(C) 2013 Gary C. Williams. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A taxonomic assessment of four species of octocorals from the northeastern Pacific Ocean (British Columbia to California) is provided. Included here are a new species of clavulariid stolonifieran Cryptophyton, a new species of the nephtheid soft coral Gersemia, an undetermined species of soft coral in the genus Alcyonium that has been referred in the literature by several other names, and a new genus is named for a plexaurid sea fan originally described in the Indo-Pacific genus Euplexaura. Discussions are included that compare the species to related taxa, or provide revisionary assessments.
Alcyonium, Cryptophyton, Gersemia, Octocorallia, northeastern Pacific, plexaurid gorgonian, soft corals, taxonomy
Recently collected material from British Columbia and California has allowed for the examination and taxonomic assessment of several shallow-water soft corals (intertidal to 20 meters in depth), as well as a plexaurid gorgonian (32-85 m).
All material examined is housed in the marine invertebrate collections of the California Academy of Sciences, preserved in 95% ethanol, and acquired from various sources. Scanning electron micrographs were made using a LEO 1450 VP SEM.
Material used for comparative purposes: Euplexaura sp., CAS 107595, Western Pacific Ocean, Palau, Neco Channel, 28 September 1996, 24 m depth, coll. Gary C. Williams, one whole specimen.
Abbreviation used in the text: CAS (California Academy of Sciences, San Francisco).
urn:lsid:zoobank.org:act:482B9A4A-2E2E-4A4A-A319-5CC211242B45
http://species-id.net/wiki/Cryptophyton_jedsmithi
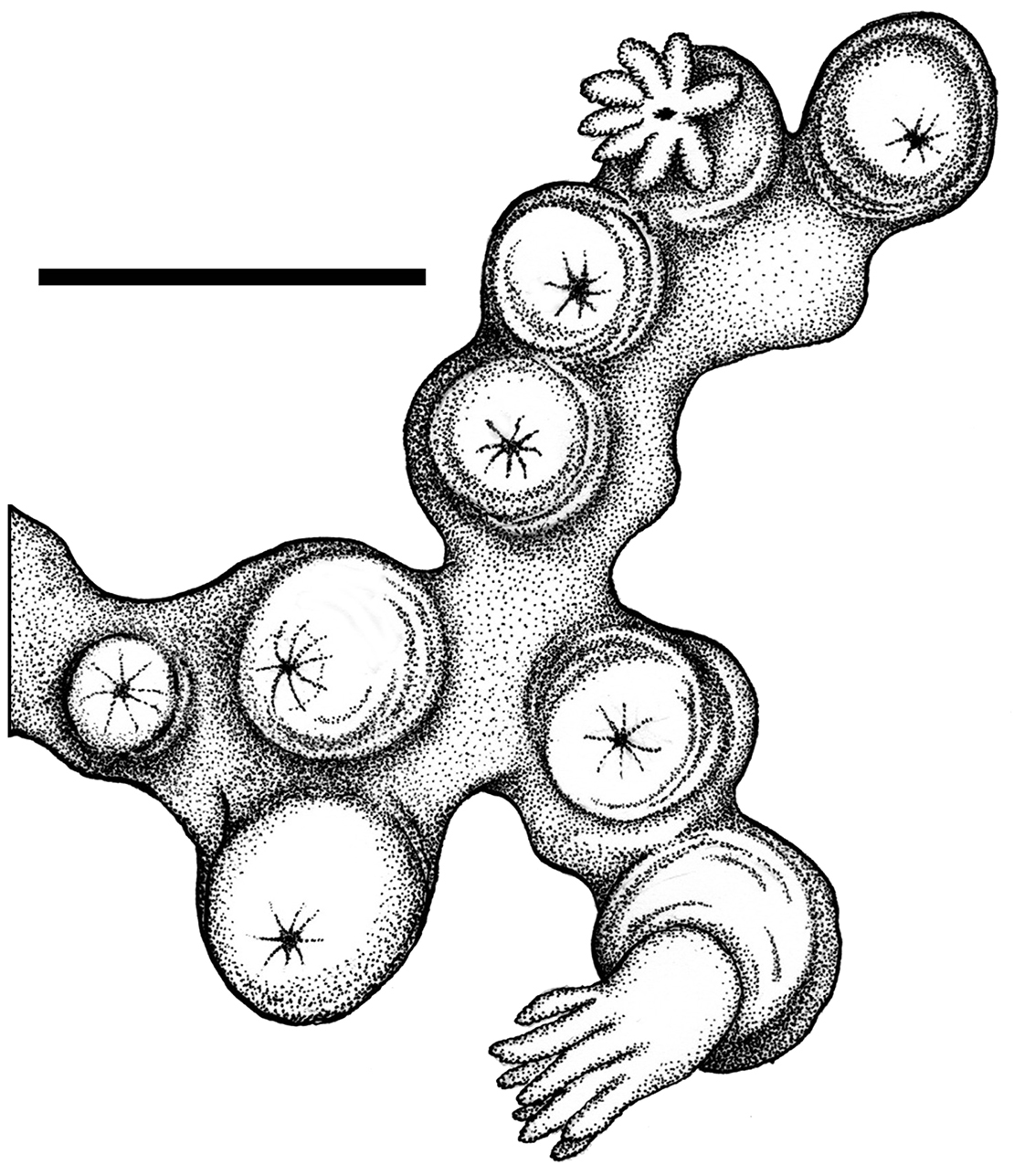
Figures 1–4, 19Stolons ribbon-like to somewhat broadened in some areas. Anthosteles hemispherical, arise directly from basal stolons, elevated stolonic bars or transverse platforms absent. Anthocodial armature absent. Sclerites of stolons and anthosteles 0.06–0.10 mm in diameter, mostly spiny balls or stellate bodies with projecting processes in three dimensions.
Holotype. CAS 177194. North America, U.S.A., California, San Diego County, San Diego, Point Loma, 32°42'N, 117°15'20"W, 12 February 2006, collector: Jeff Goddard, one specimen wet-preserved in 95% ethanol.
(Figure 19): Under a boulder in the low rocky intertidal zone at the type locality.
The species is named for Jedediah Strong Smith, American trailblazer and cartographer, who explored vast regions of western North America between 1822 and 1831, and along the Pacific Coast, including San Diego in December of 1826 (
Colonial morphology (Figures 1A, 2). The holotype consists of approximately eighty-five polyps arising from flattened basal stolons. The stolons encrust a piece of dead cheilostomatid bryozoan, 32 mm long by 20 mm wide. The surface of the bryozoan is interspersed with several calcareous tubes of a serpulid polychaete.
Polyps (Figures 1B–C). Anthosteles are moundlike, rounded, hemispherical to subcylindrical. Anthocodiae are mostly retracted within the anthosteles, although a few are emergent. The anthosteles are approximately equal in height and diameter, mostly 1–1.4 mm.
Sclerites (Figures 1D–E, 3, 4). Sclerites of the coenencyme and anthosteles resemble spiny balls or stellate bodies with projecting processes in three dimensions; 0.05 – 0.10 mm long. Sclerites are absent from the anthocodiae and polyp bodies.
Color (Figures 1A–C). Color in life: the anthosteles are pale orange and the anthocodiae are white. Wet-preserved holotype: stolons and anthosteles light grayish white, while the emergent anthocodiae are white.
Cryptophyton jedsmithi sp. n. A Wet preserved holotype (CAS 177194); scale bar = 10 mm. B–C Living holotype, details of polyps; photos courtesy of Jeff Goddard; scale bar for both = 1.5 mm. D–E Light micrographs of coenenchymal sclerites; scale bar for both = 0.10 mm.
Cryptophyton jedsmithi sp. n. A portion of the holotype, showing arrangement of nine polyps on a membranous stolon; scale bar = 3.0 mm.
Two species of the genus Cryptophyton are known, Cryptophyton goddardi Williams, 2000 and Cryptophyton jedsmithi sp. n. The two species differ in sclerite shape. Those of Cryptophyton goddardi are irregularly-shaped radiates, tuberculated rods, and shuttles (
The geographic range of Cryptophyton goddardi was originally known only from the type locality of central Oregon on the Pacific Coast of the United States, but has recently been extended southwards to southern California, and has been collected at seven locations (Figure 19), while Cryptophyton jedsmithi sp. n. is known only from the type locality – San Diego, California (Figure 19).
Cryptophyton jedsmithi sp. n. Scanning electron micrographs of coenenchymal sclerites from the holotype. Scale bar = 0.03 mm.
Cryptophyton jedsmithi sp. n. Scanning electron micrographs of coenenchymal sclerites from the holotype; scale bar = 0.03 mm. Lower right, ultrastructural detail from center of the sclerite to the adjacent left; scale bar = 0.01mm.
| 1a | Anthosteles (polyp mounds) cylindrical, up to 2.8 mm in height and 1.8 mm in diameter. Sclerites are irregulary-shaped radiates, irregular sclerites presumably derived from radiates, tuberculate rods, and shuttles; 0.06–0.18 mm long | Cryptophyton goddardi |
| 1b | Anthosteles (polyp mounds) hemispherical, approximately equal in height and diameter, mostly 1–1.4 mm. Sclerites resemble spiny balls or stellate bodies with projecting processes in three dimensions; 0.05–0.10 mm long | Cryptophyton jedsmithi sp. n. |
Figures 5–6
Alcyonium sp.
CAS 179450, Canada, British Columbia, Weynton Passage, Plumper Group of islands, Plumper Island, (50°35.501'N, 126°47.997'W), 20 m depth, 10 November 2009, collector: N. McDaniel, one whole colony. CAS 173217, Canada, British Columbia, Strait of Juan de Fuca, Swordfish Island (48°18'36.4"N, 123°34'58.4"W), 6 m depth, 28 September 2009, collectors: C. Blondeau, T. Hill, R. Van Hall, one whole colony, abundant in underwater tunnel with dynamic surge. CAS 029138, U.S.A., Alaska, Arctic Ocean, near Point Barrow, 44 m depth, 29 July 1951, collector: J. Bohlke on R/V ”Ivik”, two whole colonies.
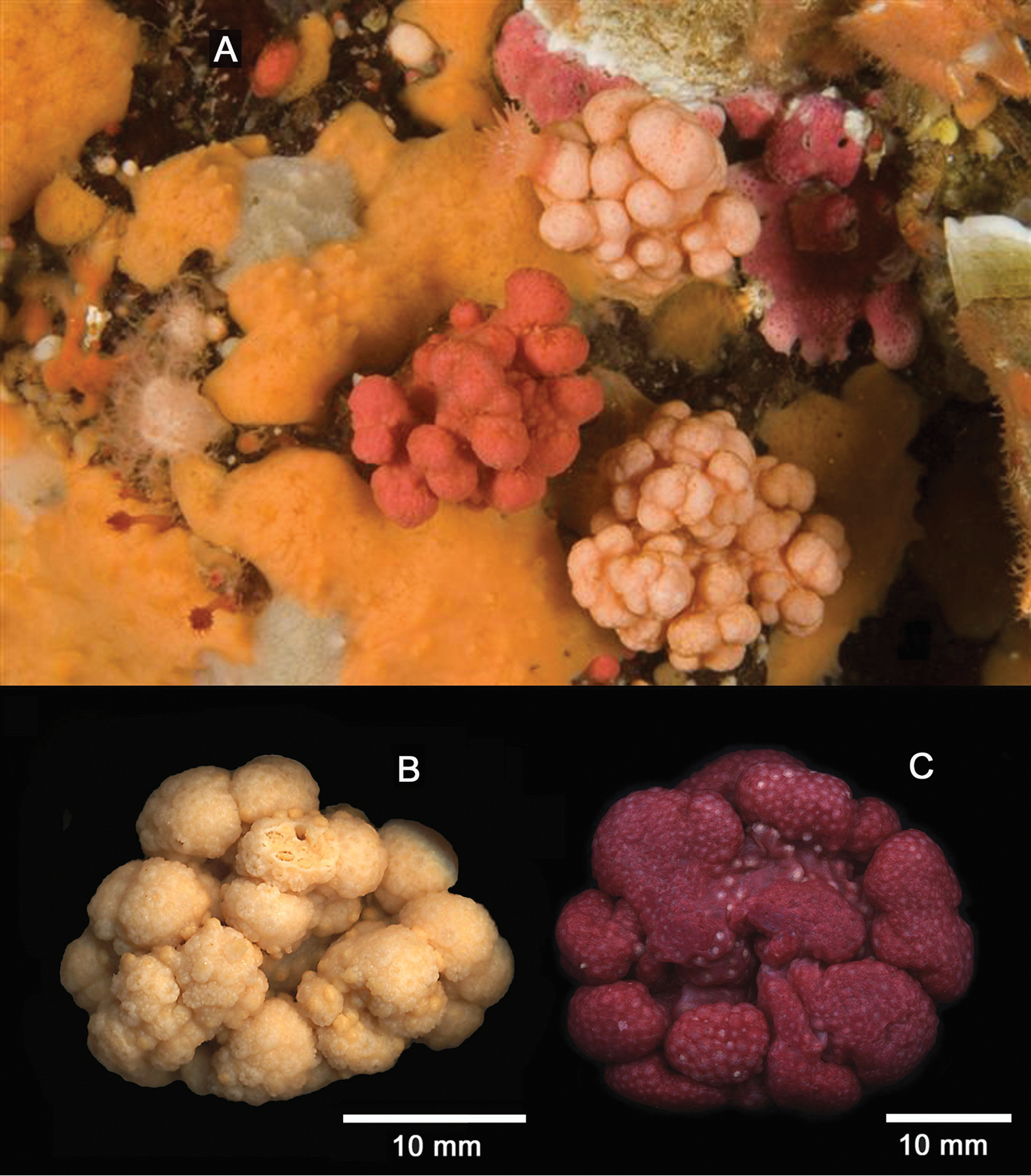
Alcyonium sp. indet. is known from the west coast of North America from Alaska south to British Columbia and California, and has been referred to as Gersemia rubiformis, Capnella rubiformis, or Eunephthya rubiformis in numerous publications (examples:
In addition, there are other ambiguous details that are relevant here. Ehrenberg’s 1834 paper is dedicated to the corals of the Red Sea, but he lists Lobularia rubiformis as inhabiting the “Mari septentrionali.” The genus Lobularia is a synonym of Cladiella, a zooxanthellate Indo-Pacific genus that is distributed in the Red Sea as well as much of the Indo-West Pacific (
Recent molecular phylogenetic evidence shows that there are two species included in Gersemia that nest in the genus Alcyonium, rather than with other nephtheids (
Alcyonium sp. indet. A Underwater photograph of orange and pale orange colonies, November 10, 2009, at Plumper Rock, Plumper Group of islands, Weynton Passage, British Columbia, Canada, GPS coordinates 50 35.495N × 126 47.998W., 20 m depth. Photo by Neil McDaniel B Wet-preserved white specimen (CAS 179450) C Wet-preserved red specimen (CAS 173217); scale bars for B and C =10 mm.
Alcyonium sp. indet. (CAS 029138). A Polyp sclerites B Coenenchymal sclerites of the polypary C Coenenchymal sclerites of the stalk. Scale bar = 0.10 mm.
urn:lsid:zoobank.org:act:C2BFCF41-3F55-43FA-9858-9FFC2543E0ED
http://species-id.net/wiki/Gersemia_lambi
Figures 7–11, 19Polyps clustered in groups on short lobes, emanating from short stalk above holdfast. Polyps tubular, curved, non-retractile, relatively large. Sclerites primarily radiates with variable ornamentation and modification of turberculation; rod-like forms also present. Colonies pink in life, white preserved.
Holotype. CAS 171939, Canada, British Columbia, Langara Island; 26 June 2004; 12 m depth, collected by Andy Lamb; one specimen. Paratypes. CAS 171940, same data as holotype, one specimen. CAS 171940, same data as holotype, one specimen.
CAS 179449, same data as holotype, 11 specimens. CAS 173218. Canada, British Columbia, east side of Kerouard Island, off south end of Queen Charlotte Island; 9 m depth, 51 54.624'N, 130 58.635'N; 7 August 2003; 20 m depth; collected by Doug Swanston; one specimen. CAS 173219. Canada, British Columbia, Queen Charlotte Islands, Kunghit Island, west side of Cape St. James; 21 May 2002; 9 m depth; collected by Danny Kent; one specimen.
(Figure 19). Shallow subtidal region from Cape Ommaney, southeast Alaska, USA (according to Neil McDaniel, pers. comm.), to central British Columbia, Canada; 9–20 m depth.
This species is named for marine naturalist and educator Andy Lamb (Vancouver, British Columbia), who collected the type material.
Colonial morphology (Figures 7B-C). The holotype measures 55 mm in length, 39 mm in width, and 35 mm in height. It is composed of dense concentrations of autozooids distributed in isolated clusters on several lobes that emanate from the stalk, which arises immediately above the basal holdfast. Each cluster usually contains approximately 5 and 15 polyps.
Polyps (Figures 7B–C, F). The polyps are monomorphic, non-retractile, tubular in shape, and vary in length and width (4.0–7.0 mm in length and 1.5–2.0 mm in width). The width of the polyps is greatest at the distal extremities. The polyps are erect and often curve upward from their bases.
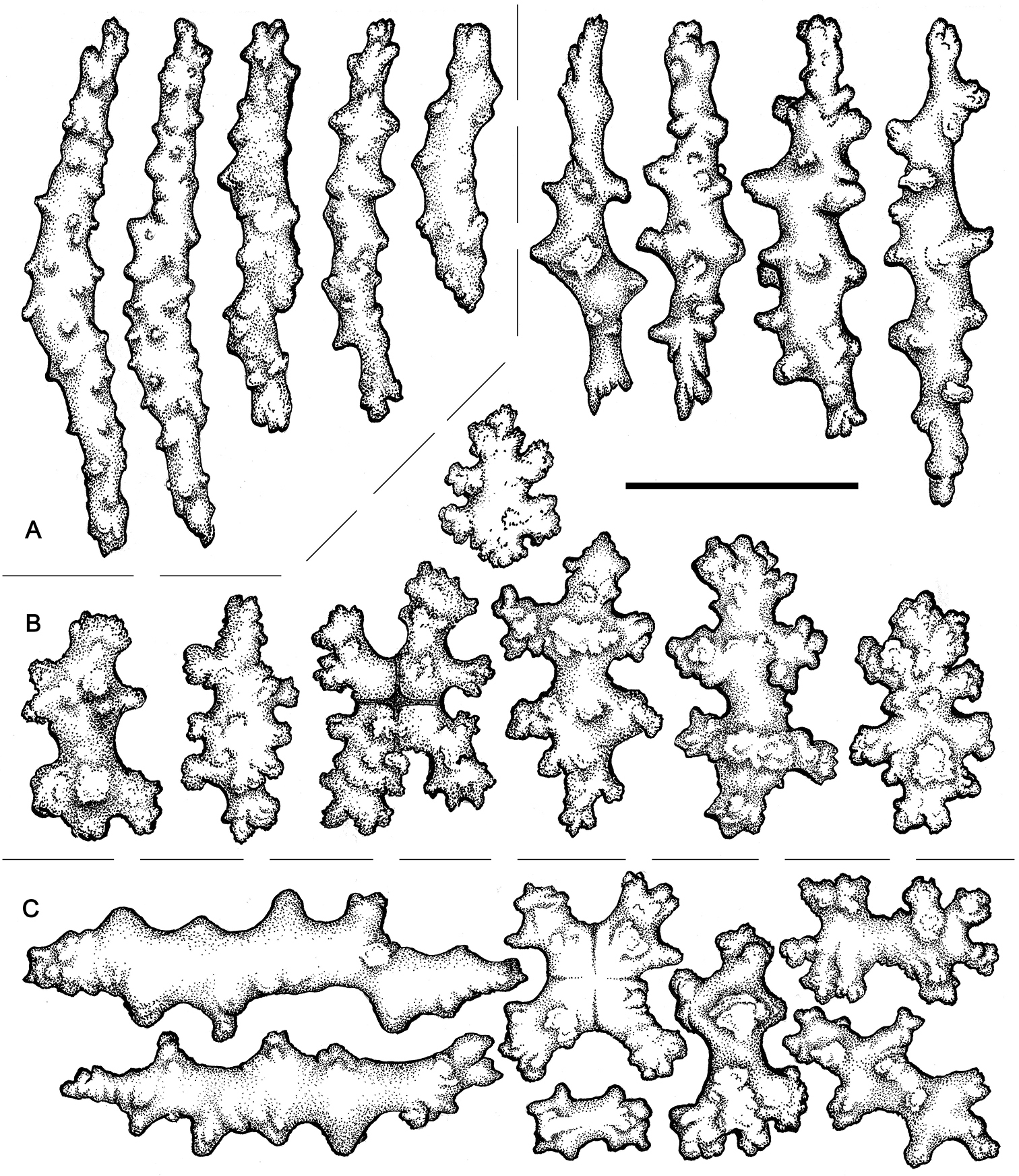
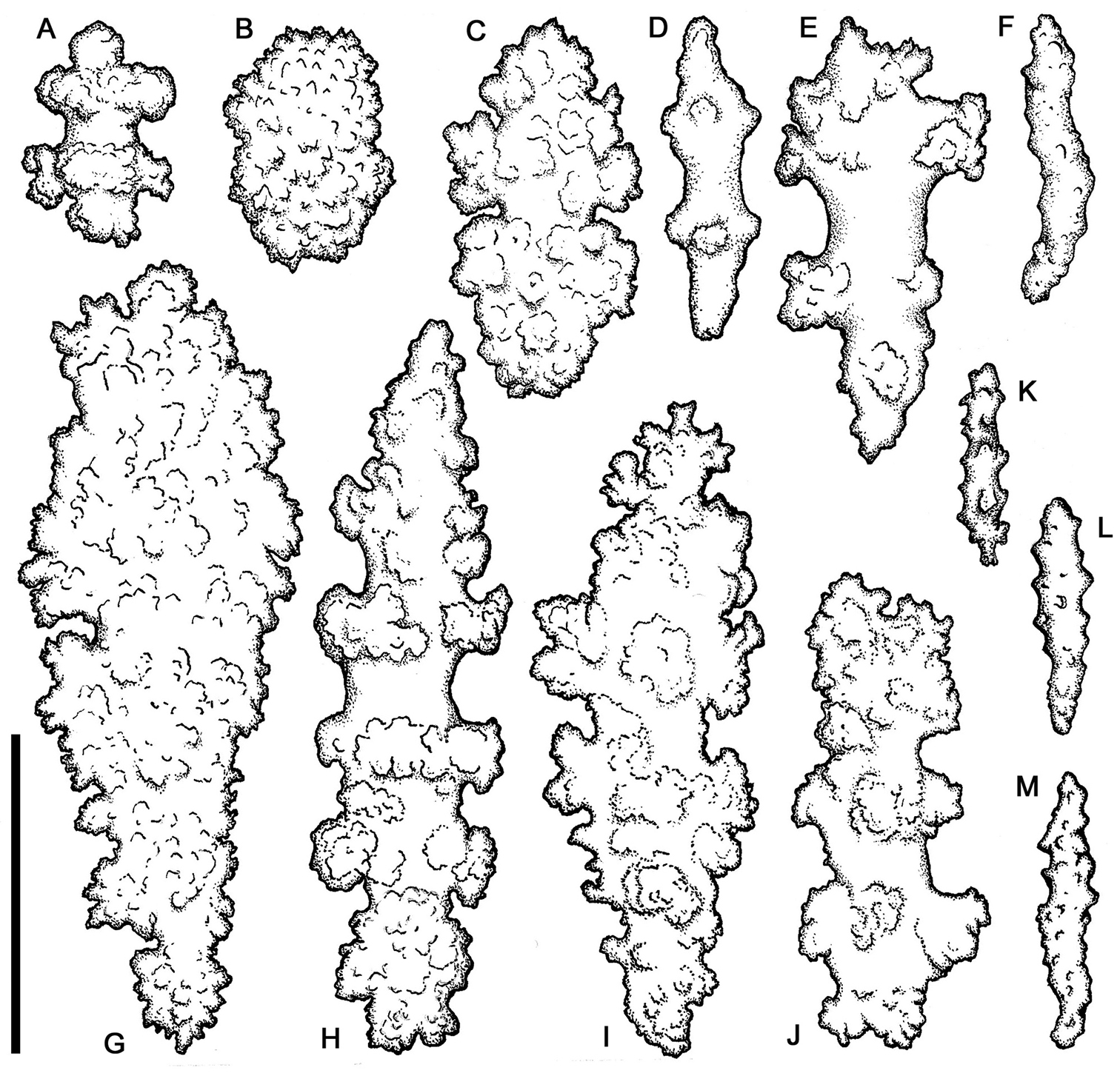
Sclerites (Figures 8–11). Coenenchymal sclerites of the polypary are primarily sharply-tuberculated radiates, 0.03–0.11 mm. Coenenchymal sclerites of the stalk are mostly variously-ornamented radiates and modified radiates, 0.03–0.12 mm long. Polyp wall sclerites abundant, uniformly and densely-distributed, 0.03–013 mm in length, mostly variably-shaped radiates and rods with a few irregularly-shaped elongate forms and crosses. Tentacle sclerites densely and uniformly distributed (not arranged en chevron), mostly radiates and rods, although a few club-shaped or approach torch-like forms are also present.
Color (Figures 7A–D, E). Colony color is pink to reddish in life, often with orange oral discs. Colonies are uniformly cream-white in color when preserved in ethanol.
Gersemia lambi sp. n. A Underwater photograph of colonies with polyps fully expanded; photo courtesy Marc Chamberlain B Wet-preserved holotype, dorsal view; scale bar = 10 mm C Wet-preserved holotype, lateral view; scale bar = 10 mm D Underwater photograph of colonies with polyps retracted; photo courtesy Neil McDaniel E Underwater photograph of colonies with polyps retracted (center); photo courtesy Marc Chamberlain F Wet-preserved paratype, lateral view (CAS 171941); scale bar = 10 mm.
Gersemia lambi sp. n. Scanning electron micrographs of polyp sclerites. Scale bar = 0.04 mm.
Gersemia lambi sp. n. Scanning electron micrographs of sclerites of the polypary coenenchyme. Scale bar = 0.04 mm.
Gersemia lambi sp. n. Scanning electron micrographs of stalk sclerites. Scale bar = 0.04 mm.
Gersemia lambi sp. n. Scanning electron micrographs of stalk sclerites. Scale bar = 0.04 mm.
The only other species known in the genus Gersemia from the Pacific Coast of North America is Gersemia juliepackardae Williams & Lundsten, 2009. Although coenenchymal sclerites of the two species are predominantly eight radiates, Gersemia juliepackardae and Gersemia lambi sp. n. differ markedly in surface feature characters. Those of Gersemia juliepackardae are narrow with slender medial waists and relatively rounded tubercle tips (
This species is distributed from Washington to southern California, while Gersemia lambi is known from southern Alaska to British Columbia. The two species differ markedly in their bathymetric distributions. Collected material of Gersemia juliepackardae is recorded between 888 to 1600 meters in depth, although video images record the species from 520–2034 meters (
Key to the species of Gersemia from the west coast of North America
| 1a | Alcohol-preserved polyps cylindrical, straight, 4.5–5.5 mm long by 1.2–1.5 mm wide. Sclerites of the distal half of polyps are red, all other sclerites colorless. Color of preserved colonies white with pink distal regions of polyps | Gersemia juliepackardae |
| 1b | Alcohol-preserved polyps tubular in shape, often curved, 4.5-8.0 mm long by 1.5–2.0 mm wide. All sclerites are colorless. Color of preserved colonies is white throughout | Gersemia lambi sp. n. |
urn:lsid:zoobank.org:act:72A0D6D2-C439-4B23-875A-1AAEF04B4C2E
http://species-id.net/wiki/Chromoplexaura
Figures 12–17, 19Plexaurid gorgonians. Colonies tall, erect, planar. Branching lateral from single basal stem. Upper branches relatively sparse, slender, elongate, mostly slightly curved. Retracted polyps as numerous low rounded protuberances all round surfaces of branches and stem. Sclerites mostly robust spindles and radiates, some ellipsoid to sub-spherical in shape.
Euplexaura marki Kükenthal, 1913.
The generic name is derived from the Greek chroma (color), and the gorgonian generic name Plexaura, in reference to the vivid color of the colonies.
The genus Euplexaura (Figures 12D, 18) is an Indo-Pacific genus (eastern Africa to the central Pacific) of 37 named species (
A comparison is also warranted between Chromoplexaura and two plexaurid genera that share some superficial similarities – Thesea with 31 described species from the Atlantic Ocean (
Forty five genera of the holaxonian family Plexauridae are currently considered valid (
Currently, the evidence based on molecular data does not support the family as being a monophyletic one, but rather has shown many genera dispersed throughout richly-populated phylogenetic trees, and do not exhibit close affinities as a group. In some cases, plexaurid genera have even appeared associated with genera in other families (
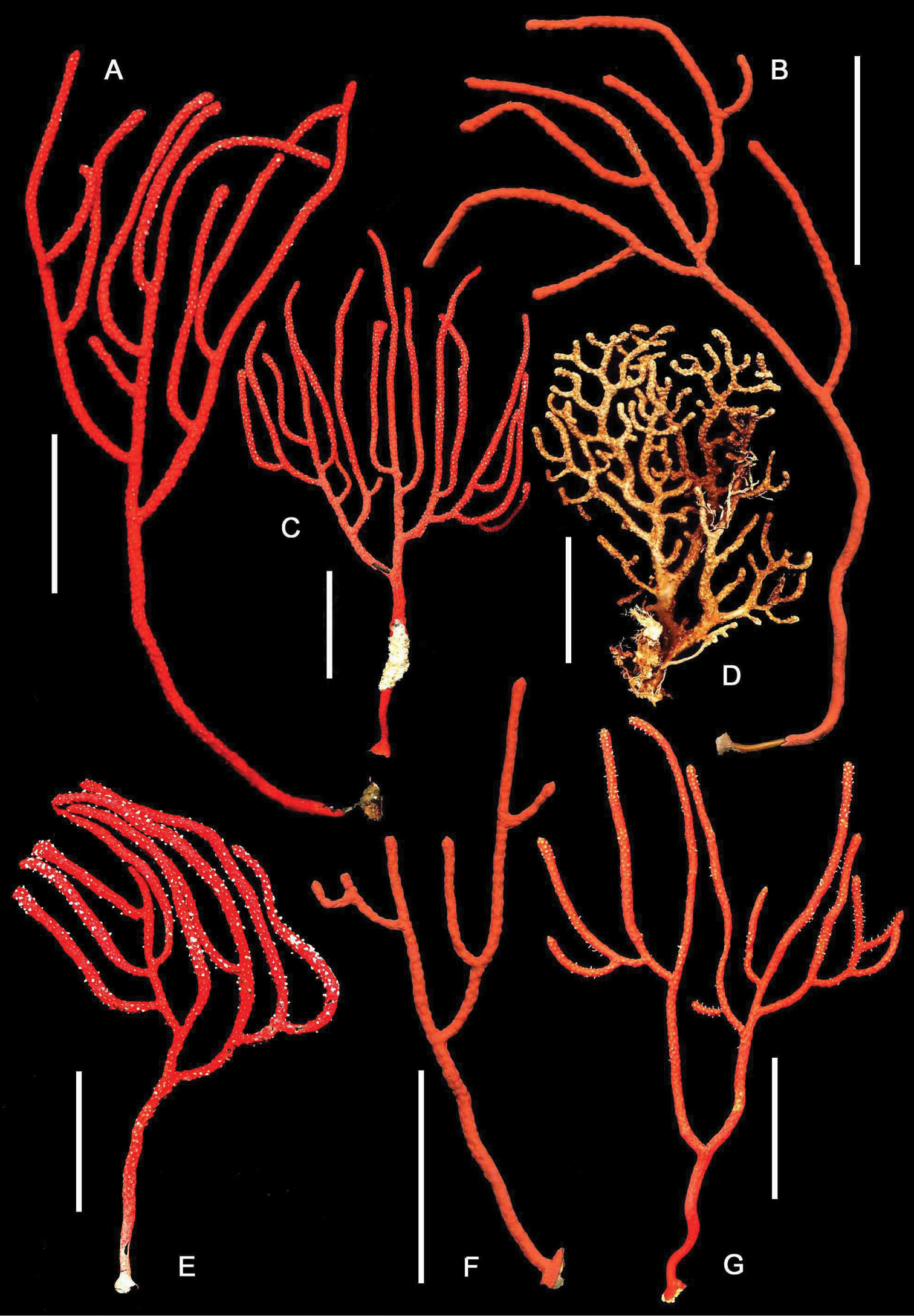
Plexaurid gorgonians, entire colonies. A, C Chromoplexaura marki (CAS 173222) B, F, G Chromoplexaura marki (CAS 096746) D Euplexaura sp. (CAS 107595.) E Chromoplexaura marki (CAS 168895). Scale bars = 50 mm.
Chromoplexaura marki, two living colonies in situ. A Detail of two gorgonians B Wide angle view showing the area inhabited by the colonies. Rittenburg Bank, Gulf of the Farallones National Marine Sanctuary, California, 83 m depth. Photo courtesy: NOAA (National Oceanic and Atmospheric Administration).
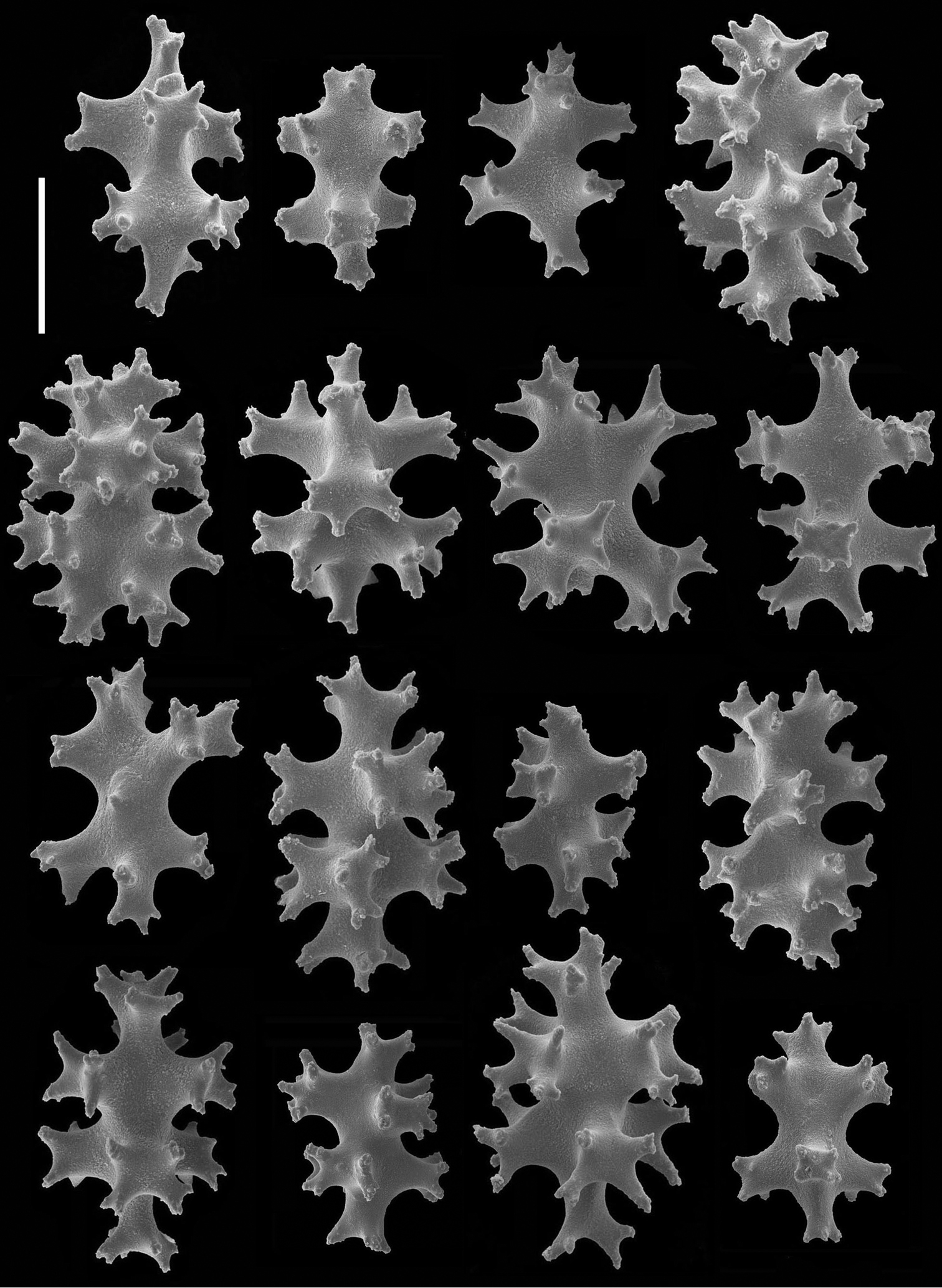
Chromoplexaura marki (CAS 096746). Scanning electron micrographs of coenenchymal sclerites. Scale bar = 0.04 mm.
Chromoplexaura marki (CAS 096746). Scanning electron micrographs of coenenchymal sclerites. Scale bar = 0.04 mm.
Chromoplexaura marki (CAS 168895). Scanning electron micrographs of the polyp sclerites. Scale bar = 0.04 mm.
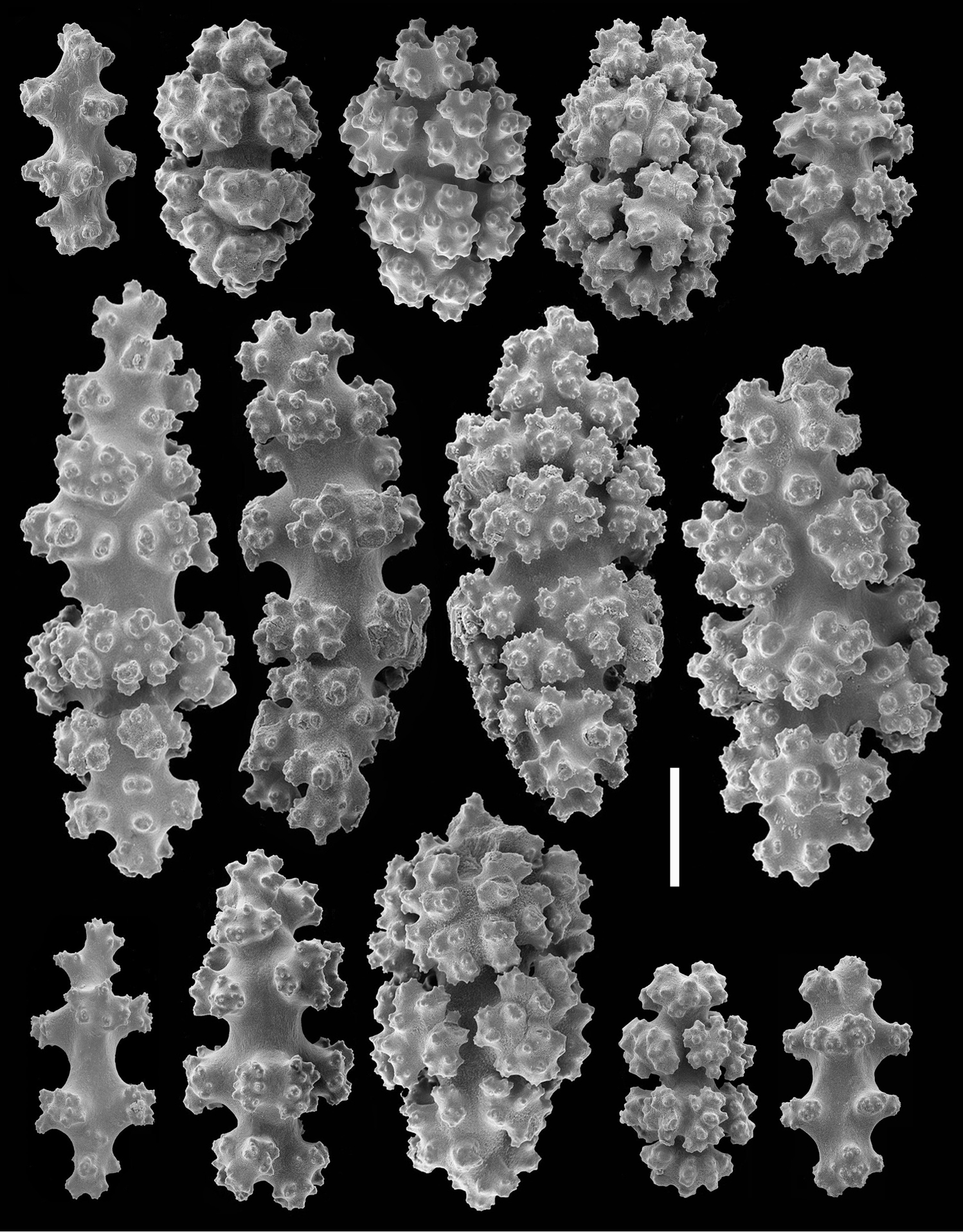
Chromoplexaura marki. Variation in sclerite form. A, B, D, E, F, H, L, M (CAS 096766) C, G, I, J, K (CAS 173222). Scale bar = 0.10 mm.
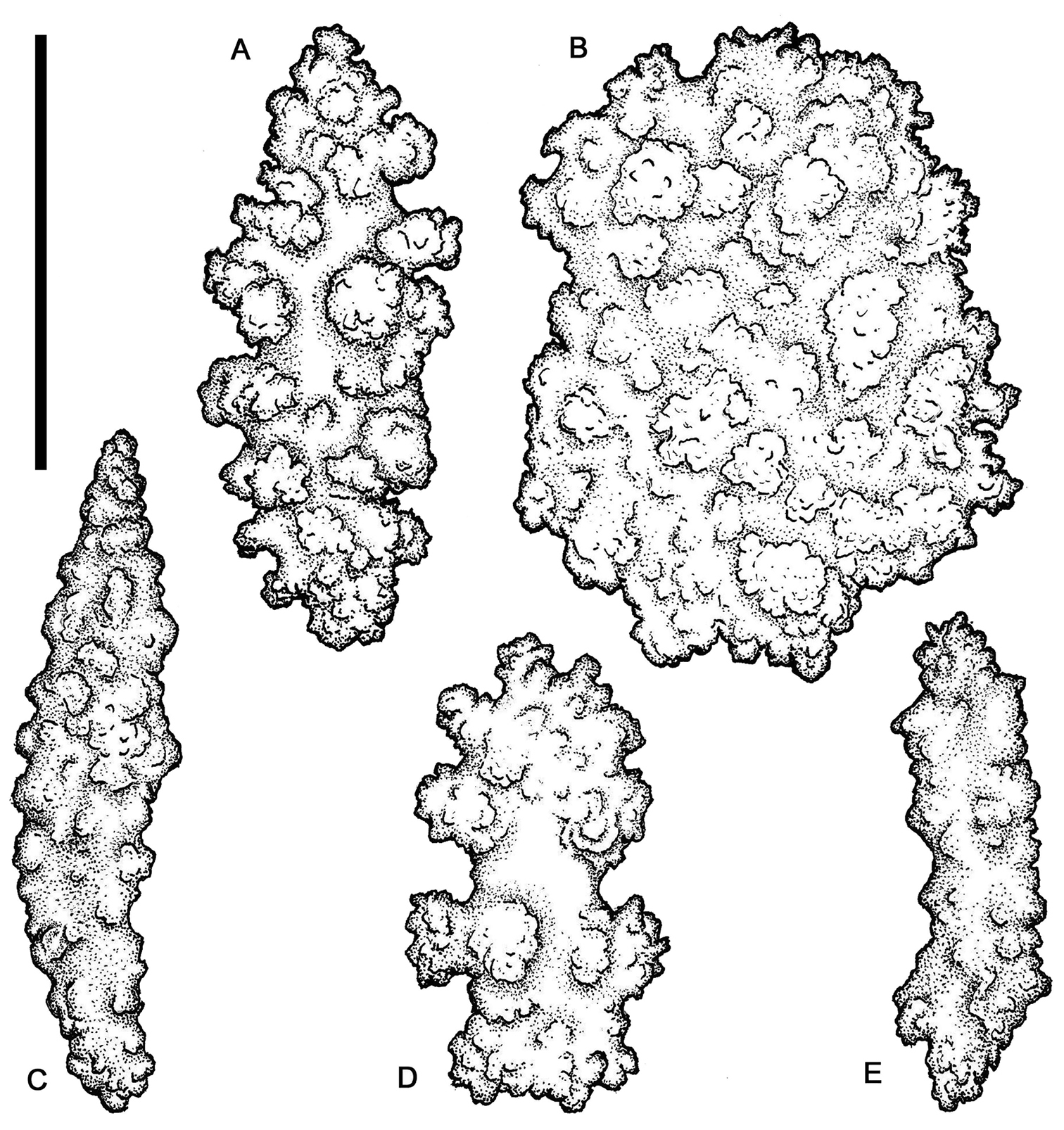
Euplexaura sp. (CAS 107595). A, B, D Coenenchymal sclerites C, E Polyp sclerites. Scale bar = 0.10 mm.
Key to the genera Chromoplexaua and Euplexaura
| 1a | Colony color red, formed by permanent red coloration of sclerites. Sclerites are mostly radiates and spindles, <0.10–0.25 mm long. One species, temperate Eastern Pacific (California to Oregon) | Chromoplexaura |
| 1b | Colony color highly variable, often formed by alcohol-soluble pigments, sclerites colorless. Sclerites are robust ovals to subsperoids and plump spindles, >0.10–0.30 mm long. Approximately 36 described species, tropical Indo-West Pacific (East Africa to Western Pacific) | Euplexaura |
http://species-id.net/wiki/Chromoplexaura_marki
Figures 12–17Euplexaura marki
CAS 096746, California, Monterey Carmel Bay off San Jose Creek Beach (Monastery Beach), 38 m depth, 20 May 1962, coll. Dennis Sullivan, five whole colonies. CAS 173222, California, Monterey Bay, Carmel Bay (Monterey Bay Marine Sanctuary), 32 m depth, 22 September 2010, coll. Karen Grimmer, two whole colonies. CAS 168895, California, (Gulf of the Farallones National Marine Sanctuary, Rittenburg Bank), 85 m depth, 8 October 2012, coll. National Oceanic and Atmospheric Administration, one whole colony.
Colonial morphology (Figures 12–13): The predominantly proteinaceous central axis has a hollow core. The main stem above the holdfast varies from 50–120 mm in length. The ultimate branches measure 10–115 mm in length by 2.5 –4.0 mm wide. The distal extremities are acute to rounded and often slightly swollen compared to the uniform width of the rest of the branches.
Polyps (Figures 12–13). Most of the polyps are fully retractile and form low rounded to hemispherical protuberances that are distributed on all sides of the branches. Some polyps are partially exserted and are <1.0 mm in width. Autozooid walls with eight longitudinal rows of densely-set, more-or-less en chevron sclerites that give rise to narrow points in the middle of each tentacle.
Sclerites (Figures 14–17). The coenenchymal sclerites are radiates, robust spindles, and ovoid forms with highly variable tuberculation, 0.06-0.24 mm in length (Figures 14, 15, 17A–E, G–J). Some are robust and subspherical to ellipsoid with numerous and less well-pronounced tubercles. The sclerites of the polyp wall and points are heavily tuberculated spindles and rods, 0.04 mm–0.09 mm in length (Figures 16; 17F, K, L, M).
Color (Figures 12–13). The color of the colonies is similar in life or preserved, the coenenchyme is uniformly- colored orange-red to vivid red, while the exsert polyps are white (Figure 11E). The coenenchymal sclerites are red-orange, while the polyp wall and points sclerites are colorless.
Distribution (Figure 19): Central Oregon to southern California; 9 to at least 90 m depth.
Biology and associated species. Several of the colonies in lot 096746, have enlargements on the branches that resemble gall-like growths, which contain epizoic barnacles of the genus Conopea (pers. comm., R. Van Syoc, California Academy of Sciences).
Map of the North American Pacific coast showing collecting localities for Cryptophyton jedsmithi sp. n. (*), Cryptophyton goddardi (●), Chromoplexaura marki (▲), and Gersemia lambi sp. n. (■). Arrows designate type localities.
In spite of the fact that the marine fauna of the west coast of the United States is relatively well known with a plethora of marine laboratories dotting the coast, as well as an abundance of well known and excellent manuals and field guides describing the fauna (
In addition, two new species of octocorals are here described from recently collected material in the intertidal zone of southern California and in shallow subtidal regions of British Columbia and southern Alaska.
I am grateful to Jeff Goddard, Neil McDaniel, Andy Lamb, Marc Chamberlain, Doug Swanston, Danny Kent, and Pauline Ridings for their observations, and for providing photographic images and octocoral material. I thank Jei-Ying Chen (California Academy of Sciences, San Francisco) for help in the preparation of scanning electron micrographs.