(C) 2013 Isabelle M. Vea. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The genus Chionaspis (Hemiptera, Diaspididae) includes two North American species of armored scale insects feeding on Pinaceae: Chionaspis heterophyllae Cooley, and Chionaspis pinifoliae (Fitch). Despite the economic impact of conifer-feeding Chionaspis on horticulture, the species diversity in this group has only recently been systematically investigated using samples from across the group’s geographic and host range. This paper provides morphological recognition characters for four new species that were recently hypothesized to exist on the basis of molecular evidence. The new species, here described, are Chionaspis brachycephalon Vea sp. n., Chionaspis caudata Vea sp. n., Chionaspis sonorae Vea sp. n. and Chionaspis torreyanae Vea sp. n. One of the new species, Chionaspis caudata Vea, has a gland spine at the apex of the pygidium, between the median lobes, unlike any other species of Chionaspis. An identification key to the species of Chionaspis feeding on pine in North America is provided.
Armored scale insects, Diaspidini, Diaspidinae, cryptic species, endemic, North America, Pinus
The armored scales insects (Hemiptera, Diaspididae) are a group of over 2500 described species of plant parasites (
However, some shortcomings of conventional morphological species descriptions are their reliance on specimens sampled from a limited number of locations, or hosts, as is often the case when identifying agricultural pests. Such limited sampling may fail to observe a range of intraspecific morphological variation across hosts and geography. More importantly, conventional species descriptions of armored scales are not often corroborated with genetic measures of species boundaries (but see
Current taxonomy of the genus Chionaspis Signoret recognizes two pine-feeding species, Chionaspis heterophyllae Cooley and Chionaspis pinifoliae (Fitch). These species are native to North America (
Aspidiotus pinifoliae was first described by Fitch as a pest of pines, “which fixes itself upon the leaves, exhausting them of their juices and then causing them to perish and fall, and the end of the limbs to die when thus defoliated” (
Chionaspis pinifoliae heterophyllae was first described by Cooley in
Since 1921 (
Most of the specimens collected by
These results highlight the possibility that even species that are well known, as in the case of pest species, may be more diverse than previously thought (i.e. contain cryptic species). It is not immediately clear how best to assign taxonomic status to cryptic species (but see
Field collection and slide mounting of all specimens were accomplished using the protocols described by
Slide mounted type specimens have been deposited at the National Insect Collection, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City (CNIN), the United States National Entomological Collection (Coccoidea collection) at the U.S. National Museum of Natural History (USNM), USA and the University of Massachusetts Insect Collection, Amherst, MA, USA (UMAM). Genomic DNA from all types, as well as lots supplying the type material (additional specimens in-situ on host tissue) from the study of
urn:lsid:zoobank.org:act:45770622-189F-47C8-A6B3-369B5BDDF3EB
http://species-id.net/wiki/Chionaspis_brachycephalon
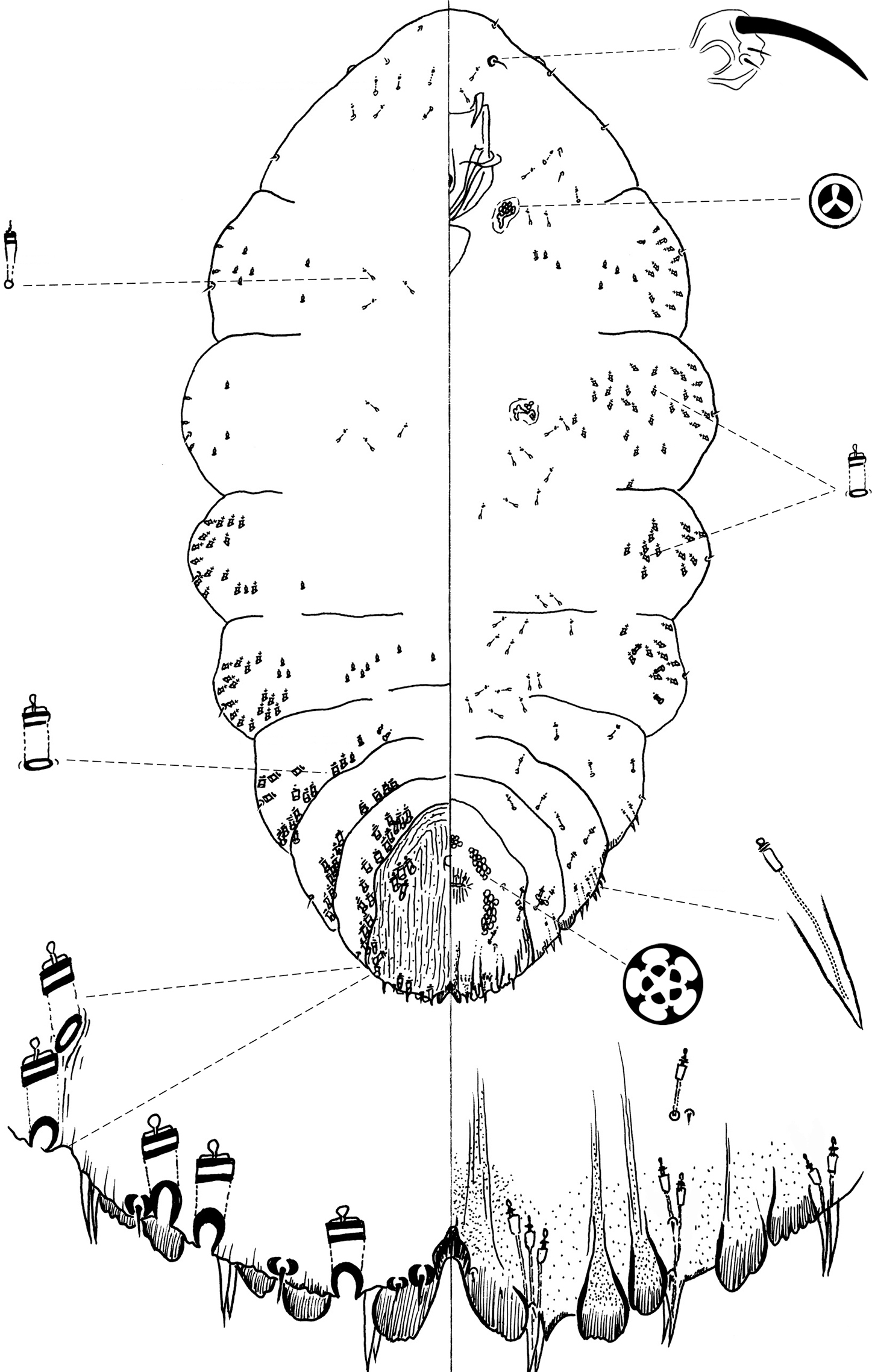
Figure 1Type locality: Mexico, Durango state, Navios, 23°53.95'N, 105°2.83'W, on needle of Pinus cooperi Blanco, 24 September 2007, R. Gwiazdowski and A. Garcia Arévalo coll.
Holotype adult female, slide-mount in balsam. Original label: “D1765A, Mexico, Durango, Resturante “Los Pinos”, Navios, 1.ix.2007, 23°53' 56.9"N, 105°02'49.6"W, R. Gwiazdowski, A. Garcia Arévalo, Pinus cooperi ”, deposited at CNIN.
Paratype: Adult female, slide-mount in balsam. D1765B, same collection data as holotype, deposited at USNM.
Other material examined: Adult female, slide-mount in balsam. Original label “D1718A, Mexico: Mexico, Hwy 95 South of Tres Marias, 1.ix.2007, 19°01'37.5"N, 99°12'35.2"W, R. Gwiazdowski, D. Gernandt, Pinus pseudostrobus Lindl.”, deposited at UMAM. Adult females on separate slides, D1718C, D and F, same collection data as D1718A, deposited at CNIN.
Chionaspis brachycephalon Vea differs from other Chionaspis by the following combination of characters (Table 1): small head, gland spine formula variable from 1-1-1 to 2-2-2 (median: 2-2-2), microduct formula also variable from 2-2-2 to 3-3-4 (median: 3-2-2); numerous marginal gland spines on abdominal segments 3 to 5, absent from abdominal segment 1 and 2; variable number of notches present on all pygidial lobes.
Field characters: All pine-feeding Chionaspis discussed here, including Chionaspis heterophyllae and Chionaspis pinifoliae, are indistinguishable by eye in the field. The adult female for all species possesses a white oystershell-shaped and slightly convex cover, with the amount of posterior expansion varying according to the diameter of host needles. Body elongate, color varying from yellow when immature to reddish brownish in specimens containing eggs, with lateral protrusion on the anterior abdominal segments. Found on needles.
Slide-mounted adult female (Figure 1), broadest at metathorax, with thoracic segments lobed laterally, prothorax becoming narrower towards the anterior, ending with a pointed head, giving the appearance of a reduced, shrunken head; length of holotype 1.33 mm, range (n= 6) 0.85 – 1.33 mm; maximum width of holotype: 0.63 mm; range (n=6) 0.45 – 0.66 mm.
Pygidium: Lobes. Posterior margin with 3 pairs of definite lobes (L1, L2 and L3), fourth pair (L4) appearing as series of low, sclerotized points; paraphyses absent. L1 separated by space 0.31 – 0.6 (0.4) times width of lobes, with a heavily sclerotized yoke, lateral and medial margins of L1 diverging from base to apex, medial margin convex with notches towards apex, lateral margin entire; L2 bilobed, smaller than L1, medial lobule larger than lateral, sometimes with notches, lateral lobule minutely notched; L3 bilobed, lateral lobule shorter than medial lobule, with minute notches, in some specimens appearing membranous or obsolete. Gland spines. Gland spine formula varying from 1-1-1 to 2-2-2 (2-2-2) (microduct formula varying from 2-2-2 to 3-3-4 (3-2-2)), gland spines projecting beyond L1; with 1 – 2 (2) gland spines on abdominal segment 5; without gland spines between L1. Ducts. Large macroducts in submedian area of segments 5 and 6 (with 5 – 10 (8) on segment 5 and 4 – 7 (5) on segment 6); in submarginal areas of segment 5 (with 8 – 10 (9)); marginal area of segments 5 to 7 (with 1 on segment 7, 2 on segment 6, 2 – 3 (2) on segment 5); absent on segment 8. Largest macroduct on segment 7 (between L1 and L2) 15 – 17.5 (17.5) μm long. Pygidial microducts always on venter in submarginal areas of segment 5 to 7, with 2 – 4 (2) ducts on segment 5, 1 – 3 (2) ducts on segment 6 and 1 ducts on segment 7 ; pygidial microduct absent from dorsum. Pores. Perivulvar pores with 5 loculi, in 5 groups, 1 median with 9-15 (13) pores, 2 anterolateral with 19 – 33 (23) pores, 2 posterolateral with 19 – 33 (25) pores. Anal opening. Located 6.8 – 10.2 (8.4) times length of anal opening from base of median lobes, diameter 15 – 20 (17.5) μm.
Dorsal setae: 2 setose on L1, 1 setose (15 μm) between lobules of L2 and L3 lobes. Ventral setae: 1 small on median lobe, 1 marginal at base of each gland spine cluster and 1 in submarginal area of each segment, 2 in submedian area of segment 6, half as long as dorsal setae; 2 pairs of setae in a row anterior to the vulva.
Prepygidium: Gland spines. Near each body margin on segments 3 and 4, absent from segment 1 and 2; with 2 – 6 (4) on segment 3 and 2–5 (4) gland spines on segment 4, all protruding from margin..Ducts. Macroducts of 2 sizes; large macroducts in submedian and submarginal areas of abdominal segments 3 and 4. Small macroducts in submedian area of any or all of segments 2 to 4, and in marginal areas from meso- or metathorax to segment 3. Prepygidial microducts present on venter and dorsum from segment 1 to 4, sparsely distributed.
Cephalothorax: Microducts present on venter and dorsum with a slight concentration around thoracic spiracles.Perispiracular pores with 3 loculi, anterior spiracles with 3 – 5 (5) pores, posterior spiracles with 1 – 5 (2) pores. Eyes represented by small sclerotized area, located on body margin at level near anterior clypeolabral shield. Antennae each with 1 long seta and 2 minute setae, distance between antennae 42.5 – 90 (57.5) μm.
Etymology. The epithet brachycephalon is a noun, derived from Greek, meaning “short head”, from brachy- short + cephalon head. The epithet refers to the head shape of this species, which appears smaller than that of other pine-feeding Chionaspis.
Diagnostic morphological characters for six species of pine-feeding Chionaspis.
| Features | Chionaspis pinifoliae (Fitch) | Chionaspis heterophyllae Cooley | Chionaspis brachycephalon Vea, sp. n. | Chionaspis caudata Vea, sp. n. | Chionaspis sonorae Vea, sp. n. | Chionaspis torreyanae Vea, sp. n. |
| Margins of prothorax | slightly convergent towards anterior | slightly convergent towards anterior | sharply convergent towards anterior | slightly convergent towards anterior | slightly convergent towards anterior | slightly convergent towards anterior |
| Gland spine formula | 1-1-1 | 1-1-1 | 2-2-2 | 2-2-1 | 1-1-1 | 2-2-2 |
| Microduct formula | 1-1-1 | 1-1-1 | 3-2-2 | 2-2-1 | 1-1-1 | 2-2-2 |
| Shape of the median lobes (L1) | basally slightly diverging, then parallel sided | diverging throughout | diverging throughout | parallel sided | medial margin parallel sided to mid margin then diverging | basally slightly diverging, then parallel sided |
| Gland spine between L1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Gland spines on segment 5 | 1 | 1 | 1 – 2 (2) | 0 | 1 | 1 |
| Gland spines on segment 4 | 1 – 3 | 1 – 3 | 2–5 (4) | 1 – 2 (1) | 1 – 4 (2) | 1 – 2 (2) |
| Gland spines on segment 3 | 2-7 | 2-5 | 2 – 6 (4) | 1 – 7 (4) | 4 – 7 (5) | 2 – 4 (3) |
| Submedial macroducts on segment 6 | 2 – 6 (3) | 2 – 4 (3) | 4 – 7 (5) | 3 – 4 (3) | 4 – 5 (5) | 3 – 6 (4) |
| L1 margin | Entire or medial notches | Lateral and medial notches | Medial notches | Entire (rarely notched) | Notches on diverging part of medial margin | Entire (rarely one notch) |
| L2 margin | Entire | Entire or with a few small notches | Sometimes notched | Medial lobule slightly notched, lateral lobule entire | Entire | Entire |
| L3 | Entire | Inner lobule entire or with a few small notches, outer lobule strongly notched | Inner lobule with minute notches, outer lobule notched and obsolete | Medial lobule entire, lateral lobule recessed, and notched | Entire or with slight notches | Entire |
Chionaspis brachycephalon Vea sp. n., adult female.
urn:lsid:zoobank.org:act:CEE3C93A-372F-40B2-A1E1-B778BC1500F4
http://species-id.net/wiki/Chionaspis_caudata
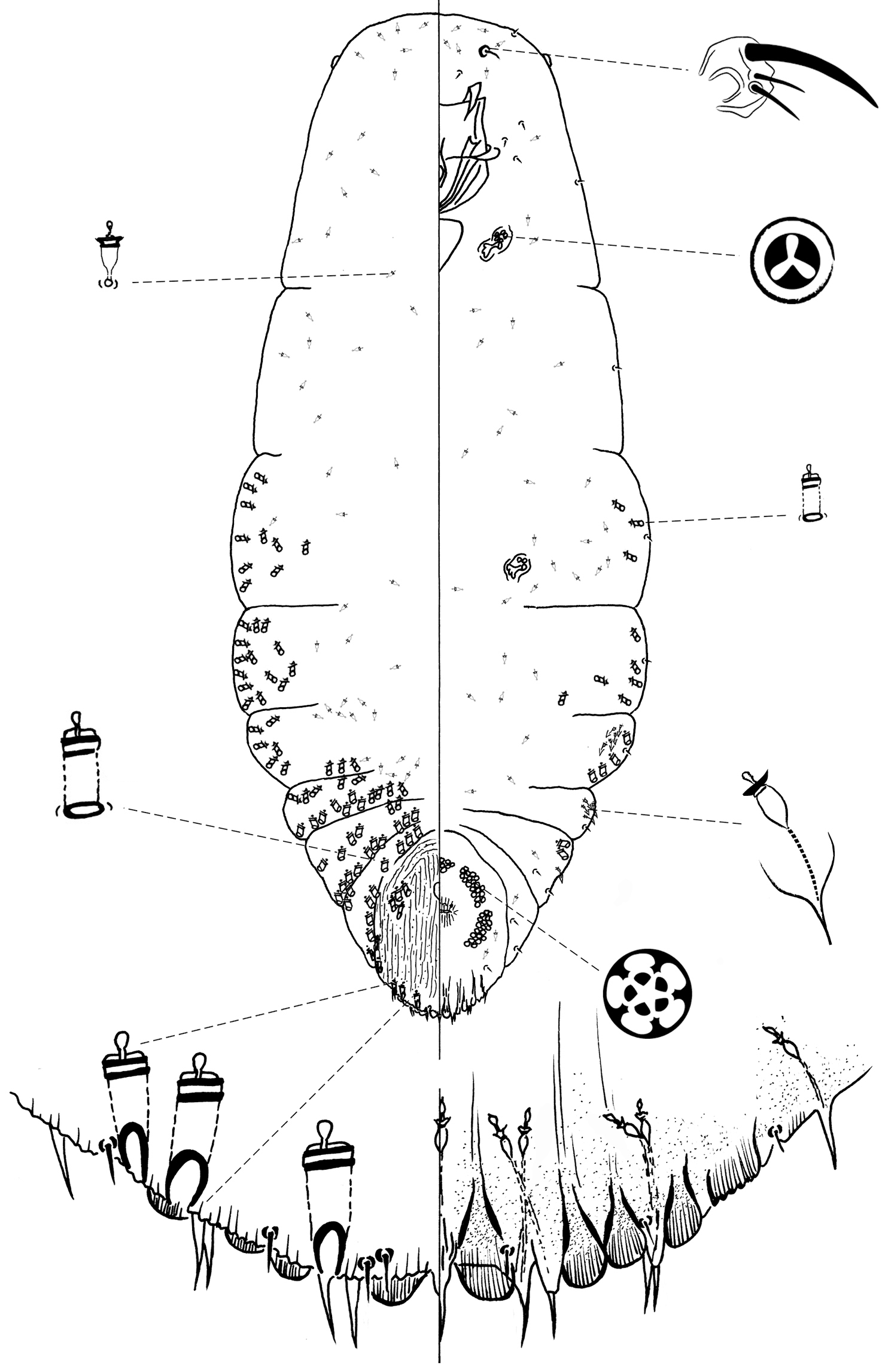
Figure 2Type locality: Mexico, Oaxaca state, Oaxaca, 16°11.99'N, 96°31.52'W, on needle of Pinus patulata longipedunculata (Loock ex Martínez), 28 August 2007, R. Gwiazdowski and M. Dahlberg coll.
Holotypeadult female, slide-mount in balsam. Original label “D1703D Mexico: Oaxaca, Oaxaca, Hwy 175, 28.viii. 2007, 16°11'59"N, 96°31'30.9"W, R. Gwiazdowski, M. Dahlberg, Pinus patulata longipedunculata”, deposited at CNIN.
Paratypes: adult female, slide-mount in balsam, D1703A, same information as the holotype, deposited at USNM. Adult female, slide mount in balsam, D1703G, same collection data as the holotype, deposited at USNM. Adult females on separate slides, D1703F, H, I, J, K and L, same information as D1703D, deposited UNAM for all but D1703F, deposited at CNIN.
Other material examined: Adult female, slide mount in balsam. Original label “D1702A, Mexico: Oaxaca, Oaxaca, Hwy 175, 28.iii.2007, 16°10'31.3"N, 96°30'24.2"W, R. Gwiazdowski and M. Dahlberg, Pinus pseudostrobus oaxacana”, deposited at UMAM. Adult female, slide mount in balsam. Original label “D2275C, Mexico, Xalapa, HWY 131, ~2.6 km N of Atzalan, 15.i.2009, Pinus chiapensis”; adult female, slide mount in balsam. Orginal label “D2292C, Mexico, Guerrero, ~30 km, North E of Atoyac de Alvarez along road perpendicular to HWY 200, 20.i.2009, Pinus chiapensis”; adult female, slide mount in balsam, D2292D, same collection data as D2292C; adult female, slide mount in balsam. Original label “D2296B. Mexico, Chiapas, ~13.5 km North of Chamula, 24.i.2009, Pinus chiapensis”; adult female, slide mount in balsam, D2296C, same collection data as D2296B; all additional material deposited at UMAM.
Chionaspis caudata Vea differs from other Chionaspis with the following combination of characters (Table 1): median lobes (L1) unyoked, parallel-sided, with a single gland spine between them, subtended by a microduct; submedian microducts absent on abdominal segment 7; gland spine absent on abdominal segment 5; head square-shaped, body with an extended thorax relative to other pine-feeding Chionaspis.
Field characters: All pine-feeding Chionaspis reported here, including Chionaspis heterophyllae and Chionaspis pinifoliae are indistinguishable by eye in the field. See the description above for Chionaspis brachycephalon Vea.
Slide-mounted adult female (Figure 2), spindle-shaped and elongate, slightly lobed to parallel-sided laterally; length of holotype 1.75 mm, range (n= 11) 1.38 – 2.03mm; maximum width of holotype: 0.61 mm; range (n=11) 0.48 – 0.7mm.
Pygidium: Lobes. Posterior margin with 3 pairs of lobes (L1, L2 and L3), fourth pair (L4) appears as series of low, sclerotized points; paraphyses absent. L1 separated by a space 0.6 – 1 (0.73) times width of lobes, without a yoke, lobes completely separated, lateral margins parallel-sided, entire, rarely notched; L2 bilobed, smaller than L1, lobules subequal, inner lobule slightly notched, outer lobule entire; L3 bilobed, medial lobule similar to L2, lateral lobule recessed and serrated. Gland spines. Gland spine formula varying from 1-1-1 to 3-3-2 (2-2-1) (microduct formula varying from 1-1-1 to 3-3-1 (2-2-1)), with always 1 gland spine between L1, subtended by 1 microduct; gland spine on segment 5 always absent. Gland spine microduct slender with a relatively developed collar at apex. Ducts. Large macroducts in submedian area of segments 5 and 6 (with 4 – 6 (6) on segment 5 and 3 – 4 (3) on segment 6); in submarginal areas of segment 5 (with 4 – 8 (7) macroducts); marginal area of segments 5 to 7 (with 1 on segment 7, 2 – 3 (2) on segment 6, 2 – 3 (2) on segment 5); absent on segment 8. Largest macroduct on segment 7 (between L1 and L2) 15 – 22.5 (20) μm long. Pygidial microducts always on venter in submarginal areas of segment 5 and 6, with 1 – 2 (2) duct on segment 5 and 2-3 (2) ducts on segment 6, always absent from segment 7; pygidial microducts absent from dorsum. Pores. Perivulvar pores with 5 loculi, in 5 groups, 1 median group with 10 – 15 (13) pores, 2 anterolateral groups with 23 – 27 (25) pores, 2 posterolateral groups with 18 – 27 (24) pores. Anal opening. Diameter 15 – 22.5 (17.5) μm, located 6.7 – 11.7 (9.9) times length of anal opening from base of median lobes. Setae. Dorsal setae: 2 setose on L1, 1 spinose between lobules of L2 and L3. Ventral setae: 1 small on L1, 1 marginal at base of each gland spine cluster and 1 submarginal area of each segment, 2 on submedian aerea of segment 6, half as long as dorsal setae; 2 pairs of setae in a row anterior to the vulva.
Prepygidium: Gland spines. Near each body margin from segment 1 or 2 to 4, with 0 – 4 on segment 1, 0 – 5 (4) on segment 2, 1 – 7 (4) on segment 3 and 1 – 2 (1) gland spines on segment 4, which are short and protrude from the margin. Gland spines from segment 1 to 3 are the smallest, and never protrude from the margin. Ducts. Macroducts of 2 sizes; largest macroducts in submedian areas of abdominal segments 4 and 3. Small macroducts in submedian area of segments 3 and 4, and in submarginal areas of segments 1 to 4. Prepygidial microducts present on venter from segment 1 to segment 3, in marginal or submarginal areas from head to segments 2 to 3. Prepygidial microducts on dorsum on segments 1 to 4, often in conspicuous clusters submedially.
Cephalothorax: Small macroducts present on last thoracic segment, marginally and submarginally. Microducts present on both surfaces, evenly distributed. Perispiracular pores primarily with 3 loculi, anterior spiracles with 6 – 8 (7) pores, posterior spiracles with 2 – 3 (2) pores. Eyes represented by small sclerotized area, located on body margin at level near anterior clypeolabral shield. Antennae each with 1 long seta. Distance between antennae 122.5 – 375 (135) μm.
Chionaspis caudata Vea possesses an unusual median gland spine between the median lobes. The epithet caudata is a Latin adjective meaning tailed (caudate), derived from cauda, tail, and referring to this peculiar feature.
Chionaspis caudata Vea differs from the other species by the rather square-shaped head and noticeably longer body, the presence of a single gland spine subtended by one microduct between the median lobes, and the gland spine formula. The presence of the median gland spine is striking as this feature prevents this species from keying to the genus Chionaspis (or indeed any related genus) in available keys to genera; however, the phylogenetic analyses of
Chionaspis caudata Vea sp. n., adult female.
urn:lsid:zoobank.org:act:B04204CC-3A4F-4BC2-9BB3-25A3CAC95C44
http://species-id.net/wiki/Chionaspis_sonorae
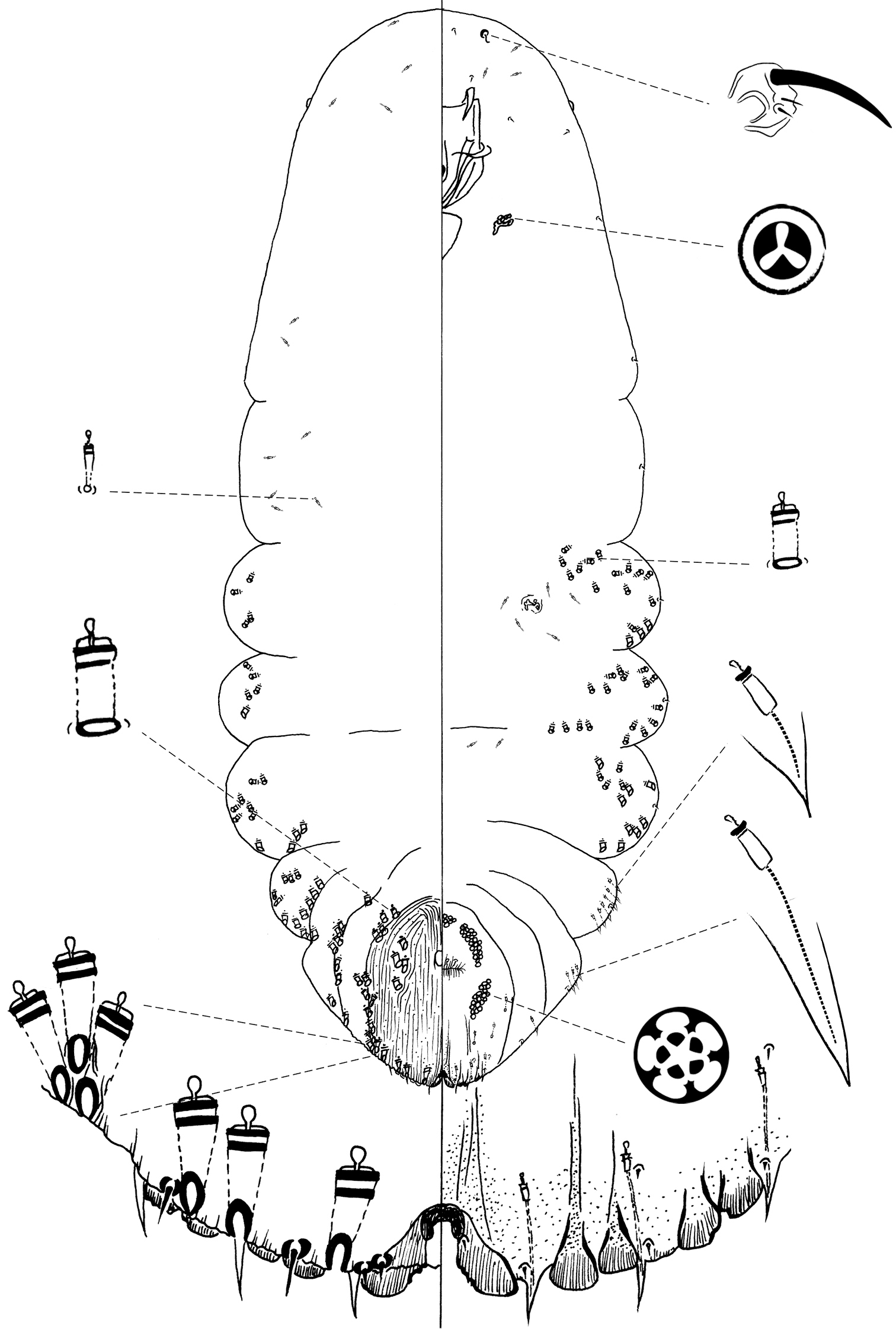
Figure 3Type locality. Mexico, Sonora state, Tecora, 28°22.45'N, 108°56.11'W, on needle of Pinus engelmannii Carr, 8 October 2007, R. Gwiazdowski, T.R. Van Devender and A. Lilia Reina coll.
Type specimens: Holotype adult female, slide-mount in balsam. Original label “D1781A, Mexico: Sonora, Yecora, 8.x.2007, 28°22'26.8"N, 108°56'06.3"W, R. Gwiazdowski, T. R. Van Devender, L.Van Devender, Pinus engelmannii (Carr.)”, deposited at CNIN.
Paratypes: Adult females on separate slides, D1781C and F same collection data as D1781A; D1781C deposited at CNIN and D1781 F deposited at UMAM.
Other material examined: Adult female, original label “D1780A, Mexico, Sonora, West of Yecora, 7.x.2007, 28°21'33.5"N, 109°01'48.3"W, R. Gwiazdowski, T. R. Van Devender, L. Van Devender, Pinus engelmannii (Carr.)”, deposited at USNM. Adult females on separate slides, D1780B, C, D, E, F and G, same collection data as D1780A, deposited at UMAM.
Chionaspis sonorae Vea is distinguishable from other Chionaspis by the combination of the following characters (Table 1): median lobe shape unusual, broad, medial margins parallel or slightly convergent in basal half, abruptly angled near midpoint, with distal half divergent, serrated; yoke horseshoe-shaped; microducts sparse.
Field characters: All pine-feeding Chionaspis reported here, including Chionaspis heterophyllae and Chionaspis pinifoliae are indistinguishable by eye in the field. See the description above for Chionaspis brachycephalon Vea.
Slide-mounted adult female (Figure 3) spindle-shaped and elongate, lobed laterally and broader posteriorly (broadest at metathorax or abdominal segment 1), length of holotype 1.29 mm, range (n=10) 1.29 – 1.83 mm; maximum width of holotype: 0.59 mm; range (n=10) 0.59 – 0.7 mm.
Pygidium: Lobes. Posterior margin with 3 pairs of definite lobes, fourth pair of lobes appearing as series of low, sclerotized points; paraphyses absent. L1 separated by space 0.3 times width of lobes, with a horseshoe-shaped yoke, lateral margins of lobes divergent, medial margin parallel from the base to midpoint, then diverging in apical half (with notches on diverging part); L2 bilobed, entire, shorter than L1, medial lobule larger; L3 slightly notched on lateral side or entire, bilobed but with outer lobule membranous, subequal or slightly smaller than inner lobule. Gland spines. Gland spine formula 1-1-1 (microduct formula 1-1-1), with 1 gland spine near each body margin of abdominal segment 5; without gland spines between L1. Ducts. Large macroducts in submedian area of segments 5 and 6 (with 4 – 7 (5) on segment 5 and 4 – 5 (5) on segment 6), in submarginal areas of segment 5 (with 5 – 7 (7)), and in marginal area of segments 5 to 7 (with 1 on segment 7, 2 on segment 6, 2 on segment 5); absent on segment 8. Largest macroduct on segment 7 (between L1 and L2) 15 – 20 (17.5) μm long. Small macroducts sparse on segment 5 (sometimes 2). Pygidial microducts on venter in submarginal areas of segment 5 to 7, with 0 – 2 (2) ducts on segment 5, 1 – 4 (2) ducts on segment 6 and 1 – 2 (2) ducts on segment 7; pygidial microducts absent from dorsum. Pores. Perivulvar pores with 5 loculi, in 5 groups, 1 median with 12 – 24 (15) pores, 2 anterolateral with 24 – 35 (28) pores, 2 posterolateral with 27 – 33 (30) pores. Anal opening. Located 7.7 – 16.3 (11) times length of anal opening from base of median lobes, diameter 10 – 17.5 (14.5) μm long. Setae. Dorsal setae: 2 setose on L1, 1 setose (~ 11 µm) between lobules of L2 and L3. Ventral setae: 1 small on L1, 1 marginal at base of each gland spine cluster and 1 on submarginal area of each segment, 2 in submedian area of segment 6, half as long as dorsal setae; 2 pairs of setae in a row anterior to the vulva.
Prepygidium: Gland spines. Near each body margin from segment 2 to 4, absent from mesothorax, metathorax and segment 1; 4 – 8 (6) on segment 2, 4 – 7 (5) on segment 3 and 1 – 4 (2) on segment 4. Gland spines on segments 3 and 4 protruding from margin and about same size as those on segment 5. Gland spines on segment 2 the smallest and never protruding from the margin. Ducts. Macroducts of 2 sizes; larger macroducts in submedian areas of abdominal segments 4 and 3. Small macroducts in submedian area of any or all of segments 3 and 4, in marginal areas from meso- or metathorax to segment 3. Prepygidial microducts almost absent on both surfaces, with a few on segment 2.
Cephalothorax: Microducts sparse on venter and dorsum, with a slight concentration around posterior thoracic spiracles and head. Perispiracular pores with 3 loculi, anterior spiracles with 5 – 6 (6) pores, posterior spiracles with 1 – 3 (2) pores. Eyes represented by small sclerotized area, located on body margin at level near anterior clypeolabral shield. Antennae each with 1 long seta and 2 minute setae, distance between antennae 60 – 117.5 (80) µm.
urn:lsid:zoobank.org:act:1EF8C3A2-CE72-420E-BE4D-9B07AD27BBA5
http://species-id.net/wiki/Chionaspis_torreyanae
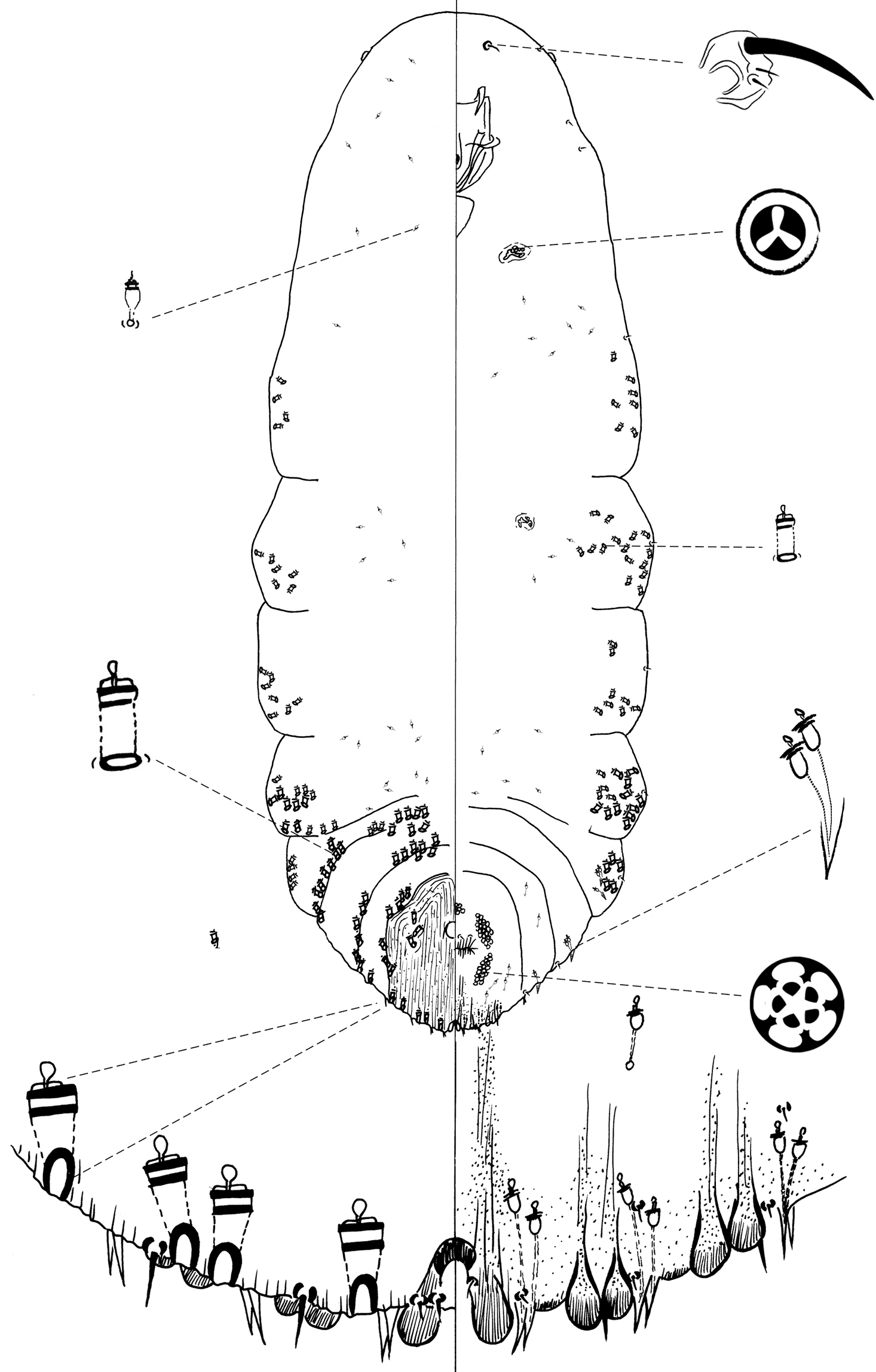
Figure 4Type location: U.S.A., California, Channel Islands, Santa Rosa Island, 33°59.09'N, 120°1.42'W, on needle of Pinus torreyana insularis Schoenherr et al., 23 January 2008, C. Greene coll.
Holotype adult female, slide-mount in balsam. Original label “D2238A, USA: California, Santa Rosa Island, 23.i.2008, 33°59'5.4"N, 120°01'25.4"W, Carolyn Greene, Pinus torreyana insularis ”, deposited at UMAM.
Paratypes: Adult females on separate slides, D2238D, E and G, same information as D2238A, deposited at USNM.
Other material examined: Adult female, slide-mount in balsam, original label “D1557A, USA: California, San Diego, 30.viii.2006, 32°56'27.2"N, 117°15'41.0"W, Rodger Gwiazdowski, Pinus torreyana”, deposited at UMAM. Adult females on separate slides, D1557D, E, F and G, same collection as D1557A, deposited at UMAM.
Adult female, slide-mount in balsam, original label “D1559A, USA: California, San Diego, 30.iii.2006, 32°55'12.9"N, 117°15'09.9"W, R. Gwiazdowski, Pinus torreyana’, deposited at UMAM. Adult female, D1559C, same collection data as D1559A, deposited at UMAM.
Adult female, slide-mount in balsam, original label “D2235A, USA: California, Santa Rosa Island, 23.i.2008, 33°59'04"N, 120°01'34.9"N, Carolyn Greene, Pinus torreyana insularis ”, deposited at USNM. DNA:AMCC: 205821. Adult female, slide-mount in balsam, original label “D2236A, USA: California, Santa Rosa Island, 23.i.2008, 33°59’4.9"N, 120°01’35"W, Carolyn Greene, Pinus torreyana insularis”, deposited at UMAM.
Adult female, slide mount in balsam, original label “D2240A, USA: California, Santa Rosa Island, 33°59'2.3"N, 120° 1'11.7"W”, Carolyn Greene, Pinus torreyana insularis”, deposited at UMAM. Adult females on separate slides, D2240C and D, same collection data as D2240A, deposited at UMAM.
Chionaspis torreyanae Vea differs from other Chionaspis with the combination of following characters (Table 1): gland spine formula 2-2-2, microduct formula 2-2-2, other abdominal gland spines usually each subtended by 2 microducts, unnotched pygidial lobes.
Field characters: All pine-feeding Chionaspis reported here, including Chionaspis heterophyllae and Chionaspis pinifoliae are indistinguishable by eye in the field. See the description above for Chionaspis brachycephalon Vea.
Slide-mounted adult female (Figure 4): spindle-shaped and elongate, lobed laterally and broader posteriorly (generally broadest at metathorax), length of holotype 1.55 mm, range (n=16) 1.15 mm – 1.65 mm; maximum width of holotype: 0.65 mm; range (n=16) 0.475 – 0.875 mm, maximum width at metathorax, rarely on first abdominal segment.
Pygidium: Lobes. Posterior margin with 3 pairs of definite lobes (L1, L2 and L3), fourth pair (L4) of lobes appear as series of low, sclerotized points; paraphyses absent. L1 separated by space 0.3 – 1 (0.6) times width of lobes, with a thick, protruding, U-shaped yoke uniting L1, lateral margins of lobes parallel, slightly diverging near apex, medial margin parallel-sided. L1 usually entire (1 minute notch may be present); L2 bilobed, smaller than L1, medial lobule always larger, both lobules entire; L3 bilobed, lateral lobule usually obsolete, or, when present, shorter than medial lobule but about equal in width. Gland spines. Gland spine formula 2-2-2 (microduct formula 2-2-2), with 1 short gland spine near each body margin on abdominal segment 5; without gland spines between median lobes. Ducts. Large macroducts in submedian area of segments 5 and 6 (with 4 – 6 (5) on segment 5 and 3 – 6 (4) on segment 6); in submarginal areas of segment 5 (with 5 – 8 (6)); in marginal area of segments 5 to 7 (with 2 – 3 (2) on segment 5, 2 on segment 6 and 1 on segment 7); absent on segment 8. Largest macroduct on segment 7 (between L1 and L2) 15 – 22.5 (20) μm long. Pygidial microducts always on venter in submarginal areas of segment 5 to 7, with 1 – 2 (1) duct on segment 5, 2 ducts on segment 6 and 1 duct on segment 7; pygidial microducts absent from dorsum. Pores. Perivulvar pores with 5 loculi, in 5 groups, 1 median with 8 – 17 (8) pores, 2 anterolateral with 20 – 27 (23) pores, 2 posterolateral with 17 – 26 (20) pores. Anal opening. Located 6.1 – 11.2 (9) times length of anal opening from base of median lobes, diameter 12.5 – 17.5 (15) μm. Setae. Dorsal setae: 2 setose on L1, 1 setose (~ 11 m) between lobules of L2 and L3. Ventral setae: 1 small on L1, 1 marginal at base of each gland spine cluster and 1 in submarginal area of each segment, 2 in submedian area of segment 6, small and short; 2 pairs of setae in a row anterior to the vulva.
Prepygidium: Gland spines. Near each body margin from segment 1 or 2 to 4, absent from mesothorax and metathorax; with 0 – 3 (0) on segment 1, 1 – 7 (3) on segment 2, 2 – 4 (3) on segment 3 and 1 – 2 (2) gland spine on segment 4 with 2 microducts extending, short and protruding from margin. Gland spines from segment 1 to 3 the smallest and never protruding from margin. Ducts. Macroducts of 2 sizes; largest macroducts in submedian and submarginal areas of abdominal segments 4 and 3. Small macroducts in submedian area of either or both of segments 3 and 4, in marginal areas from meso- or metathorax to segment 3. Prepygidial microducts sparsely present on venter and dorsum from segment 1 to 4.
Cephalothorax: Microducts sparsely present on venter and dorsum.Perispiracular pores primarily with 3 loculi, anterior spiracles with 5 – 8 (6) pores, posterior spiracles with 2 – 5 (3) pores. Eyes represented by small sclerotized area, located on body margin at level near anterior clypeolabral shield. Antennae each with 1 long seta and 2 shorter setae, distance between two antennae 65 – 135 (85) μm.
| 1 | Gland spine present between median lobes; zygosis absent between medial lobes (Figure 2) | Chionaspis caudata Vea, sp. n. |
| – | Gland spine absent between median lobes; zygosis present between median lobes | 2 |
| 2 | Head reduced, with margins converging rapidly toward anterior end of cephalothorax; gland spine cluster in 1st, 2nd, and 3rd spaces each with 2 or more microducts (Figure 1) | Chionaspis brachycephalon Vea, sp. n. |
| – | Head normally developed, rounded; gland spine cluster in 1st, 2nd, and 3rd spaces each with 1 or more microducts | 3 |
| 3 | Gland spine cluster in 1st, 2nd and 3rd spaces each with a single microduct | 4 |
| – | Gland spine cluster in 1st, 2nd and 3rd spaces each with two microducts (Figure 4) | Chionaspis torreyanae Vea, sp. n. |
| 4 | Medial margins of median lobes curving abruptly outward near midpoint between base and apex: parallel near base and becoming suddenly divergent (and slightly notched) in apical half (Figure 3) | Chionaspis sonorae Vea, sp. n. |
| – | Medial margins of median lobes not curving abruptly near midpoint: either diverging throughout their length, or parallel for most of their length and slightly diverging near apex | 5 |
| 5 | Space between median lobes, at midpoint between base and apex, > 1.5 width of median lobe; median lobes usually continually diverging throughout their length | Chionaspis heterophyllae Cooley |
| – | Space between median lobes, at midpoint between base and apex, < 1.5 width of median lobe; median lobes usually parallel for much of their length | Chionaspis pinifoliae (Fitch) |
The gland spine located between the median lobes of Chionaspis caudata Vea is unique among the species of Chionaspis.
For help with specimen collection we thank A. G. Arévalo, M. Dahlberg, D. Gernandt, C. Greene, R. Greig, J. Rull, T. R. Van Devender and A. L. Reina-G.
For logistical support we thank the Applied Entomology Group of Martin R. Aluja and Juan Rull of the Instituto de Ecología, The Gernandt lab at Universidad Nacional Autónoma de Mexico, the Ecology and Natural resource unit of the Instituto de Ecología from both Durango and Veracruz as well as Dug Miller of the USDA Systematic Entomology Lab in Beltsville, MD. For technical assistance with slide-mounting and cataloguing specimens we thank Erica Fitzpatrick, Alice Trei, and Hedda Monaghan. For comments on a previous version of this manuscript, we thank Dug Miller.
This work was supported by the National Institute for Food and Agriculture (AFRI 2009-02310), the US Department of Agriculture, and the Massachusetts Agricultural Experiment Station, under project number MAS 000941. Funding for cataloguing the specimens and data-sharing was provided by NSF (EF-1115191). Funding for RAG was provided by the National Science Foundation (DEB-0447880 and DEB-0118718) and the University of Massachusetts Natural History Collections.
Voucher information for all type specimens. (doi: 10.3897/zookeys.270.2910.app1) File format: Microsoft Word document (doc).
Explanation note: Voucher information for all type specimens; each specimen has several kinds of vouchered material. Specimens are conventionally slidemounted in balsam, and each is associated with extracted, whole genomic DNA. Additionally type lots from which the specimens have been drawn are preserved as in-situ frozen tissue associated with host plant tissue, and some specimens have DNA sequence data vouchered in GenBank. The Sample ID number directly corresponds to Sample ID number in Appendix 2.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Locality information for all type specimens. (doi: 10.3897/zookeys.270.2910.app2) File format: Microsoft Word document (doc).
Explanation note: Locality information for all type specimens; the Sample ID number directly corresponds to Sample ID number in Appendix 2. The geodetic system used for all GPS points is WGS 1984.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.