Citation: Zawierucha K, Dziamięcki J, Jakubowska N, Michalczyk Ł, Kaczmarek Ł (2014) New tardigrade records for the Baltic states with a description ofMinibiotus formosus sp. n. (Eutardigrada, Macrobiotidae). ZooKeys 408: 81–105. doi: 10.3897/zookeys.408.6612
In sixteen moss, lichen and mixed (moss/lichen) samples, collected from Estonia, Latvia and Lithuania, 291 specimens, 48 simplexes, including one exuvium with 6 eggs, and 8 free-laid eggs of eutardigrades were found. In total, 17 species, together with one new to science, were identified (all are new records for the Baltic states): Astatumen bartosi, Diphascon (Adropion) prorsirostre, D. (Diphascon) bullatum, D. (D.) pingue pingue, D. (D.) recamieri, D. (D.) rugosum, Hypsibius convergens, H. dujardini, H. cf. scabropygus, Isohypsibius ronsisvallei, I. sattleri, Macrobiotus harmsworthi harmsworthi, M. hufelandi hufelandi, Milnesium asiaticum, Milnesium tardigradum tardigradum, Minibiotus formosus sp. n. and Paramacrobiotus richtersi. The new species is most similar to Minibiotus gumersindoi, but differs from it mainly by the presence of two types of cuticular pores, the absence of a triangular or pentagonal arrangement of pores above a single large pore on legs, the presence of granulation on all legs and a different macroplacoid length sequence. In this paper we also provide photographs and morphometrics of H. cf. scabropygus.
Estonia, Europe, Hypsibius cf. scabropygus, Latvia, Lithuania, new species, Tardigrada
The Baltic States, i.e. Estonia, Latvia and Lithuania, are located on the eastern coast of the Baltic Sea, and fall within the Palearctic ecozone (
In this study we report seventeen tardigrade species, which are all new records for the Baltic States. Moreover, one of these species is also new to science. The new species belongs to the genus Minibiotus R.O. Schuster, 1980, that until 1988 contained only a single species, Minibiotus intermedius (Plate, 1888). In 1988
In addition to the description of the new species, we also provide morphometric data and photographs of Hypsibius cf. scabropygus, a rare species that belongs to a large group of hypsibiids with at least partially sculptured dorsal cuticle and pharynx with two macroplacoids and without the microplacoid.
Sixteen moss, lichen and mixed (moss/lichen) samples from trees, soil and stones were collected from 15 localities in Estonia, Latvia and Lithuania between the 29 April and the 5 May 2012 by the third author (more details below). Samples were collected and examined for tardigrades using standard methods (see
All measurements are given in micrometers [μm]. Structures were measured only if their orientation was appropriate. Body length was measured from the anterior extremity to the end of the body, excluding the hind legs. Buccal tube length and the level of the stylet support insertion point were measured according to
For species identification and differentiation, keys in
Raw data underlying the description of Minibiotus formosus sp. n. are deposited in the Tardigrada Register (
-
56°03'08"N, 24°24'10"E, ca. 33 m asl: Lithuania, Panevėžys county, Pasvalys district municipality, along the road E67, 0.5 km before the turning to Pasvalys, moss from tree and soil (slide code: LT 2422), date: 29.04.2012.
-
55°25'59"N, 24°13'32"E, ca. 59 m asl: Lithuania, Kaunas county, Kėdainiai district municipality, Truskava city, near a church, lichens from tree and moss from soil (slide code: LT 2423), date: 29.04.2012.
-
55°17'12"N, 23°58'57"E, ca. 30 m asl: Lithuania, Kaunas county, Kėdainiai district municipality, Kėdainiai city, Kranto II street; moss from wall (slide code: LT 2424), date: 29.04.2012.
-
55°17'13"N, 23°58'56"E, ca. 30 m asl: Lithuania, Kaunas county, Kėdainiai district municipality, Kėdainiai city, Paeismilgio street; moss from stone (slide code: LT 2425), date: 29.04.2012.
-
55°43'35"N, 24°21'30"E, ca. 62 m asl: Lithuania, Panevėžys county, Panevėžys district municipality, Panevėžys city, Garden Street near Holy Trinity Rector; moss from tree (slide code: LT 2440), date: 05.05.2012.
-
56°38'53"N, 23°43'18"E, ca. 7 m asl: Latvia, Zemgale region, Jelgava municipality, Jelgava city, City Park; moss from soil (slide code: ŁO 2426), date: 29.04.2012.
-
57°10'33"N, 24°50'32"E, ca. 45 m asl: Latvia, Vidzeme region, Sigulda municipality, Gutmana Cave in the Gauja National Park; moss from rocks (slide code: ŁO 2427), date: 30.04.2012.
-
56°23'55"N, 24°07'33"E, ca. 25 m asl: Latvia, Zemgale region, Bauska municipality, along Road No P103, 0.5 km from Saulaine; lichens from tree (slide code: ŁO 2428) date: 29.04.2012.
-
57°09'55"N, 24°51'03"E, ca. 73 m asl: Latvia, Vidzeme region, Sigulda municipality, Turaida city, Turaida Castle; moss from stone (slide code: ŁO 2430), date: 30.04.2012.
-
56°54'32"N, 24°08'45"E, ca. 10 m asl: Latvia, Riga Region, boundary of Ķekava municipality, along road no A2; moss from tree (slide code: ŁO 2431), date: 30.04.2012.
-
57°09'59"N, 24°50'59"E, ca. 91 m asl: Latvia, Vidzeme region, Sigulda municipality, Sigulda city, Sigulda Castle; moss from stone (slide code: ŁO 2432), date: 30.04.2012.
-
56°41'22"N, 23°47'43"E, ca. 4 m asl: Latvia, Zemgale region, Ozolnieki municipality, Ozolnieki city, about 100 m from the Ozolnieki Lake; moss from soil (slide code: ŁO 2433), date: 29.04.2012.
-
57°45'43"N, 24°20'59"E, ca. 3 m asl: Latvia, Vidzeme region, Salacgriva municipality, Salacgriva city; moss from soil, near the beach (slide code: ŁO 2434), date: 01.05.2012.
-
59°10'44"N, 24°30'06"E, ca. 59 m asl: Republic of Estonia, Harju county, Kernu Parish municipality, Road No 4, moss from tree (slide code: ES 2420), date: 04.05.2012.
-
59°10'44"N, 24°30'06"E, ca. 59 m asl: Republic of Estonia, Harju county, Kernu Parish municipality, Road No 4, moss from tree (slide code: ES 2421), date: 04.05.2012.
-
58°48'47"N, 24°24'46"E, ca. 32 m asl: Republic of Estonia, Rapla County, Märjamaa municipality, forest near Konuvere village, moss from tree (slide codes: ES 2487), date: 29.04.2012.
XV: 1 specimen.
Our specimen corresponds perfectly to the original description. Milnesium asiaticum was originally described from Kirghizstan and subsequently found in the Svalbard archipelago (
VIII: 31 specimens (including 6 simplexes) + 1 exuvium with 6 eggs.
Specimens correspond perfectly with the redescription by
XIII: 1 specimen.
Specimens correspond well with the limited original description (
XV: 11 specimens.
Although we have found only 11 specimens, we were confident in identifying them to Diphascon (Diphascon) pingue because they corresponded perfectly to the partial redescriptions by
XV: 1 specimen.
The species has previously been found in many localities, mostly in the Holarctic (
II: 3 specimens.
The species has previously been found in many localities in the Holarctic (
IX: 6 specimens (including 2 simplexes).
Belonging to the cosmopolitan convergens-dujardini complex of species (
XIV: 2 specimens (including 1 simplex), XV: 5 specimens.
Hypsibius dujardini belongs to the cosmopolitan convergens-dujardini complex of species (
XI: 1 simplex, XII: 1 specimen, XIV: 34 specimens (including 4 simplexes), XV: 24 specimens (including 6 simplexes).
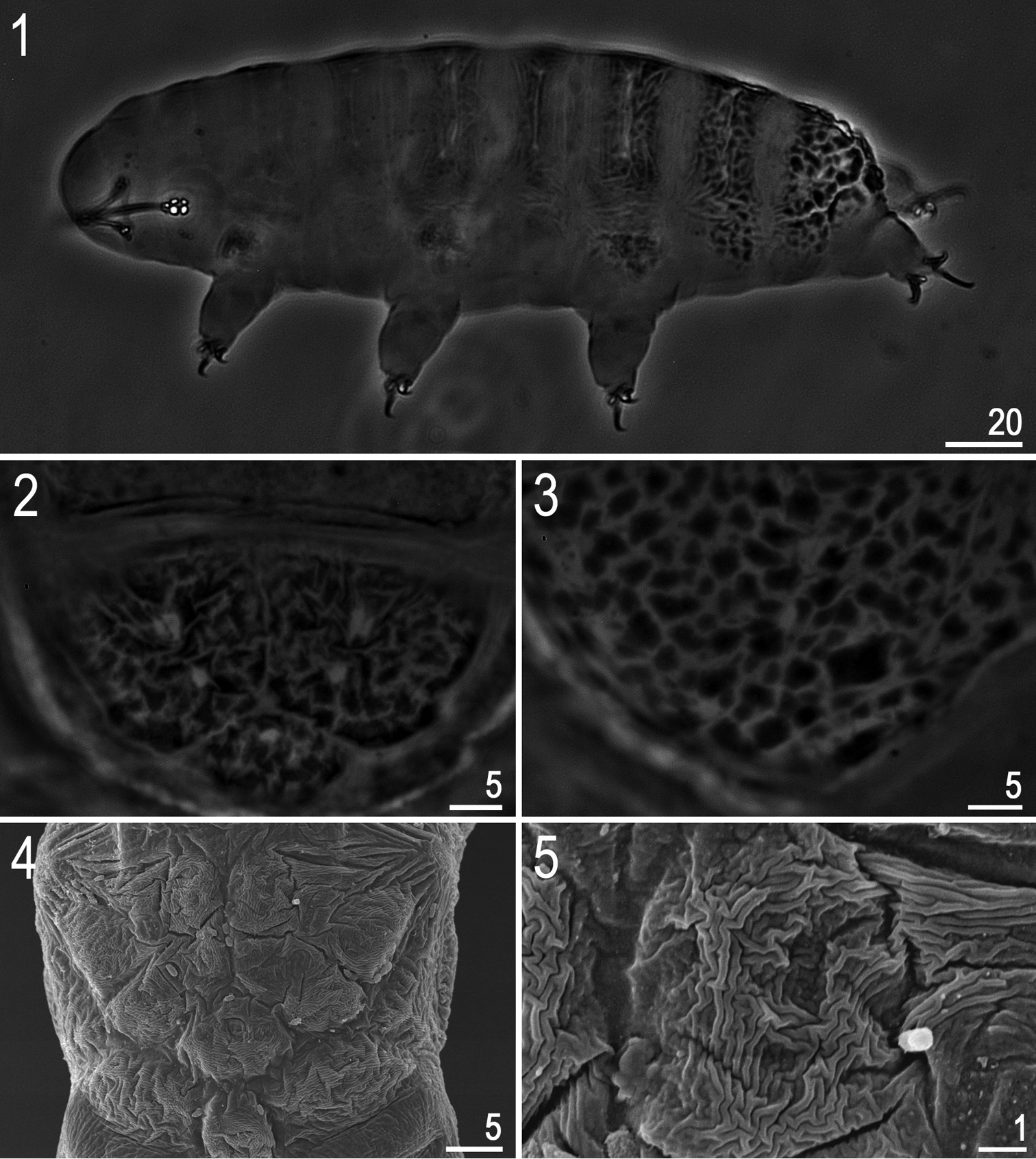
(measurements in Table 1). Adults. Body transparent/white (after preparation), eyes present in 6 of 15 (40%) specimens mounted in Hoyer’s medium (Fig. 1). Dorsal cuticle sculptured: from head to legs II without tubercles but clearly thickened, from legs II to the caudal end of the body (including legs IV) with irregular tubercles and platelets. Tubercles increasing in size from the anterior to the posterior part of the body, reaching maximum dimensions between legs III and IV, where tubercles sometimes merge and form irregular platelets (Figs 2–5). Irregular tubercles 1.0–6.0 μm in diameter. Ventral cuticle smooth (i.e. without sculpturing). Gibbosities and cuticular pores absent.
Hypsibius cf. scabropygus Cuénot, 1929: 1 habitus (dorso-lateral view) 2–4 caudo-dorsal cuticle with distinct sculpturing – tubercles and tubercles merged into platelets 5 a single caudo-dorsal platelet. (1–3: PCM, 4–5: SEM).
Measurements and pt values of selected morphological structures of Hypsibius cf. scabropygus Cuénot, 1929 mounted in Hoyer’s medium (N – number of specimens/structures measured, RANGE refers to the smallest and the largest structure among all measured specimens; SD – standard deviation).
| CHARACTER | N | RANGE | MEAN | SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | ||||||
| Body length | 14 | 183 | – | 293 | 808 | – | 1132 | 235 | 949 | 33 | 97 |
| Buccopharyngeal tube | |||||||||||
| Buccal tube length | 15 | 22.0 | – | 28.2 | – | 24.6 | – | 2.0 | – | ||
| Stylet support insertion point | 15 | 12.0 | – | 15.9 | 51.3 | – | 57.1 | 13.5 | 54.9 | 1.1 | 1.6 |
| Buccal tube external width | 15 | 1.5 | – | 2.0 | 6.5 | – | 8.0 | 1.8 | 7.2 | 0.2 | 0.5 |
| Buccal tube internal width | 15 | 0.6 | – | 0.9 | 2.1 | – | 3.4 | 0.7 | 2.7 | 0.1 | 0.4 |
| Placoid lengths | |||||||||||
| Macroplacoid 1 | 15 | 1.8 | – | 3.2 | 7.7 | – | 12.3 | 2.4 | 9.6 | 0.4 | 1.3 |
| Macroplacoid 2 | 15 | 1.7 | – | 2.7 | 7.2 | – | 11.3 | 2.2 | 8.9 | 0.3 | 1.1 |
| Macroplacoid row | 15 | 4.7 | – | 6.3 | 19.1 | – | 26.5 | 5.3 | 21.7 | 0.5 | 1.8 |
| Claw 1 lengths | |||||||||||
| External base | 14 | 2.2 | – | 4.5 | 9.1 | – | 17.2 | 3.5 | 14.1 | 0.7 | 2.1 |
| External primary branch | 14 | 4.0 | – | 8.8 | 18.2 | – | 36.1 | 6.9 | 28.1 | 1.5 | 5.0 |
| External secondary branch | 14 | 2.3 | – | 5.9 | 10.5 | – | 24.4 | 4.4 | 17.9 | 1.0 | 3.9 |
| Internal base | 12 | 2.1 | – | 4.1 | 9.3 | – | 15.5 | 3.3 | 13.4 | 0.6 | 1.8 |
| Internal primary branch | 12 | 3.8 | – | 5.9 | 16.0 | – | 23.5 | 4.8 | 19.3 | 0.6 | 2.0 |
| Internal secondary branch | 12 | 2.4 | – | 4.3 | 9.9 | – | 16.5 | 3.3 | 13.3 | 0.6 | 2.0 |
| Claw 2 lengths | |||||||||||
| External base | 11 | 3.0 | – | 5.2 | 12.4 | – | 19.9 | 4.1 | 16.7 | 0.7 | 2.2 |
| External primary branch | 13 | 6.7 | – | 10.4 | 29.7 | – | 43.7 | 8.5 | 34.7 | 1.3 | 4.4 |
| External secondary branch | 13 | 4.3 | – | 6.7 | 19.0 | – | 27.2 | 5.4 | 21.9 | 0.7 | 2.6 |
| Internal base | 10 | 2.4 | – | 4.5 | 10.9 | – | 18.9 | 3.6 | 14.6 | 0.7 | 2.2 |
| Internal primary branch | 12 | 4.0 | – | 6.7 | 17.7 | – | 27.2 | 5.4 | 22.0 | 0.9 | 2.9 |
| Internal secondary branch | 12 | 2.6 | – | 5.4 | 11.8 | – | 22.0 | 4.1 | 16.7 | 0.9 | 3.0 |
| Claw 3 lengths | |||||||||||
| External base | 9 | 2.7 | – | 6.2 | 11.9 | – | 23.8 | 4.3 | 17.3 | 1.0 | 3.5 |
| External primary branch | 9 | 7.2 | – | 10.4 | 29.3 | – | 43.7 | 8.8 | 35.7 | 1.1 | 4.4 |
| External secondary branch | 9 | 3.6 | – | 6.5 | 12.8 | – | 27.3 | 5.2 | 21.0 | 1.0 | 4.3 |
| Internal base | 11 | 2.3 | – | 4.1 | 10.5 | – | 17.2 | 3.4 | 13.9 | 0.6 | 1.9 |
| Internal primary branch | 13 | 3.8 | – | 6.5 | 17.3 | – | 27.3 | 5.4 | 21.8 | 0.9 | 3.1 |
| Internal secondary branch | 12 | 2.7 | – | 6.1 | 12.2 | – | 24.8 | 3.9 | 16.0 | 0.9 | 3.5 |
| Claw 4 lengths | |||||||||||
| Anterior base | 13 | 3.3 | – | 5.6 | 12.8 | – | 20.1 | 4.1 | 16.8 | 0.6 | 2.2 |
| Anterior primary branch | 13 | 4.4 | – | 7.5 | 19.5 | – | 31.1 | 5.9 | 24.2 | 1.1 | 3.9 |
| Anterior secondary branch | 11 | 3.1 | – | 13.2 | 13.0 | – | 47.3 | 4.8 | 18.9 | 2.9 | 9.7 |
| Posterior base | 12 | 2.7 | – | 5.4 | 12.3 | – | 21.5 | 4.5 | 18.2 | 0.9 | 3.1 |
| Posterior primary branch | 12 | 4.9 | – | 14.9 | 22.0 | – | 60.6 | 10.3 | 41.9 | 2.9 | 11.2 |
| Posterior secondary branch | 12 | 4.0 | – | 6.5 | 15.4 | – | 25.6 | 5.2 | 21.2 | 0.9 | 3.4 |
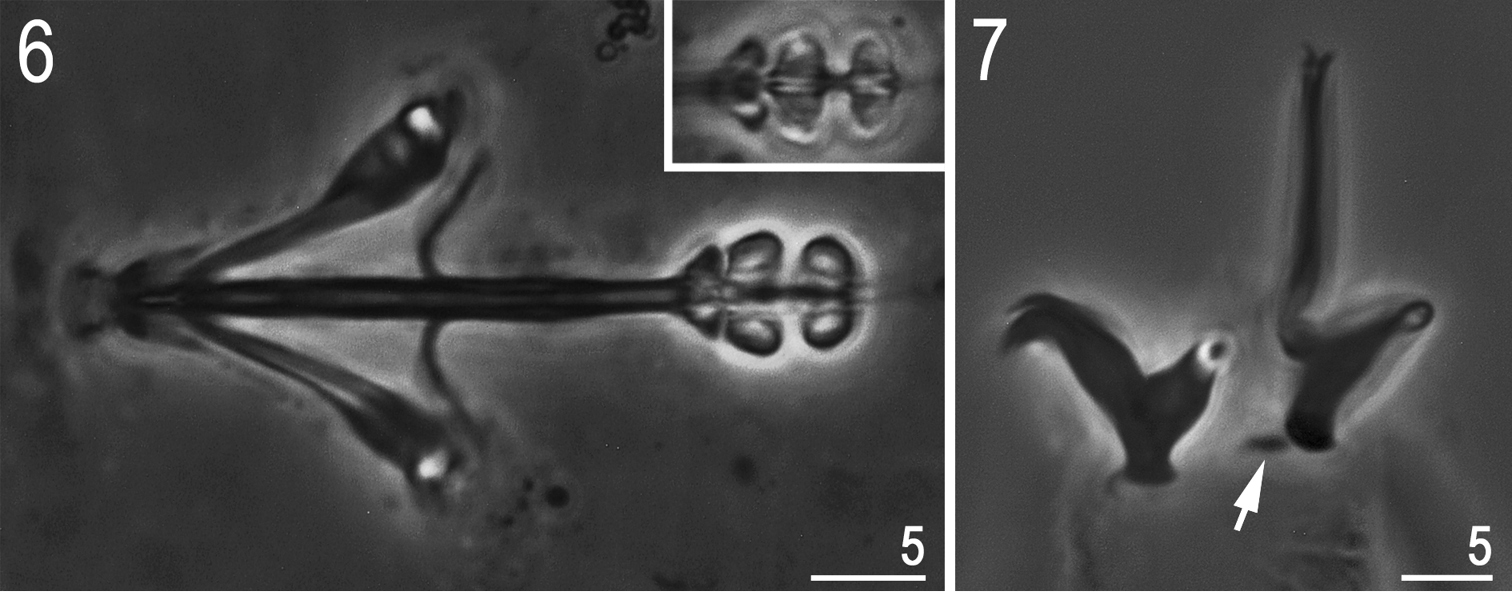
Bucco-pharyngeal apparatus of the Hypsibius type, without the ventral lamina, and with forked apophyses for stylet muscles (Fig. 6). Peribuccal lamellae absent. Teeth in the oral cavity armature absent or not visible under PCM. Pharyngeal bulb with apophyses and with two granular macroplacoids (both, without constrictions). Macroplacoid length sequence 2<1. Microplacoid and septulum absent.
Hypsibius cf. scabropygus Cuénot, 1929: 6 bucco-pharyngeal apparatus (dorso-ventral projection, ventral placoids in the insert) 7 claws IV (arrow indicates a small cuticular bar near the posterior claw). (Both PCM).
Claws of the Hypsibius type, internal claws much smaller and of a different shape than the external claws (Fig. 7). All main branches with large accessory points. Smooth, indistinct areoles under claws usually visible only on posterior claws IV. Cuticular bars under claws I-III absent but a small bar is present near the posterior claw IV (Fig. 7, arrow).
Eggs. Unknown.
Hypsibius scabropygus has been recorded from many localities, mostly in the Holarctic (
XIV: 1 specimen.
Our specimen corresponds perfectly with characters of Astatumen bartosi proposed by
III: 1 specimen.
The species has previously been reported from several, mostly European, localities in the Holarctic (
IX: 1 specimen, XI: 8 specimens (including 5 simplexes), XIV: 3 specimens, XV: 5 specimens, XVI: 1 specimen.
The species has previously been reported from many localities throughout the World, thus it is considered cosmopolitan (
XI: 1 egg, XIV: 8 specimens, 1 egg.
The species belongs to the harmsworthi group which is widely distributed across a broad range of ecosystems throughout the world (
XIV: 4 specimens, 1 egg.
The species belongs to the hufelandi group which is widely distributed across a broad range of ecosystems throughout the world (
http://zoobank.org/BDBE49B7-84CF-4FE2-BE55-A399A537DE77
http://species-id.net/wiki/Minibiotus_formosus
http://www.tardigrada.net/register/0012.htm
Tables 2 –3, Figs 8–15Holotype and 23 paratypes, 24 specimens (including 2 simplexes) and 3 unembryonated eggs).
57°10'33"N, 24°50'32"E, ca. 45 m asl: Latvia, Vidzeme region, Sigulda municipality, Gutmana Cave in the Gauja National Park; moss from rock (1 sample, slide codes: ŁO 2427/*, where the asterisk can be substituted by any of the following numbers: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12).
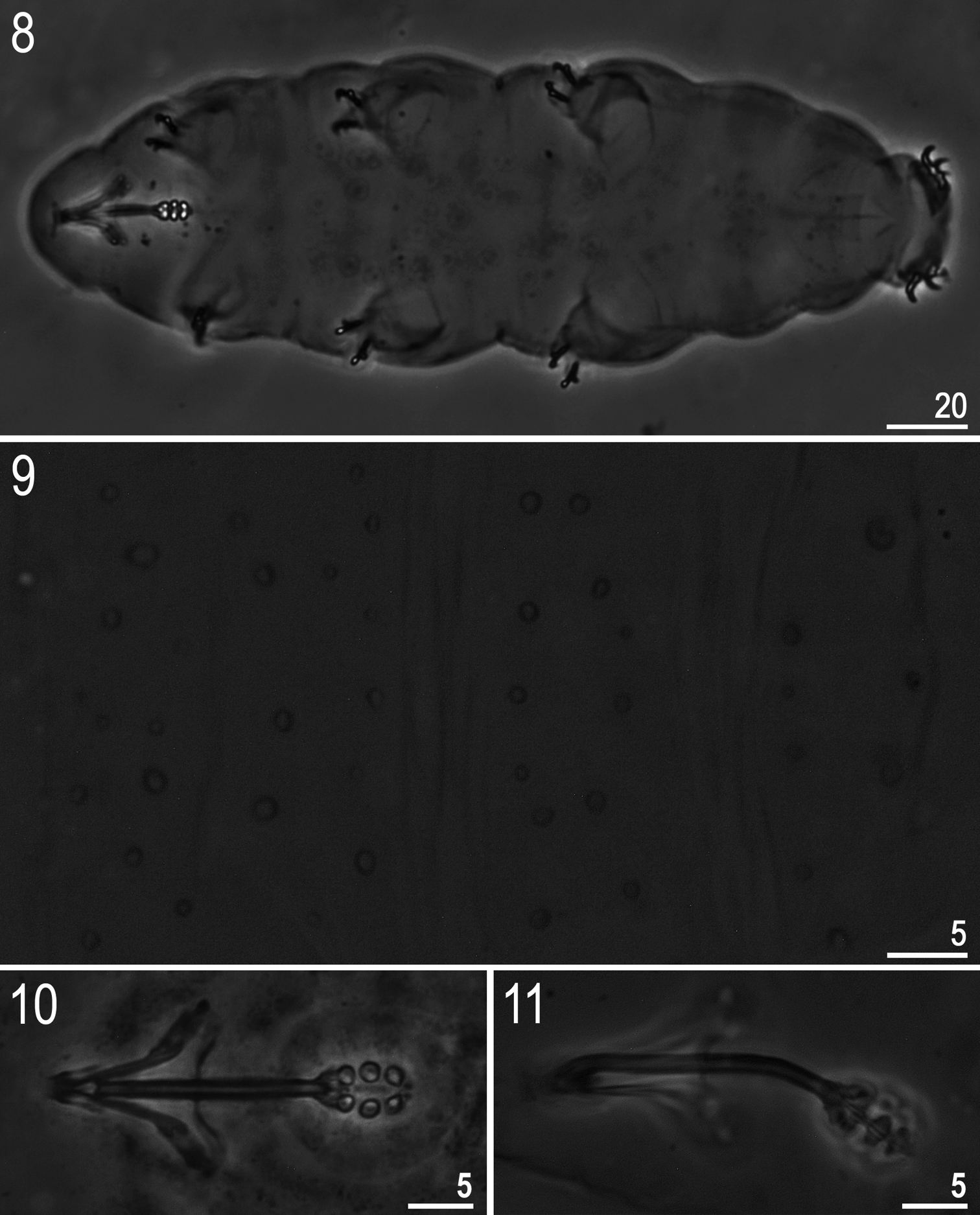
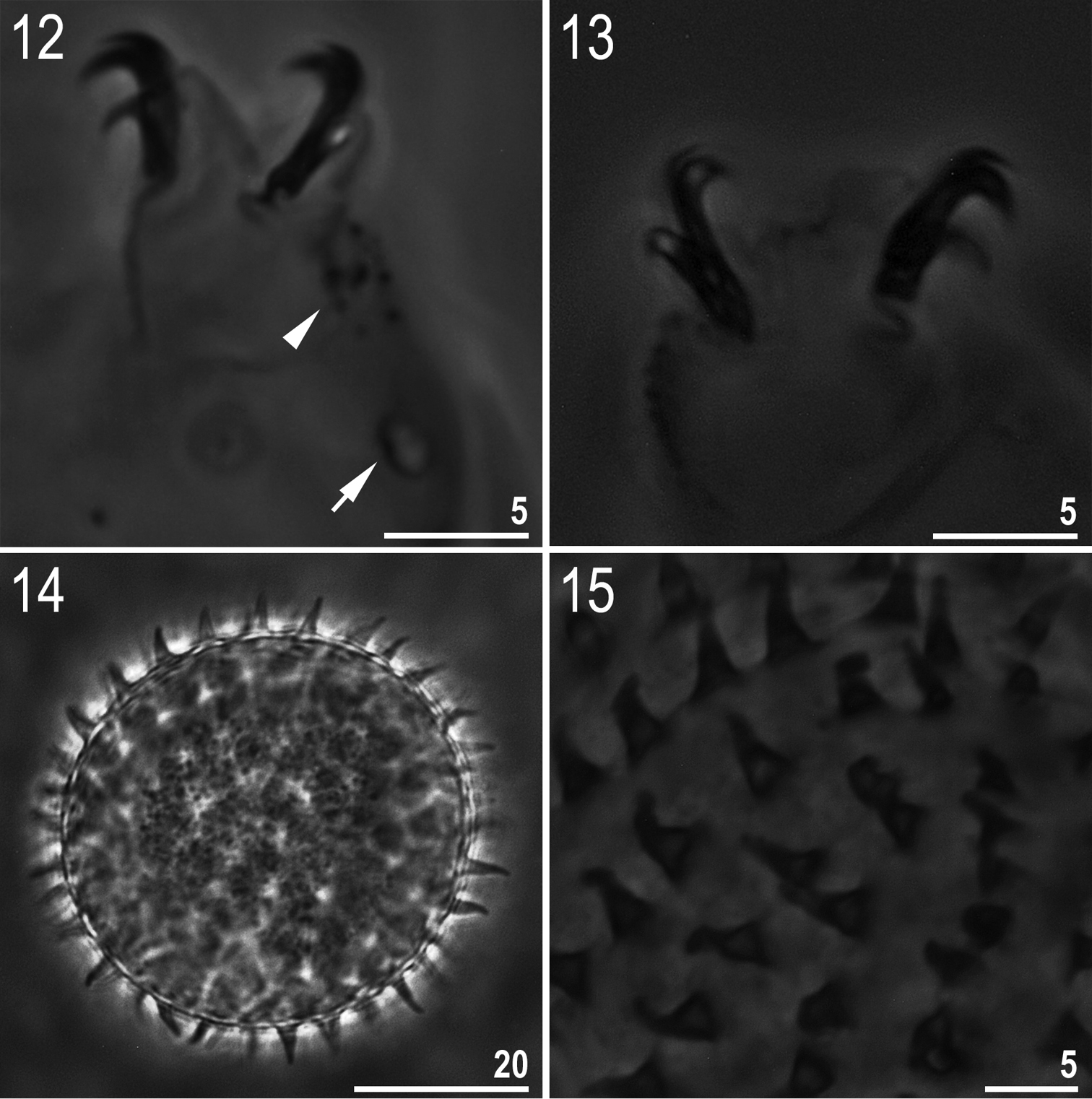
(measurements in Table 2). Body white/colourless (Fig. 8). Eyes present in 18 of 24 (75%) specimens mounted in Hoyer’s medium. Entire cuticle covered with small (0.4–1.1 μm) and large (1.9–2.5 μm) round or oval pores (Fig. 9). Pores arranged in 9–10 poorly defined transverse bands. Pores on the dorsal cuticle arranged more densely than on the ventral cuticle. A single large pore (diameter: 2.1–2.9 μm) present on external side of legs I–III (Fig. 12, arrow). A ring of pores around the mouth opening absent. Cuticle without granulation, except for legs which are all covered with fine and regular granulation (better developed on legs IV) visible only in larger specimens (Fig. 12, arrowhead).
Minibiotus formosus sp. n.: 8 habitus (holotype, ventral view) 9 dorsal cuticle with pores (holotype) 10–11 bucco-pharyngeal apparatus (10 dorso-ventral projection, paratype 11 lateral view, paratype). All PCM.
Minibiotus formosus sp. n.: 12 leg II with claws, granulation (arrowhead) and a single large pore (arrow) (holotype) 13 claws IV (paratype) 14 egg (mid-section) 15 egg surface with processes. All PCM.
Measurements and pt values of selected morphological structures of Minibiotus formosus sp. n. mounted in Hoyer’s medium (N – number of specimens/structures measured, RANGE refers to the smallest and the largest structure among all measured specimens; SD – standard deviation, ? – trait oriented unsuitably for measurement).
| CHARACTER | N | RANGE | MEAN | SD | Holotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | µm | pt | ||||||
| Body length | 7 | 113 | – | 236 | 636 | – | 1034 | 184 | 901 | 39 | 139 | 194 | 848 |
| Buccal tube | |||||||||||||
| Length | 9 | 17.7 | – | 22.9 | – | 20.3 | – | 1.7 | – | 22.9 | – | ||
| Stylet support insertion point | 9 | 9.5 | – | 12.2 | 49.5 | – | 56.2 | 10.9 | 53.8 | 0.9 | 2.0 | 12.2 | 53.3 |
| External width | 9 | 1.3 | – | 2.0 | 7.0 | – | 9.9 | 1.6 | 7.8 | 0.2 | 0.8 | 1.7 | 7.4 |
| Internal width | 9 | 0.5 | – | 0.7 | 2.4 | – | 3.4 | 0.6 | 2.8 | 0.1 | 0.3 | 0.7 | 3.1 |
| Placoid lengths | |||||||||||||
| Macroplacoid 1 | 9 | 1.3 | – | 1.9 | 6.9 | – | 8.3 | 1.5 | 7.6 | 0.2 | 0.4 | 1.8 | 7.9 |
| Macroplacoid 2 | 9 | 1.2 | – | 1.7 | 6.8 | – | 8.3 | 1.5 | 7.5 | 0.2 | 0.4 | 1.7 | 7.4 |
| Macroplacoid 3 | 9 | 1.4 | – | 2.2 | 7.4 | – | 9.6 | 1.7 | 8.2 | 0.3 | 0.6 | 1.9 | 8.3 |
| Microplacoid | 8 | 0.5 | – | 0.9 | 2.4 | – | 4.7 | 0.7 | 3.3 | 0.1 | 0.8 | 0.8 | 3.5 |
| Macroplacoid row | 9 | 4.5 | – | 6.8 | 24.3 | – | 33.7 | 5.5 | 26.9 | 0.7 | 3.0 | 5.9 | 25.8 |
| Placoid row | 8 | 5.2 | – | 7.9 | 27.1 | – | 39.1 | 6.4 | 31.0 | 0.9 | 3.9 | 6.7 | 29.3 |
| Claw 1 lengths | |||||||||||||
| External primary branch | 4 | 4.7 | – | 6.5 | 22.8 | – | 31.6 | 5.8 | 27.7 | 0.8 | 3.7 | 6.2 | 27.1 |
| External secondary branch | 3 | 3.6 | – | 4.3 | 17.5 | – | 20.9 | 3.9 | 19.4 | 0.4 | 1.7 | ? | ? |
| Internal primary branch | 6 | 4.7 | – | 6.6 | 24.3 | – | 30.4 | 5.7 | 27.4 | 0.7 | 2.4 | 6.3 | 27.5 |
| Internal secondary branch | 5 | 3.1 | – | 4.6 | 15.0 | – | 20.4 | 4.0 | 18.7 | 0.5 | 2.3 | 4.6 | 20.1 |
| Claw 2 lengths | |||||||||||||
| External primary branch | 5 | 5.2 | – | 6.9 | 25.2 | – | 34.0 | 5.9 | 28.6 | 0.6 | 3.5 | 5.9 | 25.8 |
| External secondary branch | 4 | 3.3 | – | 4.7 | 16.0 | – | 23.2 | 3.9 | 19.4 | 0.6 | 2.9 | ? | ? |
| Internal primary branch | 5 | 5.2 | – | 6.4 | 27.1 | – | 31.9 | 6.0 | 29.7 | 0.5 | 2.3 | 6.2 | 27.1 |
| Internal secondary branch | 3 | 3.5 | – | 4.1 | 18.5 | – | 20.9 | 3.9 | 19.9 | 0.3 | 1.3 | ? | ? |
| Claw 3 lengths | |||||||||||||
| External primary branch | 5 | 5.1 | – | 6.9 | 27.0 | – | 33.2 | 6.3 | 30.6 | 0.7 | 2.4 | 6.9 | 30.1 |
| External secondary branch | 6 | 3.6 | – | 4.9 | 19.0 | – | 24.1 | 4.6 | 22.1 | 0.5 | 1.8 | 4.9 | 21.4 |
| Internal primary branch | 7 | 5.1 | – | 6.6 | 25.7 | – | 32.5 | 5.9 | 29.2 | 0.6 | 2.5 | 6.4 | 27.9 |
| Internal secondary branch | 4 | 4.1 | – | 4.6 | 20.9 | – | 22.8 | 4.3 | 21.6 | 0.2 | 0.8 | ? | ? |
| Claw 4 lengths | |||||||||||||
| Anterior primary branch | 5 | 6.0 | – | 8.0 | 31.6 | – | 39.6 | 7.0 | 34.2 | 0.8 | 3.5 | ? | ? |
| Anterior secondary branch | 4 | 4.0 | – | 6.0 | 21.2 | – | 29.7 | 5.0 | 24.1 | 0.9 | 3.9 | ? | ? |
| Posterior primary branch | 6 | 6.0 | – | 7.8 | 30.7 | – | 38.4 | 7.0 | 33.9 | 0.7 | 3.1 | ? | ? |
| Posterior secondary branch | 5 | 3.9 | – | 5.5 | 20.6 | – | 27.1 | 4.8 | 23.8 | 0.6 | 2.6 | ? | ? |
Mouth antero-ventral. Ten peribuccal papulae present. Bucco-pharyngeal apparatus of the Minibiotus type (Figs 10–11). Oral cavity armature absent or not visible under PCM. Buccal tube with a poorly visible ventral lamina and with an anterior and a posterior bend (both visible in lateral view only, Fig. 11). Buccal tube walls thickened just below the stylet supports insertion point. Pharyngeal apophyses triangular, very near to the first macroplacoid. Three granular macroplacoids and a minute microplacoid present in the pharyngeal bulb. All macroplacoids of similar but not identical sizes, the macroplacoid length sequence: 2<1<3. Septulum absent.
Claws of the Macrobiotus type (Figs 12–13). Primary branches of claws with thin, but obvious accessory points detaching at the apogee of the primary branch curve. Smooth lunules present on all legs, distinctly larger under external and posterior claws. Bars and other cuticular thickenings on legs absent.
Eggs (measurements in Table 3). White/transparent, laid freely (Fig. 14). Spherical, without areolation. Processes in the shape of short, smooth, slightly flexible cones (Fig. 15). Processes are distributed on the surface of the egg close one to another but never in contact. Surface between processes smooth under PCM (Fig. 15).
Measurements of selected morphological structures of Minibiotus formosus sp. n. eggs mounted in Hoyer’s medium.
| CHARACTER | egg 1 | egg 2 | egg 3 |
|---|---|---|---|
| Diameter of egg without processes | 45.7 | 44.1 | ? |
| Diameter of egg with processes | 55.6 | 55.1 | ? |
| Process height | 4.5–5.2 | 4.8–5.2 | 4.6–5.3 |
| Process base width | 2.8–3.4 | 2.8–3.1 | 2.4–2.6 |
| Process base/height ratio | 57%–69% | 54%–65% | 47%–57% |
| Distance between processes | 2.0–2.5 | 1.9–3.9 | 1.8–2.0 |
| Number of processes on the egg circumference | 30 | 29 | 30 |
Since ventral lamina is very poorly visible, the measurements of this structure are not included in Table 2. Three unembryonated eggs have been found alongside the described specimens. Given that Minibiotus formosus sp. n. was the only Minibiotus species in the sample and because no Ramazzottius Binda & Pilato, 1986 was found in the samples, we assumed that these eggs belong to the new species.
Given that we found the composition of small and large pores in the new species beautiful, we decided to name the animal after this impression (in Latin ‘formosus’ means ‘beautiful’).
Holotype 23 paratypes and 3 eggs are deposited in the Department of Animal Taxonomy and Ecology at the Adam Mickiewicz University (Poznań, Poland).
The new species is most similar to Macrobiotus gumersindoi Guil & Guidetti, 2005, but it differs from it by: the presence of two types of cuticular pores (small and large) in the new species vs pores of a uniform size in Macrobiotus gumersindoi, the absence of a triangular or a pentagonal arrangement of pores placed above a single large pore on legs, the presence of granulation on legs, a different macroplacoid length sequence (2<1<3 in the new species vs 1=2=3 in Macrobiotus gumersindoi), and by slightly larger macroplacoids (I: 1.3–1.9 μm; II: 1.2–1.7 μm III: 1.4–2.2 μm in the new species vs 1.0 μm in Macrobiotus gumersindoi).
Other species to which Minibiotus formosus sp. n. is similar by some characteristics of adult and/or egg morphology (e.g. pores in transverse bands, eggs with conical processes), include species listed below. The new species differs specifically from:
-
Macrobiotus bisoctus (
Horning et al. 1978 ) by: the absence of trilobed and star-shaped pores (although their presence was not mentioned in the original description, they are clearly visible in Fig. 114 inHorning et al. (1978) , and by stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt≈60.3 in Macrobiotus bisoctus (according toClaxton 1998 )). -
Macrobiotus eichhorni Michalczyk & Kaczmarek, 2004 by: a different arrangement of pores on the dorsal cuticle (9–10 transverse bands in the new species vs 8 bands in Macrobiotus eichhorni), the absence of star-shaped pores, the absence of four pores around the mouth opening, the presence of a single large pore on lateral sides of legs I-III, slightly shorter buccal tube (17.7–22.9 μm in the new species vs 24.7–34.2 μm in Macrobiotus eichhorni), stylet supports inserted in more anterior position (9.5–12.2 μm [pt=49.5–56.2] in the new species vs 16.2–23.8 μm [pt=65.4–70.6] in Macrobiotus eichhorni), a different macroplacoid sequence (2<1<3 in the new species vs 2<3<1 μm in Macrobiotus eichhorni), slightly shorter placoids, and by slightly smaller external claws I–IV (compare Table 2 below and Table 1 in
Michalczyk and Kaczmarek 2004 for exact differences in dimensions of placoids and claws). -
Macrobiotus furcatus (Ehrenberg, 1859) (according to
Binda and Pilato 1992 ) by: the absence of tri- and quadrilobed cuticular pores, the presence of two types of cuticular pores (small and large in the new species vs uniformly small pores present in Macrobiotus furcatus), the presence of a single large pore on each of legs I–III, the presence of granulation on legs, the absence of the oral cavity armature, stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt≈68.4 in Macrobiotus furcatus), a different macroplacoid length sequence (2<1<3 in the new species vs 2<3<1 in Macrobiotus furcatus), and by egg processes without an obvious flexible portion (and never bifurcated). -
Macrobiotus harrylewisi Meyer & Hinton, 2009 by: the absence of tri- and quadrilobed cuticular pores, the presence of two types of pores (small and large) over the entire cuticle in the new species vs small pores present only in the anterior part of the body and large pores present only in the posterior part of the body in Macrobiotus harrylewisi, the presence of a single large pore on each of legs I–III, stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt=61.4–67.6 in Macrobiotus harrylewisi), a different macroplacoid length sequence (2<1<3 in the new species vs 2≤3<1 μm in Macrobiotus harrylewisi), a different shape of egg processes (short, single-tipped cones in the new species vs elongated, tapering cones with bulbous bases in Macrobiotus harrylewisi), a smaller diameter of eggs without and with processes (44.1–45.7 μm and 55.1–55.6 μm in the new species vs 66.1–80.0 μm and 78.2–101.9 μm in Macrobiotus harrylewisi), a slightly lower number of processes on egg circumference (29–30 in the new species vs 32–41 in Macrobiotus harrylewisi), and by smaller egg processes (4.5–5.3 μm in the new species vs 7.6–12.8 μm in Macrobiotus harrylewisi).
-
Macrobiotus jonesorum Meyer et al., 2011 by: the absence of trilobed and polygonal pores, the presence of two types of cuticular pores (small and large) in the new species vs small pores present only in the anterior part of the body, intermediate in size in the middle of the body and large pores in the posterior part of the body in Macrobiotus jonesorum), the presence of a single large pore on each of legs I-III, the presence of granulation on all legs, a slightly shorter buccal tube (17.7–22.9 μm in the new species vs 24.4–29.6 μm in Macrobiotus jonesorum), stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt=63.0–65.6 in Macrobiotus jonesorum), a slightly smaller external diameter of the buccal tube (1.3–2.0 μm [pt=7.0-9.9] in the new species vs 2.1–2.6 μm [pt=7.4–10.7] in Macrobiotus jonesorum), a different macroplacoid length sequence (2<1<3 in the new species vs 1<2<3 in Macrobiotus jonesorum), slightly shorter macroplacoids II and III (II: 1.2–1.7 μm [pt=6.8–8.6]; III: 1.4–2.2 μm [pt=7.4–9.6] in the new species vs II: 1.9–2.3 μm [pt=7.1–8.8]; III: 2.4–2.6 μm [pt=8.4–9.9] in Macrobiotus jonesorum), a slightly shorter macroplacoid row (4.5–6.8 μm [24.3–33.7] in the new species vs 7.0–8.4 μm [pt=27.0–34.4] in Macrobiotus jonesorum), the presence of a microplacoid, and by slightly shorter primary and secondary branches of external claws I–IV (compare Table 2 below and Table 2 in
Meyer et al. 2011 ). -
Macrobiotus keppelensis Claxton, 1998 by: the lack of red pigment granules, the presence of two types of cuticular pores (small and large) in the new species vs pores uniform in size (ca. 1.0 μm) in Macrobiotus keppelensis), the presence of a single large pore on each of legs I-III, a slightly shorter buccal tube (17.7–22.9 μm in the new species vs 24.9–28.4 μm in Macrobiotus keppelensis), stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt≈60.6 in Macrobiotus keppelensis), a different macroplacoid length sequence (2<1<3 in the new species vs 2=3<1 in Macrobiotus keppelensis), a slightly shorter macroplacoid row (4.5–6.8 μm in the new species vs 7.0–7.6 μm in Macrobiotus keppelensis), the lack of a membrane around egg processes, a smaller diameter of eggs with processes (55.1–55.6 μm in the new species vs 65.0–85.0 μm in Macrobiotus keppelensis), a larger number of processes on egg circumference (29–30 in the new species vs ca. 11 in Macrobiotus keppelensis), smaller egg processes (4.5–5.3 μm in the new species vs 11.0–16.0 μm in Macrobiotus keppelensis), narrower egg processes bases (2.4–3.4 μm in the new species vs 9.0–12.0 μm in Macrobiotus keppelensis), and by slightly smaller distances between egg processes (1.8–3.9 μm in the new species vs 4.0–6.0 μm in Macrobiotus keppelensis).
-
Macrobiotus orthofasciatus Fontoura et al., 2009 by: cuticular pores arranged in 9–10 transverse bands (11 transverse bands present in Macrobiotus orthofasciatus), the absence of tri- and quadrilobed cuticular pores, the presence of two types of pores (small and large) in the new species vs all pores of similar size in Macrobiotus orthofasciatus, the presence of a single large pore on each of legs I–III, the presence of granulation on all legs, stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt=66.5–67.8 in Macrobiotus orthofasciatus), a different shape of egg processes (short, single tip cones without a membrane in the new species vs screw-like processes with a membrane and six areoles in Macrobiotus orthofasciatus), a slightly larger number of processes on egg circumference (29–30 in the new species vs ca. 24 in Macrobiotus orthofasciatus), and by smaller distances between egg processes (1.8–3.9 μm in the new species vs 6.4–6.9 μm in Macrobiotus orthofasciatus).
-
Macrobiotus poricinctus Claxton, 1998 by: cuticular pores arranged in 9–10 transverse bands (8 transverse bands in Macrobiotus poricinctus), the presence of two types of pores (small and large) in the new species vs uniform pore size in Macrobiotus poricinctus), the presence of a single large pore on each of legs I–III, stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt≈59.5 in Macrobiotus poricinctus), a different macroplacoid length sequence (2<1<3 in the new species vs 2=3<1 in Macrobiotus poricinctus), a different shape of egg processes (short, single-tipped cones without a membrane in the new species vs. screw-like processes within a membrane in Macrobiotus poricinctus), the absence of granulation on egg shell, a larger number of processes on egg circumference (29–30 in the new species vs 18–20 in Macrobiotus poricinctus), slightly smaller egg processes (4.5–5.3 μm in the new species vs 6.5–7.0 μm in Macrobiotus poricinctus), and by smaller distances between egg processes (1.8–3.9 μm in the new species vs 6.0–8.0 μm in Macrobiotus poricinctus).
-
Macrobiotus pustulatus (Ramazzotti, 1959) by: the absence of triangular and polygonal pores, the presence of two types of cuticular pores (small and large) in the new species vs small pores present only in the anterior part of the body, intermediate in size in the middle of the body and the large pores in the posterior part of the body in Macrobiotus pustulatus), the presence of a single large pore on each of legs I–III and, egg processes without a filiform bristle.
-
Macrobiotus ramazzottii Binda & Pilato, 1992 by: pores arranged in bands, the presence of two types of pores (small and large) in the new species vs universal pores size in Macrobiotus ramazzottii), the presence of a single large pore on each of legs I–III, the absence of the oral cavity armature, stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt=68.2–68.3 in Macrobiotus ramazzottii), a different macroplacoid length sequence (2<1<3 in the new species vs 3<2<1 in Macrobiotus ramazzottii), and by a lower number of processes on egg circumference (29–30 in the new species vs ca. 34–41 in Macrobiotus ramazzottii).
-
Macrobiotus subintermedius (Ramazzotti, 1962) by the presence of cuticular pores, the presence of granulation on all legs, and by fully developed lunules (only small open lunules present in Macrobiotus subintermedius).
-
Macrobiotus vinciguerrae Binda & Pilato, 1992 by: pores arranged in bands, the absence of tri- and quadrilobed pores, the presence of two types of pores (small and large) in the new species vs uniform pore size in Macrobiotus vinciguerrae), the presence of a single large pore on each of legs I–III, the absence of the oral cavity armature, a larger mean body size (184 μm in the new species vs 380 μm in Macrobiotus vinciguerrae), stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt=66.1–68.7 in Macrobiotus vinciguerrae), a different macroplacoid length sequence (2<1<3 in the new species vs 2<3<1 μm in Macrobiotus vinciguerrae), a smaller diameter of eggs without and with processes (44.1–45.7 μm and 55.1–55.6 μm in the new species vs ca. 76.4 μm and ca. 88.0 μm in Macrobiotus vinciguerrae), a slightly larger number of processes on egg circumference (29–30 in the new species vs ca. 26 in Macrobiotus vinciguerrae), egg processes without flexible filaments, smaller egg processes (4.5–5.3 μm in the new species vs ca. 8.2 μm in Macrobiotus vinciguerrae), and by narrower bases of egg processes (2.4–3.4 μm in the new species vs ca. 5.0 μm in Macrobiotus vinciguerrae).
-
Macrobiotus weglarskae Michalczyk et al., 2005 by: the absence of bi-, trilobed and star-shaped pores, the presence of two types of pores (small and large) in the new species vs uniform pore size in Macrobiotus weglarskae), the absence of 3–5 large triangular or irregularly shaped pores on the caudo-dorsal cuticle above hind legs, the presence of a single large pore on each of legs I-III, a different shape of egg processes (short, single tip cones without a membrane in the new species vs. screw-like processes within a membrane in Macrobiotus weglarskae), a slightly larger number of processes on egg circumference (29–30 in the new species vs ca. 24 in Macrobiotus weglarskae), and by slightly wider bases of egg processes (2.4–3.4 μm in the new species vs 1.6–2.0 μm in Macrobiotus weglarskae).
-
Macrobiotus xavieri Fontoura et al., 2009 by: the absence of trilobed pores, the presence of two types of pores (small and large) in the new species vs all pores of similar size in Macrobiotus xavieri), the presence of a single large pore on each of legs I–III, the presence of granulation on all legs, a smaller body size (113–236 μm in the new species vs 275–410 μm in Macrobiotus xavieri), stylet supports inserted in a more anterior position (pt=49.5–56.2 in the new species vs pt=66.1–67.9 in Macrobiotus xavieri), a different macroplacoid length sequence (2<1<3 in the new species vs 2<3<1 in Macrobiotus xavieri). shorter macroplacoids (I: 1.3–1.9 μm [pt=6.9–8.3]; II: 1.2–1.7 μm [pt=6.8–8.6] III: 1.4–2.2 μm [pt=7.4–9.6] in the new species vs I: 3.6–4.5 μm [12.7–13.8]; II: 2.9–3.6 μm [10.3–11.1] III: 3.0–3.9 μm [pt=10.9–11.9] in Macrobiotus xavieri), a shorter microplacoid (0.5–0.9 μm [pt=2.4–4.7] in the new species vs 1.5–2.0 [5.0–6.2] in Macrobiotus xavieri), a shorter macroplacoid row (4.5–6.8 μm [pt=24.3–33.7] in the new species vs 9.8–12.6 μm [pt=35.6–38.5] in Macrobiotus xavieri), a shorter placoid row (5.2–7.9 μm [pt=27.1–39.1] in the new species vs 10.9–13.9 μm [39.6–43.3] in Macrobiotus xavieri), a different shape of egg processes (short, single-tipped cones in the new species vs long cones with bi- or multi-tipped tips in Macrobiotus xavieri), egg shell and processes without granulation, a smaller diameter of eggs without and with processes (44.1–45.7 μm and 55.1–55.6 μm in the new species vs 56.0–79.0 μm and 80.0–99.2 μm in Macrobiotus xavieri), a larger number of processes on egg circumference (29–30 in the new species vs 20–23 in Macrobiotus xavieri), smaller egg processes (4.5–5.3 μm in the new species vs 10.6–19.0 μm in Macrobiotus xavieri), and by slightly narrower bases of egg processes (2.4–3.4 μm in the new species vs 3.7–6.6 μm in Macrobiotus xavieri).
XI: 2 specimens (including 1 simplex) and 1 egg.
Paramacrobiotus species (until recently a collection of species within Macrobiotus) can be divided into three groups: areolatus, huziori and richtersi, with respect to the combination of two traits: the presence/absence of the microplacoid in the pharynx and the type of egg areolation. Paramacrobiotus richtersi, considered cosmopolitan, is recognised as the nominal species for a group of very similar taxa that require careful taxonomic examination of adults and egg morphology for correct identification. In the last decade many new species of this group have been described from various localities (e.g.
KZ would like to express sincere thanks to Małgorzata Kuźnik for her help in preparing figures.