Citation: Lackner T, Gomy Y (2014) Sarandibrinus, a new genus of Saprininae subfamily from Madagascar (Coleoptera, Histeridae) (Second contribution to the knowledge of the Histeridae of Madagascar). ZooKeys 427: 109–125. doi: 10.3897/zookeys.427.4799
Sarandibrinus araceliae, a new genus and species of the Saprininae subfamily is described from southern Madagascar. The new taxon exhibits autapomorphic characters for the Saprininae subfamily and is unusual especially for its large and deep prosternal foveae and the shape of spiculum gastrale. The description is accompanied by color habitus images, SEM micrographs, mouthparts and antenna line drawings and drawings of the male genitalia. Key to the genera of the Saprininae of Madagascar and the adjacent archipelagos is given. Hypocaccus (Baeckmanniolus) rubiciliae (Lewis, 1899) is newly reported from Madagascar and Hypocaccus (Nessus) perparvulus (Desbordes, 1916) is new to Mauritius.
Sarandibrinus araceliae, nouvelle espèce d'un genre nouveau de la sous-famille des Saprininae, est décrite du sud de Madagascar. Le nouveau taxon présente des caractères autapomorphiques pour les Saprininae spécialement par la présence de grandes fovéoles prosternales et par la forme de son spiculum gastrale. La description est accompagnée de photographies d'habitus en couleurs, de micro-photographies au microscope à balayage (SEM) et de dessins des antennes, des pièces buccales et des genitalia. Une clé des genres de Saprininae de Madagascar et des archipels voisins est donnée. Hypocaccus (Baeckmanniolus) rubiciliae (Lewis, 1899) est signalé comme nouveau pour Madagascar et Hypocaccus (Nessus) perparvulus (Desbordes, 1916), comme nouveau pour l'île Maurice.
Sarandibrinus, Coleoptera, Histeridae, Saprininae, taxonomy, Madagascar
With only 7 genera and 22 species (see Table 1) of the Saprininae subfamily reported from the fourth largest island of the Earth, Madagascar, including the adjacent archipelagos of Comoros, Mascarene Islands and Seychelles (
Distribution of the members of the Saprininae subfamily on Madagascar (Mad), Seychelles (Sey), Comoros (Com), and Mascarene (Masc) archipelagos. Taxa marked with * are endemic for the area in question and taxa marked with † are new for the islands/archipelagos in question.
| Mad | Sey | Com | Masc | ||
|---|---|---|---|---|---|
| HISTERIDAE Gyllenhal, 1808 | |||||
| Saprininae Blanchard, 1845 | |||||
| 1 | Euspilotus (Hesperosaprinus) modestus (Erichson, 1834) | - | - | - | + |
| 2 | Euspilotus (Neosaprinus) rubriculus (Marseul, 1855) | - | - | - | + |
| 3 | Gnathoncus sp. | + | - | - | - |
| 4 | Hypocacculus (s. str.) metallescens (Erichson, 1834) | - | - | - | + |
| 5 | Hypocaccus (Nessus) perparvulus*† (Desbordes, 1916) | + | - | - | + |
| 6 | Hypocaccus (Nessus) vulturnus (Reichardt, 1932) | - | - | - | + |
| 7 | Hypocaccus (Nessus) sp. | + | - | - | - |
| 8 | Hypocaccus (s. str.) brasiliensis (Paykull, 1811) | + | + | - | + |
| 9 | Hypocaccus (Baeckmanniolus) disjunctus (Marseul, 1855)* | + | + | + | - |
| 10 | Hypocaccus (s. str.) sp. | + | - | - | - |
| 11 | Hypocaccus (Baeckmanniolus) rubiciliae (Lewis, 1899)† | + | - | - | - |
| 12 | Malagasyprinus caeruleatus* (Lewis, 1905) | + | - | - | - |
| 13 | Malagasyprinus diana* (Lackner & Gomy, 2013) | + | - | - | - |
| 14 | Malagasyprinus perrieri* (Lackner & Gomy, 2013) | + | - | - | - |
| 15 | Saprinus (s. str.) basalis Fairmaire, 1898 | + | - | - | - |
| 16 | Saprinus (s. str.) chalcites (Illiger, 1807) | - | - | - | + |
| 17 | Saprinus (s. str.) cupreus Erichson, 1834 | + | - | - | - |
| 18 | Saprinus (Phaonius) erichsonii (Marseul, 1855) | + | + | - | + |
| 19 | Saprinus (s. str.) fulgidicollis* Marseul, 1855 | + | - | - | - |
| 20 | Saprinus (s. str.) sp. | + | - | - | - |
| 21 | Saprinus (s. str.) spendens (Paykull, 1811) | + | + | - | - |
| 22 | Sarandibrinus araceliae* sp. n. | + | - | - | - |
| Total | 17 | 4 | 1 | 8 |
All dry-mounted specimens were relaxed in warm water for several hours or overnight, depending on the body size. After removal from original cards, the beetles were side-mounted on triangular points and observed under a Nikon 102 stereoscopic microscope with diffused light. Some structures were studied using methods described by
CAS California Academy of Sciences, San Francisco, USA (D. Kavanaugh);
CYG Yves Gomy collection, Nevers, France;
TLAN Tomáš Lackner collection, temporarily housed at NCB Naturalis, Leiden, Netherlands.
Abbreviations used in measurements:
PEL Length between anterior angles of pronotum and apices of elytra;
APW Width between anterior angles of pronotum;
PPW Width between posterior angles of pronotum;
EL Length of elytron along sutural line;
EW Maximal width between outer margins of elytra.
Sarandibrinus araceliae sp. n.
Small, castaneous, shining Saprininae; basal half of elytra glabrous, apical half covered with rugulose-lacunose punctation. Head comparatively large, frontal and supra-orbital striae absent, sensory structures of antennal club in form a single ball-shaped vesicle situated on internal distal side of the antenna under the apical slit-like orifice, pronotum wholly punctate, pronotal hypomeron ciliate; prosternal foveae large, round and deep. Meso- and metaventrite as well as first visible abdominal ventrite wholly punctate; spiculum gastrale only slightly constricted laterally instead of possessing a clear ‘head’ and ‘stem’ (sensu
By the absent frontal and supra-orbital striae this taxon can be confused with two other sympatric genera: Gnathoncus Jacquelin du Val, 1858 (differing from it by the cuticular colour as well as presence of prosternal foveae) or Euspilotus Lewis, 1907, differing from it likewise by the metallic dorsum, ciliate pronotal epipleuron (both species of Euspilotus known from the region have glabrous pronotal hypomeron) or coarsely punctate frontal disk, with punctures forming elongate rugae (both Euspilotus species known from the region have their frontal disks covered with scattered punctures, never forming elongate rugae. The best differentiating character between the three taxa is probably the number of vesicles inside the antennal club: Gnathoncus possesses five, Euspilotus two or three, respectively, while Sarandibrinus has only one vesicle. The single vesicle character is present also in the sympatric genera Hypocaccus, Hypocacculus and Malagasyprinus, but the vesicle is pear-shaped vs. ball shaped in Sarandibrinus. Hypocaccus, Hypocacculus and Malagasyprinus furthermore, possess frontal and supraorbital striae, while Sarandibrinus lacks both. By the large oblique and deep prosternal foveae this taxon can be confused with the recently described genus Malagasyprinus Lackner & Gomy, 2013, differing from it by the cuticular colour (castaneous vs. dark blue/green) as well as absence of frontal and supra-orbital striae (Malagasyprinus possesses supra-orbital striae and its frontal stria is widely interrupted and prolonged onto clypeus). See also Key to the genera of the Saprininae from Madagascar (below).
The series of the new taxon was collected by sifting the litter in spiny forest (thicket) as well as by flight intercept (or yellow pan, Malaise) traps in desert scrub forest.
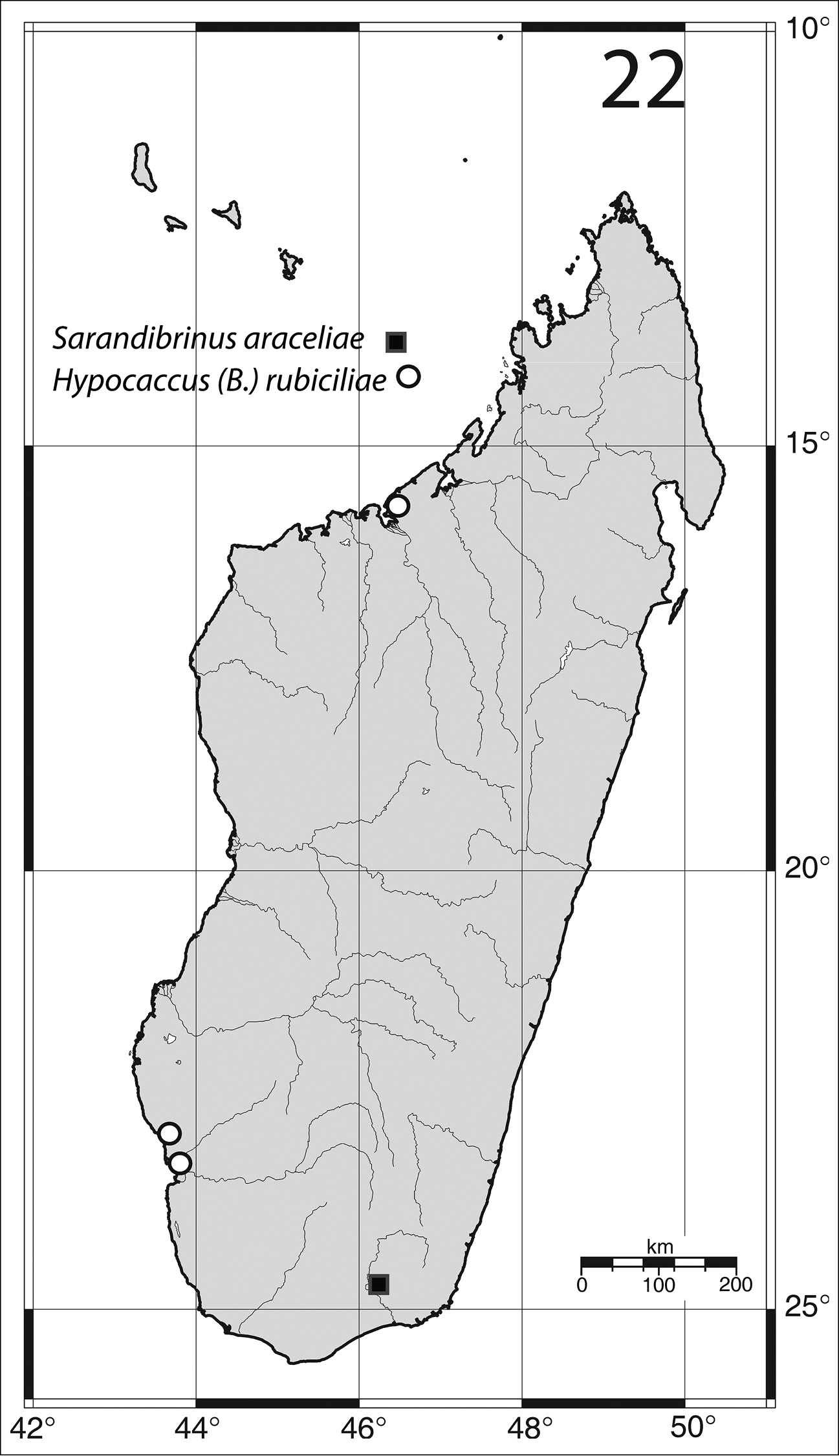
Madagascar, Toliara Province (Fig. 22).
The name of this new genus has been formed using the word “Sarandib” – one of the ancient names given by Arabs to the far-flung island that later became Madagascar and “rinus” – the two final syllabus of the name “Saprinus” to demonstrate its position as belonging to the Saprininae subfamily.
Madagascar, Toliara prov., Mahavelo Forest; Ifaty (Fig. 22).
Holotype, ♂, side-mounted on a rectangular mounting card, male genitalia extracted and glued to the same card as specimen, with the following labels: “♂” (hand-written); followed by: “MADAGASCAR: Toliara / Prov., Forêt de Mahavelo / Isantoria River, elev 115 m / 5.5. km 37°NE Ifotaka / 31.i.2002” (printed); followed by: “25°45'13"S, 46°9'5"E / coll: Fisher, Griswold et al. / Calif. Acad. of Sciences / sifted litter - spiny forest / thicket, code: BLF5278” (printed); followed by: “CASENT / 8065358” (printed); followed by: “Sarandibrinus / araceliae sp. n. / Det. T. Lackner & Y. / Gomy 2014 HOLOTYPE” (red, hand-written label) (CAS). Paratypes: 1 ♂ & 4 ♀♀, same data, but: “CASENT / 8065359”; “CASENT / 8065357”; “CASENT / 8065361”; “CASENT / 8065360” (two female paratypes, “8065360” & “8065361” are sputter-coated with gold); 1 ♀, with the following labels: “MADAGASCAR: Prov. / Toliara; Ifaty / 23°09'S, 43°37'E / 17–22 Sept. 1993” (printed); followed by: “Flight intercept / yellow pan trap in / Malaise trap in / desert scrub forest” (printed); followed by: “W. E. Steiner & R. Andriamasimanana / collectors” (printed); followed by: “Sarandibrinus / araceliae sp. n. Det. / T. Lackner & Y. Gomy / 2014 PARATYPE” (red, hand-written label); 1 ♀, with the following labels: MADAGASCAR: "Toliara Prov. / Parc National d'Andohahela / Forêt de Manantalinjo 33.6 km / 63° ENE Amboasary, 7.6 km / 99° E Hazofotsy, 12-16 I 2002" (printed); followed by: "24°49'1"S, 46°36'36"E / coll: Fisher, Griswold et al. / California Acad. of Sciences / sifted litter - in spiny forest / thicket, elev. 150m BFL4810" (printed); followed by: "CASENT / 8065685" (printed); followed by: "Sarandibrinus / araceliae sp. nov. / Det. T. Lackner & / Y. Gomy 2014 PARATYPE" (red label, written); (1 ♂ in coll. CYG; 1 ♀ in coll. TLAN; rest of the paratypes in coll. CAS).
Body length: PEL: 2.20–2.50 mm; APW: 1.00–1.25 mm; PPW: 1.75–2.00 mm; EW: 1.75–2.20 mm; EL: 1.35–1.60 mm. Body (Figs 1–2) roundly oval, convex, elytra widest at humeri; cuticle of elytra castaneous, shining, without metallic luster; pronotum darker; body ventrally dark brown to almost black; abdominal ventrites (except for first visible) rufescent; legs, mouthparts and antennae light brown to castaneous.
1 Sarandibrinus araceliae gen. & sp. n., habitus, dorsal view 2 ditto, ventral view.
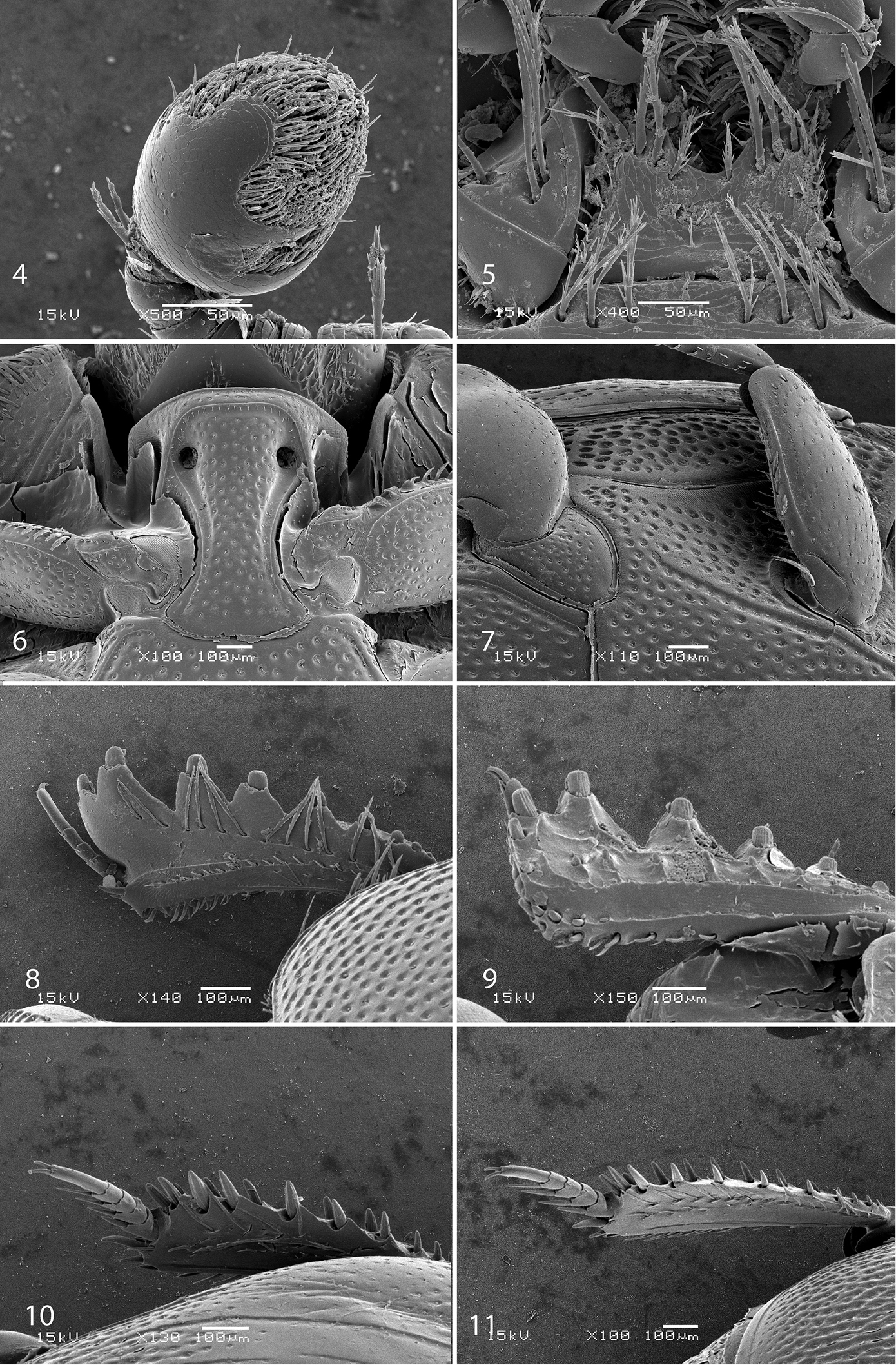
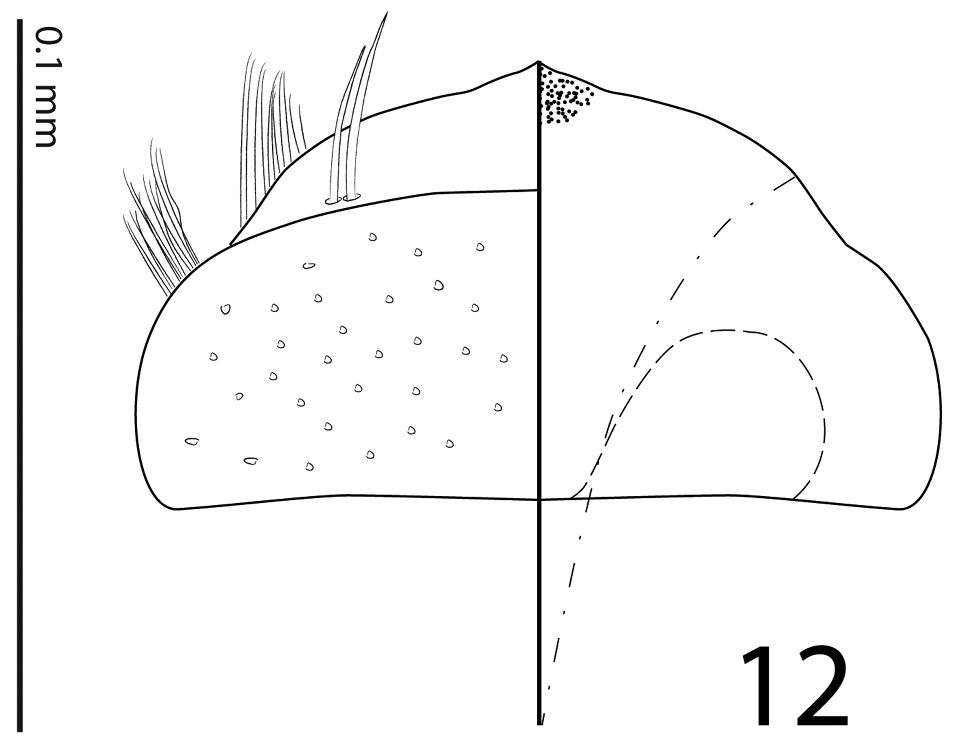
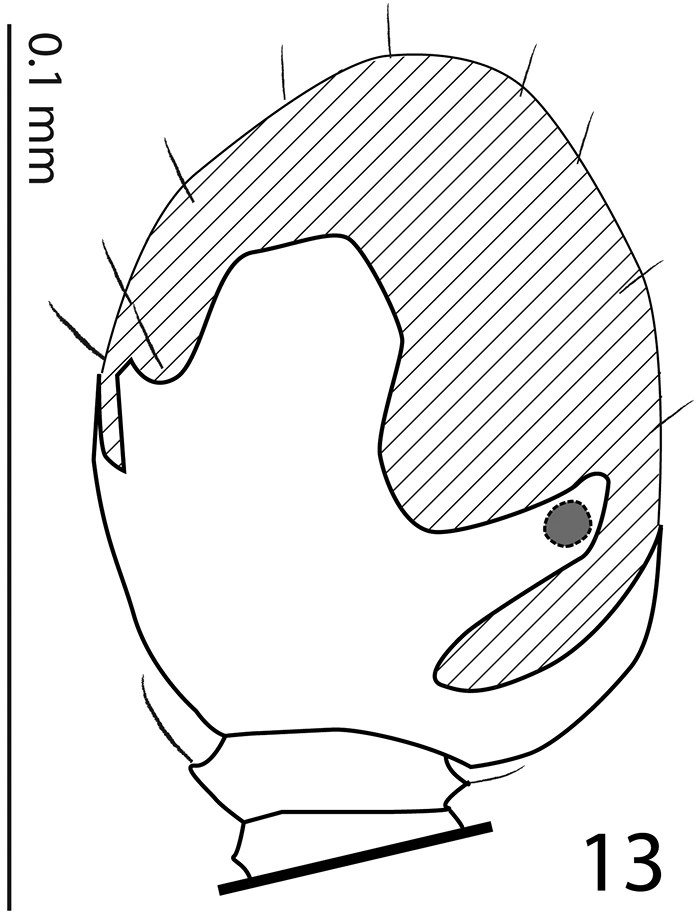
Antennal scape (Fig. 3) slightly thickened, lower margin carinate, with several rather long ramose setae and several scattered punctures; club (Fig. 4) oval, without visible articulation, dorsal surface on basal 2/3 glabrous, apical 1/3 with dense short sensilla intermingled with sparser longer erect sensilla; ventrally (Fig. 4) setose patch covers larger part of antennal club as dorsally, on each (distal and proximal) side with a single slit-like orifice; sensory structures of antennal club in form a single ball-shaped vesicle situated on internal distal side of the antenna under the slit-like orifice (Fig. 13).
Sarandibrinus araceliae gen. & sp. n., head, dorsal view.
4 Sarandibrinus araceliae gen. & sp. n., antennal club, ventro-lateral view 5 Sarandibrinus araceliae gen. & sp. n., mentum, ventral view 6 Sarandibrinus araceliae gen. & sp. n., prosternum 7 Sarandibrinus araceliae gen. & sp. n., lateral disk of metaventrite + metepisternum 8 Sarandibrinus araceliae gen. & sp. n., protibia, dorsal view 9 ditto, ventral view 10 Sarandibrinus araceliae gen. & sp. n., mesotibia, dorsal view 11 Sarandibrinus araceliae gen. & sp. n., metatibia, dorsal view.
Mouthparts: mandibles obscurely variolate, punctate, mandibular apex pointed; sub-apical tooth of left mandible not examined; labrum (Fig. 12) convex, densely imbricate, its convexity interrupted by slight median concavity; labral pits situated near anterior margin, each with two well-sclerotized long setae; terminal labial palpomere thickened, about 1.5 times as long as penultimate, its width about half its length, truncate apically; mentum (Fig. 5) trapezoidal, anterior margin deeply inwardly arcuate, on each side with four long ramose setae, lateral margins with single row of much shorter ramose setae, disk of mentum imbricate; cardo of maxilla with few short setae; stipes triangular, with three long ramose setae; terminal maxillary palpomere thickened, truncate apically, about twice times as long as penultimate; its width about half its length.
Sarandibrinus araceliae gen. & sp. n., labrum: left half depicting dorsal view and right half depicting epipharynx.
Sarandibrinus araceliae gen. & sp. n., antennal club, ventral view showing sensory structures of the antenna.
Clypeus (Fig. 3) rather short, flat, sub-quadrate, punctate, punctures linked with carinate strioles; frontal and supraorbital striae absent, in several specimens inter-linked carinate strioles appear as fragments of frontal stria; sculpture of frontal disc (Fig. 3) identical to that of clypeus; eyes flattened, almost invisible from above; occipital stria absent.
Pronotal sides (Fig. 1) moderately narrowing anteriorly; apical angles obtuse; anterior margin of pronotum broadly arcuate; pronotal depressions absent; marginal pronotal stria thin, slightly carinate and complete, somewhat weakened behind head; pronotal disc entirely covered by unusually deep and dense punctures, interspaces between them shorter than half their own diameter; pronotal hypomeron with fine dense yellow setae; scutellum well visible.
Elytral epipleura in several punctures; marginal epipleural stria fine, complete; marginal elytral stria straight, well impressed and slightly carinate, continued as weakened but complete apical elytral stria that is connected to complete sutural elytral stria. Humeral elytral stria short, deeply impressed on basal fourth, but difficult to discern due to very coarse and dense punctures and irregular strioles surrounding it; inner subhumeral stria present as a rather long medio-apical fragment; elytra with thin striae 1–4; striae in weak punctures, reaching approximately elytral half apically; fourth dorsal elytral stria basally connected with sutural elytral stria under broad arch; sutural elytral stria well-impressed and complete, in fine punctures, on apical half almost not discernible due to the extremely dense confluent punctation, apically connected with apical elytral stria, between it and elytral suture a row of fine punctures present; these fine punctures present also along entire elytral base and slightly entering elytral intervals basally; elytral humeri and flanks densely punctate, elytral disc on basal half (roughly) with large oval ‘mirror’ occupying approximately entire elytral intervals 1–4; apical half of elytra (roughly) covered with extremely coarse confluent punctures and longitudinal rugae, basally slightly entering also elytral intervals; extreme elytral apex impunctate.
Propygidium and pygidium densely and coarsely punctate, punctures separated by about their own diameter.
Anterior margin of median portion of prosternum (Fig. 6) almost straight; marginal prosternal stria present, almost complete; prosternal process between carinal prosternal striae slightly concave, with dense large setigerous punctation; carinal prosternal striae well-impressed, broadly divergent anteriorly, terminating in deep and large prosternal foveae; lateral prosternal striae carinate, sub-parallel, connected in front of apices of carinal prosternal striae, surface mesad of them with a regular row of fine microscopic setae.
Anterior margin of mesoventrite broadly inwardly arcuate; discal marginal mesoventral stria present only laterally, antero-medially obliterated; disc of mesoventrite with dense deep large punctures separated by less than their own diameter; meso-metaventral sutural stria absent; meso-metaventral suture well discernible; intercoxal disc of metaventrite in male with slight longitudinal median concavity almost indiscernible in female, completely covered by punctures identical to those of mesoventrite; lateral metaventral stria (Fig. 7) well impressed, carinate, almost straight, stopping short of metacoxa; lateral disc of metaventrite slightly concave, with punctation similar to that of metaventrite; metepisternum + fused metepimeron (Fig. 7) with even denser and deeper punctation; metepisternal stria absent.
Intercoxal disc of the first abdominal ventrite almost completely striate laterally; disc with punctation similar to that of lateral disc of metaventrite.
Protibia (Figs 8–9) slightly dilated, outer margin apically with single low tooth topped by denticle, followed by three large triangular teeth topped by denticle and another two low teeth topped by denticle; all five teeth diminishing in size gradually in proximal direction, followed by a three tiny denticles growing out directly from outer margin of protibia; setae of outer row regular, ramose, rather long; protarsal groove rather shallow; anterior protibial stria incomplete, bearing short and regular setae of intermedian row; another complimentary short stria originating approximately near tarsal insertion present; tarsal denticles absent; protibial spur (Fig. 8) short, straight, growing out from near the tarsal insertion; apical margin of protibia posteriorly with two widely separated tiny denticles; outer part of posterior surface with a sparse row of minute denticles situated on low protuberances, separated from imbricate median part of posterior surface by a definite ridge; posterior protibial stria complete, terminating in three tiny inner denticles; inner row of setae double, setae dense and short.
Mesotibia (Fig. 10) slender, outer margin with a dense row of approximately 7 prominent denticles situated inside low teeth; setae of outer row sparse, about as long as denticles themselves; setae of intermedian row shorter and finer than those of outer row, regular; posterior mesotibial stria almost complete; anterior surface of mesotibia variolate-punctate, with another row of approximately 7 denticles shorter than those of outer row; anterior mesotibial stria complete, terminating in single tiny inner anterior denticle; mesotibial spur rather long; apical margin of mesotibia with three short denticles; mesotarsomeres telescopically becoming narrower apically, each bearing two thick setae ventrally; claws of apical tarsomere slightly bent, approximately one-third its length; metatibia (Fig. 11) slenderer and longer than mesotibia, in most respects similar to it, but denticles of outer margin not growing out from inside low teeth and setae of outer row shorter than those of mesotibia.
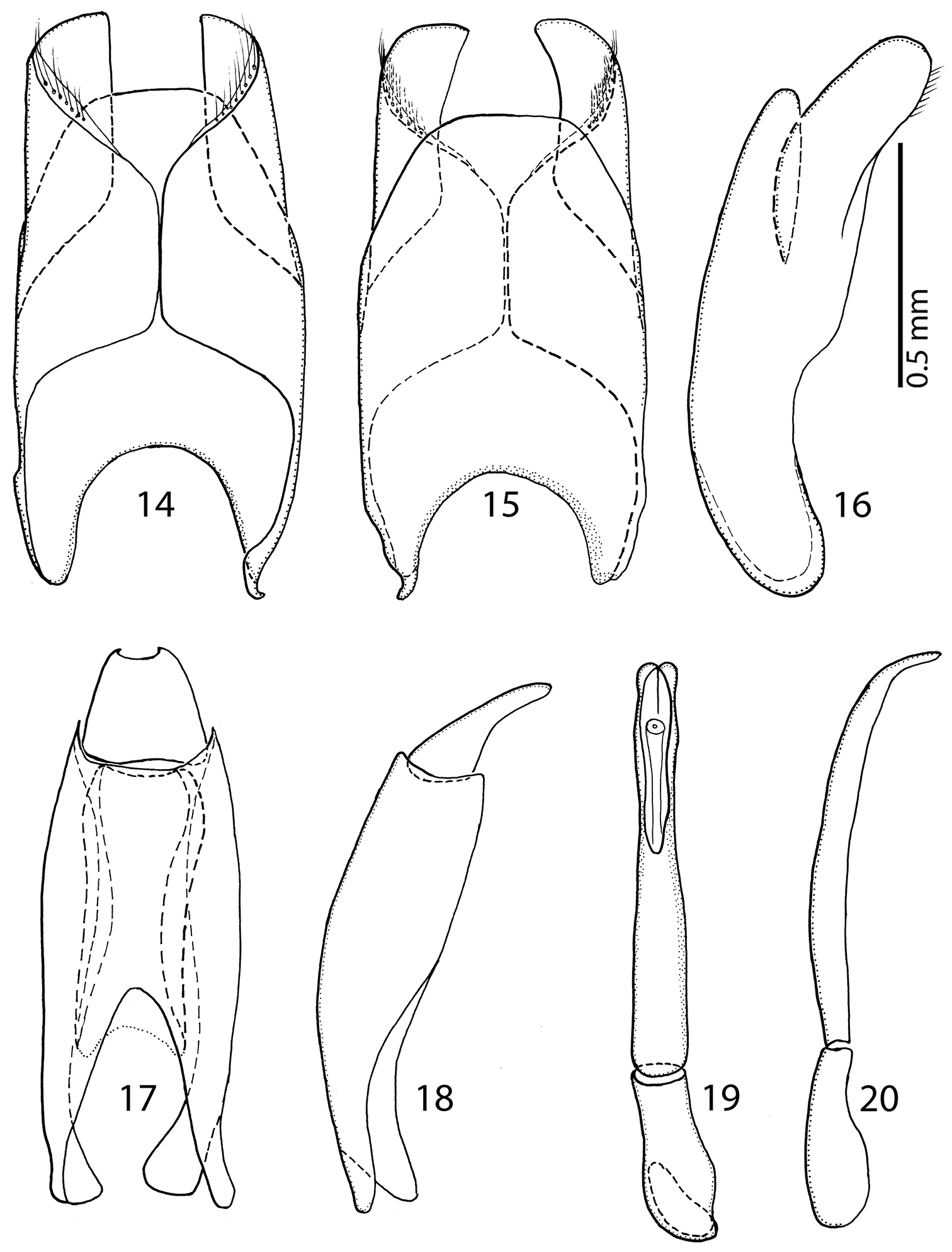
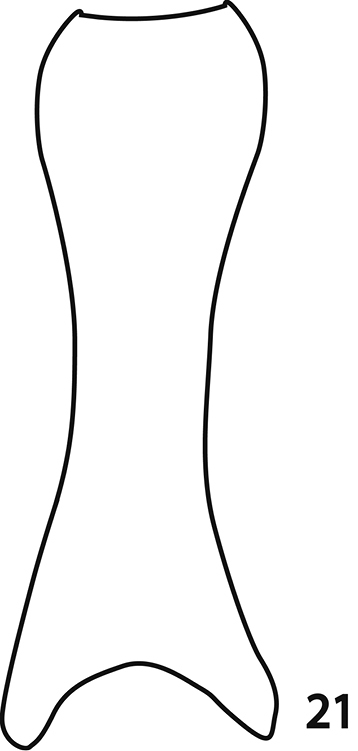
Male genitalia. Eighth sternite (Figs 14–15) apically with tiny vela adorned with a row of long, sparse setae; halves separated medially. Eighth tergite (Fig. 15) apically outwardly arcuate; eighth sternite and tergite fused laterally (Fig. 16). Ninth tergite (Figs 17–18) when compared to tenth tergite conspicuously long, acutely inwardly arcuate basally; tenth tergite (Fig. 17) apically slightly inwardly arcuate. Spiculum gastrale of both known male specimens (on basal part) damaged during the manipulation with genitalia, so we are only able to depict its ‘reconstruction’ here (Fig. 21): median part only slightly constricted, typical ‘head’ and ‘stem’ (sensu
14 Sarandibrinus araceliae gen. & sp. n., male terminalia: 8th sternite and tergite, ventral view 15 ditto, dorsal view 16 ditto, lateral view 17 Sarandibrinus araceliae gen. & sp. n., male terminalia: 9th + 10th tergites, dorsal view; spiculum gastrale, ventral view 18 Sarandibrinus araceliae gen. & sp. n., male terminalia: 9th + 10th tergites, lateral view 19 Sarandibrinus araceliae gen. & sp. n., male terminalia: aedeagus, dorsal view 20 ditto, lateral view.
Sarandibrinus araceliae gen. & sp. n., male terminalia: reconstruction of the spiculum gastrale, ventral view.
This species is dedicated to Mrs. Araceli Cancino, wife of the famous Belgian comics illustrator Midam, in appreciation of the publication of the three volumes of “Carnets de Grrreeny”.
Hypocaccus (Baeckmanniolus) rubiciliae (Lewis, 1899)
Described from Tanzania, reported also from the Republic of South Africa (Natal) (
Distribution of Sarandibrinus araceliae gen. & sp. n. and Hypocaccus (Baeckmanniolus) rubiciliae Lewis, (1899) on the Island of Madagascar.
Madagascar south-west: 21 exs., Toliara, “Plage de La Batterie”, 19.vii.1969, under algae (Y. Gomy leg.). 5 exs., Toliara env., “Fitsitika”, 20.vii.1969, beach, under algae (Y. Gomy leg.). Madagascar North: 22 exs., Majunga, “Village touristique”, 29.VII.1968, beach, under coastal wrack (Y. Gomy leg.).
Hypocaccus (Nessus) perparvulus (Desbordes, 1916)
Described from Madagascar, new to Mauritius.
Mauritius, 1 ex., Pointe aux Sables, 15.i.1971, in a coop (Y. Gomy leg.).
(For the sake of wider usage this key contains also taxa known to occur on the nearby archipelagos of Mascarene Islands, Comoros and Seychelles.)
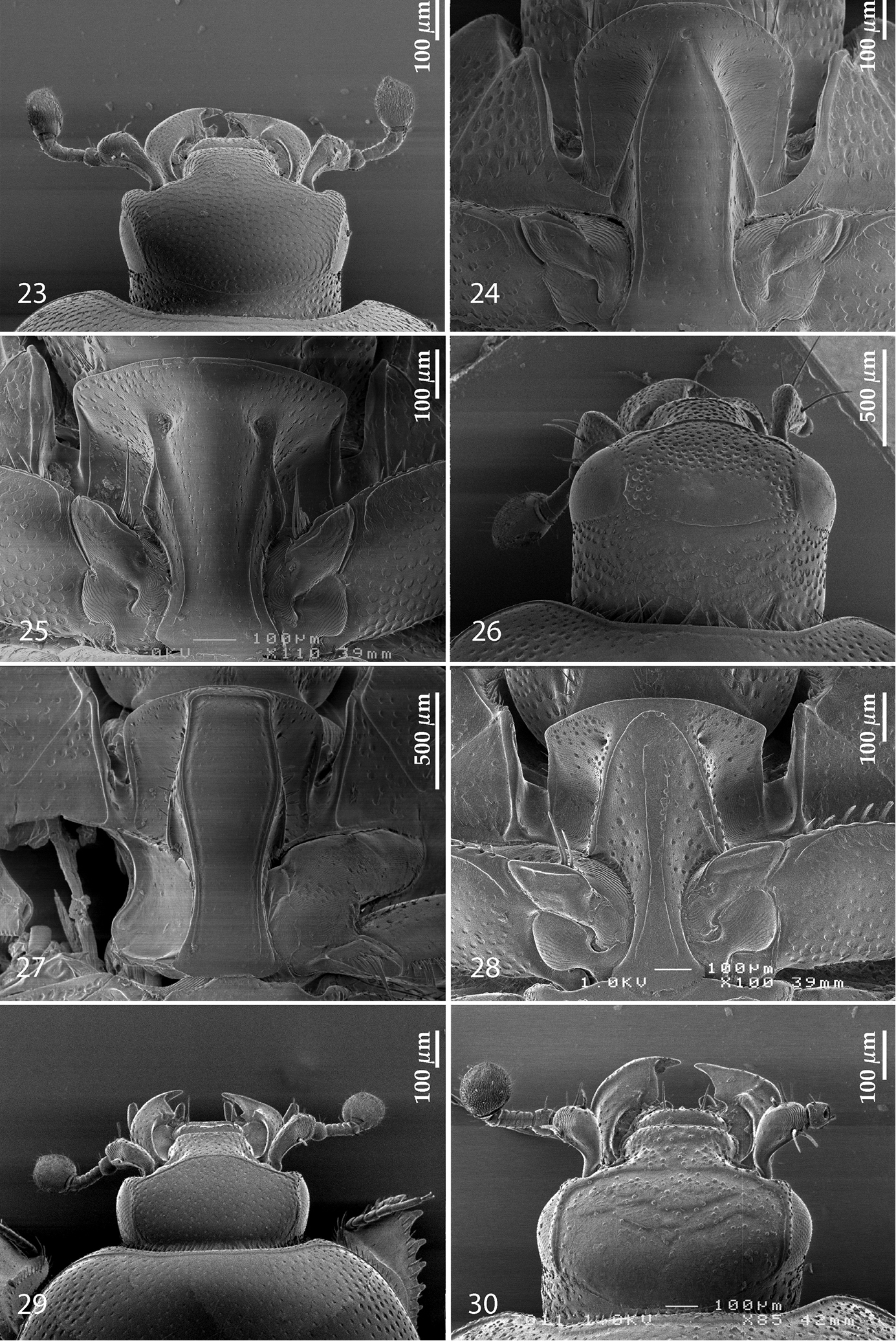
| 1 (6) | Head without any trace of frontal or supra-orbital striae (Fig. 3) | |
| 2 (3) | Frons very densely and coarsely punctate (Fig. 3); punctures forming elongate rugae | Sarandibrinus gen. n. |
| 3 (2) | Frons with fine, scattered punctation; punctures never forming elongate rugae (Fig. 23) | |
| 4 (5) | Carinal prosternal striae joined anteriorly (Fig. 24); elytra with characteristic short hooked appendix between fourth dorsal and sutural elytral striae, marginal elytral stria double | Gnathoncus Jacquelin Du Val, 1858 |
| 5 (4) | Carinal prosternal striae divergent anteriorly (Fig. 25), in some cases joined by a deep sulcus; elytral disk between fourth dorsal elytral and sutural elytral striae without a hooked appendix; marginal elytral stria single | Euspilotus Lewis, 1907 |
| 6 (1) | Frontal and/or supra-orbital striae present (Fig. 26) | |
| 7 (8) | Prosternal foveae absent (Fig. 27) | Saprinus Erichson, 1834 |
| 8 (7) | Prosternal foveae present (Fig. 28) | |
| 9 (10) | Frontal stria widely interrupted medially, prolonged onto clypeus (for fig. see |
Malagasyprinus Lackner & Gomy, 2013 |
| 10 (9) | Frontal stria complete (Fig. 29), prosternal foveae small to moderately large (Fig. 28), never deep; punctation of dorsum never forming elongate rugae | |
| 11 (12) | Frons (Fig. 29) with distinct punctation, without obvious chevrons or transverse rugae. Body size from 1.50–2.00 mm | Hypocacculus Bickhardt, 1914 |
| 12 (11) | Frons (Fig. 30) smooth with chevrons or with more or less regular transverse rugae. Body size usually larger than 2.00 mm | Hypocaccus Thomson, 1867 |
23 Euspilotus (Neosaprinus) rubriculus (Marseul, 1855), head, dorsal view 24 Gnathoncus rotundatus (Kugelann, 1792), prosternum 25 Euspilotus (Hesperosaprinus) modestus (Erichson, 1834), prosternum 26 Saprinus (Saprinus) fulgidicollis Marseul, 1855, head, dorsal view 27 ditto, prosternum 28 Hypocaccus (Hypocaccus) brasiliensis (Paykull, 1811), prosternum 29 Hypocacculus (Hypocacculus) metallescens (Erichson, 1834), head, dorsal view 30 Hypocaccus (Hypocaccus) brasiliensis (Paykull, 1811), head, dorsal view.
The newly erected genus exhibits a mixture of plesiomorphic and apomorphic characters. Among the plesiomorphic ones (sensu Lackner, unpublished) is undoubtedly the absence of both supra-orbital and frontal striae; these striae are without exception absent with the taxa near the root of the recently performed phylogenetic analysis aimed at resolving the relationships of the higher taxa of the Saprininae subfamily (Lackner, unpublished).
The presence of large, deep oblique prosternal foveae is undoubtedly an apomorphic character (as it is present mainly among more ‘derived’ Saprininae; see also Discussion in
The curiously shaped spiculum gastrale (Fig. 21), resembling that of a monotypic Namibian endemic Paraphilothis mirabilis Vienna, 1994 (Lackner, unpublished) presents an interesting question here, seen from the phylogenetic standpoint. It is likely that such a structure evolved twice independently within the Saprininae subfamily, as the two taxa Sarandibrinus and Paraphilothis are not related according to our analyses. Paraphilothis mirabilis was recovered closer to the root of the cladogram, distant from the above-mentioned clade of taxa possessing a single pear-shaped vesicle in their antennal club. Paraphilothis has been observed to possess four ball-shaped vesicles inside its antennal club (Lackner, unpublished). Emerging ideas of the higher phylogeny of the Saprininae subfamily suggest that there remains still a significant work to be performed.
Last, but not least, we should probably mention the unusual collecting conditions of this new taxon. Although Saprininae are very plastic in their ecologies (Lackner, forthcoming), there has not been so far any record of collecting a member of the Saprininae by the method of litter sifting or yellow pan trapping. It is obvious that the island of Madagascar, known for its high endemism among the living organisms has still a lot to offer from its store.
The wife of senior author Pepina Artimová is thanked for the help with Adobe Illustrator CS5. Thanks are due to Petr Baňař (Brno, Czech Republic) for the map of Madagascar used in this paper. We thank F. Slamka (Bratislava, Slovakia) for his help with the habitus photograph of Sarandibrinus araceliae. The curators of the institutes mentioned above are duly acknowledged for their help with the specimens. We are indebted to two anonymous reviewers as well as to editor of the Histeroidea section for ZooKeys whose critical reading and remarks provided much better output. This research was supported by the Internal Grant Agency (IGA n.20124364) Faculty of Forestry and Wood Sciences, Czech University of Life Sciences Prague, Czech Republic.