Citation: Williams KA, Villet MH (2014) Morphological identification of Lucilia sericata, Lucilia cuprina and their hybrids (Diptera, Calliphoridae). ZooKeys 420: 69–85. doi: 10.3897/zookeys.420.7645
Hybrids of Lucilia sericata and Lucilia cuprina have been shown to exist in previous studies using molecular methods, but no study has shown explicitly that these hybrids can be identified morphologically. Published morphological characters used to identify L. sericata and L. cuprina were reviewed, and then scored and tested using specimens of both species and known hybrids. Ordination by multi-dimensional scaling indicated that the species were separable, and that hybrids resembled L. cuprina, whatever their origin. Discriminant function analysis of the characters successfully separated the specimens into three unambiguous groups – L. sericata, L. cuprina and hybrids. The hybrids were morphologically similar irrespective of whether they were from an ancient introgressed lineage or more modern. This is the first evidence that hybrids of these two species can be identified from their morphology. The usefulness of the morphological characters is also discussed and photographs of several characters are included to facilitate their assessment.
Greenbottle blowflies, keys, morphology, discriminant analysis
The use of maggot debridement therapy (MDT) in South Africa has gained interest in the past decade (
Several identification keys have been produced either specifically for Lucilia sericata and Lucilia cuprina, or for larger suites of Luciliinae or Calliphoridae that included these two species (
A complicating factor is the known and widespread existence of natural hybrids of these species (
Twenty-four specimens of Lucilia sericata, Lucilia cuprina and their hybrids (Table 1) were chosen from specimens that had been sequenced for 28S, COI and Per genes (
Specimens previously identified by molecular markers (
| Species | Specimen | Country of origin |
|---|---|---|
| Lucilia cuprina | C_EGT_01 | Egypt - Alexandria |
| Lucilia cuprina | C_SA_BFN_01 | South Africa – Bloemfontein |
| Lucilia cuprina | C_SA_BFN_02 | South Africa – Bloemfontein |
| Lucilia cuprina | C_SA_BRT_01 | South Africa – Britstown |
| Lucilia cuprina | C_SA_BRT_02 | South Africa – Britstown |
| Lucilia cuprina | C_SA_DBN_12 | South Africa – Durban |
| *Lucilia cuprina | C_SA_DBN_01 | South Africa – Durban |
| *Lucilia cuprina | C_SA_DBN_06 | South Africa – Durban |
| *Lucilia cuprina | C_SA_NEL_01 | South Africa – Nelspruit |
| *Lucilia cuprina | C_SA_NEL_02 | South Africa – Nelspruit |
| *Lucilia cuprina | C_THA_03 | Thailand – Chiang Mai |
| *Lucilia cuprina | C_ZIM_02 | Zimbabwe – Matobos |
| Lucilia sericata | S_FRC_02 | France – Montferrier-Sur-Lez |
| Lucilia sericata | S_GER_01 | Germany – Kempen |
| Lucilia sericata | S_JPN_04 | Japan – Iwate |
| Lucilia sericata | S_NAM_01 | Namibia – Possession Island |
| Lucilia sericata | S_NAM_02 | Namibia – Possession Island |
| Lucilia sericata | S_SA_CT_01 | South Africa – Cape Town |
| Lucilia sericata | S_SA_CT_05 | South Africa – Cape Town |
| Lucilia sericata | S_SA_GHT_01 | South Africa – Grahamstown |
| Lucilia sericata | S_SA_GHT_02 | South Africa – Grahamstown |
| Lucilia sericata | S_SA_PTA_02 | South Africa – Pretoria |
| Lucilia sericata | S_SA_WTB_02 | South Africa – Witbank |
| Lucilia sericata | S_USA_01 | United States of America – Michigan |
A total of 18 distinguishing morphological characteristics of adults of Lucilia sericata and Lucilia cuprina (Table 2) were obtained by reviewing several published sources (
Published morphological characters used to distinguish specimens of Lucilia sericata and Lucilia cuprina.
| Character | Lucilia sericata | Lucilia cuprina | Analysis | |
|---|---|---|---|---|
| MDS | DFA | |||
| General | ||||
| Number of paravertical setulae or occipital bristles ( |
Usually 2+2 but up to 8+8 (not always equal numbers i.e. can be 1+2 etc.) | 1+1 | yes | no |
| Shape of postocular microtrichial pile on vertex (viewed obliquely from behind) ( |
Boundary between pale and dark areas not straight or sharply defined | Boundary straight and sharply defined | no | no |
| Width of the frontal stripe (frontal vitta) ( |
Twice as wide as a parafrontal (fronto-orbital) plate | As wide as a parafrontal (fronto-orbital) plate | yes | yes |
| Colour of the frontoclypeal membrane ( |
Light brown | Dark brown to black | yes | yes |
| Second pair of presutural acrostichals ( |
Extend at least as far as insertions of the first pair of postsutural acrostichals | Do not extend to first pair of postsutural acrostichals | yes | no |
| Number of setulae on ‘quadrat’ between discal setae and anterior margin of scutellum ( |
35–55 | 15–25 | yes | yes |
| Bristles on the scutellum ( |
Dorsal bristles distinctly smaller than lateral hairs | Dorsal bristles slightly smaller than or equal to lateral hairs | no | no |
| Number of hairs on the posterior slope of the humeral callus behind the basal setae ( |
6–8 | 0–4 | yes | yes |
| Number of hairs on the edge of the notopleuron behind the posterior notopleural seta ( |
8–16 | 2–5 | yes | yes |
| Metasternal area – sclerite midventrally between middle and hind coxae ( |
Hairy | Bare | no | no |
| Colour of the fore femora ( |
Dark metallic blue to black or dark brown | Metallic green | yes | yes |
| Contour of the last abdominal tergite ( |
Irregular depressions | Generally smooth | no | no |
| Females | ||||
| Distance between the outer and inner vertical setae of females ( |
Equal to 0.5–0.7 distance between prevertical and inner vertical setae | Equal to the distance between prevertical and inner vertical setae | yes | no |
| Size of the angle formed by the inner vertical seta relative to the prevertical and outer vertical setae of females ( |
Obtuse | Right angle | yes | no |
| Extent of metallic sheen on parafrontal sclerites of females ( |
From vertex barely to base of upper orbital seta and not enclosing bases of any frontal setae | From vertex almost to base of lower orbital seta and enclosing bases of 1 or 2 frontal setae | yes | yes |
| Males | ||||
| Shape of apical halves of cerci ( |
Broad and tapering | Slender and parallel | no | no |
| Shape of apical halves of surstyli ( |
Curved and broad | Straight and slender | no | no |
| Form of apical setae of cerci ( |
Long and wavy | Minute and straight | no | no |
Each specimen was scored against the 15 characters (Table 2). Each character was then evaluated for its effectiveness in discriminating between the species and its practical value for identification, first univariately and qualitatively, and then multivariately and quantitatively using non-metric multi-dimensional scaling (MDS) in PAST3 (
To explore the diagnosibility of the hybrids, a discriminant function analysis (DFA) was performed using PAST3 (
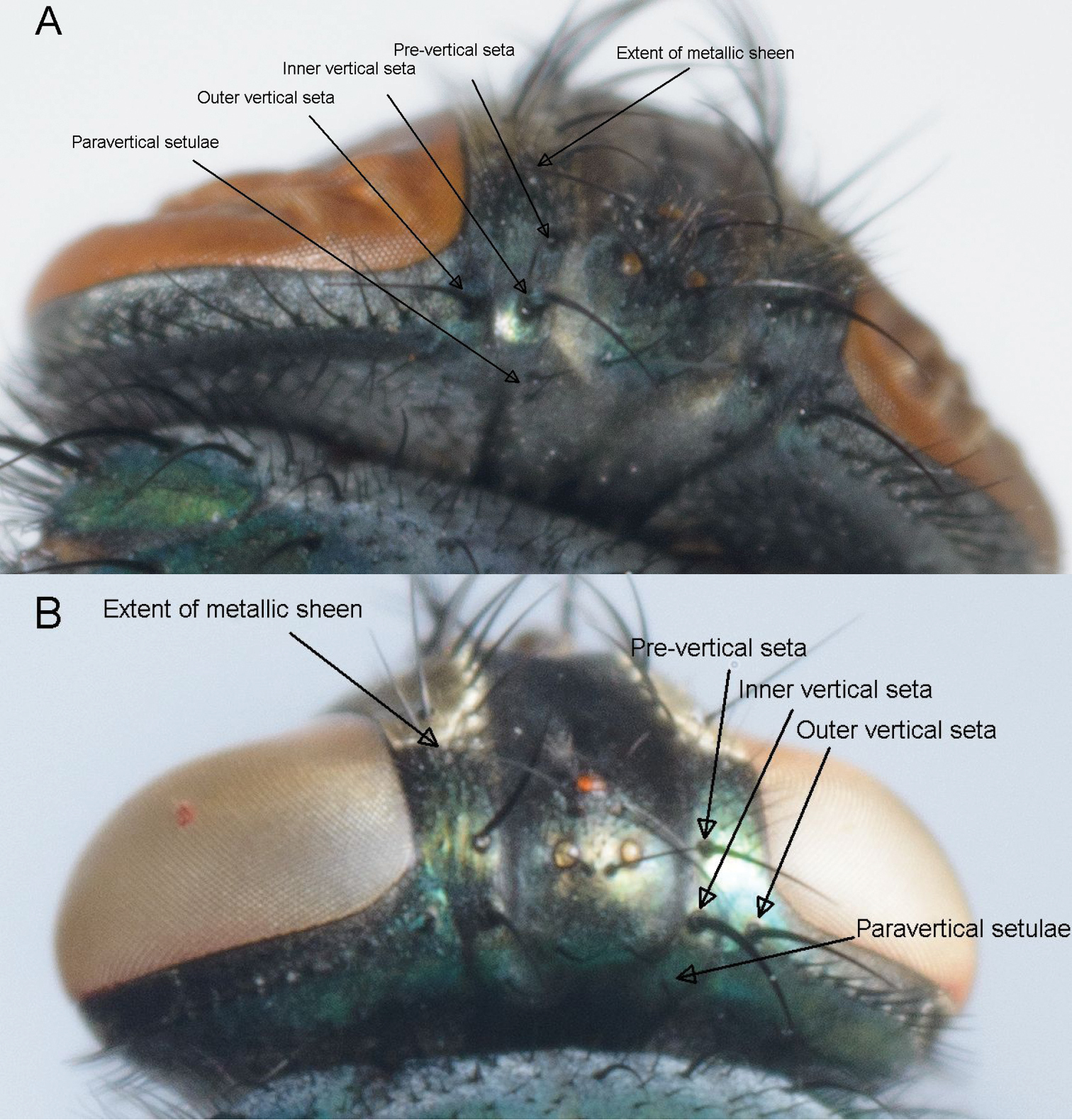
The number of paravertical setulae or occipital bristles (Table 2; Figure 1). This character was relatively consistent and reliable, but it is not easily viewed and scored if the specimens have been kept in ethanol. The hybrid specimens all keyed out as Lucilia cuprina. This character was left out of the DFA analysis due to lack of variation within Lucilia cuprina.
Paravertical setulae, distance between the outer and inner vertical setae, the size of the angle at the inner vertical triangle and extent of metallic sheen on parafrontal sclerites. Lucilia sericata (A) and Lucilia cuprina (B).
The shape of the postocular microtrichial pile on the vertex (Table 2) (
The relative positions of the three vertical setae (Table 2; Figure 1) that form a triangle on either side of the ocellar triangle in females (
The angle formed by the three vertical setae (Table 2; Figure 1). This character is consistent and easily seen even if the setae have fallen out as they have sockets, which are easily visible. Due to lack of variation within species and the hybrids being identified as Lucilia cuprina, this character was also excluded from the discriminant function analysis but it was included in the MDS analysis.
The extent of the metallic sheen on the parafrontal sclerites of females (Table 2 and Suppl. material 1; Figure 1). This character is easier to observe in dried specimens than ethanol-preserved specimens and there is some variation. The division between the two species is not absolute – there is some overlap within this character but it was not specific to the hybrids. It was included in both the DFA and MDS analyses.
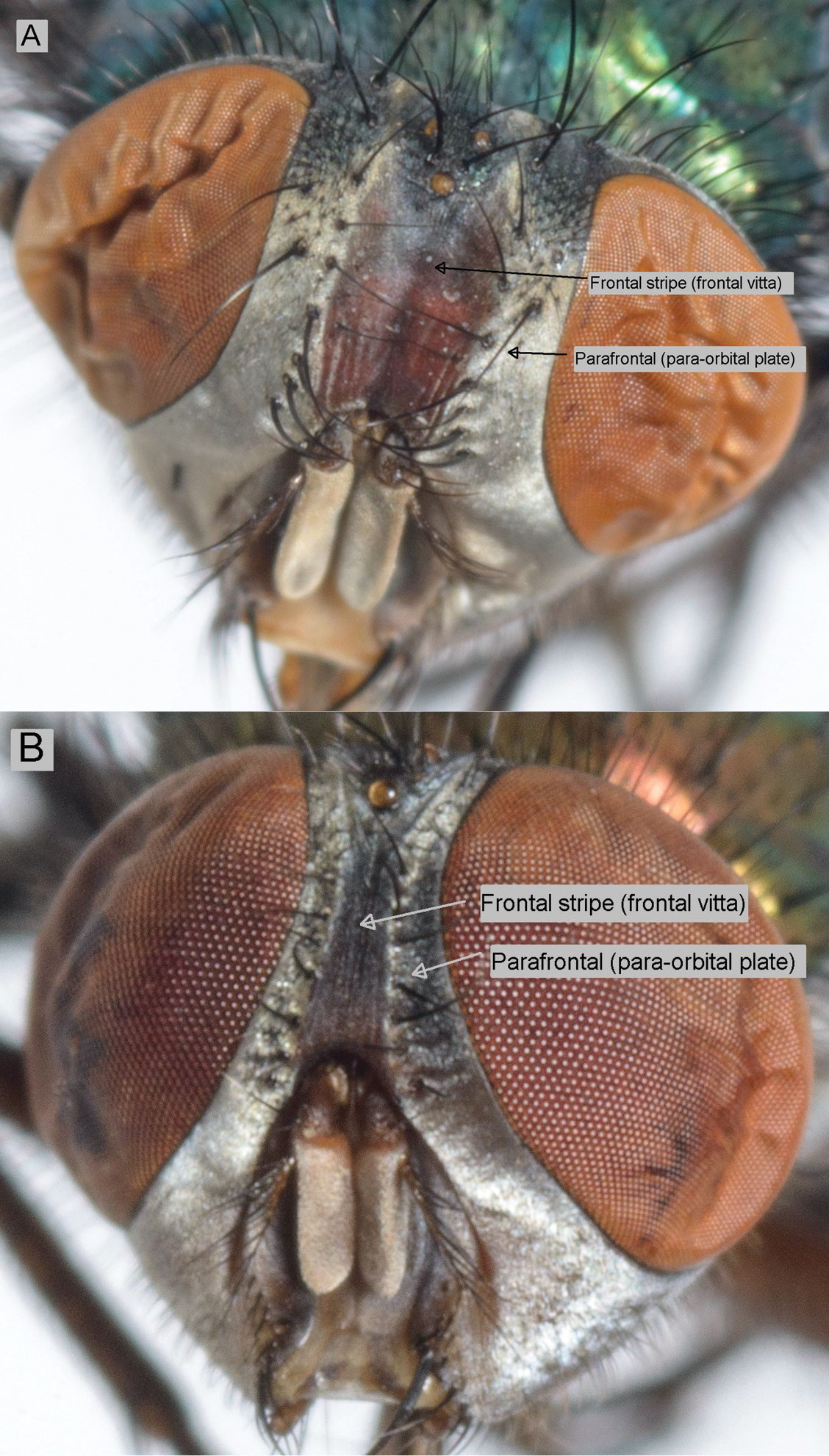
The relative width of the frontal stripe (frontal vitta) (Table 2 and Suppl. material 1; Figure 2).
Frontal stripe – Lucilia sericata (A) and Lucilia cuprina (B).
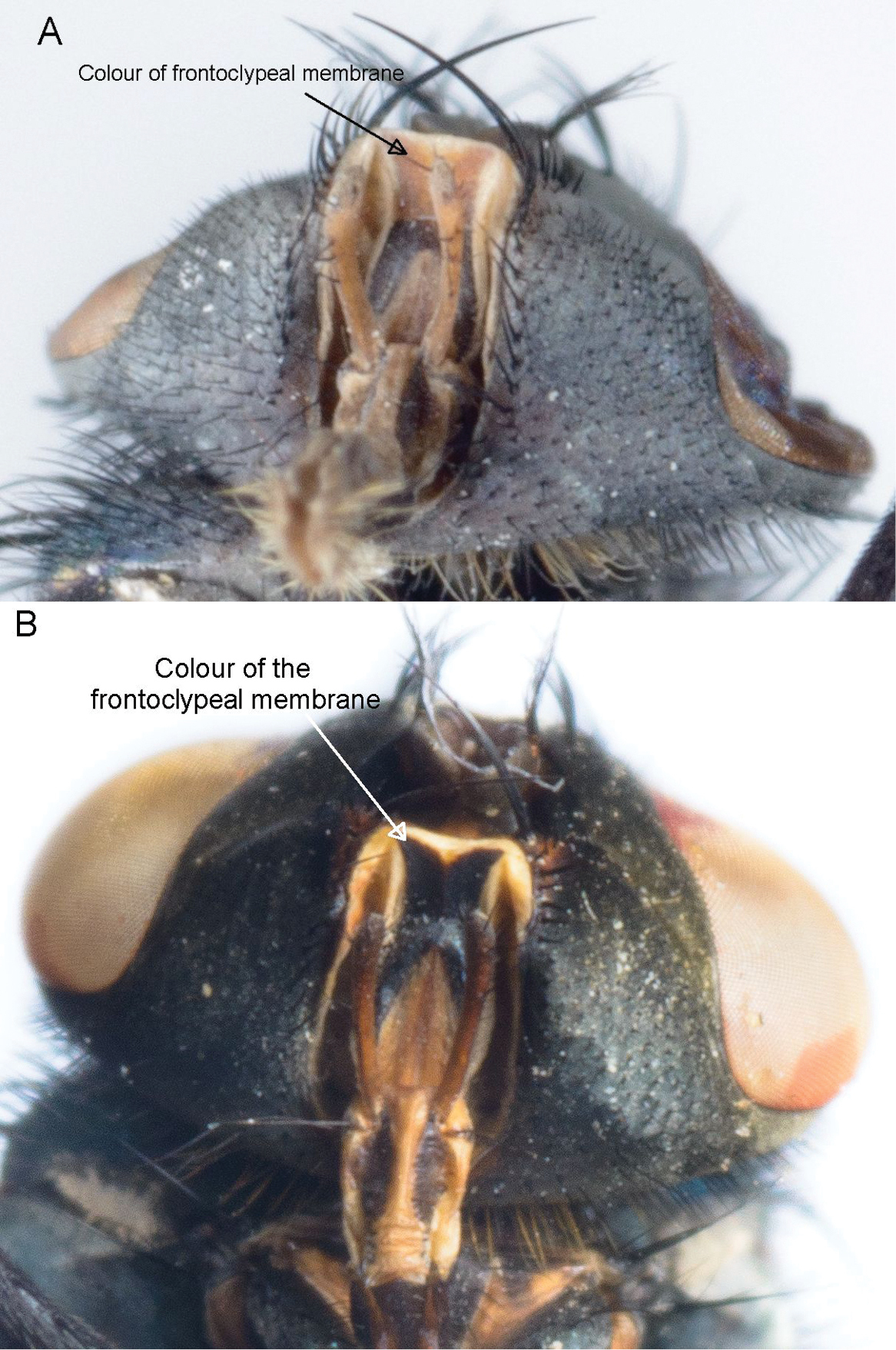
The colour of the frontoclypeal membrane (Table 2 and Suppl. material 1; Figure 3). It was not always easily visible if the proboscis was not extended but it could usually be viewed by either manipulating the proboscis or viewing the specimen from a lateral angle (
Colour of the frontoclypeal membrane. Lucilia sericata (A) and Lucilia cuprina (B).
The length of the second pair of presutural acrostichals (Table 2) is a character that is easier to see in well-preserved specimens (
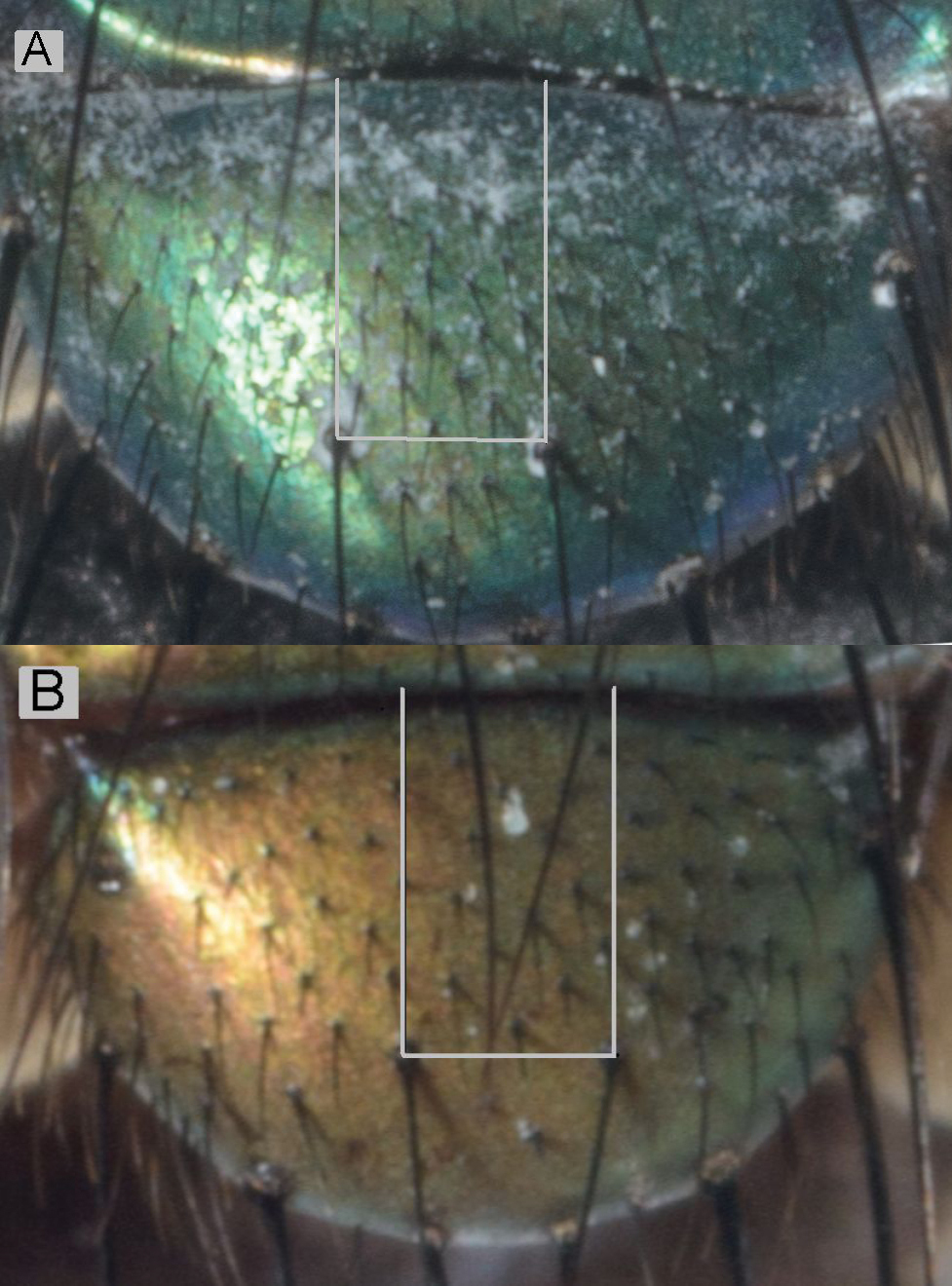
The number of setae on the scutellum (Table 2 and Suppl. material 1; Figure 4) in the ‘quadrat’ demarcated by the discal setae and the anterior margin of the scutellum represents the axis in the discriminant analysis that separated Lucilia sericata and Lucilia cuprina (
Number of setae on ‘quadrat’ between the anterior margin and discal setae on the scutellum. Lucilia sericata (A) and Lucilia cuprina (B).
The length of the bristles on the scutellum (Table 2 and Suppl. material 1) describes the length of the hairs between the two anterior bristles on the lateral margin of the scutellum in relation to the length of the hairs on the dorsal surface of the scutellum (
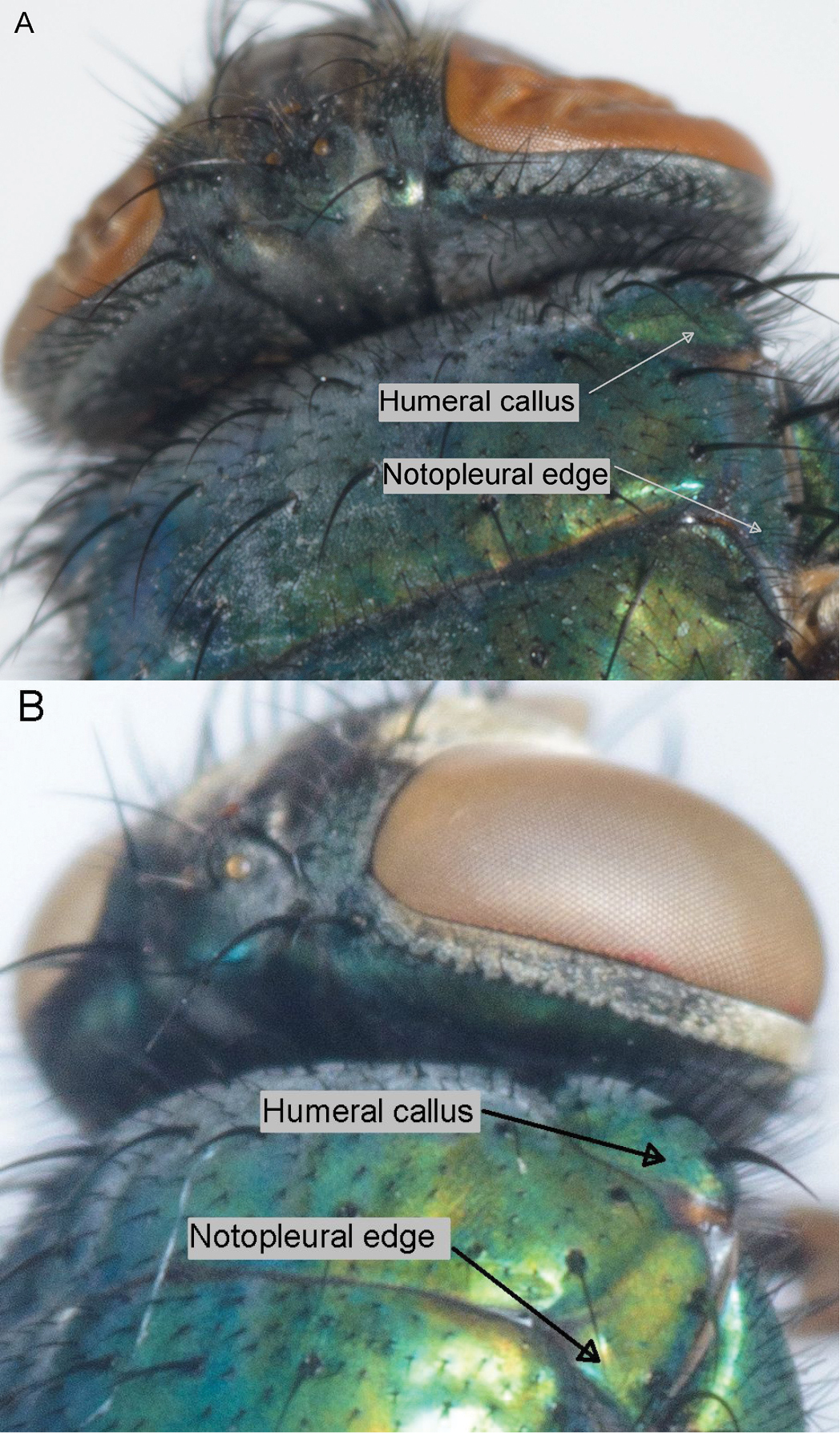
The hairiness of the posterior slope of the humeral callus (Table 2 and Suppl. material 1; Figure 5) behind the basal setae is a reliable character in separating Lucilia sericata and Lucilia cuprina even though there is variation within species in the number of hairs. The hybrids tended to have more hairs than the pure Lucilia cuprina specimens, but there was still overlap in the numbers of hairs between the hybrids and pure Lucilia cuprina.
Posterior slope of the humeral callus behind the basal setae and the posterior edge of notopleuron behind the posterior notopleural seta. Lucilia sericata (A) and Lucilia cuprina (B).
The number of hairs on the edge of the notopleuron (Table 2 and Suppl. material 1; Figure 5). Both the hairs on the notopleuron and the humeral callus are relatively easy to observe although ethanol-preserved specimens need to be dried so that the small hairs are visible. It is another reliable character in separating Lucilia sericata from Lucilia cuprina despite variation in the number of hairs within species. The hybrids showed no discernable difference in numbers of hairs from Lucilia cuprina.
The hairs on the metasternal area (Table 2), which is the sclerite mid-ventrally between the middle and hind coxae, are exceedingly difficult to view if the legs are not set appropriately to facilitate this.. All of the specimens that we examined were preserved in ethanol and it was not easy to view the metasternal area and this character was therefore not analysed.
The colour of the fore femora (Table 2 and Suppl. material 1) has long been used as a character to identify Lucilia sericata and Lucilia cuprina (
The contour of the last abdominal tergite (Table 2) is applicable only to dried specimens (
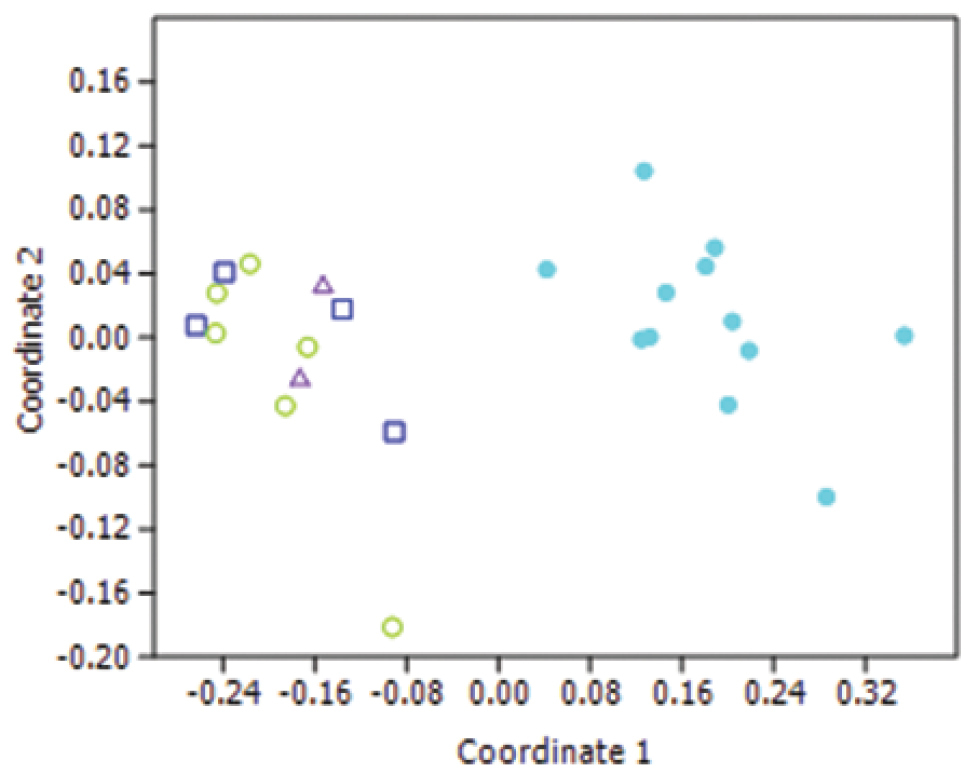
Superficially, the hybrid specimens were identified as Lucilia cuprina when keyed out using any of the published keys. There were no obvious differences in the morphology of the hybrids. When the characters were analysed using MDS, the hybrid specimens were not separated from the Lucilia cuprina specimens (Figure 6).
Non-metric Multi-Dimensional Scaling plot using a Manhattan distance metric using 11 characters. Light blue solid circles = Lucilia sericata, Green open circles = Lucilia cuprina, dark blue squares = introgressed hybrids, purple triangles = modern hybrids.
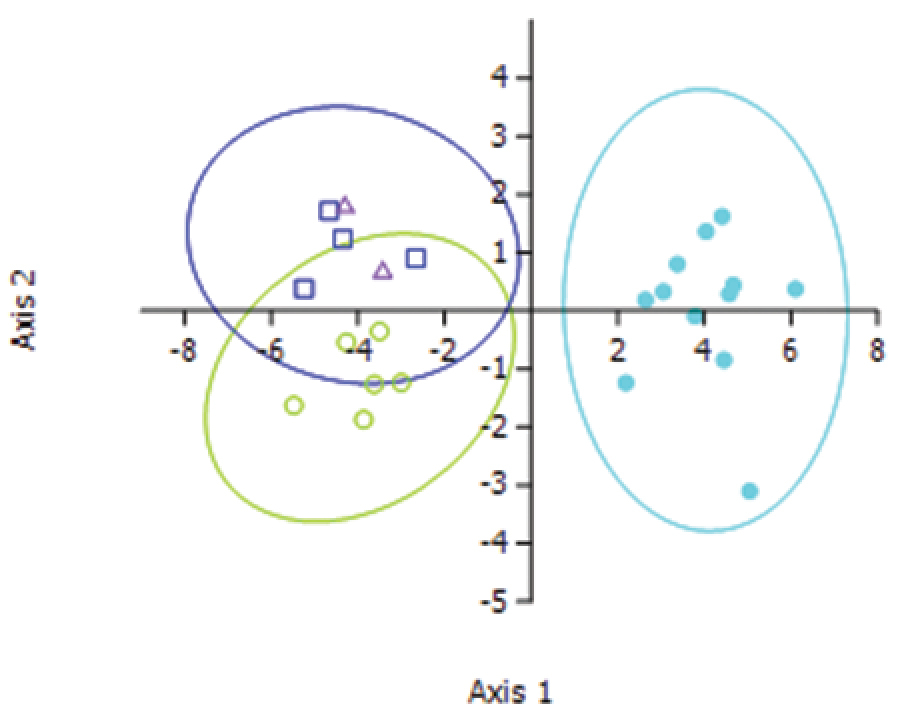
However, the ordination plot of the DFA (Figure 7) clearly shows three groups – Lucilia sericata, Lucilia cuprina and hybrids. The most influential characters were the number of setae on the scutellum (Root 1) and the number of hairs on the humeral callus (Root 2) (Table 3). It is not obvious in the morphology that there is a difference between the pure and hybrid strains, but statistically one can separate the hybrids from the pure Lucilia cuprina specimens.
Ordination plot of the first two roots of the discriminant function analysis using seven characters. Ellipses represent 95% confidence regions. Light blue solid circles = Lucilia sericata, Green open circles = Lucilia cuprina, dark blue squares = introgressed hybrids, purple triangles = modern hybrids.
Eigen vectors and values for the first two roots of the discriminant function analysis.
| Character | Root 1 | Root 2 |
|---|---|---|
| Number of setulae on ‘quadrat’ demarcated by discal setae and anterior margin of scutellum | 1.5822 | 0.0324 |
| Number of hairs on edge of notopleuron behind posterior notopleural seta | 0.5576 | 0.3300 |
| Number of hairs on posterior slope of humeral callus behind basal setae | 0.4216 | 0.9066 |
| Colour of fore femora | 0.2591 | -0.2023 |
| Relative width of frontal stripe (frontal vitta) | 0.1551 | 0.0104 |
| Extent of metallic sheen on parafrontal sclerites of females | 0.0519 | -0.0697 |
| Colour of frontoclypeal membrane | -0.1551 | -0.0104 |
| Eigenvalue | 18.5560 | 0.7406 |
Due to the greater number of female flies in the molecular study from which we chose our specimens, we did not include any males. Therefore the male genitalia characters are not discussed in detail. It is not possible to properly view the male genitalia without dissecting them and this is not ideal for non-entomologists such as medical doctors who are using these flies for MDT as one needs experience to dissect out the genitalia. It is possible to correctly identify these flies without using the male genitalia by using the other characters described in Table 2.
The DFA unambiguously separated the Lucilia cuprina specimens from the hybrids and it was statistically significant. This was not noted in previous studies where hybrids were identified only through molecular techniques (
The introgressed and modern hybrids were not separated in the DFA ordination plot (Fig. 6).
Introgressed and modern hybrids of Lucilia sericata and Lucilia cuprina can be statistically recognized using the characters described in this paper.
Four of the characters were consistently successful at separating Lucilia sericata and Lucilia cuprina (number of paravertical setulae or occipital bristles, distance between the outer and inner vertical setae of females, size of the angle at the inner vertical in triangle joining pre-, outer and inner vertical setae of females, second pair of presutural acrostichals) with little variation within the characters. The number of setae on the scutellum and the number of hairs on the humeral callus and notopleuron are also useful characters although they did show variation within species. It is advisable to use a combination of several characters to identify these two species as no single character was sufficient to separate Lucilia sericata and Lucilia cuprina.
We thank Georg Goergen, Ashley Kirk-Spriggs, Nicky Lunt, Mervyn Mansell, Hideharu Numata, Cameron Richards, Kiyoshi Saigusa, Kabkaew Sukontason, Tarek Tantawi and Robyn Tourle for providing us with specimens, Emil von Maltitz for taking photographs. Funding was provided by the National Research Foundation (NRF) of South Africa. Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Research Foundation.
Character-taxon matrix
Kirstin A. Williams, Martin H. Villet
Data type: Species data
Explanation note: Character-taxon matrix used in the MDS and DFA analyses
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.