Citation: Sullivan JB, Pogue MG (2014) The Disphragis notabilis (Schaus) species-group in Costa Rica (Lepidoptera, Notodontidae). In: Schmidt BC, Lafontaine JD (Eds) Contributions to the systematics of New World macro-moths V. ZooKeys 421: 21–38. doi: 10.3897/zookeys.421.7351
The four described taxa in the Disphragis notabilis (Schaus) species-group are reviewed, including the types and their dissected genitalia. Disphragis hemicera (Schaus), stat. rev., is elevated to species rank, D. normula (Dognin) is retained as a synonym of D. notabilis, D. sobolis Miller is confirmed as distinct from D. hemicera, and D. bifurcata sp. n., is newly described. Both D. hemicera and D. bifurcata occur in Costa Rica. The known ranges of the other species are outlined. Defining characters of each species are presented and a key to species is provided. Unusual variation in the genitalia is noted.
Taxonomy, genitalic variation
The name Disphragis notabilis (Schaus), described from French Guiana, has been applied to prominent moths throughout Central and South America. Miller described Disphragis sobolis from Ecuador and indicated that genitalic characters reveal yet another member of the complex in Ecuador (
Photographic methods used herein are described in
BMNH Natural History Museum, London, UK
INBio Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica
JBS J. Bolling Sullivan collection, Beaufort, North Carolina, USA
USNM National Museum of Natural History, Washington, District of Columbia, USA
| 1 | Socii short, 1/6 length of valva and upcurved below a short, almost hood-like uncus | 2 |
| – | Socii long, ½ length of valva, below triangular “dunce cap” uncus | 3 |
| 2 | Phallus with two plates of flattened spines at base of sclerotized portion | Disphragis hemicera |
| – | Phallus with a parallel extension at base of sclerotized portion | Disphragis sobolis |
| 3 | Socii large, bifurcated at tip | Disphragis bifurcata |
| – | Socii smaller, tapering to single point with ventral spines | Disphragis notabilis |
The female type specimen of Heterocampa notabilis Schaus was named from French Guiana. Both Heterocampa hemicera Schaus (male) from Costa Rica and Heterocampa normula Dognin (male) from Peru are listed as synonyms of Disphragis notabilis by
http://zoobank.org/0944967F-1CB1-48C6-9702-0B8FA0D8E9B9
Figs 1, 10, 14, 18, 22Holotype male: Costa Rica, Reserva Hitoy Cerere (9.404°N, 83.015°W), Limon Province, 354’, 1–4 July 2008, J. Bolling Sullivan. INBio. Paratypes: 11♂, 3♀: 4♂, same data as holotype (JBS-2094, JBS-3053); 1♀, 22 March 2003, Monty Wood (JBS-3030); 1♂, Costa Rica, Est. Biol. La Selva (10.26°N, 84.01°W), Heredia Province, 50–150 m, 21–30 June 2003, Monty Volovsic (JBS-3040), 2♂, 29 Aug. –2 Sept. 2003, J. Bolling Sullivan (JBS-3038); 2♂, Costa Rica, Upata Estacion San Gerardo (10.89°N, 85.38°W), Alajuela Province, 550 m, 17–21 July 2006, J. Bolling Sullivan, B. Espinosa (JBS-3035); 1♂, Costa Rica, Puriscal Chires, Mastatal (N9.411; W-84.220), San Jose Province, 400 m, 16–18 Oct. 2011, J. Bolling Sullivan; 1♂, Costa Rica, Verugua Rainforest Campamento (9.553°N, 83.112°W), Limon Province, 400–500 m, 12–16 March 2010, J. Bolling Sullivan (11-CRBS-2066), (JBS-5427); 1♀, Costa Rica, Tuis, 2500’, June, W. Schaus 1910-110. (BM-); 1♀, Costa Rica, Cashi, 8–10 1912 (Lankester), Rothschild Bequest, B. M. 1939-1. (BM-). (USNM, BMNH, JBS, INBio)
The name bifurcata refers to the bifurcate tip of the socii, which is diagnostic.
Maculation characters can usually be used to separate Disphragis bifurcata and Disphragis notabilis from the other two members of the complex. Forewing color is a warm brown, not mottled or brownish gray as in Disphragis sobolis and Disphragis hemicera. Additionally, the male antennal pectinations are shorter in Disphragis bifurcata and Disphragis notabilis. Males of Disphragis bifurcata are easily distinguished by the large upturned and bifurcated socii in the male genitalia. In males of Disphragis notabilis the socii usually have a single point at the apex, with many spines arising from the ventral edge. Females must be identified by maculation and geography; Disphragis bifurcata occurs in Central America and central and western Colombia.
Male. (Figs 1, 10) Head–labial palps upturned, mahogany brown on basal segment, medial segment with cream scaling along distal margin, particularly near the terminus, and apical segment mostly cream scaled with scattered brown scales. Denuded medial segment 2.4× length of apical segment. Eye round, large, surrounded tightly with scaling. Front scaling mostly cream with scattered brown scales. Vertex with additional brown scales among white scaling. Scape with cream and brown scaling, cream extending onto antennal shaft for about 10–14 segments. Antenna bipectinate basally for 30 segments, then with minute basal seta on segments to tip (68 segments). Longest rami 0.44 mm. Thorax a blend of fine brown and cream scales giving a tan appearance. Metathorax bearing a central white spot with row of darker brown scales anteriorly. Abdomen with appressed brown scaling. Forewing (17.5 mm N = 10) elongate, rounded apically and with broad tan subcostal streak from base of wing to apex. Streak encloses chocolate reniform spot and has several slightly darker brown lines crossing obliquely from costa. Basal dash below streak paralleling costa. White streak below basal dash; warm brown patch distal to white streak bordered by white; wavy antemedial (AM) and postmedial (PM) lines. Chocolate shading from middle of forewing below costal streak and forming a wedge to margin (below costal streak to anal angle). Weak gray crescent on lower half of margin. Hind wing fuscous with darker margin and veins, weak darker brown anal markings almost a spot at anal angle. Underside of forewing fuscous, anal margin and cell yellowish. Basal 3/4 of hind wing yellowish, margin brown and well differentiated. Legs a mixture of brown and white scales, appearing almost yellowish, with white scales forming rings at distal end of tarsal segments. Tibial spines 0-2-4. Male genitalia (Figs 14, 18) (8 dissections). Uncus an extended triangle, apex rounded with setae arranged almost in marginal rows. Tegumen broad, longer than vinculum. Socii extending from base of uncus as two large upcurved arms, scythe-like, apex bifurcate. Occasionally tip may be subdivided farther with arrowhead-like plates embedded near apex (visible at higher magnification). However, plates do not form ventral spine-like projections as in Disphragis notabilis. Gnathos absent, anal tube membranous. Valva elongated with costal half sclerotized, anal half membranous and enveloping deciduous scent hairs. Valva apex rounded, sclerotized costal half of valva with broad anal projection distally and sharper but rounded and more heavily sclerotized projection basally. Vinculum broad, short, rounded to saccus. Phallus long, narrow with subbasal keel, proximal half unsclerotized, ductus entering medially. Distal half of phallus sclerotized, enlarged basally at junction with membranous half, and with small teeth-like spines ventrally and laterally on basal half. Vesica emerges dorsally from aedeagus, forming a membranous tube that turns to parallel aedeagus and then to left with no major diverticula. A lightly sclerotized sliver-like cornutus often visible and often with small peg-like cornuti where vesica turns left. Eighth tergite broadly rounded, slightly sclerotized and crenulated medially at distal end. Eighth sternite lightly sclerotized, broadly rounded with well-defined, broad notch medially. Small sac-like flap in middle of sclerite, anterior end of sclerite with two broad, rounded projections with medial V-shaped notch. Ctenophores absent. Female. (Fig. 10). Female similar to male only larger (Forewing 21.3 mm, n = 3) and with fasciculate antennae. Female genitalia (Fig. 22) (3 dissections). Papillae anales bluntly rounded, slightly setose. Extension of 9th tergite forming dorsal flap. Anterior apophysis short, 25% as long as posterior apophysis. Genital plate small, elongate, consisting of a bifurcated middle phalanx with lateral “wings” from base. Ductus bursae slightly shorter than corpus bursae, narrow and tending to twist, unsclerotized. Corpus bursae egg shaped, with large signum on dorsal surface. Signum shield-like, about half as long as corpus bursae. Signum egg shaped with stippled lateral flanges anterior to midpoint. Proximal margin lightly sclerotized and faintly stippled.
Disphragis notabilis complex holotypes. 1 Disphragis bifurcata, male holotype 2 Disphragis hemicera, male holotype 3 Disphragis normula, male holotype 4 Disphragis notabilis, female holotype.
Five barcoded specimens exhibit two haplotypes that differ from each other by a maximum of 0.15%. They differ from Disphragis hemicera by a minimum of 5.61%, from Disphragis notabilis by a minimum of 1.26%, and from Disphragis sobolis by a minimum of 5.78%. The most common haplotype (11-CRBS-2066) is:
AACCTTATATTTCATTTTTGGAATTTGAGCAGGAATAGTAGGAACCTCTTTAAGTCTTCTAATTCGTGCTGAATTAGGAACCCCCGGGACTTTAATTGGAGATGATCAAATTTATAATACTATTGTAACAGCTCATGCTTTCATTATAATTTTTTTTATAGTAATACCTATTATAATTGGAGGATTTGGAAATTGATTAGTACCTTTAATATTAGGAGCCCCAGACATAGCTTTCCCACGAATAAATAATATAAGTTTTTGATTATTACCTCCTTCTTTAATACTTTTAATTTCGAGAAGTATTGTAGAAAATGGAGCAGGAACAGGATGAACAGTTTACCCACCACTGTCATCTAATATTGCTCATAGAGGAAGCTCTGTTGATTTAGCCATTTTTTCCCTTCACTTAGCTGGTATTTCATCAATTTTAGGGGCTATTAATTTTATCACAACAATTATTAATATACGATTAAATAATATATCTTTTGATCAAATACCTTTATTTGTGTGAGCTGTAGGAATTACTGCTTTTTTACTTTTACTTTCTCTCCCAGTTCTAGCTGGAGCTATTACTATACTTTTAACTGATCGTAATTTAAATACATCTTTTTTTGACCCTGCAGGGGGAGGAGATCCTATTTTATACCAACATTTATTT
Known from Guatemala to Colombia (Anchicaya, Valle, and the Magdalena Valley), and probably extending south into northern Ecuador.
This species occurs at lower altitudes and moderate elevations (1000 m) where it occurs with Disphragis hemicera.
Costa Rica.
Maculation will usually separate Disphragis hemicera and Disphragis sobolis from Disphragis notabilis and Disphragis bifurcata. Their appearance is mottled, grayish brown with a distinct dark band next to the PM line. Males may be distinguished by the shape of the phallus, which in Disphragis sobolis has a distinct dorsal projection. Females can be separated by the shape of the genital plate, which in Disphragis sobolis is bifurcate at the distal tip and in Disphragis hemicera has a middle phalanx with lateral “wings” from the base. Geographic distribution also separates Disphragis hemicera from Disphragis sobolis, with Disphragis hemicera in Central America and western Colombia, and Disphragis sobolis along the western slopes of the Andes.
Male. (Figs 2, 5) Head–labial palp upturned, mahogany brown on basal segment, medial segment with cream scaling along distal margin particularly near the terminus, and apical segment mostly cream scaled with scattered brown scales. Denuded medial segment is 3.2× length of apical segment that is shortened relative to Disphragis bifurcata. Eye round, large, surrounded tightly with scaling. Front scaling mostly cream with scattered brown scales. Vertex with additional brown scales among cream scaling. Scape with cream and brown scaling, cream scaling extending onto antennal shaft for about 14–18 segments. Antenna bipectinated basally for 33 segments, then with minute basal seta on segments to apex (73 segments). Rami noticeably longer than in Disphragis bifurcata, longest rami 0.53 mm. Thorax a blend of brown and cream scales giving a tan appearance. Metathorax with a central white spot with row of darker brown scales anteriorly. Abdomen with appressed brown scaling. Forewing (17.9 mm, n = 10) elongate, rounded apically and with broad light brown subcostal streak from base of wing to apex. Streak encloses chocolate reniform spot and has several slightly darker brown lines crossing obliquely from costa. Basal dash below streak perpendicular to thorax. White streak below dash; warm brown patch distal to white streak bordered by white; AM and PM lines wavy. Distinct brown line bisecting warm brown patch. Chocolate shading from middle of forewing below costal streak and forming a wedge to margin (below costal streak to anal angle). Gray crescent on lower half of margin with distinct brown band inward to PM line. Hind wing uniformly fuscous with brown anal markings forming something of a spot at anal margin. Light streak along anal edge. Underside of forewing fuscous with yellowish subapical crescent along costa. Basal half of hind wing yellowish, no well-differentiated margin. Legs a mixture of brown and white scales appearing somewhat yellowish with white scales forming rings at distal end of tarsal joints. Tibial spines 0-2-4. Male genitalia (Figs 15, 19) (12 dissections). Uncus lightly sclerotized and rounded, turning 90 degrees ventrally and forming a rounded, setose pad. Socii small, upturned and pointed slightly, blade-like. Tegumen broad, triangular, similar in size to vinculum. Valve elongated rounded at apex and costal half sclerotized. Anal half of valve membranous and enclosing deciduous hair-like scent scales. Distal third of valve enlarged dorsally ending abruptly with shelf-like narrowing. Second narrowing of sclerotized subcostal area 1/3 distance from base, a rounded projection less shelf-like that distal projection, but more heavily sclerotized. Juxta shovel shaped with handle toward aedeagus. Vinculum rounded to saccus. Aedeagus long, narrow and with basal 2/3 membranous, subbasal keel present. Distal 1/3 sclerotized with two prominent toothed plates at junction with membranous portion. Vesica tube-like emerging dorsally then turning 90 degrees forward to plane of phallus. Distinct lateral diverticulum to left of midpoint. Cornuti absent. Ctenophore on pelt absent. Eighth tergite broadly rounded, slightly sclerotized and crenulated medially at distal end. Sternite lightly sclerotized with “happy face” consisting of two membranous flaps for “eyes” and a broad anterior one for “mouth.” Anterior edge tapered to blunt, indented terminus. Female. (Fig. 5). Female similar to male only larger (Forewing 21.0 mm, n = 5) and with fasciculate antennae. Female genitalia (Figs 23, 26–29) (10 dissections). Papillae anales bluntly rounded, slightly setose. Extension of 9th tergite forming dorsal flap in Disphragis bifurcata greatly reduced to small crescent in Disphragis hemicera. Anterior apophysis short, 25% as long as posterior apophysis. Genital plate small, slightly elongate, consisting of a middle phalanx with lateral “wings” from base. Phalanx usually shorter than in Disphragis bifurcata. Tip of phalanx variable, usually blunt but can be indented or bifurcate. Ductus bursae slightly shorter than corpus bursae, narrow and tending to twist, membraneous. Corpus bursae egg shaped with large signum on dorsal surface. Signum shield-like, about half as long as corpus bursae. Signum egg shaped with stippled lateral flanges below midpoint. Proximal margin lightly sclerotized and faintly stippled.
Disphragis notabilis complex holotype genitalia. 5 Disphragis hemicera, male holotype (USNM-49851) a valve b phallus 6 Disphragis normula, male holotype (USNM-49852) a valve b phallus 7 Disphragis notabilis, female holotype (USNM-49853) 8 Disphragis hemicera male holotype (USNM-49851) tergites 9 Disphragis normula male holotype (USNM-49852) tergites.
Disphragis notabilis complex adults. 10 Disphragis bifurcata a male (Costa Rica) b female (Costa Rica) 11 Disphragis hemicera a male (Costa Rica) b female (Costa Rica) 12 Disphragis sobolis a male (Ecuador) b female (Peru) 13 Disphragis notabilis a male (French Guiana b female (Brazil).
Disphragis notabilis complex male valves and phalli. 14 Disphragis bifurcata (JBS-3035) a valve b phallus 15 Disphragis hemicera (JBS-3037) a valve b phallus 16 Disphragis sobolis (BMNH-NOTO1964) a valve b phallus 17 Disphragis notabilis (BMNH-NOTO1968) a valve b phallus.
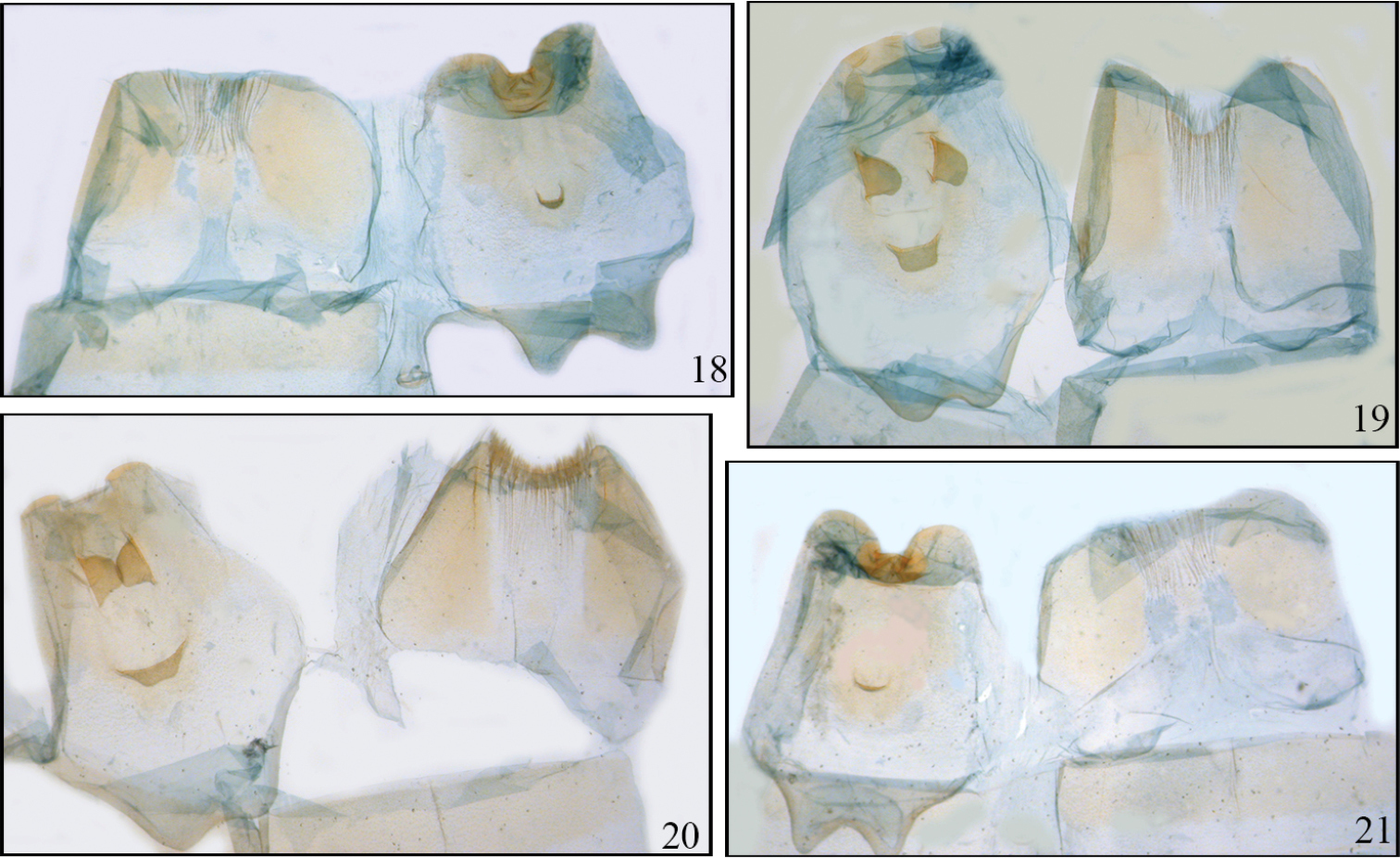
Disphragis notabilis complex male tergites. 18 Disphragis bifurcata (JBS-3035) 19 Disphragis hemicera (JBS-3037) 20 Disphragis sobolis (BMNH-NOTO1964) 21 Disphragis notabilis (BMNH-NOTO1968).
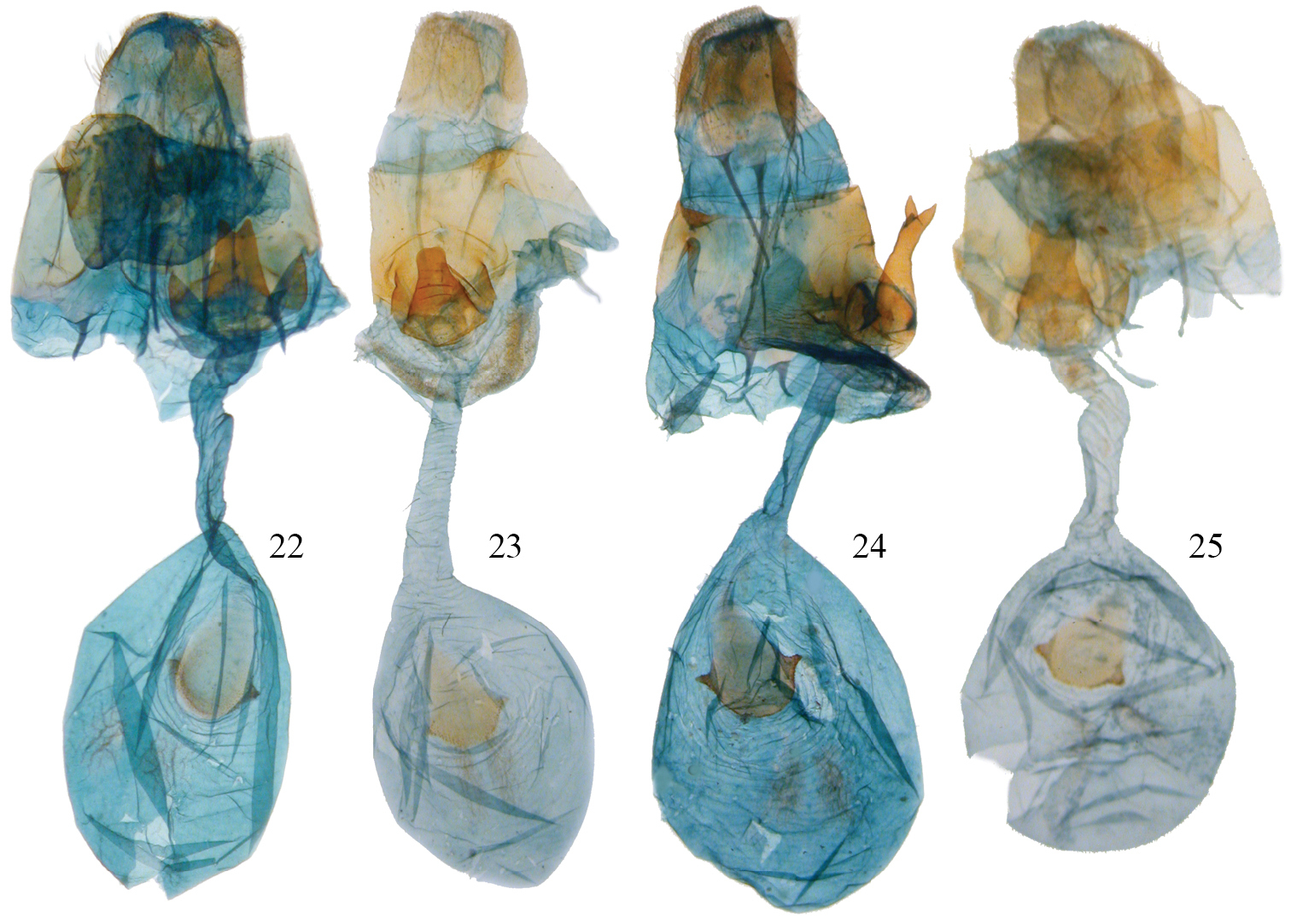
Disphragis notabilis complex female genitalia. 22 Disphragis bifurcata (BMNH-NOTO1984) 23 Disphragis hemicera (JBS-3049) 24 Disphragis sobolis (BMNH-NOTO1988) 25 Disphragis notabilis (BMNH-NOTO1972).
Variable genital plates of Disphratis hemicera from Costa Rica. 26 Costa Rica (JBS-3049) 27 Costa Rica (JBS-3041) 28 Costa Rica (JBS-3050) 29 Costa Rica (JBS-3046).
Fifty eight barcoded specimens exhibit seven haplotypes that differ from each other by a maximum of 0.30%. They differ from those of Disphragis bifurcata by a minimum of 5.61%, from Disphragis notabilis by a minimum of 5.65%, and from Disphragis sobolis by a minimum of 6.13%. The most common haplotype (11-CRBS-2519) is:
AACTTTATATTTTATTTTTGGAATTTGAGCAGGAATAGTAGGAACTTCTTTAAGTCTTTTAATTCGTGCTGAATTAGGAACCCCCGGGACTTTAATTGGAGATGATCAAATTTATAATACTATCGTAACAGCTCATGCTTTTATTATAATTTTTTTTATAGTTATACCTATTATAATTGGAGGATTTGGAAATTGATTAGTCCCTTTAATACTAGGAGCACCAGATATAGCTTTCCCACGAATAAATAATATAAGTTTTTGACTATTACCCCCTTCTTTAATACTTCTAATTTCAAGAAGTATTGTAGAAAATGGAGCTGGTACAGGATGAACAGTTTATCCCCCACTGTCATCAAATATTGCTCACGGAGGAAGCTCTGTTGATTTAGCTATTTTTTCCCTTCATTTAGCGGGTATTTCCTCAATTTTAGGGGCTATTAATTTTATTACAACAATTATTAATATACGATTAAATAATATATCTTTTGATCAAATACCTTTATTTGTATGAGCTGTAGGAATTACTGCTTTTCTACTTTTACTTTCACTCCCAGTATTAGCTGGAGCTATTACTATACTTTTAACCGATCGTAATTTAAATACATCTTTTTTCGACCCTGCTGGGGGAGGAGATCCTATTTTATACCAACATTTATTT
Disphragis hemicera occurs throughout Costa Rica at moderate altitudes. It is found south along the western coast of Colombia and may extend to the west coast of Ecuador. The northern limits are unknown but it probably occurs at least into Nicaragua.
Disphragis hemicera is by far the most common member of the group in Costa Rica and appears to be absent below 500 m. At moderate altitudes both Disphragis hemicera and Disphragis bifurcata occur together.
Disphragis notabilis: French Guiana; Disphragis normula: Peru
Maculation characters can usually be used to separate Disphragis notabilis and Disphragis bifurcata from Disphragis hemicera and Disphragis sobolis. Disphragis notabilis and Disphragis bifurcata are warm brown, not mottled or brownish gray like Disphragis hemicera and Disphragis sobolis. The male antennal pectinations are shorter in Disphragis notabilis than in Disphragis hemicera and Disphragis sobolis. Males of Disphragis notabilis are easily distinguished by their moderately wide socii, which taper to a single point with many ventral spines. In males of Disphragis bifurcata the socii are much broader and are bifurcate at the upturned apex. Females must be sorted by maculation and geography. Disphragis notabilis is Amazonian in distribution whereas Disphragis bifurcata occurs from central and western Colombia north into Central America.
Male. (Fig. 13) Head–palp upturned, mahogany brown on basal segment, medial segment with cream scaling along distal margin particularly near terminus; apical segment mostly cream with scattered brown scales. Denuded medial segment 4.1× length of apical segment. Apical segment reduced in size relative to other species in complex. Eye round, large, surrounded tightly with scaling. Front scaling mostly cream with scattered brown scales. Vertex with additional brown scales among white scaling. Scape with cream and brown scaling, white scaling extending onto antennal shaft for about 10–14 segments. Antenna bipectinate basally for 29 segments then with minute basal setae on segments to apex (71 segments). Longest rami 0.34 mm, shortest of all species. Thorax a blend of brown and cream scales giving a tan appearance. Metathorax bearing a central white spot with row of darker brown scales anteriorly. Abdomen with appressed brown scaling. Forewing (17.0 mm, n = 10) elongate, rounded apically and with broad tan subcostal streak from base of wing to apex. Streak encloses chocolate reniform spot and has several slightly darker brown lines crossing obliquely from costa. Basal dash below streak perpendicular to thorax, abbreviated relative to that of Disphragis bifurcata. White streak below dash; warm brown patch distal to white streak bordered by white; AM and PM lines wavy. Chocolate shading from middle of wing below costal streak and forming a wedge to margin (below costal streak to above mid point of margin). Weak gray crescent on lower half of margin. Warm brown from patch expanded almost to margin and reducing size of chocolate wedge seen in Disphragis bifurcata. Hind wing fuscous with darker margin, weak darker brown anal markings almost forming a spot. Underside of forewing fuscous, anal margin and cell yellowish. Basal 3/4 of hind wing yellowish, margin brown and moderately differentiated. Legs a mixture of brown and white scales appearing almost yellowish with white scales forming rings at distal end of tarsal joints. Tibial spines 0-2-4. Male genitalia (Figs 17, 21) (13 dissections). Uncus an extended triangle, rounded apex with setae arranged almost in marginal rows. Tegumen broad, longer than vinculum. Socii extending from base of uncus as two upcurved arms, scythe-like with small, spine-like projections on ventral surface. Degree of spination variable from several to many extending down to angle of socius. Gnathos absent, anal tube unsclerotized. Valve elongated with costal half sclerotized, anal half membranous and enveloping deciduous scent hairs. Valve apex rounded, sclerotized costal half of valva with broad anal projection distally and sharper shelf-like projection basally. Vinculum broad, short and rounded to saccus. Aedeagus long, narrow with basal phallus, proximal 60% unsclerotized with ductus entering medially. Distal 40% of aedeagus sclerotized, enlarged basally at junction with membranous half, and with raised mound of spines ventrally about 1/3 distal from junction. Vesica emerges dorsally from aedeagus, an unsclerotized tube with a long dorsal diverticulum. Cornuti absent. Eighth tergite broadly rounded, slightly sclerotized and crenulated medially at distal end. Eighth sternite lightly sclerotized, broadly rounded with well-defined, broad notch medially, usually broader than in Disphragis bifurcata. Small sac-like flap in middle of sclerite usually in form of narrow crescent, anterior end of sclerite with two broad, rounded projections with medial V-shaped notch. Ctenophores absent on pelt. Female. (Figs 4, 13). Female similar to male only larger (Forewing 20.9 mm, n = 6) and with fasciculate antennae. Female genitalia (Fig. 25) (5 dissections). Papillae anales bluntly rounded, slightly setose. Extension of 9th tergite forming dorsal flap. Anterior apophysis short, 25% as long as posterior apophysis. Genital plate small, elongate, consisting of a bifurcated middle phalanx with lateral “wings” from base. Phalanx somewhat longer than in Disphragis bifurcata. Ductus bursae slightly shorter than corpus bursae, twice as wide as in Disphragis bifurcata and tending to twist, unsclerotized. Corpus bursae egg-shaped with large signum on dorsal side. Signum shield-like, about half as long as corpus bursae. Signum egg shaped with stipulated lateral flanges below midpoint. Proximal margin lightly sclerotized and faintly stippled.
Two barcoded specimens exhibit 2 haplotypes that differ from each other by 0.30%. They differ from those of Disphragis hemicera by a minimum of 5.65%, from Disphragis bifurcata by a minimum of 1.26%, and from Disphragis sobolis by a minimum of 4.78%. One haplotype (11-MISC-302) is:
AACTTTATATTTCATTTTTGGAATTTGAGCAGGAATAGTAGGAACCTCTTTAAGTCTTCTAATTCGTGCTGAATTAGGAACCCCCGGGACTTTAATTGGAGATGACCAAATTTATAATACTATCGTAACAGCTCATGCTTTCATTATAATTTTTTTTATAGTAATACCTATTATAATTGGAGGATTTGGAAATTGATTAGTACCTTTAATATTAGGAGCCCCAGACATAGCTTTCCCACGAATAAATAATATAAGTTTTTGATTATTACCTCCTTCTTTAATACTTTTAATTTCAAGAAGTATTGTAGAAAATGGAGCAGGAACAGGATGAACAGTTTACCCACCACTGTCATCTAATATTGCCCATAGAGGAAGCTCTGTTGATTTAGCCATTTTTTCCCTTCACTTAGCCGGTATTTCATCAATTTTAGGGGCTATTAATTTTATCACAACAATTATTAATATACGATTAAATAATATATCTTTTGATCAAATACCTTTATTTGTATGAGCTGTAGGAATTACTGCTTTTTTACTTTTACTTTCTCTTCCAGTTCTAGCTGGAGCTATTACTATACTTTTAACTGATCGTAATTTAAATACATCTTTTTTTGACCCTGCAGGGGGAGGAGATCCTATTTTATACCAACATTTATTT
This species occurs throughout the Amazon basin from western Venezuela eastward and southward to at least Bolivia.
Disphragis notabilis is by far the most common member of the group in South America, however, earlier references to this species should be confirmed in light of the additional species described here.
Ecuador.
Male. (Fig. 12) Head–labial palpus upturned, mahogany brown on basal segment, medial segment with cream scaling along distal margin, particularly near the terminus, and apical segment mostly cream scaled with scattered brown scales. Denuded medial segment 2.6× length of apical segment. Eye round, large, surrounded tightly with scaling. Front scaling mostly cream with scattered brown scales. Vertex with additional brown scales among cream scaling. Scape with cream and brown scaling, white scaling extending onto antennal shaft for about 14–18 segments. Antenna bipectinated basally for 33 segments then with minute basal seta on segments to tip (68 segments). Rami noticeably longer than in Disphragis hemicera, longest 0.59 mm. Thorax a blend of brown and cream scales giving a tan appearance. Metathorax bearing a central white spot with row of darker brown scales anteriorly. Abdomen with appressed brown scaling. Forewing (19.3 mm, n = 5) elongate, rounded apically and with broad light brown subcostal streak from base of wing to apex. Streak encloses chocolate reniform spot and has several slightly darker brown lines crossing obliquely from costa. Brown scaling throughout as well as several black streaks. Basal dash below streak perpendicular to thorax and greatly reduced in length. White streak below dash; warm brown patch distal to white streak bordered by white; AM and PM lines wavy. Distinct brown line bisecting warm brown patch. Chocolate shading from middle of forewing below costal streak and forming a wedge to margin (below costal streak to anal angle) more extensive than in Disphragis hemicera. Prominent gray crescent on lower half of margin with distinct brown band inward to PM line. Hind wing uniformly fuscous with brown anal markings almost a spot. Light streak along anal edge. Underside of forewing fuscous with yellowish subapical crescent along costa. Basal half of hind wing yellowish, no well-differentiated margin. Legs a mixture of brown and white scales, appearing almost yellowish with white scales forming rings at distal end of tarsal joints. Tibial spines 0-2-4. Male genitalia (Figs 16, 20) (5 dissections). Uncus lightly sclerotized and rounded, turning 90 degrees ventrally and forming a much smaller setose pad than in Disphragis hemicera. Socii small but 2× larger than in Disphragis hemicera, upturned and pointed slightly, blade-like. Tegumen broad, triangular similar in size to vinculum. Valve elongated, rounded at tip and costal half sclerotized. Anal half of valve membranous and enclosing deciduous hair-like scent scales. Distal third of valve considerably enlarged dorsally then gradually narrowing. Second narrowing of sclerotized subcostal area 1/3 distance from base, a rounded projection, more heavily sclerotized. Juxta shovel shaped with handle toward aedeagus. Vinculum rounded to saccus. Aedeagus long, narrow and with basal 2/3 membranous, aedeagus present. Distal 1/3 sclerotized with prominent basal process. Vesica tube-like emerging dorsally then turning 90° to plane of aedeagus. Distinct lateral diverticulum to left of midpoint. Cornuti absent. Ctenophore absent on pelt. Eighth tergite broadly rounded, slightly sclerotized and crenulated medially at distal end. Eighth sternite lightly sclerotized with “happy face” consisting of two membranous flaps for “eyes” and a broad anterior one for “mouth.” Anterior edge tapers to blunt, indented terminus. Female. (Fig. 12) Female similar to male only larger and with fasciculate antennae. Female genitalia (Fig. 24) (3 dissections). Papillae anales bluntly rounded, slightly setose. Extension of 9th tergite forming dorsal flap in Disphragis bifurcata and Disphragis notabilis greatly reduced to small crescent in Disphragis sobolis. Anterior apophysis short, 25% as long as posterior apophysis. Genital plate small, elongated, consisting of a middle phalanx with lateral “wings” from base. Phalanx longer than in other related species. Tip of phalanx forming a Y-shape. Ductus bursae slightly shorter than corpus bursae, narrow and tending to twist, unsclerotized. Corpus bursae egg shaped with large signum on dorsal surface. Signum shield-like, about half as long as corpus bursae. Signum egg shaped with stipulated lateral flanges below midpoint. Proximal margin lightly sclerotized and faintly stippled.
One specimen has been barcoded and differs from that of Disphragis hemicera by a minimum of 6.13%, from Disphragis bifurcata by a minimum of 5.78%, and from Disphragis notabilis by a minimum of 4.78%. The haplotype (11-MISC-495) is:
AACTTTATATTTTATTTTTGGAATTTGAGCAGGAATAGTAGGAACCTCTTTAAGTCTCCTAATTCGTGCTGAATTAGGAACCCCCGGGACTTTAATTGGAGATGATCAAATTTATAATACTATTGTAACAGCTCATGCTTTTATTATAATTTTTTTTATAGTAATACCCATTATAATTGGAGGATTTGGTAATTGATTAGTTCCTCTAATATTAGGAGCTCCAGATATAGCTTTCCCACGAATAAATAATATAAGTTTTTGATTATTACCCCCCTCTCTAATACTTTTAATTTCAAGAAGTATTGTAGAAAATGGAGCAGGAACAGGATGAACAGTTTACCCCCCACTGTCATCAAACATTGCTCATAGAGGAAGATCTGTTGATTTAGCTATTTTTTCCCTTCACTTAGCAGGTATTTCATCAATTTTAGGAGCTATTAATTTTATTACAACAATTATTAATATACGATTAAATAACATATCTTTTGATCAAATACCTTTATTTGTTTGAGCTGTAGGAATTACTGCTTTTTTACTTTTACTCTCTCTTCCAGTATTAGCAGGAGCTATTACTATATTATTAACCGATCGTAATTTAAATACATCTTTTTTTGACCCCGCTGGGGGAGGAGATCCTATTTTATATCAACATTTATTT
This species appears to be limited to the eastern slopes of the Andes from Bolivia to Villavicencio, Colombia.
Disphragis sobolis was recently described from Ecuador; the species appears to have a much greater geographical range and occurs to almost 3000 m. The lower altitude limits of its range are undefined, as is the southern boundary.
The Disphragis notabilis complex is typical of many neotropical species. When studied in detail, they frequently are found to consist of a number of very similar species that do have structural differences, can be separated by barcoding, and occupy different altitudes or geographic ranges. The correct generic placement of this complex is not in Disphragis (as placed by
A second difficulty encountered in this study was the hyper-variation of the female genital plate in Disphragis hemicera (Figs 26–29). This intraspecific variation (in individuals with identical barcodes) has also been seen in Didugua Druce (JBS, unpubl. data) and makes the delineation of species and their defining characters with female specimens extremely difficult. Fortunately, such extreme variation has not been observed in males.
The exact distribution of the four species of the Disphragis notabilis complex found in Colombia (and probably Ecuador) remains to be elucidated but should highlight the individual habitat requirements of each species. Neither larvae nor foodplants are known. The geographical area where Disphragis bifurcata and Disphragis notabilis come into contact should be particularly interesting because the two species differ by 1.4% in their barcodes, a magnitude lower than between most congeneric species. However, there are a number of characters that separate the two species and characters do not seem to intergrade in individuals from central Colombia and western Venezuela.
We would like to thank Jocelyn Gill, Don Lafontaine, and Chris Schmidt at the Canadian National Collection. Jocelyn for help with the illustrations, Don and Chris for suggestions regarding the manuscript. Paul Hebert of the University of Guelph, Guelph, Canada allowed the use of unpublished barcode data. Dan Janzen and Winnie Hallwachs shared unpublished barcode and life history data. Paul Thiaucourt shared his genitalic preparations and extensive knowledge of notodontids. Bernardo Espinosa at INBio enthusiastically helped collect many of the specimens used in this study.