Citation: Yoshida T, Hirowatari T (2014) Taxonomic notes on Cryptamorpha sculptifrons Reitter (Coleoptera, Silvanidae), with description of its larval morphology. ZooKeys 412: 117–129. doi: 10.3897/zookeys.412.7615
Cryptamorpha sculptifrons Reitter, 1889 is redescribed and a lectotype and paralectotype are designated. The mature larva of C. sculptifrons is described. It is hypothesized that a variety described by
Taxonomy, redescription, lectotype, variety, urogomphi
The family Silvanidae Kirby, 1837 (Coleoptera, Cucujoidea) includes two subfamilies, about 58 genera and approximately 500 species (
The genus Cryptamorpha Wollaston, 1854 (Brontinae, Telephanini) includes 27 described species globally (
While larval morphology can provide useful information for phylogenetic studies (e.g.
In this paper, we redescribe Cryptamorpha sculptifrons and designate a lectotype and paralectotype. In addition, we describe the larval morphology of this species for the first time.
External characters were observed under a stereoscopic microscope (Olympus SZX10). Genital structures were placed on a cavity slide glass with 50% glycerol solution and observed with an optical microscope (Nikon Eclipse E400). The genitalia slide was prepared in the following steps: The removed abdomen was placed in a 200 µl PCR tube filled with 10% solution of potassium hydroxide (KOH) and kept in heated water for about seven minutes. After rinsing in 70% ethanol solution, the abdomen was dissected by cutting its lateral side using fine insect pins. The genitalia were transferred to a cavity slide glass with 50% glycerol solution for observation. After the observation, the genitalia and abdomen were mounted in Euparal on cover glasses each glued to a piece of cardboard, and pinned with the specimens.
Photographs of adults were taken with digital camera (Canon EOS 7D), and composite images were produced using automontage software Combine ZM. These images were retouched using Photoshop 6.0 (Adobe Systems Inc.).
The larvae were preserved in 70% ethanol (
Technical terms of genital structures follow
The lectotype and the paralectotype designated herein are deposited in the Natural History Museum, London (BMNH). The other specimens examined in this paper are deposited in the Ehime University Museum, Matsuyama (EUMJ) and the Entomological Laboratory, Kyushu University, Fukuoka (ELKU).
http://species-id.net/wiki/Cryptamorpha_sculptifrons
Japanese name: Semaru-hoso-hiratamushi
Figs 1–5Body length from anterior margin of clypeus to apex of elytra measured along the median line: 3.69–4.14 mm (n=14).
Coloration (Fig. 1A). Surface brown, yellowish-brown in some lighter colored specimens. Elytra brown, sometimes slightly lighter than head and pronotum, with a variable round dark macula at each posterior half; elytra of lectotype with dark maculae. Antennae unicolorous, brown, as in head and pronotum.
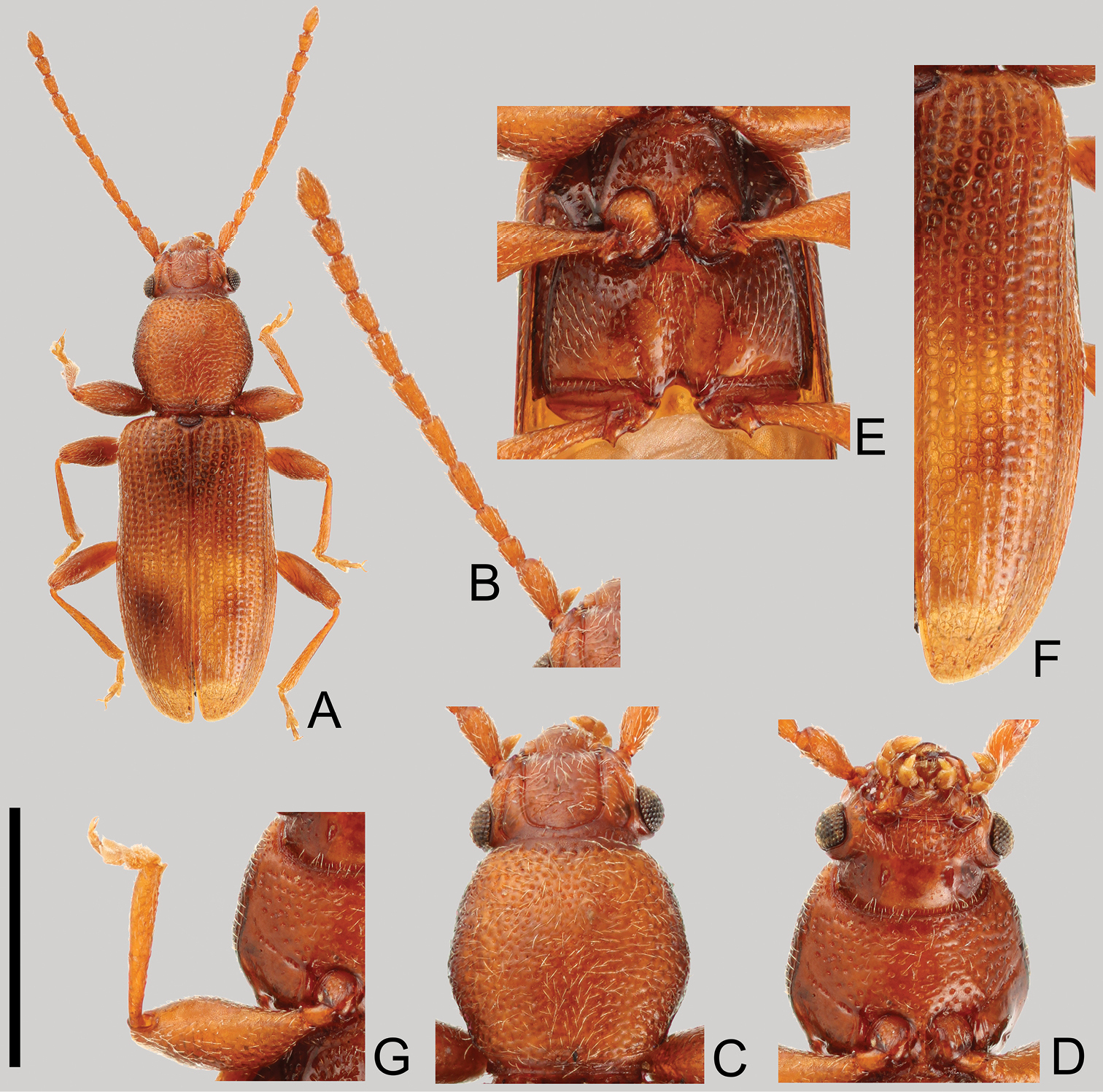
Cryptamorpha sculptifrons Reitter, 1889, lectotype, male. A Habitus, dorsal view B left antenna C head and pronotum, dorsal view D head and pronotum, ventral view E metaventrite F right elytron G right foreleg, ventral view. Scale: 2.0 mm for A and 1.0 mm for B–G.
Head (Fig. 1B, C and D). Triangular, width across eyes 0.76–0.86 mm. Temples immediately narrowed behind eyes, slightly incised at bases, with short setae except around eyes. Eyes relatively small, somewhat prominent, diameter less than half of length of head. Ventral surface with rough irregular punctuation except posterior half; dorsal surface with sparser punctuation. Antennal length 2.07–2.09 mm, 2nd antennomere short; 9th and 10th widened distally; covered with medium length semi-erect pubescence on each antennomere, 11th (apex) with short pubescence; approximate ratio of the length of each antennomere in lectotype as follows: 2.2: 1.0: 1.4: 1.7: 1.8: 1.7: 1.5: 1.5: 1.2: 1.2: 1.8 (Fig. 1B).
Prothorax (Fig. 1C and D). Rectangular, longer than wide, maximum width near middle, length 0.84–1.01 mm, width 0.89–0.95 mm in male (n=8) and 0.91–0.82 mm in female (n=5). Dorsal surface punctuated moderately densely except around posterior margin. Pubescence composed of many medium length setae on the dorsal side, and several relatively short setae on the ventral side. Each anterior angle with a few very small protuberances, with depressions around posterior angles of ventral surface. Procoxae without punctuation.
Elytra (Fig. 1F). Elongate, length 2.38–2.62 mm, combined width 1.22–1.43 mm. Punctures a little wider than interstices, scutellary striole composed of seven or eight punctures. Pubescence composed of semi-erect medium length setae.
Legs (Fig. 1E and G). Trochanter with a tooth on apical angle of inner margin. Profemora stout, maximum width near middle. Mesotibia curved inwards around apical 1/4.
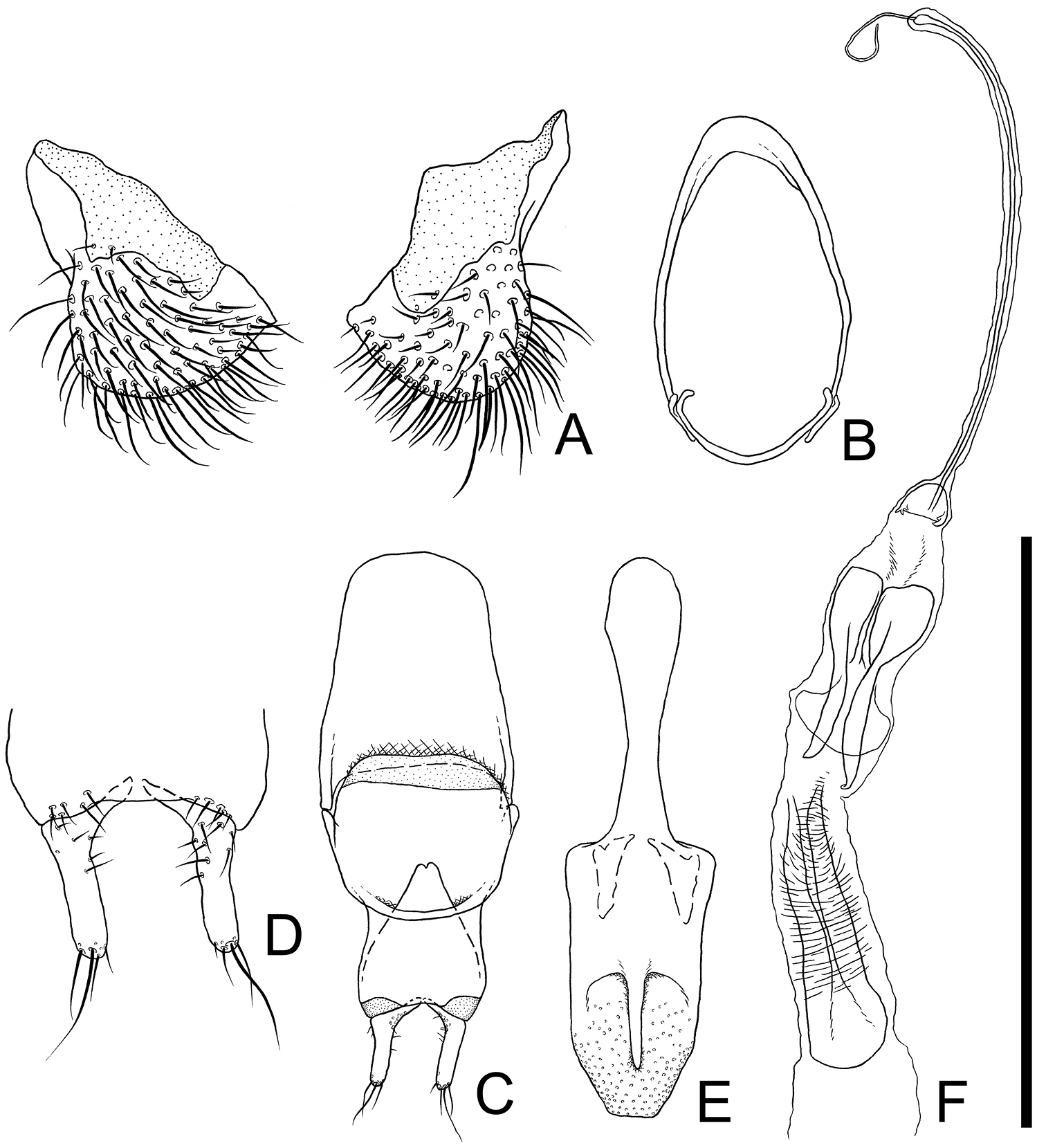
Eighth and 9th sternites (Fig. 2A and B). Eighth sternite (Fig. 2A) triangular, furcated near middle, each posterior half semicircular, covered with many medium length and some short setae, membranous around anterior portions. Ninth sternite (Fig. 2B) composed of two curved elements forming a ring: a short posterior semicircular strut strongly curved inwards at each end, connected to a long anterior element by a membrane; the long element strongly curved at middle, with a short dorsally orientated subapical process on each end, and the anterior half broadened.
Male genital organs of Cryptamorpha sculptifrons Reitter, 1889, lectotype, male. A 8th sternite, ventral view B 9th sternite C phallobase, dorsal view D parameres, ventral view E penis, dorsal view F internal sac, dorsal view. Scale: 0.5 mm for A, D and 1.0 mm for B, C, E, F.
Aedeagus (Fig. 2C–F). Parameres (Fig. 2C and D) L-shaped, relatively long, basal portions strongly curved inwards, inner dorsal margin of basal portion with several punctures, several medium length setae sparsely grouped around half of ventral surface, one or two long, a few medium length and a few short setae on each apex. Phallobase (Fig. 2C) rectangular, longer than wide, membranous around posterior angles, anterior margin of dorsal portion curled up at posterior 2/7, anterior angles protruding anterolaterally; each lateral portion strongly extended posteriorly, exposed from anterior margin of dorsal portion, connecting at posterior 1/3, membranous around anterior margin. Penis (Fig. 2E) elongate, posterior half relatively wide, somewhat flat, moderately punctuated on posterior 1/5, with blunt apex, and dorsal portion thinly extended posteriorly.
(Fig. 3). Males and females are very similar. However, males can be distinguished from females by the wider pronotum which is comparatively expanded (Fig. 3A) and the strongly impressed central area of the 7th sternite with a small protrusion around middle of posterior margin (Fig. 3B).
Sexual dimorphism of Cryptamorpha sculptifrons Reitter, 1889. A, B male C, D female. A, C Head and pronotum, dorsal view; B, D 7th sternite, ventral view. Scale: 1.0 mm.
Lectotype here designated: male, Chûzenji, Nikkô City, Tochigi Prefecture, Japan, 19–24–VIII–1881, G. Lewis leg. (BMNH). Paralectotype: 1 male, same data as lectotype (BMNH).
JAPAN: [Gunma Pref.] 2 females, Hatomachi-Pass, Katashina Village, 29–VIII–1978, Y. Hori leg. (EUMJ). [Nagano Pref.] 1 female, Mt. Kisokomagatake, 6–VII–1960, Y. Kimura leg. (EUMJ). [Yamanashi Pref.] 1 male, Takizawa-Rindô, Narusawa Village, 1–VIII–2005, T. Kurihara leg. (EUMJ). [Gifu Pref.] 2 female, 5 male & 1 ex., Nigorigo-Onsen, Gero City, 14–15–VIII–2013, N. Tsuji leg. (ELKU).
JAPAN: Honshu; China, Bhutan, India (
Syntypes of this species consisted of two specimens. We designate a brownish male specimen as lectotype, and another male yellowish-brown specimen as paralectotype, because the original description expressed their color as “testacea”, which means reddish brown color (
Head capsule width of mature larvae: 0.90–0.96 mm (n=27).
Coloration. Body white to yellowish white. First antennomere, posterior 4/5 of 2nd and anterior half of 3rd somewhat darkened. Frontal arm white. Most setae, base and posterior 2/7 of urogomphi brownish white.
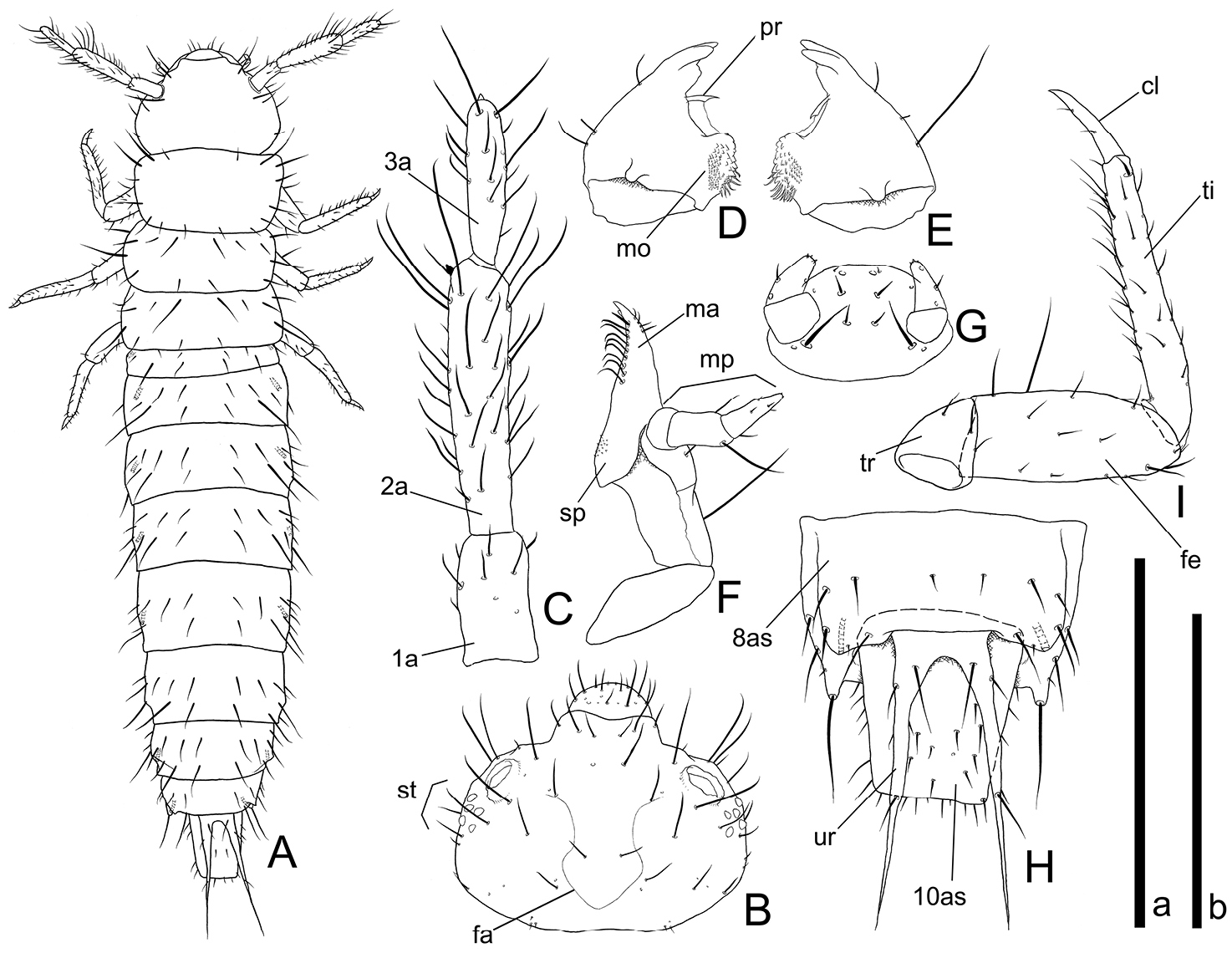
Head (Figs 4A–G, 5A and B). Rectangular, covered with medium length and long setae and several short setae. Frontal arm U-shaped (Figs 4B and 5A). Antenna (Figs 4C and 5B) moderately long; 1st antennomere stout but relatively long, apical half covered with relatively short setae; 2nd subparallel, relatively thick, twice as long as 1st, covered with many variable length setae, a short and thick conical sensorium present near apex of inner margin; 3rd less than 1.5 times as long as 1st, covered with many short and a few long setae, a stout and short seta present on apex. Mandibles (Fig. 4D and E) triangular with two acute and one blunt teeth around each apex; a prostheca on anterior 1/3 of each inner margin, anterior angles strongly pointed; molae on bases of inner margins enlarged in anterolateral direction with many minute spines; several fine spines around inner margins of posterior half; a few medium length setae and a long seta on each outer margin. Maxilla (Fig. 4F) oblong; mala diverging at apex, with several short setae on outer margin near apex, inner margin with relatively thick and long setae in a row of which the most apical seta spiniform; stipes with many moderately dense minute spines around inner margin of central dorsal areas; maxillary palpus 3-segmented; 1st spherical and short; 2nd more than twice as long as 1st, with a few long setae on apical portion; 3rd almost as long as 2nd with a few minute spines on apex and some short setae. Labium (Fig. 4G) round, covered with several short setae and a pair of relatively long setae; palpus two segmented, covered with a few short setae, a few very short spines on each apex. Six stemmata on each lateral portion (Fig. 5A).
Mature larval morphology of Cryptamorpha sculptifrons Reitter, 1889. A Habitus, dorsal view B head, dorsal view; C right antenna, dorsal view D right mandible, ventral view E left mandible, ventral view F right maxilla, dorsal view G labium, ventral view H 8th to 10th abdominal segments, dorsal view I right foreleg, dorsal view. Abbreviations: cl–claw; fa–frontal arm; fe–femur; ma–mala; mo–mola; mp–maxillary palpus; pr–prostheca; sp–stipe; st–stemmata; ti–tibiotarsus; tr–trochanter; ur–urogomphus; 1a–1st antennomere; 2a–2nd antennomere; 3a–3rd antennomere; 8as–8th abdominal segment; 10as–10th abdominal segment. Scale: a for A–H, 2.0 mm for A, 1.0 mm for B, H, 0.5 mm for C–F and 0.3 mm for G; b for I, 0.5 mm.
Mature larval morphology of Cryptamorpha sculptifrons Reitter, 1889. A Head, dorsal view B right antenna, dorsal view C 8th to 10th abdominal segments, dorsal view D left foreleg, dorsolateral view E, F urogomphi, dorsal view, basal portion (E) and middle portion (F). Scale: 0.5 mm for A–D and 0.1 mm for E, F.
Thorax and abdomen (Figs 4A, H, 5C, E and F). The shape easily deformed according to their posture. Thoraxes covered with short and medium length setae, a pair of relatively long setae on middle lateral margins, two relatively long setae on anterior angles of prothorax. Abdomen 10-segmented; 1st abdominal segment short, 2nd to 8th rectangular, wider than long, posterior angles of 8th strongly protruding, covered with some variably sized setae on each segment, a long seta on apex of each posterior angle of 8th (Fig. 4H), 9th concealed but urogomphi exposed from under posterior margin of 8th abdominal tergite, less than twice as long as 10th, deeply emarginate around base, a few medium length setae around base and a relatively short seta on middle of each branch, anterior half of each branch scaly (Figs 4H, 5C, E and F), 10th longer than wide, covered with relatively short setae.
Legs (Figs 4I and 5D). Elongate. Trochanter triangular, covered with a few medium length setae; femur relatively thick, covered with short and medium length setae and one or two long setae around inner margin; tibiotarsus elongate, curved strongly inwards around base, covered with many medium length setae and a relatively stout seta around apex; claw elongate, weakly curved inwards, apex pointed, with two short setae.
27 exs. (two specimens were examined with slide preparation, and two specimens with a SEM), Nigorigo-Onsen, Gero City, Gifu Prefecture, Japan, 14–15–VIII–2013, N. Tsuji leg. (ELKU).
According to Mr. Tsuji (pers. comm.), the mature larvae were collected from dead leaves of Southern Japanese hemlock Tsuga sieboldii with their adults.
The examined larvae were collected with adult individuals of Cryptamorpha sculptifrons. They were identified as a member of the subfamily Brontinae from the generic key to the known larvae of some cucujoid families (
Silvanid classification is in its infancy, and there is only one preliminary phylogenetic analysis for this family, treating 20 genera, based on 15 characters, by
In the key to known larvae of America North of Mexico by
Accumulation of detailed descriptions of the immature stages of more Silvanid taxa would be required for more accurate inferences on phylogenetic relationships and the completion of a more correct taxonomic key to larvae.
We wish to express our cordial thanks to Dr. Roger Booth and Dr. Beulah Garner (BMNH), Prof. Masahiro Sakai and Assoc. Prof. Hiroyuki Yoshitomi (EUMJ), Mr. Naomichi Tsuji (Faculty of Agriculture, Kyushu University, Fukuoka), Dr. Munetoshi Maruyama (the Kyushu University Museum, Fukuoka), Assoc. Prof. Satoshi Kamitani and the other members of the Entomological Laboratory (ELKU) for their valuable advice for our study and/or generous loan of material. Dr. Michael Karner (Senckenbergisches Naturforschendes Institut, Frankfurt am Main) and Sir Anthony Galsworthy (BMNH) provided us with critical comments to improve the manuscript. We also thank the member of the biosystematics laboratory (Faculty of Society and Cultural Studies, Kyushu University, Fukuoka) for permission to use their SEM.
This study was contributed from the Entomological Laboratory, Kyushu University, Fukuoka (Ser. 7, No. 10)