Citation: Nieto Nafría JM, Blackman RL, Martin JH (2014) Two more new species of Aphidura (Hemiptera, Aphididae), and a note on variation in Aphidura bozhkoae Narzikulov. ZooKeys 425: 113–130. doi: 10.3897/zookeys.425.7797
Two new species of Aphidura Hille Ris Lambers, 1956 (Hemiptera, Aphididae, Macrosiphini) are described; Aphidura libanensis sp. n. from Prunus prostrata in Lebanon, and Aphidura corsicensis sp. n. from Cerastium soleirolii in Corsica (France). Studies of Aphidura bozhkoae specimens from different localities have revealed that this species varies in its pattern of dorsal sclerotisation and other morphological characters, within and between populations. An updated key for identifying the world’s species of Aphidura is presented.
Aphidura, new taxa, descriptions, intraspecific variation, key of species
Aphidura Hille Ris Lambers, 1956 (Hemiptera, Aphididae, Macrosiphini) currently includes 22–25 subjective valid species, exhibiting a Mediterranean-Pontian-Turanian distribution with extensions to neighbouring areas and exceptionally (one species) to the Russian Far East. Many of the species are associated with Caryophyllaceae, and some feed on Prunus and related genera, indicating that there may be host alternation between Amygdaloideae (Rosaceae) and Caryophyllaceae, but their biology and life cycles are mostly unknown (
Aphidura bozhkoae Narzikulov, 1958 was described from Prunus (= Cerasus) verrucosa in Tajikistan, and has subsequently been found on various species of Prunus in the neighbouring countries Kyrgyzstan and Uzbekistan, and also in Kazakhstan, Iran and Georgia (
Specimens of the following five samples have been studied:
(1) 9 apterous viviparous females and 1 alate viviparous female: TAJIKISTAN: Cerasus verrucosa, Iskandirkul, 14-VII-1959 (3 apterae, 1 alate); same host-plant, Ziddy [Siddi in slide label by Hille Ris Lambers], 28-VI-1954 (6 apterae), N. Narzikulov leg.; Aphidura bozhkoae, Narzikulov det.; BMNH collection. Both Tajik localities are type-localities of this species (
(2) 54 apterous viviparous females and 13 alate viviparous females: TAJIKISTAN: Cerasus verrucosa, Varzov (1 km above Kondara), 24-V-1981; Cerasus erythrocarpa, Ziddy, 9-VI-1981, G. Kh. Shaposhnikov leg. (samples 7524 and 7551); Aphidura bozhkoae, G. Kh. Shaposhnikov det.; Zoologicheskiy Institut collection. Ziddy is a type-locality of this species and Varzov is near Kondara, which is also a type-locality. These specimens were dry (alcohol had completely evaporated); they were hydrated and later mounted on microscopic slides.
(3) 115 apterous viviparous females and 63 alatae viviparous females: IRAN: several host plants, localities and dates (data in
(4) 5 apterous females: LEBANON, Prunus prostrata, Jabal el Barouk, 22-V-1973, D. Hille Ris Lambers leg. (sample 754); Aphidura “nitens” manuscript name, D. Hille Ris Lambers det.; BMNH collection.
(5) 3 apterous viviparous females: CORSICA (France), Cerastium soleirolii, North slopes of Mount Cinto, 08-VII-1980, J. H. Martin leg. (sample number 3061); Aphidura “cerastii”, manuscript name, D. Hille-Ris-Lambers det.; BMNH collection.
Morphological measurements were made according to
A Leica DC digital 96 camera with IM 1000 version 1.10 software was used for the photomicrographs, which have been taken and mounted by L. M. Fernández Blanco.
Siphunculi tapering to apex or slightly swollen in a distal portion. Dorsum of metathorax to abdominal segment 6 with a discal plate (apterae). Mesosternal mammariform processes pale, flat and with spinules (apterae). Abdominal marginal tubercles usually present. Tarsal formula 3.3.3. (sometimes one tarsus with 4 setae)
from 5 specimens (Figs 1A–E). Unknown colour in life, possibly shiny black with antennae, legs and siphunculi pale brown. In mounted specimens, antennae, mesosternal mammariform processes, legs, siphunculi, abdominal segment 8 sclerotized band, cauda and genital and anal plates are yellowish brown, with very proximal and distal portions of siphunculi, antennal segment VI and very apical portion of antennal segment V somewhat darker than aforementioned structures, all in contrast with head and dorsum of thorax and most part of abdomen, which are very dark brown. Frons undulated. Head with rugosity lines and spinules (both more abundant on dorsum). Antennal cuticle imbricated. Setae on body dorsum, antennae, and most of those on legs thick, with apices blunt or slightly capitate. Mesosternal mammariform processes pale, flat and with spinules. A discal plate present (from metathorax to abdominal segment 6); prothorax, mesothorax and abdominal segment 7 with wide transverse bands, which are darker than the discal plate, abdominal segment 8 with a band paler than the others. Marginal tubercles usually present on both sides of prothorax and several abdominal segments; they are small, but the abdominal ones are relatively tall. Siphunculi tapering to apex or slightly swollen in the distal quarter, spinulose imbrication, distinct preapical incision and flange. Cauda broadly triangular. Metric and meristic features in Table 1.
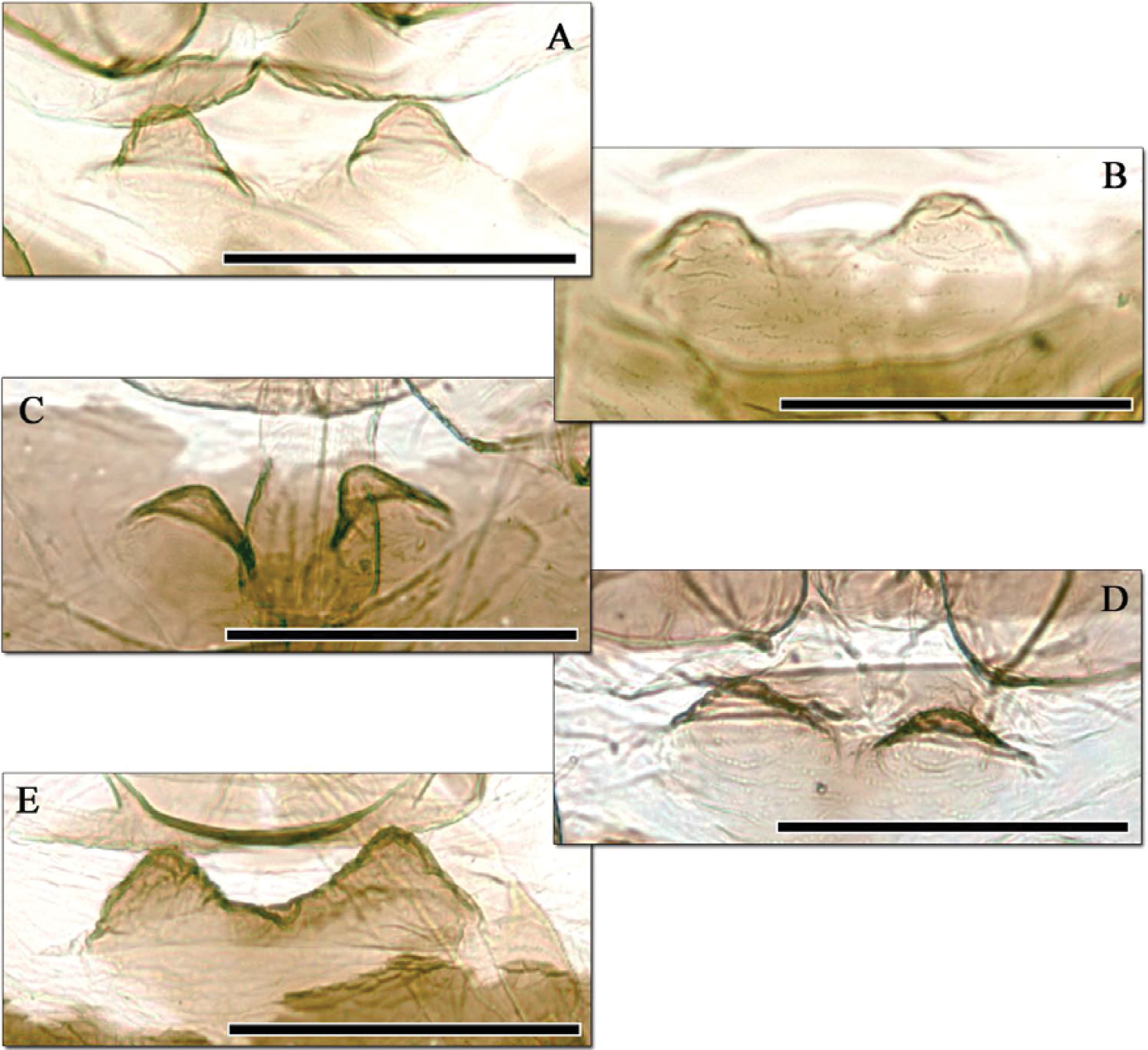
Aphidura libanensis sp. n., apterous viviparous females. A Habitus B Part of prothorax with a marginal tubercle (signalled with an arrow) C Marginal zone of abdominal segments 3 and 4, with marginal tubercles (signalled with arrows) D Mesosternum with mammariform processes (signalled with arrows) E Mesosternum with mammariform processes (signalled with arrows) of another specimen.
Metric and meristic features of apterous viviparous females of Aphidura libanensis sp. n. and Aphidura corsicensis sp. n.
| Aphidura libanensis | Aphidura corsicensis | |
|---|---|---|
| n=5 | n=3 | |
| Body [mm] | 1.805–1.938 | 2.025–2.088 |
| Antenna [mm] | 1.605–1.698 | 1.590–1.655 |
| Antenna / Body [times] | 0.87–0.93 | 0.79–081 |
| Ant. segm. III [mm] | 0.43–0.51 | 0.48–0.52 |
| Ant. segm. IV [mm] | 0.27–0.31 | 0.27–0.29 |
| Ant. segm. V [mm] | 0.20–0.24 | 0.22–0.24 |
| Ant. segm. VI base [mm] | 0.09–0.13 | 0.11–0.12 |
| Ant. segm. VI processus terminalis | 0.39–0.46 | 0.31–0.34 |
| Ant. segm. VI processus terminalis / Ant. segm. III [times] | 0.78–1.03 | 0.60–0.67 |
| Ant. segm. VI processus terminalis / base [times] | 3.56–4.89 | 2.63–3.00 |
| Ultimate rostral segm. [mm] | 0.12–0.13 | 0.15–0.16 |
| Ultimate rostral segm. / its basal width [times] | 2.00–2.27 | 2.58–2.73 |
| Ultimate rostral segm. / Ant. segm. VI base [times] | 0.96–1.39 | 1.25–1.36 |
| Hind femur [mm] | 0.52–0.56 | 0.61–0.63 |
| Hind tibia [mm] | 0.96–1.03 | 1.06–1.09 |
| Hind tibia / Body [times] | 0.52–0.56 | 0.52–0.53 |
| Hind tarsus, 2nd segm. [mm] | 0.11–0.12 | 0.10–0.11 |
| Hind tarsus, 2nd segm. / Ultimate rostral segm. [times] | 0.84–1.00 | 0.65–0.75 |
| Abdominal (segm. 2–6) marginal tubercles [number] | 0–3 | 0 |
| Siphunculus [mm] | 0.30–0.35 | 0.42–0.45 |
| Siphunculus / Body [times] | 0.17–0.18 | 0.20–0.22 |
| Siphunculus / Ant. segm. III [times] | 0.65–0.74 | 0.82–0.89 |
| Siphunculus / its basal width [times] | 3.50–4.27 | 4.83–5.60 |
| Siphuncular width, maximal / basal [times] | 0.56–0.70 | 0.61–0.82 |
| Siphuncular width, maximal / minimal [times] | 1.05–1.11 | 1.04–1.10 |
| Siphuncular minimal width / Hind tibiae, diameter at middle [times] | 1.13–1.80 | 1.00–1.18 |
| Cauda [mm] | 0.13–0.16 | 0.23–0.24 |
| Cauda / siphunculus [times] | 0.40–0.49 | 0.51–0.57 |
| Cauda / its basal width [times] | 0.87–0.97 | 1.41–1.50 |
| Setae on... | ||
| ... Frons [μm] | 25–35 | 32–37 |
| ... Frons / b. d. Ant. segm. III [times] | 1.1–1.6 | 1.3–1.5 |
| ... Vertex [μm] | 15–30 | 32–35 |
| ... Vertex / b. d. Ant. segm. III [times] | 0.7–1.4 | 1.2–1.4 |
| ... Antennal segment III [μm] | 12–15 | 10–14 |
| ... Antennal segment III / b. d. Ant. segm. III [times] | 0.6–0.7 | 0.4–0.6 |
| ... Ultimate rostral segm, [number] | 6–9 | 17–19 |
| ... Hind femur, dorsal [μm] | 12–18 | 17–25 |
| ... Hind femur, ventral [μm] | 22–28 | 25–38 |
| ... Hind tibia, dorsal, at middle [μm] | 22–33 | 27–33 |
| ... Hind tibia, dorsal / Tibial diameter (at middle) [times] | 0.5–0.8 | 0.5–0.7 |
| ... Hind tarsus, first segm. [number] | 3 (4) | 3 |
| ... Abdominal segm. 2–4 [μm] | 10–18 | 25–30 |
| ... Abdominal segm. 2–4 / b. d. Ant. segm. III [times] | 0.5–0.8 | 1.0–1.1 |
| ... Abdominal segm. 8 [μm] | 27–33 | 32–38 |
| ... Abdominal segm. 8 / b. d. Ant. segm. III [times] | 1.2–1.5 | 1.3–1.4 |
| ... Abdominal segm. 8 [number] | 4 | 3–4 |
| ... Genital plate, discal [number] | 2 | 5–7 |
| ... Genital plate, marginal [number] | 10–15 | 13–20 |
| ... Cauda [number] | 6–8 | 6–9 |
Note: Used abbreviations: Ant., Antennal; b. d., basal diameter; n. number of measured specimens; segm., segment or segments.
Holotype: apterous viviparous female, collected on Prunus prostrata Labill. (Rosaceae), Jabal el Barouk (Lebanon), 2-V-1973, Hille Ris Lambers leg.; paratypes: four apterous females collected at the same time as the holotype.
The specific name of the new species is an adjective that means inhabitant of Lebanon, in feminine.
D. Hille Ris Lambers thought that these Lebanese specimens were conspecific with others found on Prunus in Iran, which were being studied by him and G. Remaudière, and that they all belonged to an undescribed species, which was named in draft “nitens” (Remaudière's epistolary archive). Certainly these Lebanese aphids do not belong to any previously described species, but they are also not conspecific with the Iranian ones, which we believe to be Aphidura bozhkoae (see next section).
Aphidura libanensis sp. n. resembles Iranian specimens of Aphidura bozhkoae and the East Asian species Aphidura mordvilkoi in the shape of the siphunculi and the extensive and solid discal plate, but there is an important difference in the number of first tarsal setae: four setae on each first tarsal segment in Aphidura bozhkoae and Aphidura mordvilkoi, and three in Aphidura libanensis. Aphidura libanensis and Aphidura mordvilkoi can also be distinguished from one another by the number of accessory setae on the ultimate rostral segment (6–9 in Aphidura libanensis, 2–4 in Aphidura mordvilkoi) and the relative length of the processus terminalis (3.6–4.9 times base of antennal segment VI in Aphidura libanensis, 2.2–2.7 times in Aphidura mordvilkoi). Aphidura libanensis and Aphidura bozhkoae are very similar in absolute and relative lengths of most body parts, the setae of Aphidura libanensis (Table 1) are all generally longer than those of Aphidura bozhkoae (Table 2).
Metric and meristic features of apterous viviparous females of Aphidura bozhkoae from Tajikistan, collected by Narzikulov (column Tajik. [Na.]) and by Shaposhnikov (column Tajik. [Sh.], and from Iran, collected by Remaudière. In parenthesis exceptional data.
| Tajik. [Na.] | Tajik. [Sh.] | Iran [Re.] | |
|---|---|---|---|
| n = 7 | n = 13 | n = 31 | |
| Body [mm] | 1.950–2.125 | 1.925–2.250 | 1.413–2.125 |
| Antenna [mm] | 1.408–1.748 | 1.475–1.785 | 1.290–1.765 |
| Antenna / Body [times] | 0.70–0.83 | 0.70–0.88 | 0.73–0.92(1.07) |
| Ant. segm. III [mm] | 0.36–0.49 | 0.40–0.48 | 0.35–0.52 |
| Ant. segm. IV [mm] | 0.22–0.28 | 0.22–0.28 | 0.19–0.34 |
| Ant. segm. V [mm] | 0.19–0.22 | 0.18–0.24 | 0.18–0.25 |
| Ant. segm. VI base [mm] | 0.10–0.12 | 0.10–0.12 | 0.09–0.12 |
| Ant. segm. VI processus terminalis | 0.39–0.52 | 0.40–0.55 | 0.33–0.48 |
| Ant. segm. VI processus terminalis / Ant. segm. III [times] | 0.98–1.10 | 0.98–1.18 | (0.72)0.85–1.15 |
| Ant. segm. VI processus terminalis / base [times] | 3.58–4.76 | 3.86–5.00 | 2.89–4.55 |
| Ultimate rostral segm. [mm] | 0.12–0.13 | 0.13–0.15 | 0.11–0.14 |
| Ultimate rostral segm. / its basal width [times] | 1.56–2.36 | 1.92–2.64 | 1.92–2.89 |
| Hind tibia / Body [times] | 0.44–0.52 | 0.42–0.55 | 0.47–0.62 |
| Hind tarsus, 2nd segm. [mm] | 0.12–0.14 | 0.12–0.14 | 0.10–0.13 |
| Hind tarsus, 2nd segm. / Ultimate rostral segm. [times] | 0.92–1.04 | 0.89–1.00 | 0.77–1.00 |
| Abdominal marginal (segm. 2–6) tubercles [number] | 0 | 0 | 0–6 |
| Abdominal (segm. 8) spinal tubercles [number] | 0 | 0 | 0(2–3) |
| Siphunculus [mm] | 0.35–0.48 | 0.36–0.45 | 0.28–0.44 |
| Siphunculus / Body [times] | 0.17–0.22 | 0.18–0.22 | 0.17–0.25 |
| Siphunculus / Antennal segm. III [times] | 0.90–1.04 | 0.85–1.10 | 0.69–0.92 |
| Siphunculus / its basal width [times] | 3.68–5.67 | 3.60–5.25 | 3.42–6.15 |
| Siphuncular width, maximal / basal [times] | 0.60–0.73 | 0.50–0.75 | 0.53–0.85 |
| Siphuncular width, maximal / minimal [times] | 1.00–1.04 | 1.00–1.10 | 1.00–1.11 |
| Siphuncular minimal width / Hind tibiae, diameter at middle [times] | 1.14–1.50 | 1.10–1.60 | 1.06–1.64(2.00) |
| Cauda [mm] | 0.17–0.18 | 0.17–0.22 | 0.12–0.18 |
| Cauda / siphunculus [times] | 0.37–0.51 | 0.39–0.51 | 0.35–0.48 |
| Cauda / its basal width [times] | 1.08–1.21 | 1.05–1.33 | 0.87–1.33 |
| Setae on... | |||
| ... Frons [μm] | 10–14 | 7–20 | 8–13 |
| ... Frons / b. d. Ant. segm. III [times] | 0.3–0.5 | 0.3–1.0 | 0.3–0.6 |
| ... Vertex [μm] | 10–15 | 10–22 | 8–10 |
| ... Vertex / b. d. Ant. segm. III [times] | 0.5–0.6 | 0.4–1.0 | 0.3–0.6 |
| ... Antennal segment III [μm] | 7–10 | 7–10 | 6–10 |
| ... Antennal segment III / b. d. Ant. segm. III [times] | 0.3–0.4 | 0.3–0.4 | 0.3–0.5 |
| ... Ultimate rostral segm, [number] | 9–13 | 7–18 | 6–11 |
| ... Hind femur, dorsal [μm] | 10–13 | 9–18(28) | 8–13 |
| ... Hind femur, ventral [μm] | 12–20 | 12–20(35) | 10–23 |
| ... Hind tibia, dorsal, at middle [μm] | 15–24 | 17–30 | 10–23 |
| ... Hind tibia, dorsal / Tibial diameter (at middle) [times] | 0.4–0.6 | 0.4–0.8 | 0.2–0.8 |
| ... Hind tarsus, first segm. [number] | 4 | 4 | 4 |
| ... Abdominal segm. 2–4 [μm] | 9–13 | 10–15 | (5)8–10 |
| ... Abdominal segm. 2–4 / b. d. Ant. segm. III [times] | 0.3–0.5 | 0.4–0.7 | 0.2–0.5 |
| ... Abdominal segm. 8 [μm] | 10–15 | 12–20(25) | 8–15 |
| ... Abdominal segm. 8 / b. d. Ant. segm. III [times] | 0.4–0.6 | 0.6–0.9(1.1) | 0.3–0.6 |
| ... Abdominal segm. 8 [number] | 2–4 | (2)4(5) | 4 |
| ... Genital plate, discal [number] | 2(4) | 2(3)- | 2 |
| ... Genital plate, marginal [number] | 12–20 | 12–24 | 12–19 |
| ... Cauda [number] | 8–12 | 7–10 | 6–10 |
Note: Used abbreviations: Ant., Antennal; b. d., basal diameter; n. number of measured specimens; segm., segment or segments.
Siphunculi slightly swollen. Dorsum of thorax and abdomen with setiferous sclerites (apterae). Mesosternal mammariform processes pale, flat and smooth (apterae). Abdominal marginal tubercles absent. Tarsal formula 3.3.3
from 3 specimens (Fig. 2). Shiny dark green in life. In mounted specimens, head, antennae, legs, siphunculi, cauda, genital and anal plates, and thoracic and abdominal sclerites yellowish brown, with apex of antennal segments III, IV and V, antennal segment VI, very apex of tibiae, tarsi and apex of siphunculi something darker than aforementioned structures. Frons undulated. Head mostly smooth, some rugosity lines are present on dorsum. Proximal section of antennal segment III and segments IV-VI imbricated. Setae on body dorsum, antennae and most of those on legs thick with apices blunt or slightly capitate. Mesosternal mammariform processes pale, flat and smooth. Setiferous sclerites present on dorsum of thorax and abdomen, mostly with one setae and similar in size to spiracular sclerites; marginal sclerites on abdominal segments 2-4 often coalescent; those on segment 6 forming postsiphuncular sclerites; and those on segment 7 and 8 partially coalescent; sclerites on abdominal segments 6-8 with spinules. Intersegmental sclerites small. Siphunculi slightly swollen (maximal width 1.04-1.10 times minimal width of the stem), with small scales and distinct preapical incision and flange. Cauda tongue shaped. Metric and meristic features in Table 1.
Aphidura corsicensis sp. n., apterous viviparous female. A Habitus B Mesosternum with mammariform processes (signalled with arrows).
Holotype: apterous viviparous female, collected on Cerastium soleirolii Ser. ex Duby. (Caryophyllaceae), North slope of Mount Cinto, (Corsica, France), noted as more than 1100m, 08-VII-1980, J. H. Martin leg.. Paratypes: two apterous females from the same colony as the holotype.
The specific name of the new species is an adjective that means inhabitant of Corsica, in feminine.
This species was included as Aphidura sp. in the host lists and key to aphids on Cerastium by
Aphidura corsicensis can be easily distinguished from other Aphidura species by the abundant dorsal setiferous sclerites, which are not present in any other species of the genus. The host plant is not normally found below 1900m (Arthur Chater, Kew Gardens, pers. comm.) but the plant hosting Aphidura corsicensis was estimated to have been collected at around 1100-1200m (Martin).
In addition, the Tajik specimens all lack marginal or spinal tubercles, whereas in specimens from Iran small marginal tubercles may be present on some of abdominal tergites 2-6, and there may also be spinal tubercles on abdominal tergite 8 (Table 1).
When morphometric data are compared, however, the ranges of measurements and ratios for all characters agree well between countries, any slight differences being within the expected range of intraspecific variation (Table 2). Furthermore, examination of more Tajik material from the Zoologicheskiy Institut collection (Figs 3, 4), and of more apterae from Iran in the collection of the Muséum national d’Histoire naturelle, showed that the BMNH specimens constituted the extremes of a range of variation in a single species, with Iranian apterae in particular varying in the dorsal pattern of sclerotisation, pigmentation of siphunculi and shape of mesosternal processes. Further confirmation came from photomicrographs of Aphidura bozhkoae from Prunus incana in Georgia kindly provided by S. Bardjadze, which showed a similar range of variation in these characters within a single sample.
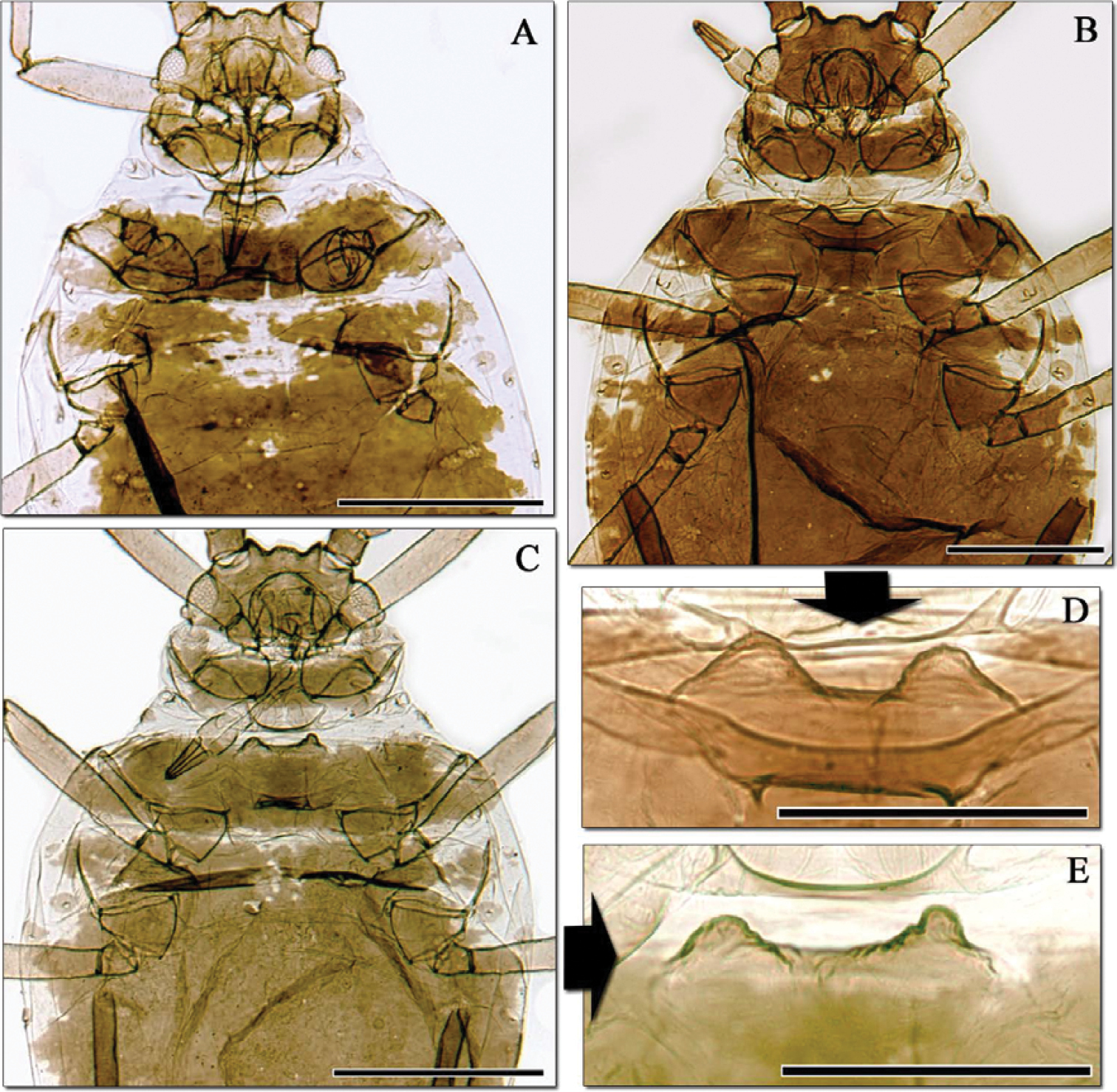
Aphidura bozhkoae apterous viviparous females from Ziddy (Tajikistan). A–C habitus, anterior part D–E Mesosternum with mammariform processes. A specimen collected in 1954 by Narzikulov B–E Two specimens collected in 1981 by Shaposhnikov. Note the different dorsal pattern and the different size and form of processes.
Aphidura bozhkoae mesosternal processes of five apterous viviparous females collected in Ziddy (Tajikistan) in 1981 by G. Shaposhnikov, same sample as the two specimens in Figure 3.
Intraspecific variation in the extent of dorsal sclerotisation is evident in other species of Aphidura, especially in those that are widely collected such as Aphidura picta. However this usually involves varying degrees of fragmentation or reduction of the entire discal plate rather than any specific part of it; in this respect the variation shown by Aphidura bozhkoae is unusual. Siphuncular pigmentation and the extent of development of the mesosternal processes are characters that have been used to discriminate between Aphidura species, but experience with Aphidura bozhkoae shows that they need to be applied carefully, especially when describing new species from small samples.
In brackets are: (1) when necessary morphological characters are included that do not have correspondence in the other proposition of the disjunctive, but which are useful to confirm identification; (2) illustration reference, from literature or in this paper; (3) host plants; and (4) distribution, with countries listed in geographical order from West to East.
| 1 | Siphunculi markedly swollen (maximal swollen width at least 1.2 times minimal stem width) | 2 |
| – | Siphunculi of different form (cylindrical, subcylindrical, tapering or slightly swollen, see |
9 |
| 2 | Most of dorsal setae placed on conical tubercles. [Dorsum without segmental pigmented sclerotisation; |
Acanthophyllum acanthophylli |
| – | Dorsal setae not placed on tubercles | 3 |
| 3 | Mesosternal processes and cauda pale | 4 |
| – | Mesosternal processes and cauda more or less pigmented, light brown to brown | 6 |
| 4 | Siphunculi dark brown, 2.3–2.7 times cauda which has 7–11 setae. Abdominal dorsum with spino-pleural patch, postsiphuncular sclerites pigmented and marginal sclerites. [Ultimate rostral segment 1.0–1.2 times second segment of hind tarsi; cauda 1.1–1.2 times its basal width; |
Aphidura nomadica |
| – | Siphunculi pale, sometimes with smoky apex, 1.6–2.2 times cauda, which has 6–7 setae. If a spino-pleural patch present then ultimate rostral segment is 1.2–1.5 times second segment of hind tarsi | 5 |
| 5 | Antennal segment VI processus terminalis at least 1.4 times antennal segment III and approximately 4 times antennal segment VI base. Longest dorsal setae on abdominal segment 2–4 approximately 3 μm. Cauda tongue-shaped. Dorsum pale with dark intersegmental sclerites. [ |
Aphidura graeca |
| – | Antennal segment VI processus terminalis at most 1.1 times antennal segment III and at most 3.1 times antennal segment VI base. Longest dorsal setae on abdominal segment 2–4 are 7–13 μm. Cauda triangular, sometimes slight constricted. Dorsum with variable sclerotisation and pigmentation, sometimes mostly pale. [ |
Aphidura amphorosiphon |
| 6 | Abdominal (or thoracic-abdominal) discal plate present, sometimes divided in transversal bands | 7 |
| – | Abdominal discal plate absent; a broken and irregularly edged spinopleural patch usually present, sometimes with bridges to marginal sclerites | 8 |
| 7 | Mesosternal processes wide and low. Longest dorsal setae on abdominal segment 2–4 are 10–11 μm. Discal plate sometimes divided in transversal bands. Siphunculus 1.6–2.0 times cauda, which has 7–11 setae. [ |
Aphidura melandrii |
| – | Mesosternal processes more or less narrow and tall. Longest dorsal setae on abdominal segment 2–4 are 10–55 μm. Discal plate always complete. Siphunculus 1.6–2.6 times cauda, which has 5–8 setae. [ |
Aphidura ornatella |
| 8 | Siphunculus 1.7–2.7 times cauda. Longest frontal setae 22–28 μm and 1.0–1.4 times basal diameter of antennal segment III. [ |
Aphidura naimanica |
| – | Siphunculus 1.5–1.7 times cauda. Longest frontal setae 35–40 μm and 1.6–1.8 times basal diameter of antennal segment III. [ |
Aphidura alatavica |
| 9 | First segment of tarsi with 4 or less often with 3 setae. [Head and prothoracic transversal band as dark as thoracic-abdominal discal plate. Siphunculi cylindrical and straight. On Rosaceae species] | 10 |
| – | First segment of tarsi usually with 3 setae, sometimes with 2; very infrequently with 4 | 11 |
| 10 | Antennal segment VI processus terminalis 2.2–2.7 times antennal segment VI base. Ultimate rostral segment with 2–5 accessory setae. Marginal tubercles usually present on abdominal segments 2–4. [ |
Aphidura mordvilkoi |
| – | Antennal segment VI processus terminalis 2.9–5.2 times antennal segment VI base. Ultimate rostral segment with 8–10 accessory setae. Abdominal marginal tubercles always absent. [Fig. 3, 4. |
Aphidura bozhkoae |
| 11 | Dorsum of thorax and abdomen with setiferous sclerites, similar in size to spiracular sclerites, and sometimes coalescing between them [Fig. 1. On Cerastium soleirolii. France: Corsica] | Aphidura corsicensis sp. n. |
| – | Dorsum of thorax and abdomen never with setiferous sclerites; other sclerotization can be present | 12 |
| 12 | Siphunculus slightly swollen with a maximal width close to 1.2 times minimal stem width and 1.6–2.0 times cauda, which is 1.5–1.8 times its basal width and has 7–11 setae; both as dark as head dorsum and thoracic and abdominal sclerotisation (a discal plate can be present). Longest dorsal setae on abdominal segment 2–4 are 10–11 μm and approximately 0.5 times basal diameter of antennal segment III. [ |
Aphidura melandrii |
| – | Characters not in above combination | 13 |
| 13 | Siphunculus at most 1.95 times cauda (which is short triangular), pale or uniformly dusky and slight swollen. Dorsum of head and mesosternal processes pale. Segmental thoracic and abdominal sclerotisation and pigmentation absent | 14 |
| – | Siphunculus at least 1.90 times cauda, both diversely shaped and coloured. Dorsum of head and mesosternal processes pale or pigmented. Thoracic and abdominal segmental sclerotisation and pigmentation rare completely absent | 15 |
| 14 | Siphunculus at least 0.26 mm, 0.6–0.95 times antennal segment III, and 1.7–1.95 times cauda, which is longer than its basal width. Mesosternal processes conspicuous. [ |
Aphidura pujoli |
| – | Siphunculus shorter than 0.20 mm, 0.41–0.56 times antennal segment III, and 1.7–1.9 times cauda, which is not longer than its basal width. Mesosternal processes sometimes inconspicuous. [ |
Aphidura pakistanensis |
| 15 | Antennal segment I at least 1.25 times its maximal width. Longest dorsal setae on abdominal segments 2–4 are 35–55 μm and 1.5–2.0 times basal diameter of antennal segment III. [Discal plate oval and dark; siphunculi weakly ornamented, smooth distad; |
Aphidura delmasi |
| – | Antennal segment I at most 1.2 times its maximal width. Longest dorsal setae on abdominal segments 2–4 at most 25 μm and 1.2 times basal diameter of antennal segment III | 16 |
| 16 | Abdomen usually with spinopleural patch and separate marginal sclerites; if a discal plate is present then it has irregular margins and frequently there are windows in spinal areas of the thoracic, if integrated, and anterior abdominal segments. Dorsal patch or plate smooth and reticulated. Siphunculi dark brown to black, subcylindrical and usually straight, 1.8–2.0 times cauda, which is broad triangular and has 10–16 setae. Ultimate rostral segment with 6–10 accessory setae. [ |
Aphidura ornata |
| – | Characters not in above combination | 17 |
| 17 | Longest setae on abdominal segments 2–4 (dorsum) and antennal segment III 3–8 μm and 0.15–0.50 times basal diameter of antennal segment III | 18 |
| – | Longest setae on abdominal segments 2–4 (dorsum) and antennal segment III 8–25 μm and 0.30–1.60 times basal diameter of antennal segment III; if they are 8 μm long then marginal abdominal tubercles present or ultimate rostral segment shorter than second segment of hind tarsi | 19 |
| 18 | Siphunculi dark brown, head dorsum, mesosternal processes and cauda brown to dark brown. Ultimate rostral segment 1.15–1.25 times second segment of hind tarsi. Cauda 1.4–1.8 times its basal width. [ |
Aphidura pannonica |
| – | Siphunculi (with smoked apex, head dorsum, mesosternal processes and cauda pale. Ultimate rostral segment as long as second segment of hind tarsi. Cauda 1.0–1.1 times its basal width. [ |
Aphidura togaica |
| 19 | Marginal tubercles on prothorax and abdominal segments 2–4 usually present and spinal tubercle on abdominal segment VIII sometimes present | 20 |
| 20 | Aphids relatively large (longer than 1.7 mm) and provided with an extensive, solid discal plate. Setae on antennal segment III, and on dorsum of head, abdominal segments 2–4 and abdominal segment 8 at least 12, 15, 10 and 27 μm respectively. [Fig. 2. On Prunus prostrate. Lebanon] | Aphidura libanensis sp. n. |
| – | Aphids relatively small (shorter than 1.4 mm) with a broken pattern of dorsal sclerotisation. Setae on antennal segment III, and on dorsum of head, abdominal segments 2–4 and abdominal segment 8 at most 8, 10, 8 and 15 μm respectively. [ |
Aphidura iranensis |
| – | Marginal and spinal abdominal tubercles absent | 21 |
| 21 | Siphunculi pale, usually as pale as most part of tibiae | 22 |
| – | Siphunculi pigmented, usually darker than most part of tibiae | 23 |
| 22 | Antennal segment VI processus terminalis 5.0–5.5 times antennal segment VI base. Cauda triangular or tongue-shaped with slight proximal constriction. Ultimate rostral segment shorter than second segment of hind tarsi. [ |
Aphidura gypsophilae |
| – | Antennal segment VI processus terminalis 2.8–4.0 times antennal segment VI base. Cauda tongue-shaped. Ultimate rostral segment 1.23–1.45 times second segment of hind tarsi. [Clypeus swollen both forward and laterally; |
Aphidura urmiensis |
| 23 | Cauda tongue-shaped, 1.40–1.80 times its basal width. Mesosternal processes more or less pigmented, usually darker than tibiae. [Thoracic and abdominal sclerotisation variable, usually a spinopleural abdominal patch with irregular edges and windows in several segments, including the posterior ones; siphunculi pigmented, but usually pale than abdominal sclerotized dorsum; |
Aphidura picta |
| – | Cauda triangular, although sometimes with a slight proximal constriction, 1.05–1.40 times its basal width. Siphunculi and mesosternal processes as pale as tibiae | 24 |
| 24 | Ultimate rostral segment 0.90–1.00 times second segment of hind tarsus, with 8–10 accessory setae. Cauda approximately 1.30–1.40 times its basal width. Longest dorsal setae on abdominal segment 2–3 are 8–11 μm and 0.3–0.5 basal diameter of antennal segment III. [ |
Aphidura massagetica |
| – | Ultimate rostral segment 1.05–1.45 times second segment of hind tarsus, with 10–16 accessory setae. Cauda approximately 1.05–1.35 times its basal width. Longest dorsal setae on abdominal segment 2–3 are 13–23 μm and 0.6–1.0 basal diameter of antennal segment III. [ |
Aphidura gallica |
We are grateful to A. Stekolshchikov (Zoological Institute, Russian Academia of Sciences, St. Petersburg) for providing us with two Tajik samples of Aphidura bozhkoae, and to S. Barjadze (Agricultural University of Georgia, Tbilisi, Georgia) for providing photomicrographs and information about apterous viviparous females of Aphidura bozhkoae from Georgia. The restoration of Tajik specimens of Aphidura bozhkoae of the Zoologicheskiy Institut collection was made by M.P. Mier Durante. Arthur Chater, then based at the Royal Botanic Gardens, Kew (UK), kindly provided the identification of the host plant of Aphidura corsicensis and the note concerning its usual altitude range.