 5 to 7 granules modified as spines | 5 |
| 2 | Longitudinal dark stripes over tergites present | 3 |
| − | Tergites densely pigmented, without longitudinal stripes | Tityus melici |
| 3 | One longitudinal dark stripe evident over tergites | Tityus stigmurus |
| − | Three longitudinal dark stripe evident over tergites | 4 |
| 4 | Prosoma predominantly dark, pedipalps and legs without spots, pectines with 25–25 teeth | Tityus aba |
| − | Prosoma with dark inverted triangle, pedipalps and legs pigmented, pectines with 23–23 teeth | Tityus martinpaechi |
| 5 | Coloration dark reddish, pedipalps and legs pigmented | Tityus kuryi |
| − | Coloration yellowish, pedipalps and legs without spots | Tityus serrulatus |
Based on male MZUFBA 2569.
Coloration: Reddish brown with numerous dark areas (Fig. 1). Carapace dark with some light-brown areas (Fig. 5). Ocular tubercle dark. Mesosoma dark in tergite VI, tergite VII with a darker central region and lighter red-brown lateral region (Fig. 1); metasomal segments I–IV reddish brown with dark areas posteriorly in the lateral region and on the submedian ventral carinae (Figs 1 and 3); a dark spot occupying almost the entire segment V (Fig. 12). Telson: vesicle reddish brown, lighter than the metasomal segment V, with two small spots at the base; aculeus with dark spots at the base, red-brown medially and blackened distally (Fig. 12). Coxosternal region yellow with black spots in the coxapophyse I and II (Fig. 3); sternite III light brown, sternites IV–VI darker with the posterior medial region light brown, sternite VII darker with medially light brown T-shaped spots (Fig. 3). Chelicerae dark with a light brown base; apex of the fingers brown. Pedipalps reddish brown with dark spots in the patella (Figs 9–10) and chela (Figs 7–8); fingers generally dark but distally light brown. Legs light brown with dark spots on tibia and tarsi (Fig. 1).
Tityus kuryi (male). 5 Carapace 6 Pectines 7–8 Chela, dorsal external and ventral views 9–10 Patella, dorsal and external views 11 Femur, dorsal view. Scale bars= 1 mm.
Tityus kuryi (male): Lateral views of the metasoma and telson. Scale bars= 1 mm.
Morphology: Carapace: anterior margin with a weak median concavity (Fig. 5); median ocular tubercle situated anterior to the center of the carapace and median eyes separated by more than one ocular diameter. Three pairs of lateral eyes; median ocular carina moderate with medium granules (Fig. 5); anterior median furrow moderately deep. Moderately granular.
Mesosoma: Tergites moderately granular with larger granules in the posterior region; presence of median carinae in all tergites. Tergites I–II with reduced carinae; in the tergites III–IV the carinae occupy the distal half and begin bifurcated and finish merged; tergite VII pentacarinate; transversal carinae present in all tergites. Pectines: pectinal teeth 24–25, basal middle lamellae of pectines not dilated (Fig. 6). Sternites weakly granular; a clear triangular zone in the posterior region of the sternite III and a reduced smooth and shiny slightly expanded triangular zone in the posterior region of the sternite V (Fig. 3). Sternite VI with two short median carinae occupying the distal half. Sternite VII with five carinae, two parallel submedian, occupying the entire sternite with a small carinae between them, and two lateral carinae restricted to the central region.
Metasoma: Metasomal segments: I with ten complete paired carinae (ventral submedian, ventral lateral, lateral inframedian, lateral supramedian and dorsal lateral with adjacent granules, the dorsal lateral has one spinoid posterior granule) (Fig. 12); II with eight complete paired carinae (inframedian lateral carinae incomplete on anterior third and present sparse granules; others are complete with adjacent granules; dorsal lateral with one spinoid granule) (Fig. 12); III with eight complete paired carinae (inframedian lateral carinae absent; others complete with adjacent granules, the dorsal lateral with three spinoid posterior granules) (Fig. 12); IV with eight complete paired carinae (inframedian lateral carinae absent; others are complete with adjacent granules; dorsal lateral with four spinoid posterior granules) (Fig. 12); V with five complete carinae with uniform and adjacent granules (inframedian lateral carinae and dorsal lateral carinae absent; two complete paired carinae: ventral submedian and ventral lateral; one ventral median carina); intercarinal surface moderately granular (Fig. 12). Telson: vesicle with five carinae (four of which vestigial and only the ventral well defined); aculeus long and strongly curved; subaculear tooth strong and romboid with two small dorsal teeth (Fig. 12).
Chelicerae: Dentition as defined by
Pedipalp: (Figs 9–11) femur with 5 carinae, the dorsointernal, dorsoexternal and externomedian carinae present median granules; ventrointernal with smaller granules and internomedian with larger granules; patella with 7 carinae, the dorsoexternal, internomedian, ventrointernal and dorsomedian carinae present median and adjacent granules; internomedian with a proximal spinoid granule (Fig. 9); dorsoexternal, externomedian and ventroexternal with small and continuous granules; chela with 9 carinae of which the dorsoexternal, dorsal secondary, dorsointemal, ventrointernal, internomedian, ventroexternal, digital, subdigital and ventromedian, all with small and continuous granules; all pedipalp surfaces moderate to weakly granular. Movable fingers with 17–17 oblique lines of granules. Trichobothriotaxy: ortobothriotaxy A–α (
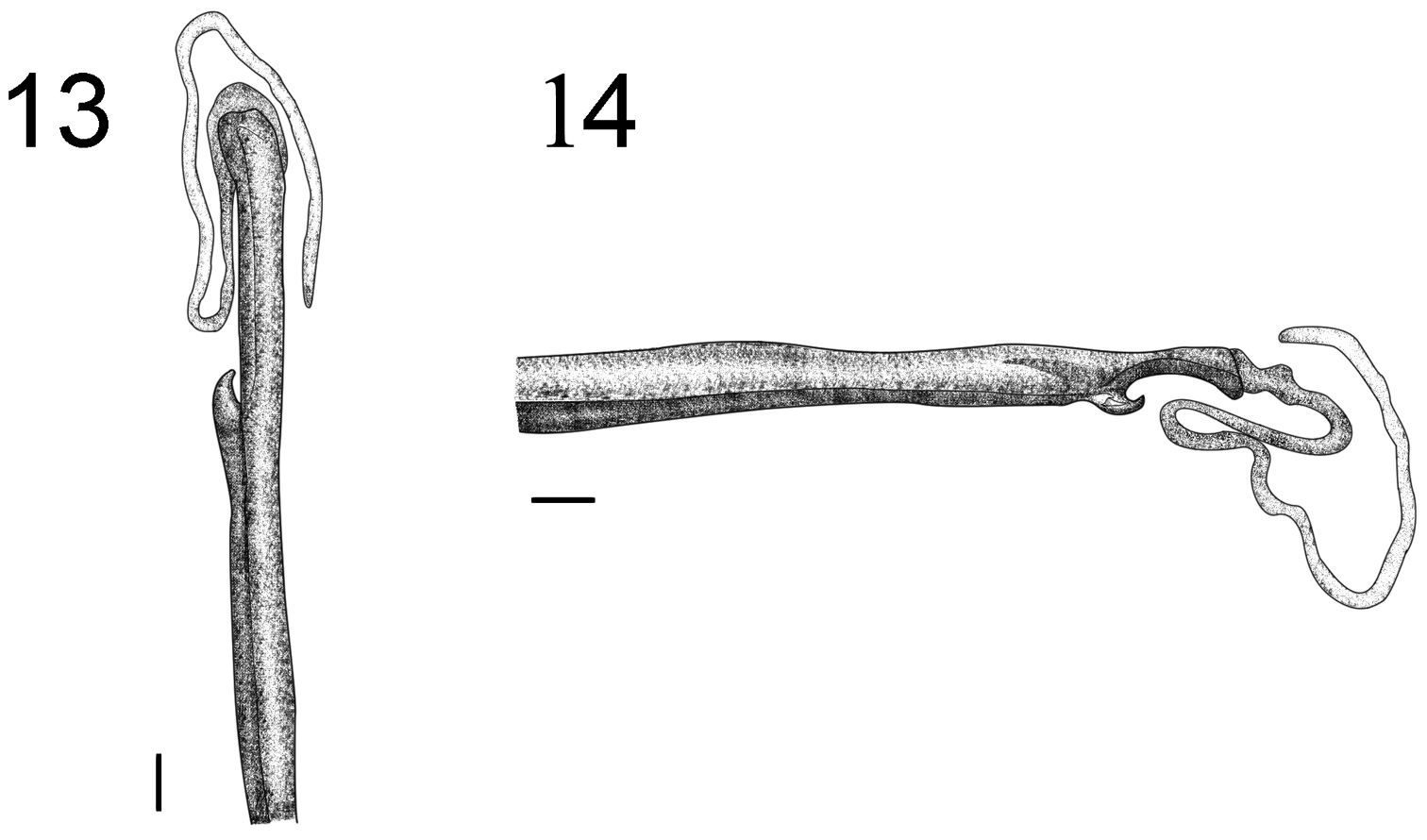
Hemispermatophore: Flagelliform, long and narrow, measuring approximately 13.5 mm, general color light brown; the trunk is trough-shaped; the flagellum is half the width of the trunk and approximately the same length (Fig. 13). Presence of three distal lobes: basal lobe, internal lobe and external lobe. The basal lobe is hook-shaped, protruding internally or externally; the internal lobe extends up to the base of the basal lobe flagellum, the external lobe extends from the medial basal lobe to the posterior third of the internal lobe (Fig. 14). The basal and external lobes are blackened.
Hemispermatophore of Tityus kuryi (male MZUFBA 2570; Palmeiras, Bahia, Brazil): dorsal (1) and lateral (2) views. Scale bars= 1 mm
Sexual dimorphism: Despite being similar to the females with regard to the coloration pattern and morphological details of the species (Figs 1–4), the male of Tityus kuryi presents metasomal segments 1.5 time more robust than those of the females (Figs 1–4; Table 1).
Measurements (in mm) of two males and seven females used to investigate the sexual dimorphism in Tityus kuryi.
| Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MZUFBA number | 2569 | 2570 | 2451 | 2505 | 2529 | 2572 | 1000 | 1602 | 2166 |
| Total Length | 72.9 | 72.9 | 60.2 | 59.2 | 58.5 | 60.8 | 67.6 | 71.5 | 78.1 |
| Carapace | |||||||||
| Length | 8.6 | 8.5 | 8.0 | 6.7 | 7.2 | 8.1 | 8.2 | 8.1 | 8.3 |
| Anterior width | 5.5 | 5.9 | 5.0 | 5.0 | 5.2 | 5.2 | 4.3 | 4.6 | 4.6 |
| Posterior width | 7.8 | 9.1 | 7.7 | 6.7 | 7.4 | 7.1 | 9.0 | 8.8 | 8.8 |
| Metasoma | |||||||||
| Segment I (length) | 7.0 | 7.9 | 5.9 | 4.4 | 5.4 | 5.2 | 5.8 | 5.5 | 6.0 |
| Segment I (width) | 6.9 | 6.3 | 4.6 | 4.1 | 4.4 | 4.3 | 5.0 | 5.0 | 4.8 |
| Segment II (length) | 9.5 | 9.1 | 6.9 | 6.2 | 6.5 | 6.6 | 6.8 | 7.1 | 7.0 |
| Segment II (width) | 7.0 | 6.8 | 4.6 | 4.2 | 4.4 | 4.5 | 5.0 | 5.1 | 4.8 |
| Segment III (length) | 10.2 | 10.1 | 7.7 | 6.8 | 7.0 | 8.2 | 7.5 | 7.6 | 7.6 |
| Segment III (width) | 7.0 | 6. | 4.9 | 4.4 | 4.3 | 4.7 | 5.1 | 5.1 | 5.0 |
| Segment IV (length) | 10.8 | 10.7 | 8.7 | 7.7 | 7.9 | 4.6 | 8.2 | 8.3 | 8.1 |
| Segment IV (width) | 6.5 | 6.7 | 4.6 | 4.2 | 4.3 | 8.2 | 5.5 | 5.1 | 5.0 |
| Segment V (length) | 10.6 | 10.4 | 8.7 | 8.4 | 8.3 | 9.1 | 9.5 | 8.8 | 9.1 |
| Segment V (width) | 5.7 | 5.8 | 4.2 | 4.0 | 4.1 | 4.2 | 4.6 | 4.1 | 4.5 |
| Vesicle | |||||||||
| Length | 8.5 | 8.7 | 8.0 | 5.9 | 7.3 | 6.9 | 6.8 | 8.2 | 8.3 |
| Depth | 2.5 | 2.4 | 2.7 | 1.5 | 1.1 | 2.6 | 2.7 | 2.7 | 2.7 |
| Pedipalp | |||||||||
| Femur (length) | 8.1 | 8.4 | 7.5 | 6.5 | 7.6 | 6.6 | 8.2 | 7.6 | 7.6 |
| Femur (width) | 2.2 | 2.2 | 2.0 | 1.8 | 1.9 | 1.8 | 2.2 | 2.5 | 2.3 |
| Patella (length) | 9.2 | 9.0 | 8.5 | 7.8 | 7.5 | 8.4 | 8.2 | 8.0 | 8.2 |
| Patella (width) | 2.8 | 2.9 | 2.8 | 2.4 | 2.8 | 2.9 | 3.2 | 3.2 | 3.2 |
| Chela (length) | 16.2 | 16.0 | 14.5 | 13.4 | 13.5 | 14.3 | 14.3 | 14.3 | 14.3 |
| Chela (width) | 3.0 | 3.0 | 2.9 | 2.7 | 2.7 | 2.9 | 3.1 | 3.1 | 3.1 |
| Movable finger (length) | 10.5 | 9.6 | 9.6 | 8.5 | 8.7 | 9.2 | 9.7 | 9.7 | 9.7 |
Known only from the Chapada Diamantina region, Bahia State, Brazil.
The male of Tityus kuryi is usually larger than the female (male: 72.93–72.95 mm; female: 58.54–78.03mm) and pectinal tooth counts varied as follows: 24–25 teeth in males and 23–26 in females. The number of principal rows of granules varied from 16–17 in both sexes. The spinoid granules on the posterior end of the dorsal lateral carinae of metasomal segments III and IV are greater in number, is larger and sharper on the left than on the right, and the counts varied as follows: 1–3 in metasomal segment III and 2–4 in metasomal segment IV.
Tityus kuryi occurs in a restricted environment of montane savannas biome named “Campos Rupestres” of the Espinhaço Range (Cadeia do Espinhaço), in rocky areas of high altitudes in the Chapada Diamantina, Bahia State. The annual average temperature there is 22–24 °C, with 36–38 °C as absolute maximum and 4.8 °C as absolute minimum. It is found at altitudes above 840 m. They can also be found near to residential areas, but always near to natural fragments hidden in debris under or between stones.
In an attempt to explain the strategies of the life history in populations of scorpions, Vandel (1928 apud
The sexual populations of Tityus serrulatus and Tityus stigmurus have a highly restricted geographic distribution, while asexual populations (parthenogenetic) of both species have a wide geographic distribution, occupying urban areas across the Country (
Although Tityus serrulatus has been known as parthenogenetic since 1962 (
New records of males of Tityus kuryi, Tityus serrulatus and Tityus stigmurus.
| Species | Registration number | Municipality and state of Brazil | Latitude (DMS) | Longitude (DMS) |
|---|---|---|---|---|
| Tityus kuryi | MZUFBA 2569 | Palmeiras, Bahia | 12°31'44"S, 41°33'32"W | |
| MZUFBA 2570 | Palmeiras, Bahia | 12°31'44"S, 41°33'32"W | ||
| Tityus serrulatus | MZUFBA 2573 | São Desedério, Bahia | 12°35'21"S, 44°98'42"W | |
| Tityus stigmurus | MZUFBA 2339 | Exú, Pernambuco | 07°30'43"S, 39°43'26"W | |
| MZUFBA 166 | Santo Estevão, Bahia | 12°25'49"S, 39°15'05"W | ||
| MZUFBA 168 | Santo Estevão, Bahia | 12°25'49"S, 39°15'05"W | ||
| MZUFBA 270 | Ruy Barbosa, Bahia | 12°17'02"S, 40°29'38"W | ||
| MZUFBA 2104 | Iraquara, Bahia | 12°14'56"S, 41°37'08"W | ||
| MZUFBA 2297 | Morro do Chapéu, Bahia | 11°33'00"S, 41°09'21"W | ||
| MZUFBA 763 | Feira de Santana, Bahia | 12°16'00"S, 38°58'00"W | ||
| CIAVE 41 | Jacobina, Bahia | 11°10'50"S, 40°31'06"W | ||
| CIAVE 23 | Lauro de Freitas, Bahia | 12°53'38"S, 38°19'37"W | ||
| CIAVE 617 | Salvador, Bahia | 12°58'16"S, 38°30'39"W | ||
| CIAVE 923 | Salvador, Bahia | 12°58'16"S, 38°30'39"W | ||
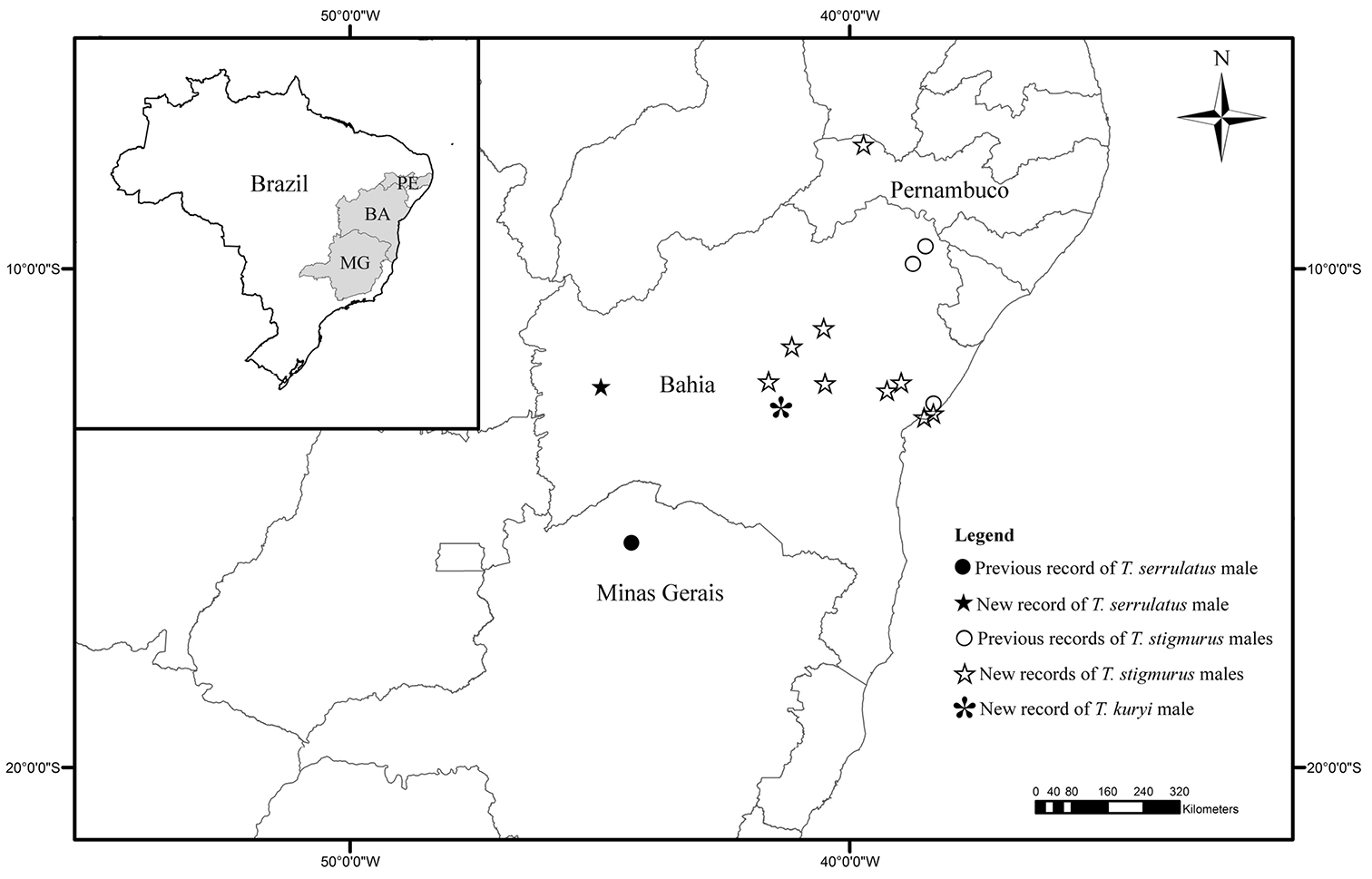
The male specimen of Tityus serrulatus used in this study is deposited in MZUFBA and came from the municipality of São Desiderio, Bahia. We can say now that sexual population of this species, previously restricted to the State of Minas Gerais (municipality Espinosa) and Southeastern Brazil (
Map showing the previous and new records of males of Tityus kuryi, Tityus serrulatus and Tityus stigmurus.
In spite of the new records of sexual populations of Tityus serrulatus and Tityus stigmurus to previously unknown areas, parthenogenesis seems to be the main reproductive strategy of these species. This assertion is primarily based on the low frequency of males of these species in three of the largest scorpion collections in Brazil (Instituto Butantan-SP, Museu Nacional-RJ and MZUFBA) and on our field experience collecting with a UV flashlight. Unlike other parthenogenetic scorpions, Tityus serrulatus and Tityus stigmurus are actually the two species better adapted to urban environments. They are regarded as a public health problem due to their rapid expansion in urban areas, their proliferation and the toxic effects of their poison (
We can emphasize here the large number of individuals analyzed as opposed to the few males found, providing evidence of the rarity of males, both in nature and in scientific collections.
The authors are grateful to Claudio Augusto Ribeiro de Souza for the help with confirmation of the dimorphism of the species. We are grateful to Silvanir Pereira Souza, Lucas Menezes Silva and Bruno Oliveira Cova for their help with the production of figures. We thank to Sonia Maria Christophe and Rafael Burguer for the permission to collect scorpions in their property. We acknowledge Leonardo Carvalho for his important suggestions and Nelson Silva for the help with English language. The authors thank the “Laboratório Central de Saúde Pública da Bahia Professor Gonçalo Moniz (LACEN-BA)”, “Laboratorio de Biomonitoramento e Ecologia de Bentos (LAMEB)”, “Laboratorio de Biomonitoramento e Ecologia de Abelhas (LABEA)” and “Laboratório de Geoecologia de Sedimentos Marinhos (GEOECO)” for providing infrastructure and equipment; and the “Fundação de Amparo e Pesquisa do Estado da Bahia (FAPESB)” for granting the financial support of two Scientific Initiation scholarships to the first author, which generated the results of this study.