Citation: Wang J, Tong X, Wu D (2014) The effect of latitudinal gradient on the species diversity of Chinese litter-dwelling thrips. ZooKeys 417: 9–20. doi: 10.3897/zookeys.417.7895
To understand the global distribution patterns of litter-dwelling thrips, a total 150 leaf litter samples were collected from 6 natural reserves located in three climatic regions, temperate, subtropical and tropical. The results showed the relative abundance of Thysanoptera was over 3.0% in 4 natural reserves from subtropical and tropical zone, and reached 5.9% in one tropical reserve, only less than Acarina and Collembola. In contrast it was only 0.3% in the warm temperate natural reserves, and no thrips were collected in a mid temperate reserve. The order on the average species numbers per plot of litter thrips was tropic > subtropics > temperate (n=25, p<0.05). Mean density of litter thrips per plots in the tropics and subtropics was significantly higher than that in the temperate region (n=25, p<0.05), but the average density was not significantly different between tropical and subtropical zones (n=25, p>0.05). The diversity of litter thrips in the tropics and subtropics was much higher than that in the temperate area based on comparsions of Shannon-Wiener diversity index (H’), Pielou eveness index (J), and Simpson dominance index (D). All of these results indicated that litter-dwelling thrips lived mainly in tropical and subtropical regions; meanwhile, species number and relative abundance increased with decreasing latitude.
Soil invertebrate, leaf-litter thrips, species diversity, latitudinal gradient, global distribution pattern, China
Global distribution patterns of organisms have become a hot research topic in recent years due to increasing concerns about the global loss of species richness (
Litter-dwelling thrips is a group of thysanopteran insects that have adopted the habitat of forest litter or surface soil where they feed only on either fungal hyphae or fungal spores during the early stages of leaf decay (
Most publications of litter-dwelling thrips species were descriptions of new genera and species, sometimes with little information on their vegetation or microhabitat associations (
To determine whether or not species diversity of litter-dwelling thrips alters at higher latitudes, we collected litter samples from six natural reserves which are located respectively in the temperate, subtropical and tropical zones, along a 4100 km latitudinal gradient in East China. The observations are also interesting from the point of view of understanding geographical scale differences in ecology, and responses of ecosystems to global warming.
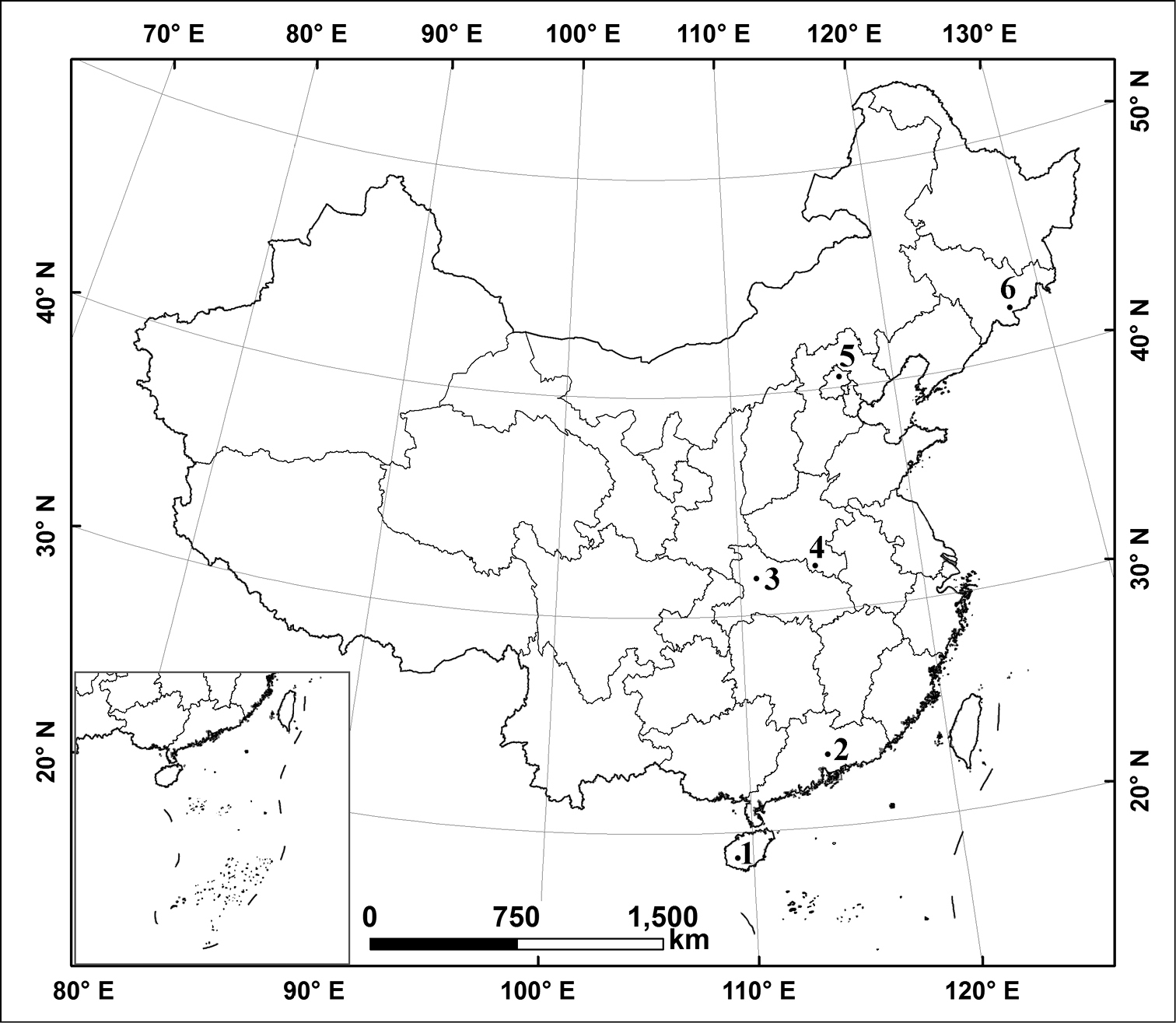
This study was conducted on six different natural reserves along a broad latitudinal gradient China (Figure 1): Wuzhishan Natural Reserve (Hainan Province), Nankunshan Natural Reserve (Guangdong Province), Shennongjia Natural Reserve (Hubei Province), Jigongshan Natural Reserve (Henan Province), Yunmengshan National Forest Park (Beijing), Changbaishan Natural Reserve (Jilin Province). Natural conditions are summarized in Table 1.
Locations of sampling stations in China. 1 Wuzhishan Natural Reserve, Hainan Province 2 Nankunshan Natural Reserve, Guangdong Province 3 Shennongjia Natural Reserve, Hubei Province 4 Jigongshan Natural Reserve, Henan Province 5 Yunmengshan National Forest Park, Beijing 6 Changbaishan Natural Reserve, Jilin Province.
Natural conditions in the locations of sampling stations.
| 1* | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Latitude (N) | 18°51' | 23°38' | 31°25' | 31°50' | 40°33' | 41°55' |
| Longitude (E) | 109°42' | 113°50' | 110°20' | 114°05' | 116°40' | 127°40' |
| Annual average temperature (°C) | 23 | 21 | 14 | 15 | 9 | 3 |
| Annual average precipitation (mm) | 2400 | 2163 | 1750 | 1119 | 700 | 680 |
| Type of forest | Tropical rain forest | Evergreen seasonal rain forest | Deciduous broad-leaf forest | Deciduous broad-leaf forest | Deciduous broad-leaf forest | Pine and broad-leaf mixed forest |
| Zone of temperature | Tropic | Subtropics | Subtropics | Subtropics | Temperate | Temperate |
| Biogeographic region | Oriental region | Oriental region | Oriental region | Palearctic region | Palearctic region | Palearctic region |
*1. Wuzhishan Natural Reserve, Hainan Province; 2. Nankunshan Natural Reserve, Guangdong Province; 3. Shennongjia Natural Reserve, Hubei Province; 4. Jigongshan Natural Reserve, Henan Province; 5. Yunmengshan National Forest Park, Beijing; 6. Changbaishan Natural Reserve, Jilin Province.
Wang et al. (2012) suggested that species diversity of litter-dwelling thrips was highest in October and December. Species diversity of litter thrips and other litter macro-invertebrates was quantitatively sampled from six natural reserves in October from 2007 to 2010. In each sampling, 5 plots (10 × 10 m) were randomly selected, and the distance between each sample plot was more than 50 m; 5 quadrate litter samples (50 × 50 cm) were selected in each plot – four in each corner and one in the center. A total of 150 litter samples were then placed in labeled plastic bags and extracted in the laboratory by means of a modified Tullgren funnel.
Litter-dwelling thrips and other soil macro-invertebrates were extracted with the modified Tullgren funnels, using 60 W bulbs suspended 10 cm above the top of the samples over 10 hrs until the litter dried and became fragile. Specimens were then preserved in 75% ethanol. All extractions were completed within 3 days. The macro-invertebrate samples were sorted to Order level, and counted under a dissecting microscope. The adults of leaf-litter thrips were identified to species, and the larval stages of thrips were separated under the category of “thrips larvae” and counted separately.
Species richness, density (individuals/m2), relative abundance and frequency were applied to indicate the diversity of litter-dwelling thrips. Relative abundance refers to the total number of specimens for a particular species divided by the total number of all litter thrips, while frequency expresses the number of individuals a species collected in a month, divided by the total number of months. A “dominant group” is defined as having a relative abundance of more than 10%; the relative abundance of “ordinary groups” is between 1% and 10%; “rare group” is less than 1%. The density of each thrips species in each month was the mean of 25 quadrate samples from 5 plots and presented as Mean ± standard errors.
Shannon-Wiener diversity index (H’) = -∑(Ni / N) ln (Ni / N)
Simpson dominance index (D) = ∑(Ni / N)2
Pielou evenness index (J) = H’ / ln S
In the above, Ni is the number of individuals of species i; N is the total number of individuals of all the species; S is the number of species (
A total of 53, 353 individuals of litter-dwelling invertebrate were collected in 150 samples, belonging to 30 groups in 10 classes under 3 phyla. Acarina and Collembola accounted for more than 10.0% of the total individuals of litter invertebrates and were considered to be “the dominant groups”. Relative abundance of litter-dwelling thrips was distinctly different in the different natural reserves (Table 2). It was a common Order of the litter invertebrate assemblage from tropical and subtropical regions, was rare an Order in temperate regions. In Wuzhishan Natural Reserve, litter thrips individuals accounting for 5.9% of the litter invertebrates, the relative abundance was maximum, only less than Acarina and Collembola.
Species composition and density (Means ± SE) of litter-dwelling thrips in 6 natural reserves from different latitude (unit: individuals/m2).
| Species | 6 | 5 | 4 | 3 | 2 | 1* |
|---|---|---|---|---|---|---|
| Acallurothrips sp. | 0.0a | 0.0a | 0.0a | 0.0a | 0.0a | 0.3±0.3a** |
| Adraneothrips russatus | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 1.0±0.7a |
| Adraneothrips chinensis | 0.0a | 0.0a | 0.0a | 3.3±3.3a | 2.3±1.2a | 3.5±1.5a |
| Apelaunothrips lieni | 0.0d | 0.0d | 5.5±2.1bc | 7.8±7.1ab | 19.3±9.5a | 1.0±0.5c |
| Baenothrips asper | 0.0a | 0.0a | 0.0a | 0.0a | 0.0a | 0.5±0.5a |
| Heliothripoides reticulates | 0.0a | 0.0a | 0.0a | 0.0a | 0.0a | 0.3±0.3a |
| Holothrips sp. | 0.0a | 0.0a | 0.0a | 0.3±0.3a | 0.0a | 0.0a |
| Holurothris morikawai | 0.0b | 0.0b | 0.0b | 0.0b | 3.0±2.7a | 0.0b |
| Hoplothrips sp. | 0.0d | 0.5±0.3bc | 2.3±2.0ab | 5.5±2.4a | 0.0d | 1.8±1.5ab |
| Hyidiothrips japonicus | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 7.5±3.3a |
| Karnyothrips flavipes | 0.0a | 0.0a | 0.0a | 0.5±0.3a | 1.0±1.0a | 0.3±0.3a |
| Psalidothrips ascitus | 0.0c | 0.0c | 42.5±26.9a | 0.0c | 12.3±5.4b | 21.3±18.2ab |
| Psalidothrips simplus | 0.0a | 0.0a | 0.0a | 0.3±0.3a | 5.0±5.0a | 0.0a |
| Psalidothrips sp. | 0.0a | 0.0a | 0.0a | 0.0a | 0.3±0.3a | 0.3±0.3a |
| Preeriella parvula | 0.0b | 2.5±2.2ab | 0.0b | 71.5±71.2a | 0.5±0.5ab | 0.0b |
| Stephanothrips japonicus | 0.0c | 0.0c | 4.3±3.3b | 22.3±8.1a | 2.5±1.1b | 1.0±0.6b |
| Terthrothrips palmatus | 0.0b | 0.0b | 0.0b | 0.0b | 0.0b | 2.0±1.2a |
| Mystrothrips flavidus | 0.0b | 0.0b | 0.0b | 0.0b | 1.3±1.3ab | 1.8±0.9a |
| Thrips sp. | 0.0b | 0.0b | 0.0b | 1.8±0.3a | 0.0b | 1.6±0.8a |
| Thrips larvae | 0.0c | 0.5±0.3c | 37±12.3ab | 14.8±10.5b | 31.8±11.7ab | 49.3±7.3a |
*1. Wuzhishan Natural Reserve, Hainan Province; 2. Nankunshan Natural Reserve, Guangdong Province; 3. Shennongjia Natural Reserve, Hubei Province; 4. Jigongshan Natural Reserve, Henan Province; 5. Yunmengshan National Forest Park, Beijing; 6. Changbaishan Natural Reserve, Jilin Province.
**Values in a row followed by the same letters indicate no significant difference at 0.05 level of probability (ANOVA, Tukey HSD) and values with standard errors.
In total, 19 species of litter-dwelling thrips (1578 individuals), representing 16 genera and 2 families, were collected during the survey period (Table 2). Most species and genera belonged to the family Phlaeothripidae (18 and 15 respectively); these phlaeothripids live as fungus-feeders in leaf litter. Of the family Thripidae 22 individuals were collected; these thripids are flower-living or leaf-feeding and only enter the litter or soil to pupate.
Species composition and density of fungus-feeding thrips were different in the six natural reserves (Table 2). In Wuzhishan Natural Reserve, 371 individuals of 14 species and 12 genera were collected in leaf litter. Among them, Psalidothrips ascitus and Hyidiothrips japonicus were dominant species, accounting for 48.6% and 17.1% of the total adult thrips individuals, respectively. Heliothripoides reticulates and Terthrothrips palmatus were collected only in this reserve. In three natural reserves located in subtropics, 10 genera and 12 species were collected: Preeriella parvula, Psalidothrips ascitus, Apelaunothrips lieni, Stephanothrips japonicus were dominant species, accounting for 33.5%, 19.9%, 15.1% and 13.5% of the total adult thrips individuals, respectively. In Yunmengshan, 14 individuals in 2 species, Preeriella parvula and Holothrips sp., were collected. No leaf-litter thrips were collected in Changbaishan Natural Reserve, the most northern of the six sites.
According to species number and individuals of each species, Shannon-Wiener diversity index, Simpson dominance index and Pielou evenness index were applied to analyze community structure of fungus-feeding thrips (Table 3). The order on the average species numbers per plot of fungus-feeding thrips was tropics > subtropics > temperate (n=25, p<0.05). Mean density of fungus-feeders per plot in tropics and subtropics was significantly higher than in temperate region (n=25, p<0.05), but the average density was not significantly different between tropical and subtropical zones (n=25, p>0.05). The diversity of fungus-feeding thrips in the tropics and subtropics was much higher than in the temperate area, based on the comparsions of Shannon-Wiener diversity index (H’), Pielou eveness index (J), and Simpson dominance index (D).
The comparison of diversity indices of fungus-feeding thrips in five natural reserves from different latitude.
| Diversity indices | 6 | 5 | 4 | 3 | 2 | 1* |
|---|---|---|---|---|---|---|
| number of species/plot | 0 | 1.2±0.4 d | 3.4±0.5 c | 5.4±0.4 b | 5.8±0.2 b | 8±0.9 a** |
| Density (mean±SE) | 0 | 3.6±2.4 b | 91.6±37.3 a | 127.9±59.9 a | 78.9±30.3 a | 93.0±22.4 a |
| No. of species | 0 | 2 | 4 | 8 | 10 | 14 |
| Shannon-Wiener Diversity index H’ | - | 1.149 | 1.6207 | 1.995 | 2.424 | 2.202 |
| Simpson's dominant index D | - | 0.476 | 0.6152 | 0.638 | 0.7492 | 0.659 |
| Pielou's equality index J | - | 0.725 | 0.698 | 0.601 | 0.7008 | 0.564 |
*1. Wuzhishan Natural Reserve, Hainan Province; 2. Nankunshan Natural Reserve, Guangdong Province; 3. Shennongjia Natural Reserve, Hubei Province; 4. Jigongshan Natural Reserve, Henan Province; 5. Yunmengshan National Forest Park, Beijing; 6. Changbaishan Natural Reserve, Jilin Province.
**Values in a row followed by the same letters indicate no significant difference at 0.05 level of probability (ANOVA, Tukey HSD) and values with standard errors.
Extant insects of the order Thysanoptera include approximately 6000 described species worldwide, classified into nine families (
In recent years, we conducted a series of investigations by quantitative sampling methods to survey the species diversity of leaf-litter thrips in China. We found these litter-dwelling thrips to be a common group of litter macro-invertebrates, with high species diversity and relative abundance in Guangdong Province of subtropical China. For example, the numbers of these thrips accounted for 3% to 13.5% of total litter macroinvertebrate individuals caught in four different forest types of two natural reserves (
In the New World, the fauna of litter thrips is represented primarily by three genera, Eurythrips, Terthrothrips and Tylothrips (
A total of five distribution patterns can be recognized among the 15 genera of litter-dwelling thrips in six nature reserves. Genera with a Pan-tropical distribution are most abundant, including the following: Acallurothrips, Adraneothrips, Baenothrips, Holothrips, Karnyothrips, Preeriella, Stephanothrips and Terthrothrips. These genera are found in the tropical and subtropical areas of Asia-Africa-America. Apelaunothrips is distributed in the tropical and subtropical areas of Asia-Africa. Hyidiothrips and Psalidothrips occur in the warmer areas of Asia and America. Heliothripoides, Holurothrips and Mystrothrips are distributed across tropical or subtropical Asia, being found in China, Japan, Korea and the India-Malaya area. Excluding the genera with cosmopolitan distributions (Hoplothrips), almost all litter-dwelling genera are found in tropical and subtropical areas. Differing from geographic distribution patterns at genus level, the 18 species of fungus-feeding thrips were divided into four distribution patterns, according to species currently distribution areas. Endemic to South China distribution: Apelaunothrips lieni, Adraneothrips chinensis and Terthrothrips palmatus. Eastern Asia distribution: Adraneothrips russatus, Holurothrips morikawai, Hyidiothrips japonicus, Psalidothrips simplus, Stephanothrips japonicus and Mystrothrips flavidus recorded in Japan and South China. Tropical Asia distribution: Heliothripoides reticulates, Psalidothrips ascitus and Preeriella parvula recorded in India-Malaya area or Japan. Tropical Asia, Africa and America disconnected distribution: Baenothrips asper and Karnyothrips flavipes. These zoogeographic analyses indicate that litter-dwelling thrips possess tropical and subtropical characteristics. Litter-dwelling thrips inhabiting forest litter usually have weak flight ability, some species are even wingless, but individual genera or even species are sometimes found in two disconnected continents. The causes have been discussed by
The species richness of litter-dwelling thrips in our quantitative investigation at six locations across a broad latitudinal gradient reveal an increase with decreasing latitude, as reported for many other taxonomic groups of invertebrates, prominent examples include termites (
We thank Prof. Zhang Weiqiu (Department of Entomology, SCAU, China) for providing taxonomic advice. Special thanks to Dr. Laurence Mound (CSIRO Entomology, Australia) for going through the early draft of the manuscript and improving the text. We are grateful to Liang Chang, Xuefeng Wang, Xin Sun, et al. (Northeast Institute of Geography and Agroecology, CAS, China), and anonymous reviewers for valuable feedback on the manuscript. This study was supported by the National Natural Sciences Foundation of China (No. 41001144 and No. 31372236) and the China Postdoctoral Science Foundation funded project (No. 2013M540262).