(C) 2013 Ralf Janssen. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
This paper reports on an abnormally developed embryo (ADE) of the common pill millipede Glomeris marginata. This ADE represents a modified case of Duplicitas posterior, in which two posterior ends are present, but only one anterior end. While the major posterior germ band of the embryo appears almost normally developed, the minor posterior germ band is heavily malformed, has no clear correlation to the single head, little or no ventral tissue, and a minute amount of yolk. The anterior end of the minor germ band is fused to the ventral side of the major germ band between the first and second trunk segment. At least one appendage of the second trunk segment appears to be shared by the two germ bands. Morphology and position of the minor germ band suggest that the ADE may be the result of an incorrectly established single cumulus [the later posterior segment addition zone (SAZ)]. This differs from earlier reports on Duplicitas posterior type ADEs in Glomeris marginata that are likely the result of the early formation of two separate cumuli.
Teratology, Diplopoda, Development, Segmentation, vasa
Abnormally developed myriapods have been reported repeatedly in the past (
The majority of data on abnormally developed myriapods comes from centipedes (
This paper reports on the rare find of an abnormally developing embryo of Duplicitas posterior in which one of the posterior ends of the embryo is minute, lacking portions of its ventral ectodermal tissue, is detached from the yolk, and is atypically connected to the major germ band. The way in which the minor germ band is connected to the major germ band, and its point of connection, suggests that both posterior germ bands may have formed from one single (disturbed) posterior cumulus.
Mature specimens of Glomeris marginata were collected in Germany (Nordrhein-Westfalen, Kreis Kleve) and cultivated in plastic boxes (22 cm × 13 cm × 5 cm) filled with decomposing leaves and moist clay. During egg deposition and embryogenesis the temperature was constantly held between 21–22°C. Eggs were manually separated from the clay egg-chambers and the chorion was removed by incubation in 2% sodium hypochloride for 1–2 minutes. Developing eggs were fixed for four hours in a mixture of 1 ml 4% formaldehyde in phosphate buffered saline pH 7.4 with 0.1% Tween-20 (PBST) and 1ml heptane. After fixation, embryos were washed in methanol and stored in methanol at -20°C for at least three weeks prior to in situ hybridization. The vitelline membrane was removed manually with fine forceps (Dumont No. 5, Fine Science Tools).
RNA isolation and cDNA synthesis were performed as per
Cell nuclei were visualized with the fluorescent dye 4-6-Diamidin-2-phenylindol (DAPI). Embryos were incubated in 1 μg/ml DAPI in phosphate buffered saline with 0.1% Tween-20 (PBST) for 30 minutes. Excess DAPI was removed by washes in PBST for at least one hour. Whole mount in situ hybridization (WISH) was performed as described in
Pictures were taken with a digital camera (Axiocam; Zeiss, Jena, Germany) attached to a dissection microscope (Leica, Heerbrugg, Switzerland). Brightness, contrast and color values were corrected using image processing software (Adobe Photoshop CS2, V.0.1 for Apple Macintosh; Adobe Systems Inc. San Jose, CA, USA).
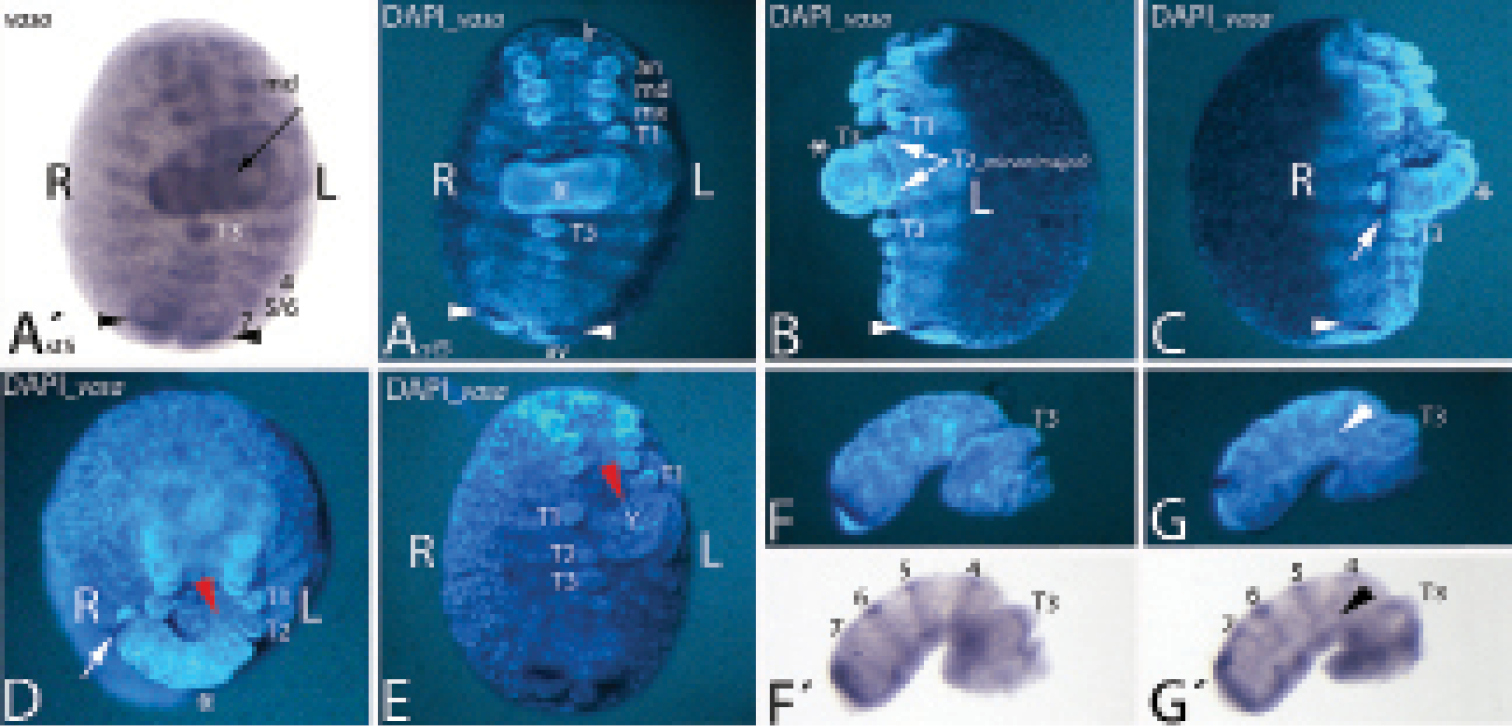
A single abnormally developed embryo (ADE) with the here-described teratological morphology was found (Fig. 1). The specimen was stained for the DEAD-box helicase vasa. It represents a developing embryo at approximately stage 5 (staging after
In the Duplicitas posterior-type embryo described here the development of the major germ band is almost normal, and its posterior part is clearly aligned with the single anterior pole (the complete head) (Fig. 1A–C). The development of the major germ band is abnormal in two respects. First, the posterior part of the major germ band is bent towards the embryo’s right side (Fig. 1A/A´). Second, all segments of the major germ band that are situated posterior to the contact point with the minor germ band are dorso-ventrally compressed, especially with respect to ventral tissue (Fig. 1A/E). This effect is more pronounced in tissue that is close to the contact point with the minor germ band. The limbs on the third trunk segment (T3), for example, are even fused and form a single broadened appendage (Fig. 1A/E). The limb buds of T4 and T5 stand unnaturally close together. The rear end of the major germ band, however, appears normal with a properly formed segment addition zone (SAZ), a pair of anal valves and an anus (Fig. 1A–E). Remarkably, while the amount of ventral tissue is reduced, the dorsal segmental units appear to have formed normally in the complete posterior part of the major germ band. This supports earlier findings that ventral and dorsal segmental patterning is decoupled (
The minor germ band is less well developed and lacks crucial parts of normally developing embryos (Fig. 1). It is fused to the left side of the major germ band between the first and the second trunk segments (Fig. 1B/E). The lateral view on the left side of the embryo reveals that at least one T2 appendage is shared by the two (major and minor) germ bands (Fig. 1B); otherwise a major germ band with four pairs (instead of three pairs) of primary outgrowing trunk limbs has to be assumed. Note that in Glomeris marginata the walking limbs of the first three trunk segments develop faster than the posterior walking limbs. Two pairs of developing limb buds are recognizable in the anterior region of the minor germ band. These represent the appendages of T3 and T4. The terminal region of the minor germ band ends in an unpaired region, which may represent the fused anal valves. This region lies posterior to the strong expression of vasa in the SAZ (Fig. 1F–G´). Unlike the situation in the major germ band, this domain of vasa expression is in a continuous transversal stripe, rather than with a ventral gap as in the major germ band and in normally developing embryos (Fig. S1). This implies that ventral tissue is lacking from the posterior end of the minor germ band. This assumption is further supported by the lack of the anus, and the fused anal valves. Tissue anterior to the SAZ may also comprise mainly dorsal tissue since in this area the expression of vasa is in continuous transverse stripes (Fig. 1F´/G´). In normally developing embryos vasa is strongly expressed in the mesoderm of dorsal segmental units, but only very weakly in the ventral mesoderm (Fig. S1). This indicates that ventral tissue may be lacking from most of the minor germ band. In the anterior part of the minor germ band, however, at least ectodermal ventral tissue must be present as indicated by the presence of the limb buds. The stripes of vasa-positive tissue in the minor germ band do not surround the complete ‘embryo’, but are discontinuous at the germ band’s dorsal side (Fig. 1G´). This means that dorsal closure has not yet happened. The number of vasa stripes in the minor germ band suggests that the same number of segments have formed in the minor and the major germ band. Four stripes of vasa can be seen posterior to T3 in the minor germ band (Fig. 1F´/G´). These stripes appear to correspond to three stripes of vasa posterior to T3 in the major germ band (Figs 1A and S1A). How is this possible? In Glomeris marginata dorsal segmental tissue corresponding to the ventral leg bearing segmental units T5 and T6 (and T7/T8) first develops as separate haplosegmental units, but subsequently the dorsal units fuse to form the diplosegments (
Among all described Glomeris marginata ADEs, this specimen is the only one that possibly developed two separate posterior ends from one posterior cumulus. In Glomeris marginata all segments posterior to T1 form from the SAZ that develops from the cumulus, and all segments anterior to (and including) T1 form from the blastoderm, the regio germinalis (
An unusual abnormally developed embryo of the type Duplicitas posterior. A´–E Whole-mount embryo. Anterior is towards the top. F–G´ Separated minor germ band. Posterior is to the left. A´ shows the bright-field photography of the embryo stained for vasa. Ventral view. Arrow points to stripe of expression in the minor germ band. A DAPI corresponding to A´. The asterisk marks the minor germ band. Arrowheads point to strong expression of vasa near the rear end. B Lateral view on left side. Asterisk and arrowhead as in A. Arrows point to T2 appendages. C Lateral view on right side. Asterisk and arrowhead as in A. Arrow points to strong expression of vasa in the minor germ band. D Ventral view. Embryo tilted towards beholder. Asterisk and arrow as in C. Red arrowhead points to contact point of minor germ band with major germ band. E Ventral view. Minor germ band was surgically removed. Red arrowhead as in D. F/G Separated minor germ band. Lateral views at different angles. F´/G´ Bright-field photographs corresponding to F/G. Arrowhead in G/G´ points to lack of dorsal vasa expression. Abbreviations: 4 to 7 vasa expression in the dorsal segmental units four to seven; 5/6 vasa expression in the fused dorsal tissue (diplosegment) aligned with T5 and T6; an antenna; L left side of embryo; md mandible; mx maxilla; R right side of embryo; T1-T2 first to third walking limb; Y yolk.
I would like to thank native English speaker Illiam Jackson for proofreading of the manuscript and correcting grammar and spelling.
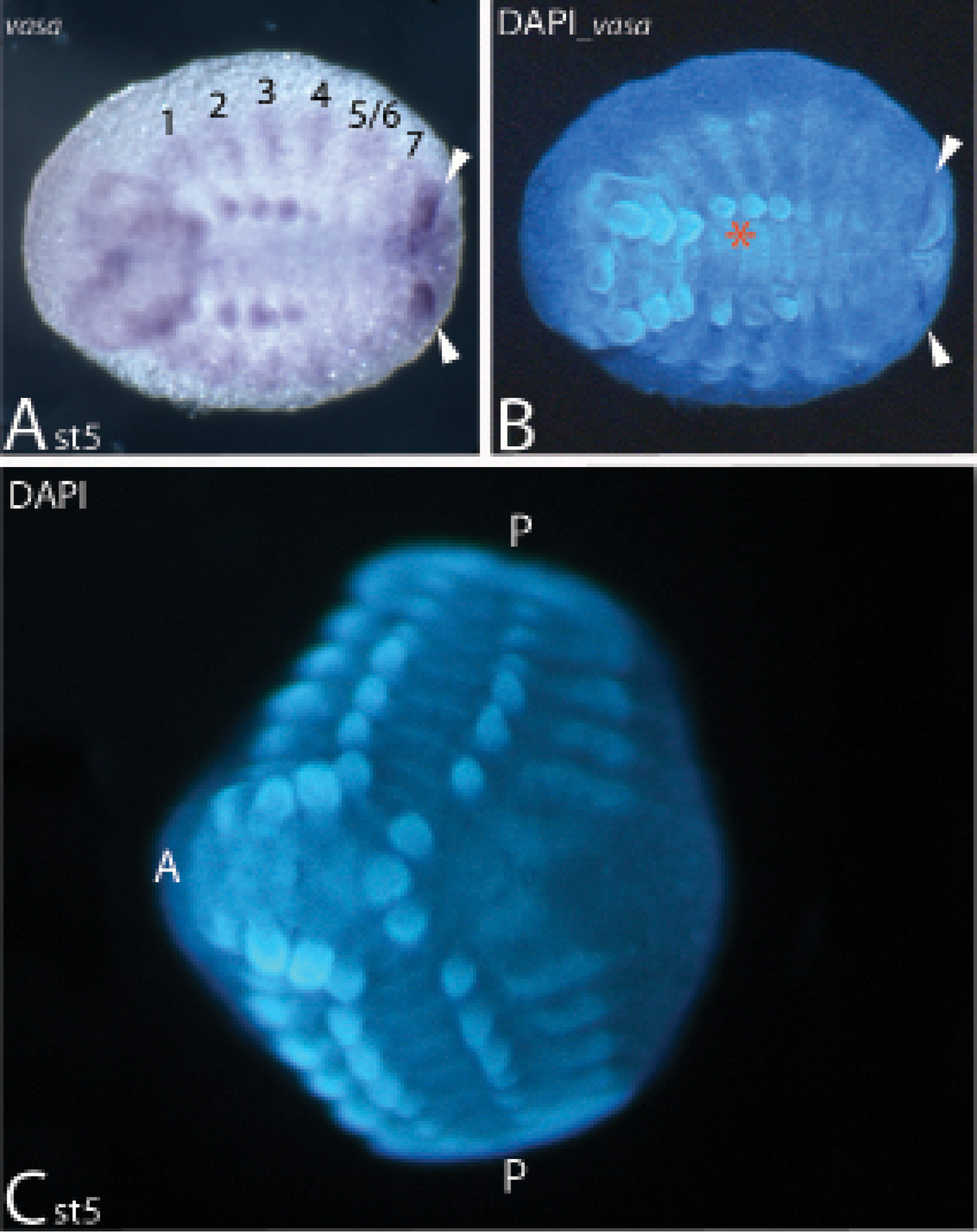
Expression of vasa (A/B) and a typical abnormally developed embryo (ADE) of Duplicitas posterior type (C).In all panels anterior is to the left. A/B Bright-field and corresponding DAPI-stained embryo. White arrowheads mark strong expression of vasa anterior to the anal valves. Red asterisk marks the position where the minor germ band is connected to the major germ band in the presented ADE (cf. Fig. 1). C Typical Duplicitas posterior-type embryo. Abbreviations: 1 to 7, expression of vasa in dorsal segmental units corresponding to ventral trunk segments one to seven; 5/6, first diplosegmental unit corresponding to ventral trunk segments five and six; A anterior pole (head); P posterior pole (anal valves).
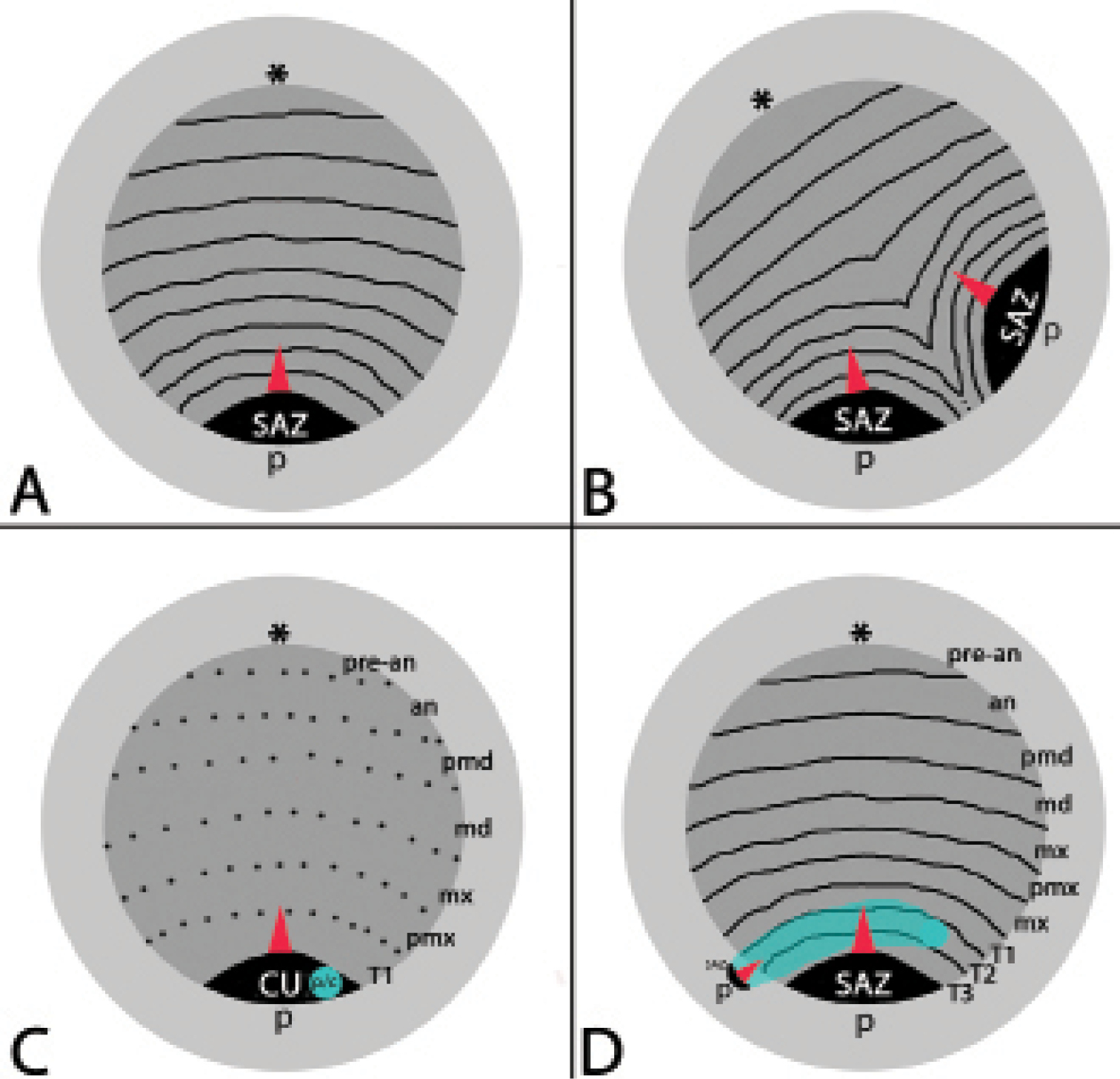
Schematic representation of normal development (A), ‘normal’ Duplicitas posterior type development (B), and the rare modified case of Duplicitas posterior reported in this paper (C/D). Light grey: yolk; dark grey: (major) germ band; transverse black lines: segmental boundaries; dashed lines: future segmental boundaries; light blue: minor germ band. Asterisks mark the anterior pole. Red arrowhead point to the direction in which new segments are added from the segment addition zone (SAZ). Abbreviations: an antennal segment; c cumulus of minor germ band; cu cumulus of major germ band; md mandibular segment; mx maxillary segment; p/c posterior (p) end of minor germ band and cumulus (c); pmd premandibular segment; pmx, postmaxillary segment; pre-an pre-antennal area; SAZ segment addition zone; T1-T3 first to third trunk segment.