(C) 2013 Andrés H. Vélez-Bravo. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The male genital sclerites of cockroaches of genus Muzoa Hebard 1921 are described for first time and the new species Muzoa curtalata sp. n. is described and ilustrated. A dichotomous key to identify the species of genus Muzoa is given.
Colombia, Dichotomous key, Male genital sclerites, Muzoa curtalata Vélez sp. n.
The genus Muzoa is distributed in Central and South America (
The detailed study of the genital sclerites of cockroaches performed by
Therefore, in this work the genus Muzoa is rediagnosed, now adding, morphological characters related to the male genital sclerites, and additionally, one new species is described for the genus.
Observations of external morphological characters were made with Leica MS5 and MZ16 stereomicroscopes (magnification 10–64× and 7–115×), equipped with an ocular graticule for measurements of lengths and ratios. Drawings were prepared with Digital Camera Leica EC3 attached to the compound scope. Based on the digital image, illustrations were made with an illustration software, in order to highlight features of taxonomic significance. The methods for dissecting male genitalia followed
The morphological terminology followed Torre-Bueno (
The descriptions of the male genital sclerites were made based on the permanent slides (belonging to the holotypes) and fresh material preserved in glycerine. Illustrations were made based on fresh material.
The insect Collection codens are in accordance with
ANSP USA, Pennsylvania, Philadelphia, Academy of Natural Sciences.
CIB Colombia, Medellín, Centro de Investigaciones Biológicas.
MUJ Colombia, Bogotá D.C., Pontificia Universidad Javeriana, Museo Javeriano de Historia Natural, Laboratorio de Entomología.
UNAB Colombia, Bogotá D.C., Museo Entomológico de la Facultad de Agronomía, Universidad Nacional de Colombia.
USNM USA, Washington D.C., National Museum of Natural History.
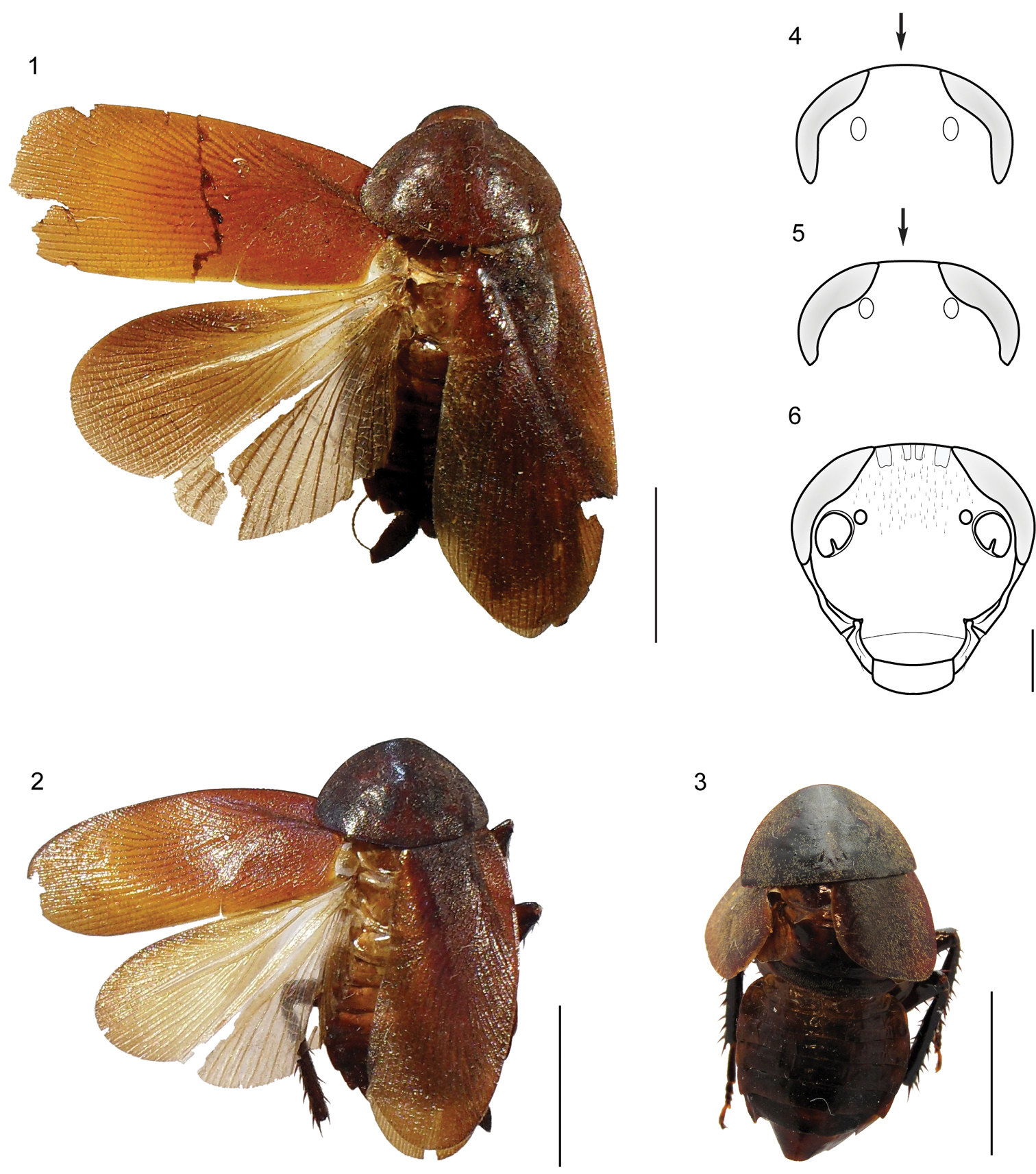
| 1 | Cockroaches with tegmina and membranous wings well developed, completely covering the supra-anal plate (Figs 1, 2) | 2 |
| – | Cockroaches with tegmina and membranous wings shortened, reaching just the first abdominal tergite (Fig. 3). Lateral extension of L2a elongate and extended dorsally over the process “via” (Fig. 16); apex of the region R2 of the genital sclerite R (right phallomere), broad and truncated (Fig. 20) | Muzoa curtalata Vélez sp. n. (Colombia) |
| 2 | Head vertex convex (Fig. 4). Styli short, four times longer than wide. Process “via” slender, long and curved (Fig. 14). Lateral extension of L2a extending over the process “via” (Fig. 14). | Muzoa simplex (Colombia) |
| – | Head vertex straight (Fig. 5). Styli long, five times longer than wide (Fig. 12). Process “via” short, thick and straight (Fig. 15). Lateral extension of L2a not extending over the process “via” (Fig. 15) | Muzoa madida (Colombia, Costa Rica) |
Although the original descriptions of Muzoa madida (Fig. 2) and Muzoa simplex (Fig. 1) are extensive and detailed (see
1–3 Habitus (dorsal) of the species of genus Muzoa. 1 Muzoa simplex Hebard, 1921, holotype male (ANSP) 2 Muzoa madida Rehn, 1930, holotype male (ANSP). 3 Muzoa curtalata sp. n., holotype male (MUJ). Scale bar 1 cm. 4–6 Heads (ventral) of the species of genus Muzoa 4 Muzoa simplex 5 Muzoa madida 6 Muzoa curtalata sp. n. The arrow is indicating the shape of the vertex. Scale bar 1 mm.
Species of medium size (24–27 mm male, 20–26 mm female), with body dark brown. The legs and antennae are entirely brown. Pronotum and tegmina reddish brown.
Head triangular and with big reniform eyes, extending antero-laterally beyond to the antennal socket, eyes are not globose; intraocular distance of the same length than distance between ocellar fenestra; face globose; gena and pleurostoma undivided, at least externally, so that the subgenal suture only present in the inner margin of pleurostoma; subantenal suture ending next to the inferior margin of the eye; the face and gena bare. Antennae filiform and setosas along their length; the first flagellar segment of the same length that the pedicel.
Pronotum parabolic, with its cephalic margin convex and the caudal margin truncated. In either sex, both pairs of wings are developed surpassing slightly the apex of the cercus, except in Muzoa curtalata sp. n. in which the males are brachypterous. Fore wings with the base of the remigium narrower than the base of vanal region (vannus) and the apex rounded; with discoidal sector longitudinal. Tegmina and pronotum densely covered with fine silky pubescence. Legs long and slender; cephalic coxa with a diagonal carina; antero-ventral margin of the front femur without spines heavy, only with short and heavy setae and with three terminal spines; postero-ventral margin of the hind femur with terminal spine; tarsomeres 1-4 with pulvilli, the first metatarsomere with its pulvilli covering only a 1/3 of its length; tarsal claws simple and symmetrical; arolium present.
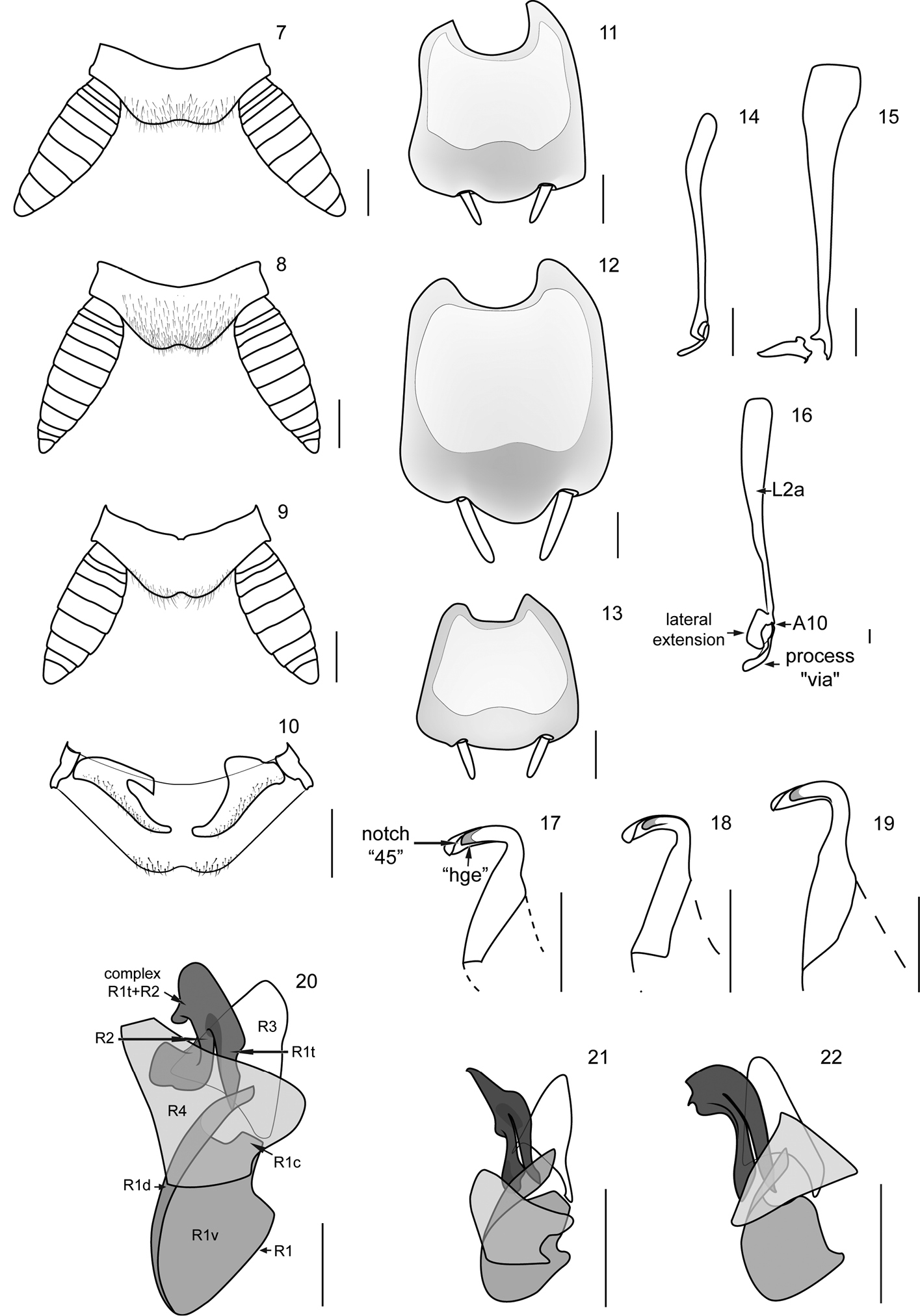
Abdomen often convex and short; first tergite unspecialized. Supra-anal plate tranverse and with the caudal margin produced and bilobed (Figs 7, 8). Cerci long, thick and subspatulate, composed of 9 to 12 segments; last segment small and narrower than the remaining segments. Ventrally, right paraproct specialized and transverse, this is articulated directly with the lateral margin of the supra-anal plate throughout its right lateral margin. Male subgenital plate symmetric, with styles similarly shaped (Figs 11, 12). Internally, attached to this plate, is located the membranous pouch with genital sclerites L2, L3, and R (right phallomere).
Genital sclerites.The male genital sclerites of the species of the genus Muzoa are formed by sclerites L2 (Figs 14, 15), L3 (Figs 18, 19), and R (right phallomere) (Figs 21, 22).
Genital sclerite L2 thin and elongated. Sclerotized region L2a and process “via” separated but closely articulated (Articulation 10 (A10), see
Hook “hla” of sclerite L3 with the typical shape observed in most Ectobiidae and Blaberidae species, with distal area narrow and elongated; in addition to the notch “45”, the hook also exhibit groove “hge” along of its lower margin (Figs 17-19). In ventral view, basal area of hook “hla” longer than its apical area; left lateral margin of basal area, straight. Membranous tube of hook “hla” not covered by setae.
Genital sclerite R (right phallomere) formed by the sclerotized regions R1, R2, R3 and R4 (Figs 20–22). Region R1 as a large and bulky structure at the caudal region of sclerite R; subregion R1v broader than subregion R1d, which is a longitudinal narrow and elongated band, extending along the left lateral margin of R1; in species like Muzoa madida and Muzoa simplex this band does not reach the caudal margin of R1. Subregion R1d passing over the complex R1t+R2, surpassing its farthest right margin; its size varies among species. As in the other genera of Nyctiborinae, regions R1 and R3 articulated by the lower right corner of R3 and the upper right corner of R1. In all species of Muzoa the upper right corner of R1 (R1c) slightly projected (Figs 20–22). Subregion R1t is not fused with other areas of R1. Left arm of the complex R1t+R2 thicker than right arm, varying from apically rounded in Muzoa simplex to pointed in Muzoa madida. Complex R1t+R2 similar in size to region R3, located on its left margin, below the subregion R1d. Apex of R1t and R2 extending beyond caudal margin of R3.
Region R3 as a slightly sclerotized plate articulated by its lower right corner to R1c. This plate is longer than wide and its apex is rounded (Figs 20–22).
Region R4 as an elongated dorsal plate, covering R1 and R1t+R2 complex in part (Figs 20–22).
Supra-anal plate (dorsal), subgenital plate (vental), and male genital sclerites of the species of genus Muzoa. Muzoa simplex Hebard, 1921: 7 Supra-anal plate 11 Subgenital plate 14 Median sclerite L2 (dorsal) 18 Hook “hla” of L3 (ventral) 21 Right sclerite R (dorsal). Muzoa madida Rehn, 1930: 8 Supra-anal plate 12 Subgenital plate 15 Median sclerite L2 (dorsal) 19 Hook “hla” of L3 (ventral) 22 Right sclerite R (dorsal). Muzoa curtalata sp. n. (Holotype): 9 Supra-anal plate 10 Supra-anal plate (ventral) with the paraprocts 13 Subgenital plate 16 Median sclerite L2 (dorsal) 17 Hook “hla” of L3 (ventral) 20 Right sclerite R (dorsal) (sclerotized regions R1 [subregions R1c, R1d, R1v, R1t], R2, R3, R4). Scale bar 1 mm.
Material examined.
| Species | Country | Locality | Method of collecting | Date | Collector’s names | Condition of specimen | Repository |
|---|---|---|---|---|---|---|---|
| Muzoa madida | Colombia | Chocó, Nuquí, Jurubida, Emberá community. Edge of primary forest. | Shannon-165W | 23 Oct. 1995 | R. Vélez | Male. Pinned | CIB. Used to illustrate the genital sclerites |
| Muzoa madida | Costa Rica | Limón, forest near La Emilia. In dense second growth forest, 304 m. | 15 Sep. 1937 | Rehn | Male. Pinned | ANSP. Holtype 5481 | |
| Muzoa madida | Costa Rica | Turrialba. 900 m. | Heyne, Berlin-Wilm | Male. Pinned | USNM. | ||
| Muzoa simplex | Colombia | Boyacá, Muzo | Sep. 1919 | A. María | Male. Pinned | ANSP. Holotype 9295 | |
| Muzoa simplex | Colombia | Chocó, Nuquí, Jurubida, Emberá community. Edge of primary forest | Shannon-165W | 23 Oct. 1995 | R. Vélez | 2 Male. Pinned | CIB. Used to illustrate the genital sclerites |
| Muzoa simplex | Colombia | Cundinamarca, Bogotá | Jan. 1934 | Guevara | 2 Male.<br/> Pinned | USNM. | |
| Muzoa simplex | Colombia | Cundinamarca, Tibacuy, Ins. Pol. Cumaca. 4°21'N, 74°27'W, 1647 m. | 14 Nov. 1993 | Valderrama | Male.<br/> Pinned | UNAB. |
urn:lsid:zoobank.org:act:0B136466-60A0-44D8-8DF8-528FDE38D010
http://species-id.net/wiki/Muzoa_curtalata
Figures 3, 6, 9, 10, 13, 16, 17, 20Colombia, Valle del Cauca, Tuluá, Juan María Céspedes botanical garden, 4.029214, -76.160409, 1100 m, E. Amat leg. 24–31 Aug 1996.
Holotype male, pinned, with genitalia in a separate microvial. Original label: “Colombia. Valle. Mun. Tuluá. Jardín Botánico “Juan María Céspedes” 1100 m.s.n.m. E. Amat leg. 24–31 Ago 1996” MUJ – BLA - 025.
This species belongs to the genus Muzoa by: 1) Pronotum parabolic, with the caudal margin truncated; 2) antero-ventral margin of the cephalic femur without spines; 3) tarsal claws simple and symmetrical; 4) first abdominal tergite unspecialized; 5) supra-anal plate tranverse, with caudal margin produced and bilobed; 6) cerci long, thick and subspatulate; 7) male subgenital plate symmetric, with styles similarly shaped; 8) genital sclerites: process “via”, of the genital slcerite L2, finger-shaped and 9) hook “hla” of the genital sclerite L3, with groove “hge” along its lower margin. Muzoa curtalata differs from Muzoa madida and Muzoa simplex for its brachypterous condition. Muzoa curtalata is more closely related to Muzoa simplex for having a long lateral extension of L2a, which covers part of the process “via” (Figs 14, 16), different to Muzoa madida, in which the lateral extension is shorter and never covers part of the process “via” (Fig. 15).
Species of medium size (19.8 mm), with body dark brown; legs and antennae entirely brown. Pronotum and tegmina reddish brown (Fig. 3).
Head triangular and with big reniform eyes, extending antero-laterally beyond the antennal sockets; intraocular distance equal to distance between ocellar fenestra (1.3 mm) and lesser than distance between antennal sockets (2.0 mm) (Fig. 6); face globose; gena and pleurostoma undivided, at least externally, so that subgenal suture only present on the inner margin of pleurostoma; subantennal suture ending next to inferior margin of eye; face with many short setae on the frons, gena and remaining of face bare.
Pronotum parabolic, with cephalic margin convex and caudal margin truncated. Brachypterous. Fore wings coriaceous, lacking distinct veins; apex truncated, not surpassing the first abdominal tergite. Hind wings slightly developed, with reduced venation. Tegmina and pronotum covered densely with fine silky pubescence. Legs long and slender; cephalic coxa with a diagonal carina; antero-ventral margin of the front femur without spines heavy, only with short and thick setae, with three terminal spines; antero-ventral margin of middle and posterior femur with five and six spines correspondingly, postero-ventral margin with four and five spines respectively; tarsomeres 1-4 with pulvilli, first metatarsomere with its pulvilli covering only 1/3 of its length; tarsal claws simple and symmetrical; arolium present.
First abdominal tergite unspecialized. Supra-anal plate transverse, with caudal margin produced and bilobed (Fig. 9); cerci long, thick and subspatulate, composed of nine segments; last segment shorter and narrower than remaining segments (Fig. 9); ventrally, right paraproct transverse and claw-shaped (Fig. 10), articulated directly with the lateral margin of supra-anal plate through its right lateral margin. Subgenital plate symmetric, with styli similary shaped (Fig. 13). Internally, subject to this plate is located the membranous pouch with genital sclerites L2 (Fig. 16), L3 (Fig. 17), and R (right phallomere) (Fig. 20).
Genital sclerites. Genital sclerite L2 thin and elongated. Sclerotized region L2d and the process “via” separated but closely articulated (A10). Process “via” finger-shaped, slender and long (Fig. 16). Region L2a slightly sclerotized, at least four times length of “via”, with a lateral extension extending over the process “via”.
Hook “hla” of the genital sclerite L3 with distal area elongated; in addition to the notch “45”, with the groove “hge” along its lower margin (Fig. 17). Basal area of “hla” longer than its apical area, left lateral margin of basal area straight.
Genital sclerite R (right phallomere) formed by sclerotized regions R1, R2, R3 and R4 (Fig. 20). Region R1 as a large and bulky structure at the caudal region of sclerite R; subregion R1v much wider than subregion R1d, which is a narrow and elongated band, extending along left lateral margin of R1; subregion R1d projected over the apex of R1t (Fig. 20). R1c slightly projected, articulated to the lower right corner of R3 (Fig. 20). Subregion R1t is not fused with other areas of R1. Both arms of the complex R1t+R2 have more or less the same length. Left arm of the complex R1t+R2 thick, irregularly shaped, projected towards the left. Complex R1t+R2, similar in size to region R3, located on the left corner of the region R3, below the projection of R1d. Apex of R1t and R2 extended beyond the caudal margin of R3.
Region R3 as a nearly triangular, slightly sclerotized plate articulated by its lower right corner to R1c; apex of R3 rounded (Fig. 20).
Region R4 as a wide dorsal plate, covering R1 and R1t+R2 complex in part (Fig. 20).
Measurements(mm). Body length 19.8; pronotum maximum length × width 6.2 × 10.5; tegmen length × width 7.0 × 5.9; interocular width 1.3; interantennal sockets width 2.0.
curtus (L) = short, alatus (L) = winged. The name refers to the short tegmina of this species.
North of South America in the department of Valle del Cauca, Colombia.
Special thanks to Jennifer C. Girón (UPRM-INVCOL) for their comments and corrections to this work. To Dr Jon K. Gelhaus and Sr Jason Weintraub (ANSP), to Sr Juan Zuluaga (CIB), to Dr Giovanny Fagua (MUJ) and Dr Floyd Shockley (USNM) for the loan of Nyctiborinae specimens. Visits to USNM collection was funded by the Franz Lab (University of Puerto Rico). Jessup and McHenry Awards made possible to visit ANSP collection.