Citation: Muir AI, Hossain MdMM (2014) The intertidal polychaete (Annelida) fauna of the Sitakunda coast (Chittagong, Bangladesh), with notes on the Capitellidae, Glyceridae, Lumbrineridae, Nephtyidae, Nereididae and Phyllodocidae of the “Northern Bay of Bengal Ecoregion”. ZooKeys 419: 1–27. doi: 10.3897/zookeys.419.7557
Of seven species of polychaetous annelids collected from the intertidal zone of Sitakunda coast, Chittagong, Bangladesh, five were new records for the country. The seven are listed, with brief notes on these, some previously recorded! species and others housed in the collections of the Natural History Museum, London. Keys are given to the recorded species of Phyllodocidae, Nereididae, Lumbrineridae, Nephtyidae and Capitellidae of the “Northern Bay of Bengal Ecoregion”, and to the recognised species of Glyceridae from the Bay of Bengal. The worms in this Ecoregion are subject to the outflows of the Irrawaddy, Ganges, Hooghly and Mahanadi Rivers, and many of them are known to be freshwater tolerant.
Taxonomy, Polychaeta, new records, keys, Odisha, West Bengal, Myanmar
There has long been an emphasis on taxonomy in marine studies, for example
Polychaete annelids are a major group within the soft bottom macro-invertebrates (
The polychaete fauna of Bangladesh is little studied, despite the importance of marine resources to the country. The largest identification works for the littoral and shallow-water polychaetes of the Indian Ocean area are
The present study therefore aims to provide further information on the taxonomy of polychaetes in Bangladesh waters at two sites on the Sitakunda Upazila coast, north of the city of Chittagong (see Table 1), one of which is affected by ship-breaking activity on the shore.
Details of the sampling sites.
| Site | Name & location | Substratum | Remarks |
|---|---|---|---|
| 1 | Muradpur, 22°35'02"N, 91°34'09"E | Silty-muddy with fine grain of sand (towards sea side) | Relatively undisturbed site along with planted mangroves (relatively high Organic Carbon and Organic Matter compared to other site) |
| 2 | Madambabirhat, 22°30'56"N, 91°43'44"E | Sandy cum muddy | Highly polluted & disturbed area due to Ship Breaking Activities in intertidal zone of the coast (low OC & OM) |
The “Northern Bay of Bengal Ecoregion” of the “Bay of Bengal Province” of the “Western Indo-Pacific Realm” was devised by
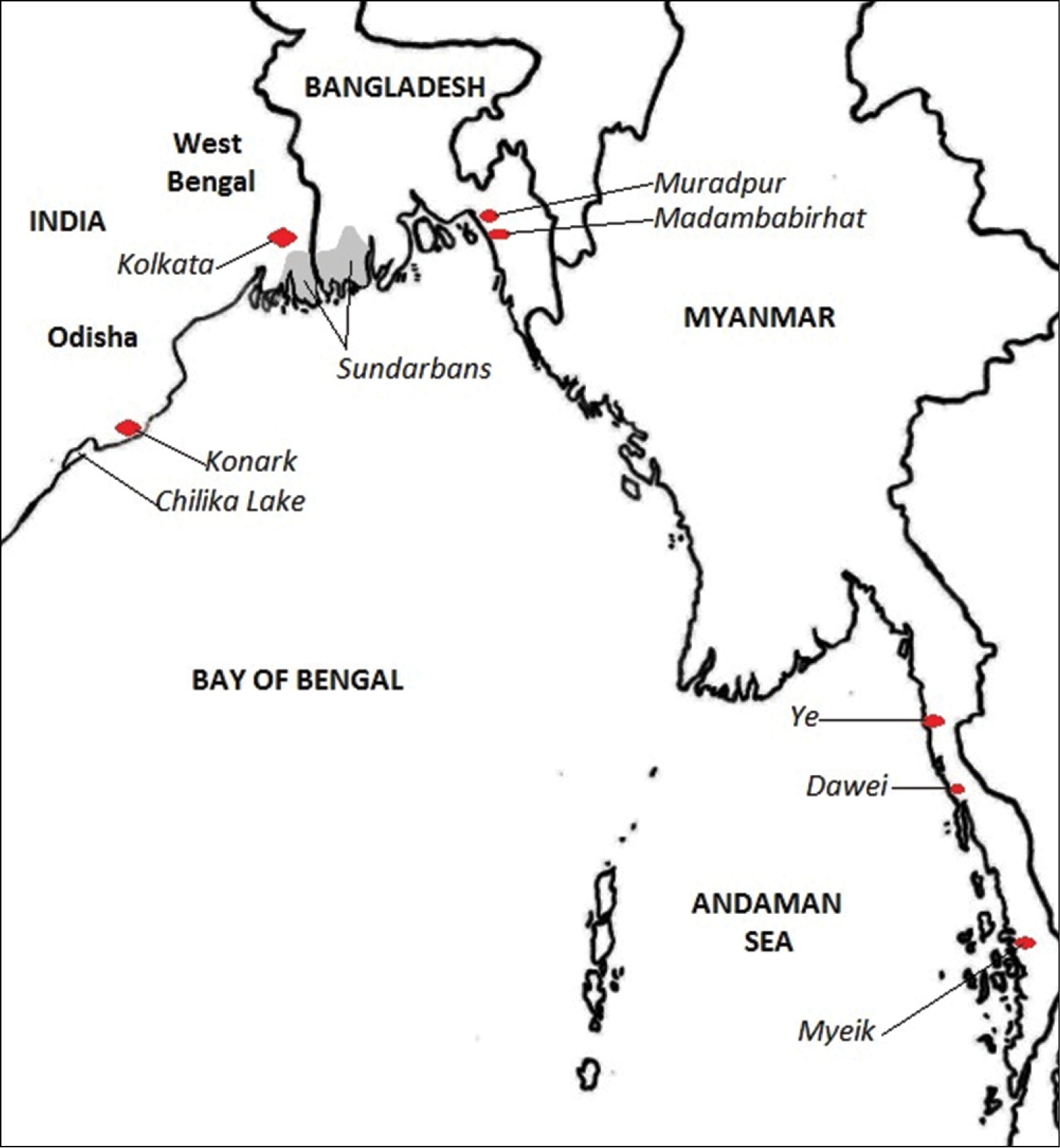
In this paper, the new specimens from Bangladesh are compared with the same families of polychaetes reported from the “Northern Bay of Bengal Ecoregion”, including the entire coast of Myanmar and the entire Odisha coast (to include the freshwater polychaetes of Chilka Lake). Important localities are shown in Figure 1.
Map of localities mentioned in the text.
Quantitative samples were collected between April 2007 and November 2008, but the present paper only deals with the taxonomic details of polychaetes collected at those sites. Samples were collected from the intertidal zone by using a hand-held corer with a depth penetration of 15 cm. The collected samples were washed through a 0.5 mm mesh hand sieve with filtered water at the collection point to separate animals from sediment. The materials retained on the sieve were placed in plastic vials to which 5% formalin was added for fixing the organisms, and labelled. The vital stain Rose Bengal was added to the vials to help in sorting the organisms from debris. In the laboratory the materials were poured into a round transparent Petri dish and separated from debris using needle, brush and magnifying glass. Then the organisms were preserved in 75% ethyl alcohol for identification. An Olympus compound microscope with video facility was used and relevant keys (
Because there are so few records from Myanmar, some specimens deposited in the Natural History Museum, London, by Professors G.E. Gates (Judson College, Rangoon) and F.J. Meggitt (University College, Rangoon) between 1931 and 1938, and only partially published by
Identification keys are given in this paper, but any identifications made using them should be checked against good descriptions or reliably identified specimens, because not only may new records or even new species be found, but some of the older reports cited here may have been mis-identifications or represent cryptic species (it is interesting that the type locality of Capitella capitata is West Greenland (
The taxonomy and systematics of the Annelida have been rapidly changing in recent years. It must be recognised that the classifications used in publications such as
Polychaetous annelids are often regarded as a marine group (albeit with some freshwater tolerant species), but it should be noted that non-marine species also exist (see
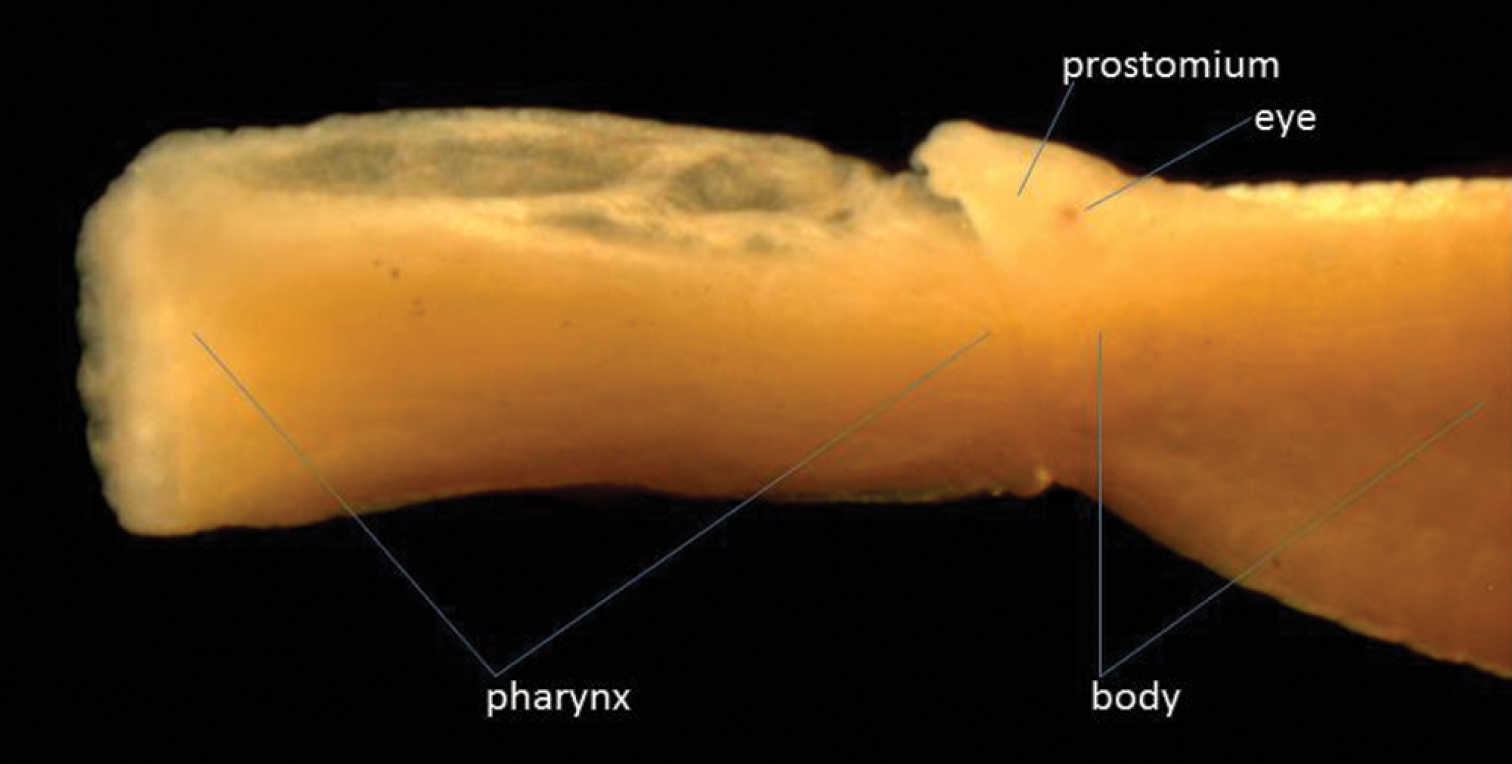
One specimen was found: length 15 mm, width 0.75 mm for 92 segments, but anal cirri missing. Anteriorly the height of the segments is 1mm, but posteriorly the body becomes dorso-ventrally flattened. This specimen has two pairs of tentacular cirri on the first segment, the dorsal being shorter than the ventral ones (they are both, however, small and difficult to see). The first chaetae are on the second segment. The pharynx is everted, showing a smooth surface and a ring of 12 large subglobular papillae around the opening (Fig. 2). The dorsal cirri are small and rounded, compressed against the side of the body. The ventral cirri, distally rounded, are almost as long as the chaetal lobe anteriorly, but slightly longer posteriorly. The tip of the acicula is just emergent from the chaetal lobe in the anterior part of the body, but in the posterior part of the body is much more protuberant. The specimen is colourless in alcohol except for some brown markings dorsally by the pygidium.
Lateral view of anterior end of Eteone cf. delta with pharynx extended.
This specimen, especially the structure of the pharynx, displays similarities to Eteone delta Wu & Chen, 1963, which is known from the Yangtze delta, the Pearl River and Zhangjiang estuary, China (
This is a new record for Bangladesh, no members of the family Phyllodocidae being recorded by
Two specimens from Maungmagaun, Myanmar, in the Natural History Museum, London, (NHMUK ANEA 1935.1.31.34 and NHMUK ANEA 1937.1.4.4) have been identified as Phyllodoce castanea by C.C.A. Monro. On both of these specimens many of the head appendages are missing or regenerating, but the identifications are probably correct. The species is now known as Nereiphylla castanea (see synonymy in
Two species of Eteone are recorded from West Bengal (
In Odisha, Anaitides madeirensis, Eteone (Mysta) ornata and Eteone barantollae have been recorded from estuaries by
These species from northern Bay of Bengal waters can be keyed out as follows, but any identifications must be checked against reliable descriptions as many other species are known from the Indo–Pacific area.
| 1 | Two pairs of tentacular cirri | 2 |
| – | Four pairs of tentacular cirri | 4 |
| 2 | Pharyngeal surface smooth, but terminates in a ring of 12 large subglobular papillae | Eteone cf. delta Wu & Chen, 1963 |
| – | Pharyngeal surface distally with rows of swollen papillae | 3 |
| 3 | Pharynx with five distal rows of swollen papillae | Hypereteone barantollae (Fauvel, 1932) |
| – | Pharynx with three to four rows of swollen papillae. Body with three rows of dark spots | Mysta ornata Grube, 1878 |
| 4 | Median antenna present on head | Eulalia magalaensis Kinberg, 1866 |
| – | Median antenna absent | 5 |
| 5 | Segments 1 and 2 fused, but not forming a collar; Pharynx with small, irregularly distributed, papillae | Nereiphylla castanea (Marenzeller, 1879) |
| – | Segment 1 covered dorsally by the posterior part of the prostomium, but not fused to segment 2; Pharynx with 12 longitudinal rows of papillae proximally and 6 rugose bands distally | Phyllodoce madeirensis Langerhans, 1880 |
The pharynx is often not everted in preserved material, but the jaws and any paragnaths/papillae present may be seen by making a mid-ventral cut backwards from the mouth, cutting through the ventral surface of the pharynx as well as the body wall for several segments, and folding the resulting flaps to the side to reveal the complete jaw apparatus.
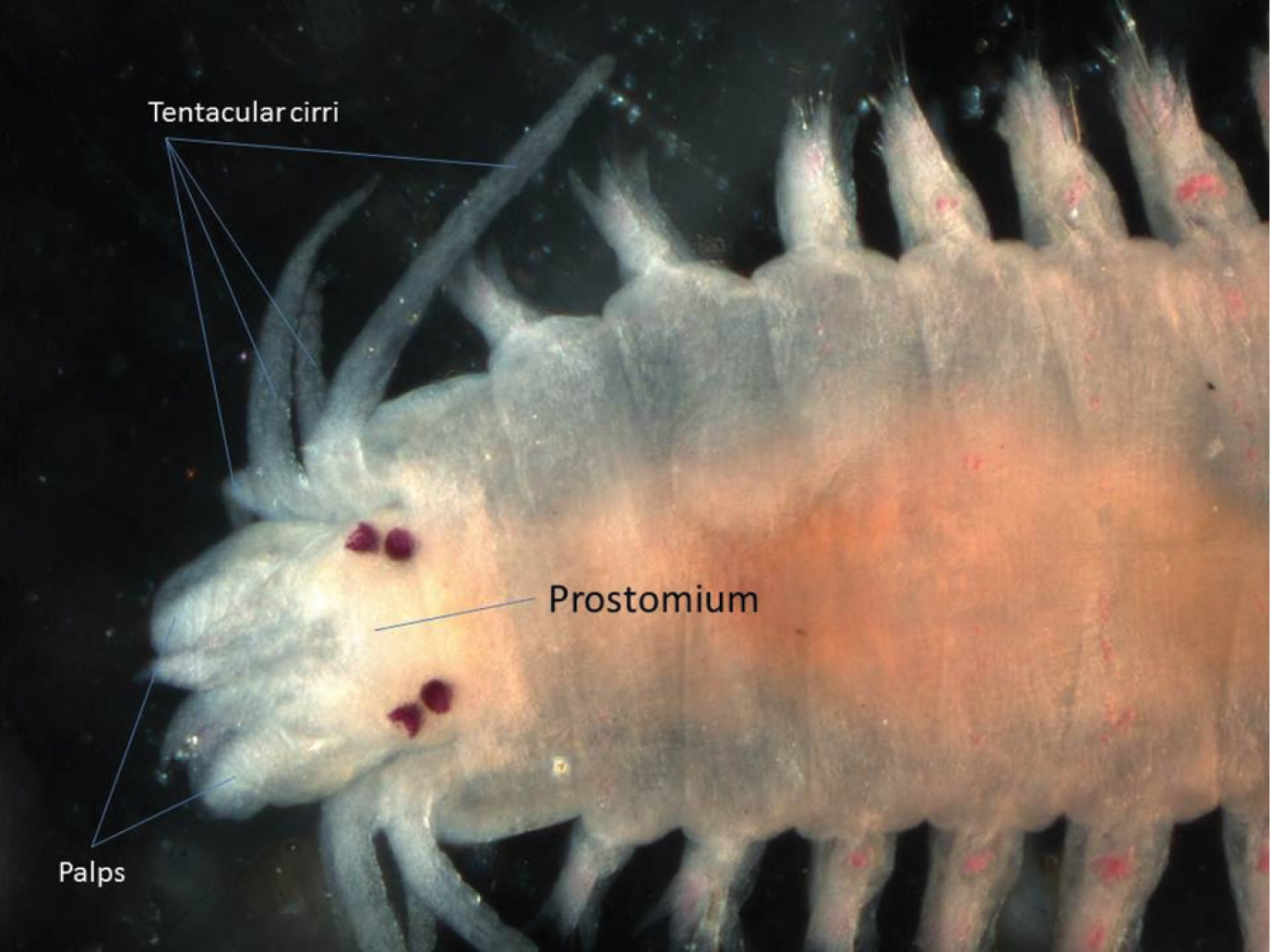
One specimen was found. This species could be regarded as a typical nereidid, having paragnaths on the pharynx and four pairs of tentacular cirri (Fig. 3). The arrangement of the paragnaths agrees with that depicted by
Dorsal view of anterior end of Neanthes chingrighattensis.
This species has no paragnaths on the pharynx and only three pairs of tentacular cirri (Fig. 4). It is, however, not a member of the genus Namanereis because it has parapodia with two distinct branches (notopodium and neuropodium) each with chaetae. Members of Namanereis only have one parapodial lobe with chaetae. This species is not mentioned in
Dorsal view of anterior end of Lycastonereis indica.
The collections from Myanmar in the Natural History Museum, London, include: NHMUK ANEA 1933.3.18.43, NHMUK ANEA 1937.1.4.16-42 Ceratonereis burmensis Monro, 1937, Types; NHMUK ANEA 1931.6.22.70, NHMUK ANEA 2014.7 Namalycastis abiuma species group; NHMUK ANEA 1931.6.22.67-69 Namalycastis multiseta Glasby, 1999, Types; NHMUK ANEA 1931.6.22.71-73 Neanthes meggitti Monro, 1931, Types; NHMUK ANEA 1933.3.18.5-14, NHMUK ANEA 1935.1.31.6, NHMUK ANEA 1935.1.31.7-8 Nereis falcaria; NHMUK ANEA 1933.3.18.15-16 Nereis sp.; NHMUK ANEA 1933.3.18.1-4, NHMUK ANEA 1937.3.10.10-11 Nereis zonata; NHMUK ANEA 1937.3.10.12 Perinereis cultrifera?; NHMUK ANEA 1937.1.4.43-44 Perinereis nuntia; NHMUK ANEA 1932.11.25.5, NHMUK ANEA 1937.1.4.45-66 Perinereis singaporiensis; NHMUK ANEA 1932.11.25.2-3, NHMUK ANEA 1933.3.18.44-46, NHMUK ANEA 1935.1.31.16-18, NHMUK ANEA 1937.1.4.67-68 Pseudonereis trimaculata; NHMUK ANEA 1933.3.18.32-33 Tylonereis bogoyawlenskyi.
Many of these species have had their names changed for taxonomic reasons, or are otherwise worthy of comment.
The genus Ceratonereis has been revised by
Leonnates jousseamei has been synonymised with Leonnates indicus by
Nereis falcaria was reduced to a subspecies of Nereis jacksoni by
Perinereis nuntia has been studied by
The very similar species Pseudonereis trimaculata and Pseudonereis variegata have been kept separate by
The relevant species mentioned above can be keyed out as follows, but any identifications must be checked against reliable descriptions as many other species are known from the Bay of Bengal and other Indo–Pacific areas.
| 1 | Three pairs of tentacular cirri. Paragnaths absent from pharynx | Lycastonereis indica Rao, 1981 |
| – | Four pairs of tentacular cirri. Paragnaths present or absent | 2 |
| 2 | Paragnaths absent from pharynx | 3 |
| – | Paragnaths present on pharynx | 16 |
| 3 | Branchiae present dorsally on some notopodia | 4 |
| – | Branchiae absent from notopodia | 8 |
| 4 | Cluster of branchial filaments below dorsal cirrus on about segments 8 to 40 | 5 |
| – | Dorsal cirrus develops into bipinnate branchia on some middle segments | 6 |
| 5 | Branchiae as row of simple filaments, later developing into two whorls of filaments, on segments 10–38 | Dendronereides gangetica Misra, 1999 |
| – | Cluster of branched branchiae below dorsal cirrus on segments 8 to 40 | Dendronereides heteropoda Southern, 1921 |
| 6 | Chaetae include homogomph and heterogomph spinigers. Papillae present on both rings of pharynx | Dendronereis dayi Misra, 1999 |
| – | Chaetae all homogomph spinigers. Papillae absent or only on oral ring of pharynx | 7 |
| 7 | Pharyngeal papillae absent. All branchiae bipinnate. Anterior neuropodia with 5–6 lobes | Dendronereis arborifera Peters, 1854 |
| – | Papillae present on oral ring of pharynx. First three pairs of branchiae pectinate, the rest bipinnate. Anterior neuropodia with 10–12 lobes and an inferior ligule (number decreasing posteriorly) | Dendronereis aestuarina Southern, 1921 |
| 8 | Parapodia clearly biramous. All chaetae spinigers | 10 |
| – | Parapodia clearly biramous. Spinigers and homogomph falcigers present | 9 |
| – | Parapodia with no deep separation between notopodium and neuropodium. Spinigers and heterogomph falcigers present | 12 |
| 9 | Falcigers present on all neuropodia | Leonnates indicus Kinberg, 1866 |
| – | Falcigers not present on anterior neuropodia | Leonnates persicus Wesenberg-Lund, 1949 |
| 10 | Neurochaetae include homo-, sesqui- and heterogomph spinigers | Ganganereis sootai Misra, 1999 |
| – | All chaetae homogomph spinigers | 11 |
| 11 | Neuropodia trilobed anteriorly, bilobed posteriorly | Tylonereis bogoyawlenskyi Fauvel, 1911 |
| – | All neuropodia bilobed | Tylonereis fauveli Southern, 1921 |
| 12 | Body widest mid-anteriorly (chaetigers 9–20). Sub-neuroacicular chaetae heterogomph spinigers and falcigers | 15 |
| – | Body with uniform width anteriorly, tapering in far posterior region | 13 |
| 13 | Sub-neuroacicular chaetae heterogomph falcigers and heterogomph spinigers | 14 |
| – | Sub-neuroacicular chaetae heterogomph falcigers | Namanereis quadraticeps (Blanchard in Gay, 1849) species group |
| 14 | Prostomium 1.3–2.3× wider than long. Usually less than 10 sesquigomph spinigers in neuropodial supra-acicular fascicle in midbody | Namalycastis abiuma (Grube, 1872) species group |
| – | Prostomium 2.4× wider than long or even wider. 10-30 sesquigomph spinigers in neuropodial supra-acicular fascicle in midbody | Namalycastis multiseta Glasby, 1999 |
| 15 | Antennae minute, not reaching tip of palpophore. Heterogomph falcigers with boss extremely prolonged. Jaw with 2-3 subterminal teeth + 2-4 ensheathed proximally | Namalycastis fauveli Rao, 1981 |
| – | Antennae more or less reaching tip of palpophore. Heterogomph falcigers with boss not prolonged. Jaw with 2-5 subterminal teeth + 3-5 ensheathed proximally | Namalycastis indica (Southern, 1921) |
| 16 | Groups V, VI, VII and VIII with no paragnaths | Composetia burmensis (Monro, 1937) |
| – | Groups V, VI, VII and VIII with paragnaths | 17 |
| 17 | Group VI = at least one transverse paragnath | 18 |
| – | All paragnaths in the shape of cones or small dots | 22 |
| 18 | Group VI = one transverse bar | 19 |
| – | Group VI = two transverse bars | Perinereis singaporiensis Grube, 1878 |
| – | Groups V and VI have a continuous row of transverse bars | Perinereis nuntia (Savigny, 1818) species group |
| 19 | Group V absent | Perinereis cavifrons Ehlers, 1920 |
| – | Group V = 1-3 paragnaths | 20 |
| 20 | Group I = 1-3 paragnaths | 21 |
| – | Group I = 6-12 paragnaths in an irregular group | Perinereis nigropunctata (Horst, 1889) |
| 21 | Groups II-IV arranged in clusters | Perinereis cultrifera (Grube, 1840) |
| – | Groups II-IV arranged in regular comb-like rows | 33 |
| 22 | One simple falciger present in posterior notopodia | Nereis onychophora Horst, 1918 |
| – | Compound notopodial falcigers present posteriorly | 23 |
| – | Notopodial falcigers absent posteriorly | 26 |
| 23 | Groups VII and VIII as a single row except in juveniles where it may be double | Nereis falcaria (Willey, 1905) |
| – | Groups VII and VIII as an irregular band two to four deep | 24 |
| 24 | Apices of notopodial falcigers with 1-3 large teeth | Nereis persica Fauvel, 1911 |
| – | Apices of notopodial falcigers smooth or lightly serrate | 25 |
| 25 | Group I = 0, Group III = 5 in single transverse row, Groups VII and VIII = a broad band with an anterior row of large paragnaths and two to three posterior rows of smaller ones | Nereis jacksoni Kinberg, 1866 |
| – | Group I = 1-3, Group III = 12-22 in a transverse group, Groups VII and VIII = 2 rows, the posterior with smaller, more numerous paragnaths | Nereis zonata Malmgren, 1867 |
| – | Group I = 1-3, Group III = about three rows totalling 20-30, Groups VII and VIII = three or four irregular rows | Nereis lamellosa Ehlers, 1868 |
| 26 | Falcigerous chaetae entirely absent | Neanthes chingrighattensis Fauvel, 1932 |
| – | Some falcigers present in neuropodia | 27 |
| 27 | Paragnaths of basal ring forming a continuous band which is broad ventrally | 28 |
| – | Paragnaths of V and VI separate, Groups VII and VIII forming a band 2-4 deep | Neanthes willeyi (Day, 1934) |
| – | Paragnaths of V absent, Groups VII and VIII forming a band of one to three rows | 30 |
| 28 | Group I = 1 paragnath | Neanthes operta Stimpson, 1856 |
| – | Group I = 4-12 paragnaths, which may be small | 29 |
| 29 | Groups V, VI, VII and VIII form a complete broad band of several rows of paragnaths | Neanthes caudata (Chiaje, 1841) |
| – | Group V = 4-6 rather large paragnaths, Group VI = 5-6 paragnaths in a round cluster, Groups VII and VIII form 3-4 irregular rows of very large and small cones | Neanthes meggitti Monro, 1931 |
| 30. | Group VI = 1 paragnath | 31 |
| – | Group VI = several paragnaths (may be minute) | 32 |
| 31 | Group VI = 1 paragnath; Group IV = 6-12 large paragnaths | Neanthes glandicincta (Southern, 1921) |
| – | Group VI = 1 large, oval, paragnath; Group IV = a wedge of about 20 paragnaths | Neanthes mossambica Day, 1957 |
| 32 | Group I = 6-10 paragnaths, Group II = 18-20 paragnaths, Group III = a transverse band of 3-4 rows of paragnaths | Neanthes chilkaensis (Southern, 1921) |
| – | Group I = 1 paragnath, Group II = 6 paragnaths, Group III = 11 paragnaths | Neanthes reducta (Southern, 1921) |
| 33 | Group V = 1-3 paragnaths. Neuropodia of anterior chaetigers with ventral ligule shorter than the acicular ligule. Dorsal cirrus attached terminally in the last quarter of the body | Pseudonereis trimaculata Horst, 1924 |
| – | Group V = 1 paragnath. Neuropodia of anterior chaetigers with ventral ligule as long as the acicular ligule. Dorsal cirrus terminal in the last few chaetigers only | Pseudonereis variegata (Grube, 1857) |
The Lumbrineridae used to be regarded as part of the family Eunicidae (e.g.
The pharynx is usually not everted in preserved material, but the maxillae may be seen by making a lateral cut (not a mid-ventral cut as used for nereidids) backwards from the side of the mouth, cutting through the side of the pharynx as well as the body wall for several segments, and folding the resulting flap to the side to reveal the complete jaw apparatus. If it is necessary to dissect the pharynx in this way, the anterior chaetae should be studied first.
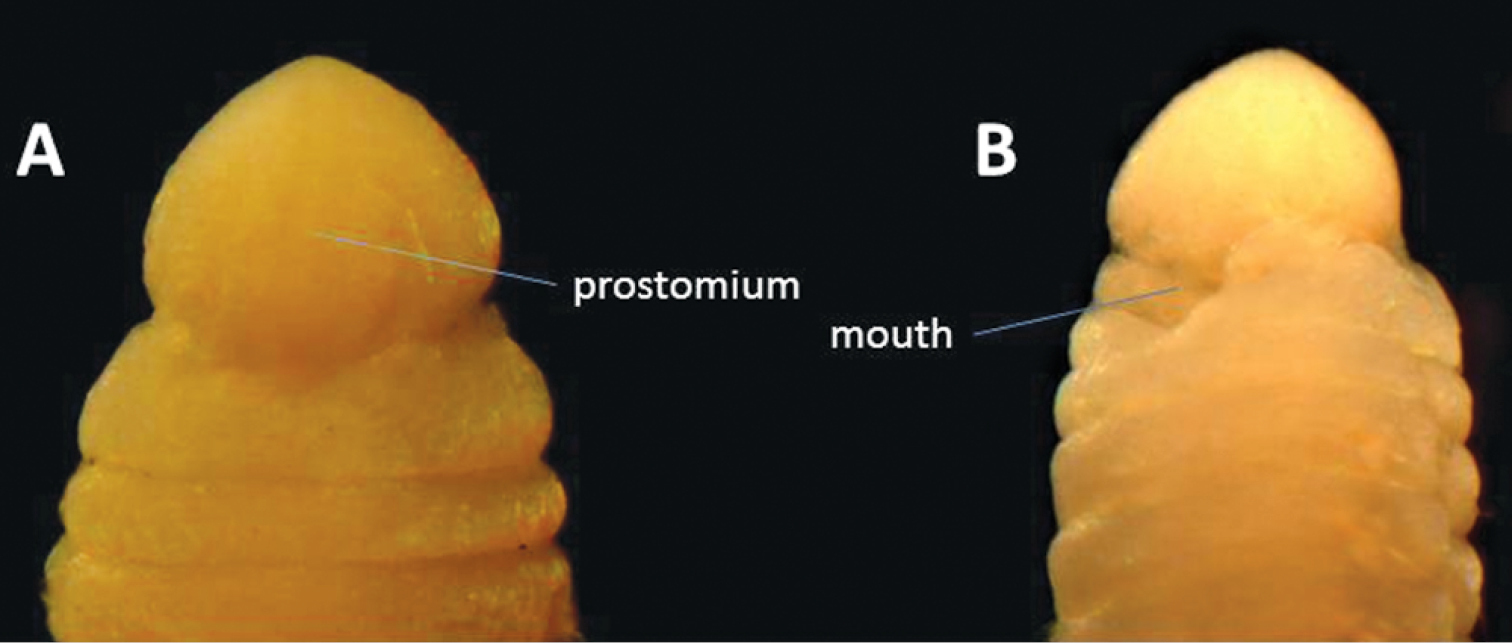
Six specimens of this species (Fig. 5) were found. The species, originally published as Lumbriconereis malayensis, was redescribed by
Anterior end of Gesaneris malayensis. A Dorsal view B Ventral view.
Previous records of lumbrinerids in Bangladesh are Lumbrinereis heteropoda heteropoda (Marenzeller, 1879) and Lumbrinereis tetraura (Schmarda, 1861), recorded by
The collections of the Natural History Museum, London, contain a previously unpublished specimen (NHMUK ANEA 1937.3.10.15) collected by Prof. F.J. Meggitt at Maungmagaun, Myanmar, which can be identified using the key in
In Odisha, the species Lumbrineris heteropoda, Lumbrineris notocirrata and Lumbrineris polydesma have been recorded from coasts and estuaries, while Lumbrineris polydesma and Lumbrineris simplex were found in Chilka Lake (
Lumbrineris heteropoda has been transferred to the genus Kuwaita by
Lumbrineris tetraura is accepted as a member of the genus Lumbrineris by e.g. George and
Lumbrineris simplex, having no hooked chaetae and no antennae, is better placed in the genus Arabellonereis.
Lumbrineris notocirrata has been transferred to the genus Ninoe by
The species Lumbriconereis pseudobifilaris Fauvel, 1932, has been recorded from 250 fathoms (about 450 m) depth off Akyab, Myanmar (
Lumbrinereis and Lumbriconereis are incorrect spellings of the generic name Lumbrineris. The northern Bay of Bengal ecoregion species of Lumbrineridae can be keyed out as follows, but any identifications must be checked against reliable descriptions as many other species are known from the Bay of Bengal and other Indo–Pacific areas.
| 1 | Three antennae present | Kuwaita heteropoda (Marenzeller, 1879) |
| – | Antennae absent | 2 |
| 2 | Hooded hook chaetae absent | Arabellonereis simplex (Southern, 1921) |
| – | Hooded hook chaetae presen | 3 |
| 3 | Simple hooded hooks only | 4 |
| – | Compound hooded hooks present anteriorly, as well as simple hooded hooks along the body | Lumbrineris sphaerocephala (Schmarda, 1861) |
| 4 | Lateral mouth pads present. Branchiae present in posterior part of body, as a small dorsal knob or transparent vesicle, a little above the base of the parapodium | Ninoe notocirrata (Fauvel, 1932) |
| – | Lateral mouth pads absent. Branchiae absent | 5 |
| 5 | Hooks start about chaetiger 29 or 30 | Lumbrineris polydesma Southern, 1921 |
| – | Hooks start about chaetiger 1-4 | 6 |
| 6 | Maxillary apparatus with five pairs of maxillae. Maxilla IV completely pigmented. Hooks with entire main tooth and crest of smaller teeth above it | Scoletoma tetraura (Schmarda, 1861) |
| – | Maxillary apparatus with four pairs of maxillae. Maxilla IV with whitish central area. Hooks with furcate main tooth and crest of smaller teeth above it | Gesaneris malayensis (Rullier, 1969) |
A posterior fragment of a glycerid was found in this collection, but it is unidentifiable to species. It is, however, a new record for Bangladesh because the fragment appears to bear branchiae on the parapodia, whereas Glycera lancadivae Schmarda, 1861, the only species in
The collections of the Natural History Museum, London, contain a specimen of Glycera cinnamomea (NHMUK ANEA 1938.5.7.45) which was collected from Investigator station 549, at a depth of 24 fms (43.89 m), off Mergui Harbour (= Myeik, near the mouth of the Tanintharyi river, Myanmar), identified by
Glycera convoluta, Glycera lancadivae, Glycera longipinnis, Glycera rouxii, and Glycera tesselata have been reported by
| 1 | All parapodia uniramous, notopodia absent. Ailerons rod-like | Hemipodia simplex (Grube, 1857) |
| – | Parapodia biramous after (usually) first two, notopodia with simple capillary chaetae. Ailerons with a more or less triangular or deeply incised base | 2 |
| 2 | Proboscideal papillae without terminal fingernail structure | 3 |
| – | Proboscideal papillae with terminal fingernail structure | 7 |
| 3 | One postchaetal lobe in all parapodia | 4 |
| – | Two postchaetal lobes at least on parapodia of mid-body | 6 |
| 4 | In mid-body, notopodial prechaetal lobes shorter than neuropodial lobes. Branchiae absent | Glycera lapidum Quatrefages, 1866 |
| – | In mid-body, prechaetal lobes of about same length or notopodial lobes longer. Branchiae present or absent | 5 |
| 5 | Digitiform proboscideal papillae without ridges. Ailerons with deeply incised bases. Simple digitiform branchiae situated termino-dorsally on parapodia | Glycera sphyrabrancha Schmarda, 1861 |
| – | Conical proboscideal papillae with about 5–20 transverse ridges. Ailerons with slightly arched bases. Branchiae absent | Glycera oxycephala Ehlers, 1887 |
| 6 | Ailerons with deeply incised bases. Postchaetal lobes short, rounded. Branchiae absent | 7 |
| – | Ailerons with interramal plate and triangular bases. Parapodia of mid-body with slender triangular notopodial and distinctly shorter rounded neuropodial postchaetal lobes. Retractile branchiae situated medially on anterior side of parapodia | 8 |
| 7 | Digitiform proboscideal papillae with straight, median, longitudinal ridge | Glycera tesselata Grube, 1863 |
| – | Digitiform proboscideal papillae with about 6-20 transverse ridges | Glycera brevicirris Grube, 1870 |
| 8 | Parapodia with slender triangular notopodial and distinctly shorter, rounded, neuropodial postchaetal lobes; simple digitiform retractile branchiae | Glycera nicobarica Grube, 1868 |
| – | Parapodia with two slender triangular postchaetal lobes of about same length or notopodial lobes only slightly longer than neuropodial lobes; digitiform retractile branchiae with 1-2 rami | Glycera unicornis Savigny, 1818 |
| 9 | Parapodia of mid-body with two slender triangular postchaetal lobes of about same length | 10 |
| – | Parapodia of mid-body with slender triangular notopodial and shorter, more or less rounded, neuropodial postchaetal lobes | 11 |
| 10 | Parapodia without branchiae | Glycera onomichiensis Izuka, 1912 |
| – | 1–5 digitiform branchial rami situated dorsally on parapodial bases | Glycera cinnamomea Grube, 1874 |
| 11 | In mid-body and posterior parapodia neuropodial postchaetal lobes more or less rounded. Simple digitiform branchiae situated termino-dorsally on parapodia | 12 |
| – | In posterior parapodia neuropodial postchaetal lobes as long as notopodial lobes and equally slender triangular. Simple digitiform branchiae situated medio-dorsally on parapodia | Glycera posterobranchia Hoagland, 1920 |
| 12 | All biramous parapodia with two postchaetal lobes. Proboscideal papillae with long, medium or short stalk | 13 |
| – | In anterior parapodia only one, medially inserted slender triangular postchaetal lobe. Proboscideal papillae with short stalk | Glycera macrobranchia Moore, 1911 |
| 13 | Proboscideal papillae with long stalk | 14 |
| – | Proboscideal papillae with medium-length or short stalk | 15 |
| 14 | Stalk without ridges. Ailerons with pointed triangular bases | Glycera alba (Müller, 1776) |
| – | Stalk with numerous ridges. Ailerons with triangular bases | Glycera natalensis Day, 1957 |
| 15 | Proboscideal papillae with short stalk. Prostomium consisting of about 11–15 rings. Ailerons with triangular bases | Glycera tridactyla Schmarda, 1861 |
| – | Proboscideal papillae with medium-length stalk. Prostomium consisting of about 19–28 rings. Ailerons with pointed triangular bases | Glycera africana Arwidsson, 1899 |
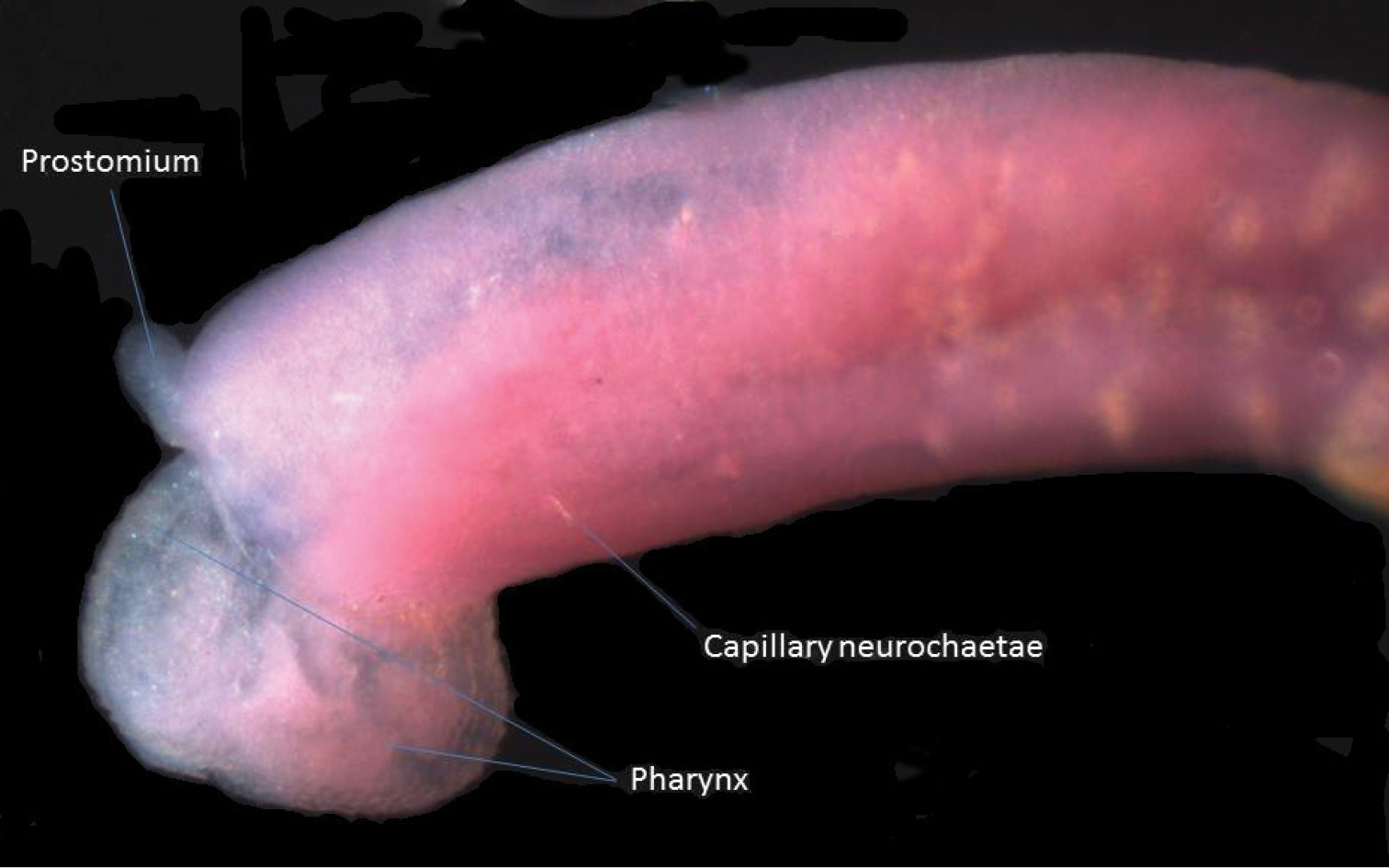
The single 6.5 mm long worm found (Fig. 6) is probably this species, which has been well described by
Dorsal view of semi-transparent specimen of Micronephtys oligobranchia with partially everted pharynx.
Nephtys oligobranchia is the only nephtyid recorded by
Nephtys oligobranchia and Nephtys polybranchia Southern, 1921, have both been recorded from West Bengal (
Nephtys oligobranchia is recorded from the Baitarani River, Odisha, by
The northern Bay of Bengal ecoregion species of Nephtyidae can be keyed out as follows, but any identifications must be checked against reliable descriptions as many other species are known from the Bay of Bengal and other Indo–Pacific areas.
| 1 | Interramal cirri (branchiae) large and curving in towards the body, especially anteriorly | Aglaophamus dibranchis (Grube, 1878) |
| – | Interramal cirri poorly developed | 2 |
| 2 | Interramal cirri absent from posterior half of body | Micronephtys oligobranchia (Southern, 1921) |
| – | Interramal cirri continuing more or less to end of body | Nephtys polybranchia Southern, 1921 |
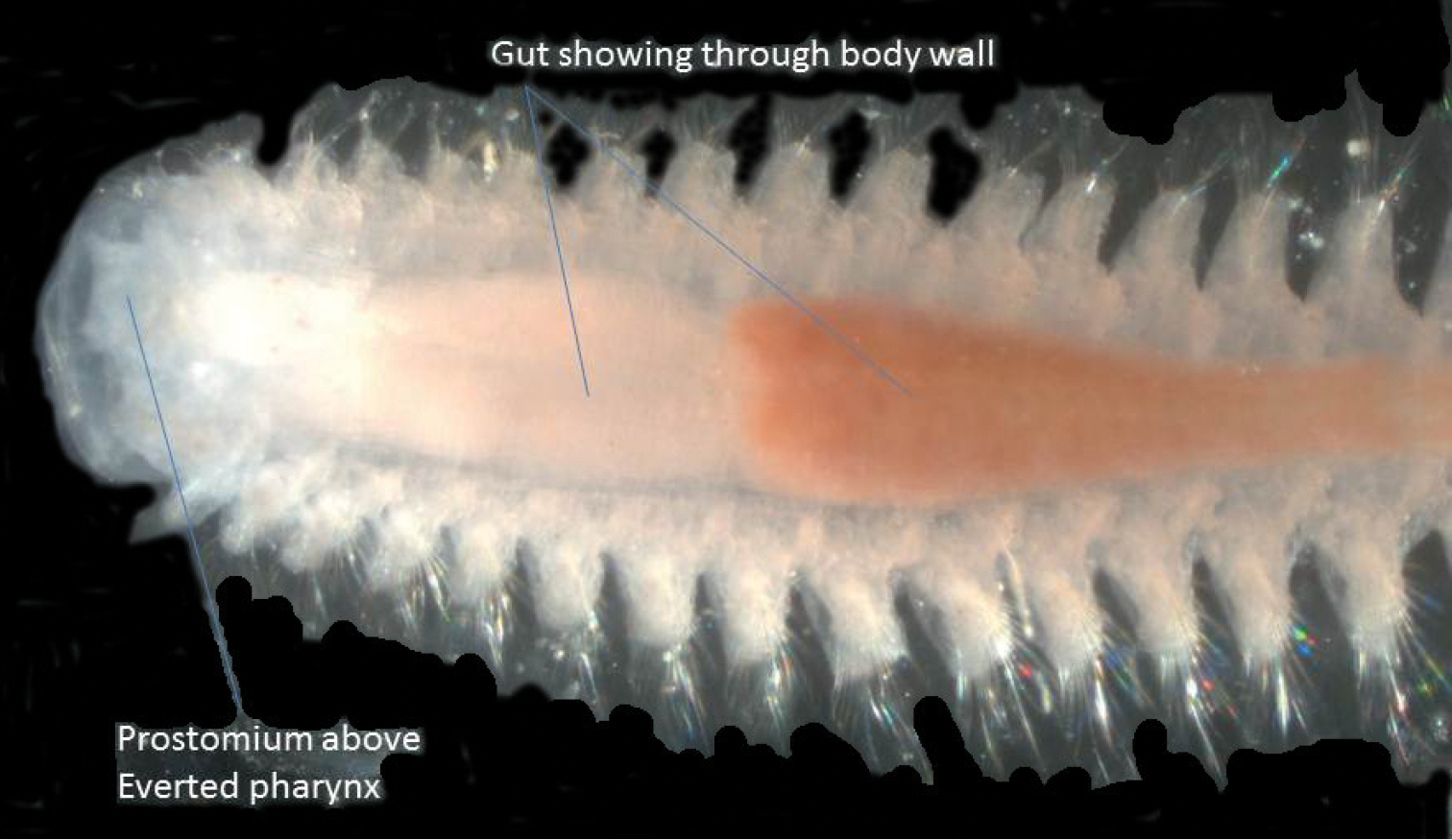
Specimens of Capitellidae, having no head appendages and usually no obvious parapodial lobes, can easily be mistaken for oligochaetes (e.g.
Lateral view of anterior end of Heteromastus filiformis with everted pharynx.
This is a new record according to
The Natural History Museum, London, has a specimen of Notomastus near latericeus Sars, 1851 sensu
Matla bengalensis Stephenson, 1908, was described as a new genus and species of oligochaete from West Bengal, but is now (
In Odisha, five species of capitellid have been reported (
There follows a key to the reported species of capitellid from the Northern Bay of Bengal ecoregion, but any identifications must be checked against reliable descriptions as many other species are known from the Bay of Bengal and other Indo–Pacific areas. In particular,
Most keys to capitellid genera start with the number of segments in the thorax. There may be a sudden change in size or shape of the segments at the start of the abdomen, but in many cases it is easier to make a temporary whole mount of the specimen and count the segments with capillary chaetae.
| 1 | Posterior abdominal segments with stout acicular chaetae; pygidium with two stout, conical, diverging anal cirri | Scyphoproctus armatus (Fauvel, 1929) |
| – | Posterior abdominal segments with hooded hook chaetae; anal cirri absent | 2 |
| 2 | 4-7 anterior segments with capillary chaetae | 3 |
| – | 11-13 anterior segments with capillary chaetae | 6 |
| 3 | Thorax with 9 segments, all with chaetae | Capitella capitata (Fabricius, 1780) |
| – | Thorax with 12 segments, the first achaetous | 4 |
| 4 | 4 anterior segments with capillary chaetae | Parheteromastus tenuis Monro, 1937 |
| – | 5 anterior segments with capillary chaetae | 5 |
| – | 6 anterior segments with capillary chaetae | Barantolla sculpta Southern, 1921 |
| 5 | Abdominal hooks with longer anterior shaft than posterior shaft (node posterior to middle of shaft), two rows of teeth above main fang; posterior abdomen with expanded notopodial lobes | Heteromastus similis Southern, 1921 |
| – | Abdominal hooks with longer posterior shaft than anterior shaft (node anterior to middle of shaft), three teeth above main fang; posterior abdomen with expanded neuropodial lobes | Heteromastus filiformis (Claparède, 1864) sensu Day, 1967 |
| 6 | 11 anterior segments with capillary chaetae | 7 |
| – | 13 anterior segments with capillary chaetae | Dasybranchus caducus (Grube, 1846) |
| 7 | Abdomen with anterior two or more segments with mixed fascicles of capillary chaetae and hooded hooks | Mastobranchus indicus Southern, 1921 |
| – | No segments with mixed chaetal fascicles | Notomastus near latericeus Sars, 1851 |
Of the seven taxa identified above, five are new records for Bangladesh. This shows that the polychaete fauna of Bangladesh is still not well known.
Some earlier records of polychaetes will have to be re-studied before the fauna list of the Bangladesh area is complete, as names will change for taxonomic or nomenclatural reasons. An example of this is the genus Talehsapia, reported from the Hooghly estuary and South 24-Parganas, West Bengal by
It is notable that some species reported from Bangladesh have very wide reported distributions – the same species being reported from both Bangladesh and northern Europe may be the result of misidentification or an unrecognised cryptic species. It is also notable that
While the surface salinity in the open part of the Bay of Bengal oscillates from 32–34.5‰, in the coastal region it varies from 10–25‰ and at the river mouths the surface salinity decreases to 5‰ or even less (
Further work on the occurrance and abundance of the macrobenthic fauna and ecology of the Sitakunda coast, Chittagong, will be published in the future (Hossain and M. Belal in prep.).
AIM would like to thank Dr. Chris Glasby, of the Museum and Art Gallery of the Northern Territory, Darwin, Australia, for a useful discussion regarding Lycastonereis indica, and Ms. Lenka Neal (NHM) for her great help with taking photomicrographs. MMMH thanks Young Power Social Action (YPSA), Bangladesh, for their financial support to do this research.