(C) 2013 Ante Vujić. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new hoverfly species, Cheilosia barbafacies Vujić & Radenković sp. n. (Diptera, Syrphidae), is described and distinguished from the closely related species Cheilosia pascuorum Becker, 1894, based on material collected from the mountains of the Balkan Peninsula. Diagnostic characteristics and an identification key for the members of the proxima group of Cheilosia s.str., including the new taxon, are provided.
Diptera, Syrphidae, Cheilosia barbafacies, proxima group , new species
Cheilosia Meigen, 1822 is the largest Palaearctic hoverfly (Diptera, Syrphidae) genus with nearly 300 species listed by
This genus belongs to the monophyletic tribe Rhingiini of the subfamily Eristalinae, as a sister group of Eumerini (
The subgeneric classification of Cheilosia has been changed from
All known species undergo larval development in specific plants or fungi, although some species feed on a wide range of plants. There is only one known exception, the species of the subgenus Neocheilosia Barkalov, 1983, which feed on sap and cambium of coniferous trees. One of the most serious pests of genus Cheilosia, is the species Cheilosia vulpina (Meigen, 1822) that infested 50% of artichoke (Cynara scolymus) crops in Northern France during the 1980s (
These blackish hoverflies without mimetic features still cause identification troubles for taxonomists, due to the existence of many morphologically similar taxa with variable characters. There is no key through which all European species of Cheilosia can be identified. Recently, attempts have been made to stabilise the nomenclature of western European Cheilosia species, by dealing with small groups of closely related species (
After detailed analysis of published material under the name Cheilosia pascuorum Becker, 1894 from the Balkan Peninsula (
List of species of Cheilosia belonging to the proxima group and their distribution.
| Species name |
Species distribution, from |
|---|---|
| Cheilosia balkana | Alps (Italy), Balkans (Montenegro, Serbia, Slovenia). |
| Cheilosia barbafacies sp. n. | Dinaric mountains on the Balkan Peninsula |
| Cheilosia gigantea | Fennoscandia south to the Alps; Germany eastwards through northern and central Europe together with northern Italy and the Balkans (Slovenia, Bosnia and Herzegovina, Serbia, Montenegro, FRY Macedonia, Bulgaria) into European parts of Russia, and from Ukraine to the Caucasus; in Siberia from the Urals to the Pacific coast. |
| Cheilosia ingerae | Northern Norway, Sweden and Finland. |
| Cheilosia pascuorum | Alps (France, Germany, Switzerland, Austria), Romania, parts of European Russia, the Balkans (Serbia, Montenegro). |
| Cheilosia proxima | Fennoscandia south to the Pyrenees and the mountainous regions of Spain; Britain eastwards through much of Europe, the Balkans (Slovenia, Croatia, Bosnia and Herzegovina, Serbia, Montenegro, FRY Macedonia, Greece, Bulgaria) into Turkey and European parts of Russia; in Siberia from the Urals to Kamchatka. |
| Cheilosia rufimana | From Finland, Denmark and Belgium eastwards through mountainous regions of central Europe to the Balkans (Serbia, Bulgaria); Ukraine; Kazakstan; Asiatic Russia. |
| Cheilosia velutina | Fennoscandia south to Spain; from Ireland eastwards through much of Europe into Russia and through Siberia to the Pacific coast. |
| Cheilosia vulpina | Denmark to the Pyrenees and northern Spain; from England eastwards through central Europe to the central and southern parts of Russia as far as western Siberia. |
The characters used in the key, descriptions, and drawings employ the terminology established by
The specimens under study were collected by sweep netting. To study male genitalia, specimens were relaxed and the genitalia were extracted using an insect pin with a hooked tip.
Genitalia were cleared by boiling individually in tubes of water-diluted KOH pellets for 5 min. This was followed by brief immersion in acetic acid to neutralize the KOH and immersion in ethanol to remove the acid. Samples were stored in microvials containing glycerol. Drawings were made with an FSA 25 PE drawing tube attached to a binocular microscope. Measurements were taken with an eye piece graticule or micrometer.
All the studied material, including type material, has been deposited at the Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad, Serbia (FSUNS).
Chilosia Agassiz, 1846
Cartosyrphus Bigot, 1883
Chilomyia Shannon, 1922
Chaetochilosia Enderlin, 1936
Dasychilosia Enderlin, 1936 Proxima species group
Diagnosis. Eyes pale haired; antennal pits separated; vertex grey dusted; central prominence rounded and more protruding than lateral corner of subcranial cavity, in lateral view; face at the level of central prominence less wide than half width of head. Probasisternum of protorax not fused with adjacent sclerites; anterior anepisternum bare; scutellum with black, exceptionally yellow, marginal setae; katepisternum with upper and lower hair patches connected or narrowly divided, entirely dusted; legs predominantly black, except tibiae usually paler on both ends; front coxa without lateral tooth; last tarsomere of front leg unmodified; in females, some hairs on hind tibiae longer, at least more than half of its width. Sternites of abdomen entirely grey dusted; male genitalia: gonostylus with a characteristic dorsal lobe (Figs 1, 2), neither S-shaped (Fig. 3A) nor sickle-shaped (Fig. 4D, 4E).
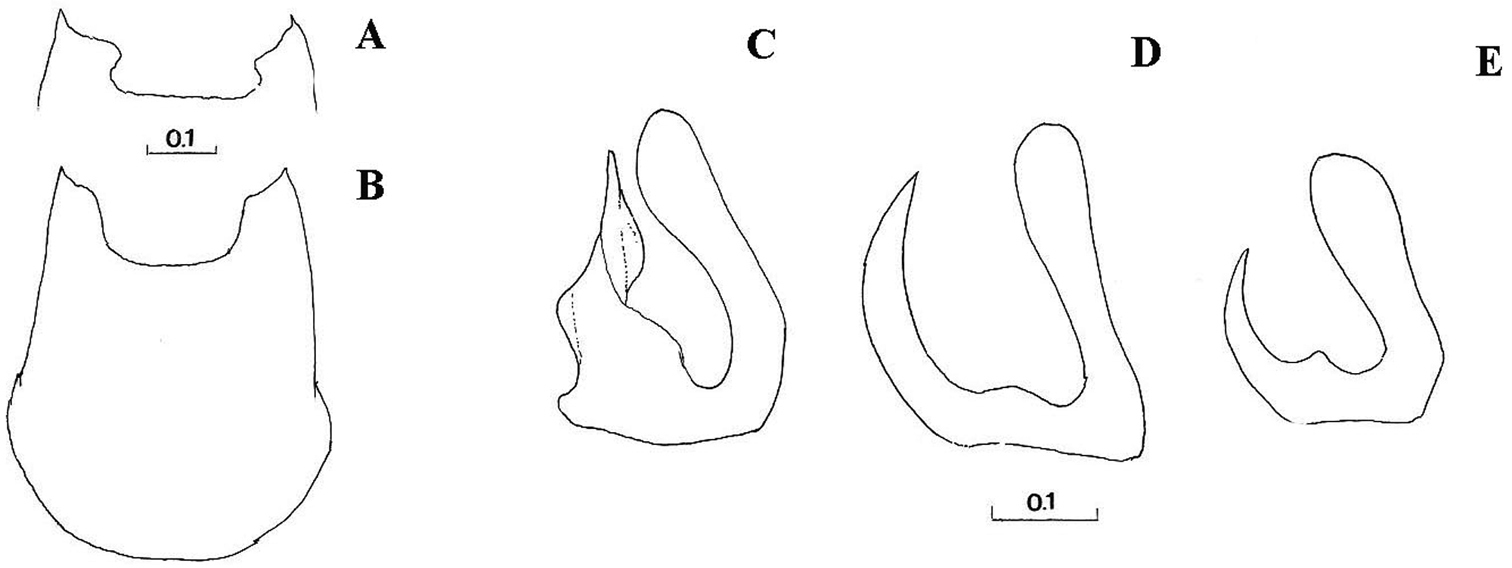
Comments. This group is related and morphologically similar to the following Cheilosia s.str. species: Cheilosia barbata Loew, 1857, Cheilosia naruska, Cheilosia aerea Dufour, 1848, and variabilis group of species, i.e. Cheilosia melanopa (Zetterstedt, 1843), Cheilosia redi, Cheilosia honesta Rondani, 1868, Cheilosia variabilis (Panzer, 1798). Nevertheless, all of them can be distinguished by a combination of characters: Cheilosia barbata has a S-shaped gonostylus (Fig. 3A) and females have less dusted sternites, undusted central part of the katepisternum and a narrower frons with parallel sides; in Cheilosia aerea, hairs on the anterior anepisternum are present and sternites are less dusted in some specimens and populations; in Cheilosia naruska, sternites are undusted except for slightly dusted anterior and posterior margins; males in variabilis group have sickle-shaped gonostylus (Fig. 4D, 4E) and females have very short and adpressed hairs on hind tibiae.
urn:lsid:zoobank.org:act:9F2ABB52-652B-4652-AD7C-8ACB4D8579C8
http://species-id.net/wiki/Cheilosia_barbafacies
Figs 1A, 2A, 2B, 4A, 4B, 5–7, 8A, 8C, 9–11, 12A, 12BMONTENEGRO: Durmitor, Škrčko-Sušički basen, 43˚11'7"N, 19˚3'28"E, broad-leaf forest, 25 June 1995, A. Vujić leg.
Holotype ♂, in excellent condition. MONTENEGRO. Original label: “Durmitor YU / Skakala 25.06.’95. / leg. Vujić.” 43°10'16"N; 18˚59'56"E (FSUNS 05768).
Paratypes, in excellent condition. MONTENEGRO: ♂ Original label: “007. Durmitor / Skrcka jezera / 5.07.1983.”43°8'8"N; 19°0'56"E (published in
MALE (Figs 1A, 2A, 2B, 4A, 4B, 5, 6C, 8A, 8C, 9A, 11A, 12A, 12B).
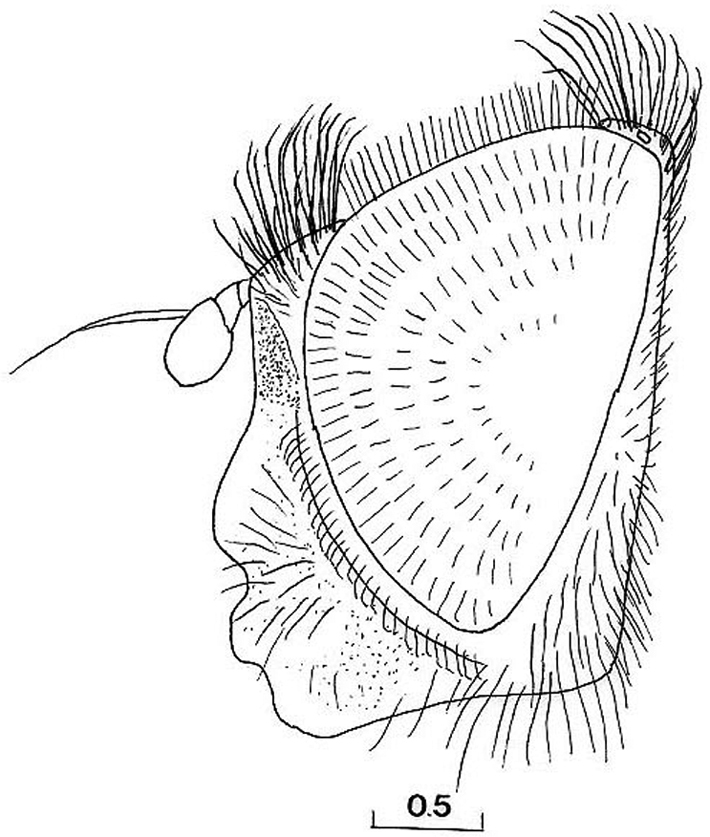
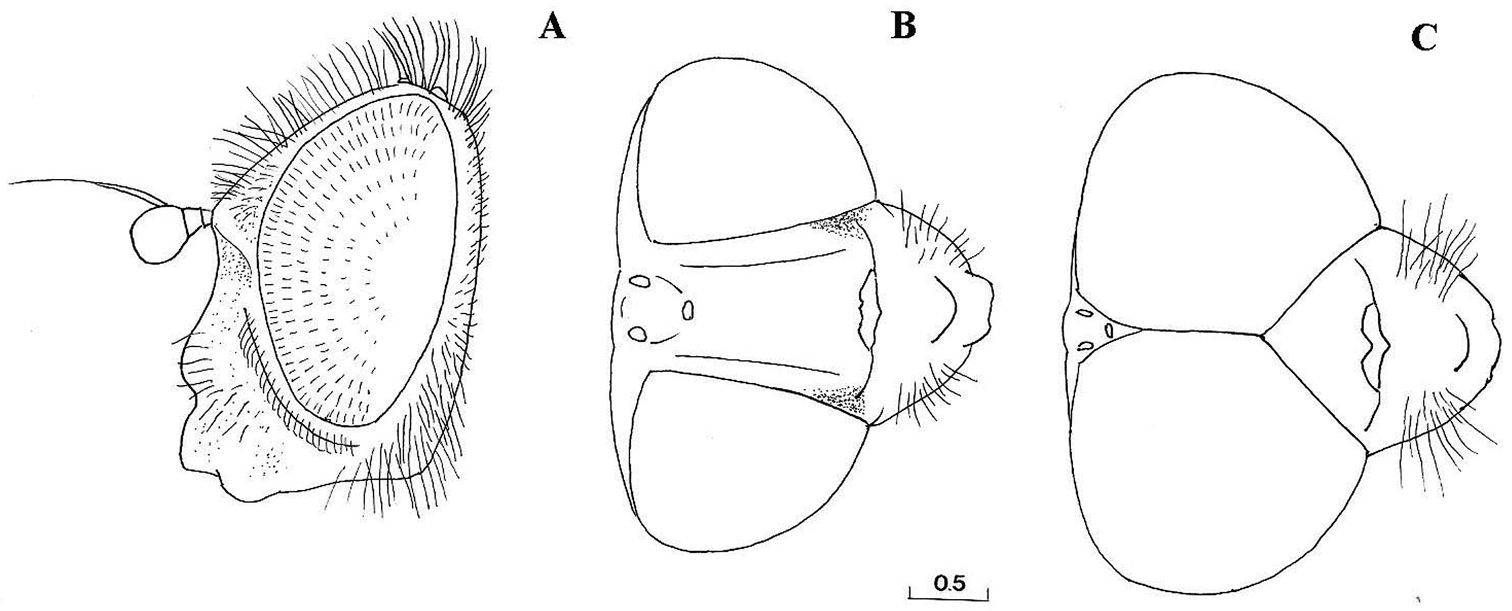
Head: Face with long, predominantly pale hairs, central prominence rounded (Fig. 5); orbital stripe with short, pale hairs. Frontal triangle small, undusted, covered with black hairs; eye contiguity longer than frontal triangle (Fig. 6C). Eyes completely covered with greyish hairs. Occiput narrow, white-grey dusted. Antennae dark, third antennal segment from dark-brown to reddish; arista bare and short (Fig. 11A). Clypeus dusted.
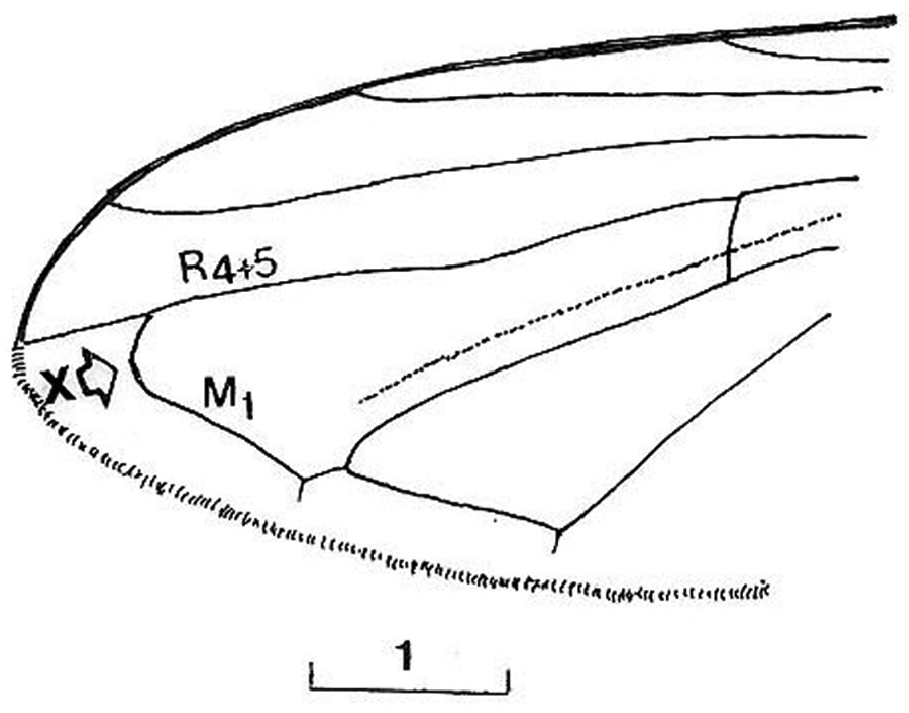
Thorax: Scutum with dark-olive shine, laterally slightly dusted, covered with long, pale and black hairs (Fig. 9A); central disc shining, with fine puncturation. Scutellum covered with long hairs and numerous longer black hairs on posterior margin (Fig. 9A). Pleurae dusted, covered with predominantly pale hairs mixed with black hairs on anepisternum and anepimeron; katepisternum continuosly pilose. Wing brownish, with dark veins, completely covered with microtrichia; vein M1 meeting vein R4+5 at an obtuse angle (Fig. 7: x). Calypters yellowish-grey. Haltere yellowish-grey with dark capitulum. Legs dark, except pale apex of femora, basal 1/3–1/4 and apical 1/5–1/6 of tibiae and ventral surface of tarsi on fore and middle legs; hairs on legs predominately pale mixed with black.
Abdomen: Tergites shining, except the whole tergite 2 and dull central area on tergites 2 and 3, which extends from anterior margin of tergite 2 to basal 6/7 of tergite 3, leaving the posterior margin of tergite 3 shining; tergites covered with erected, pale hairs, except few black hairs on posterior half of tergite 4 and on pregenital segments. Sternites grey dusted covered with pale hairs.
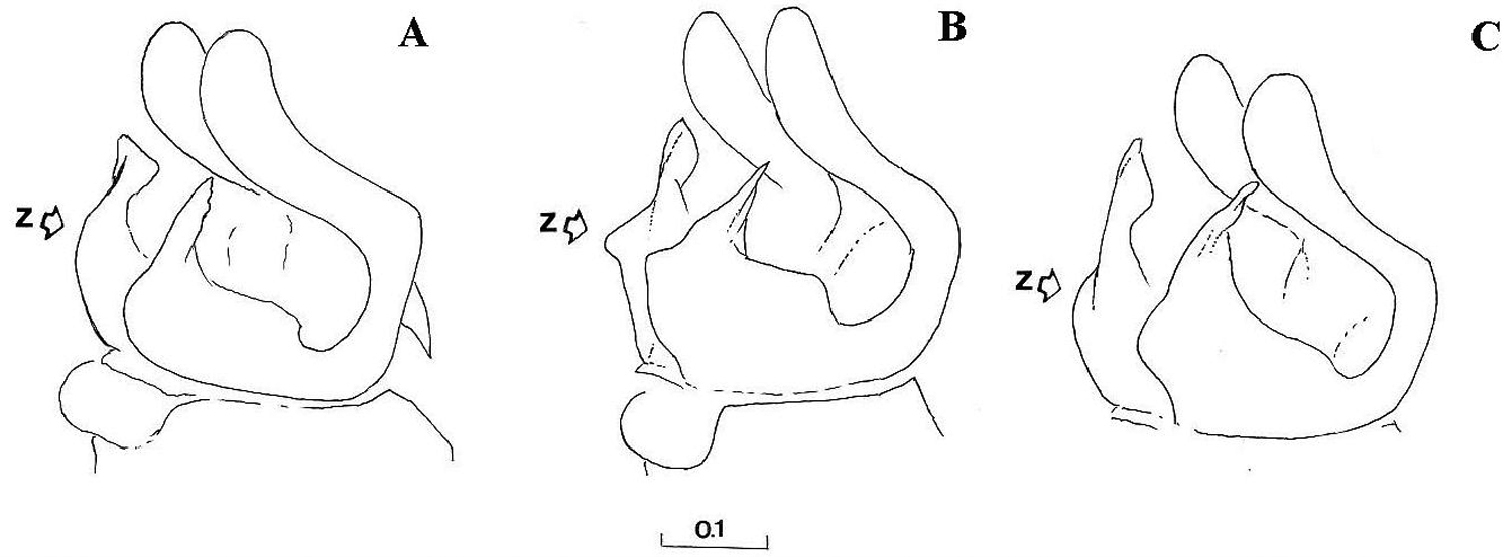
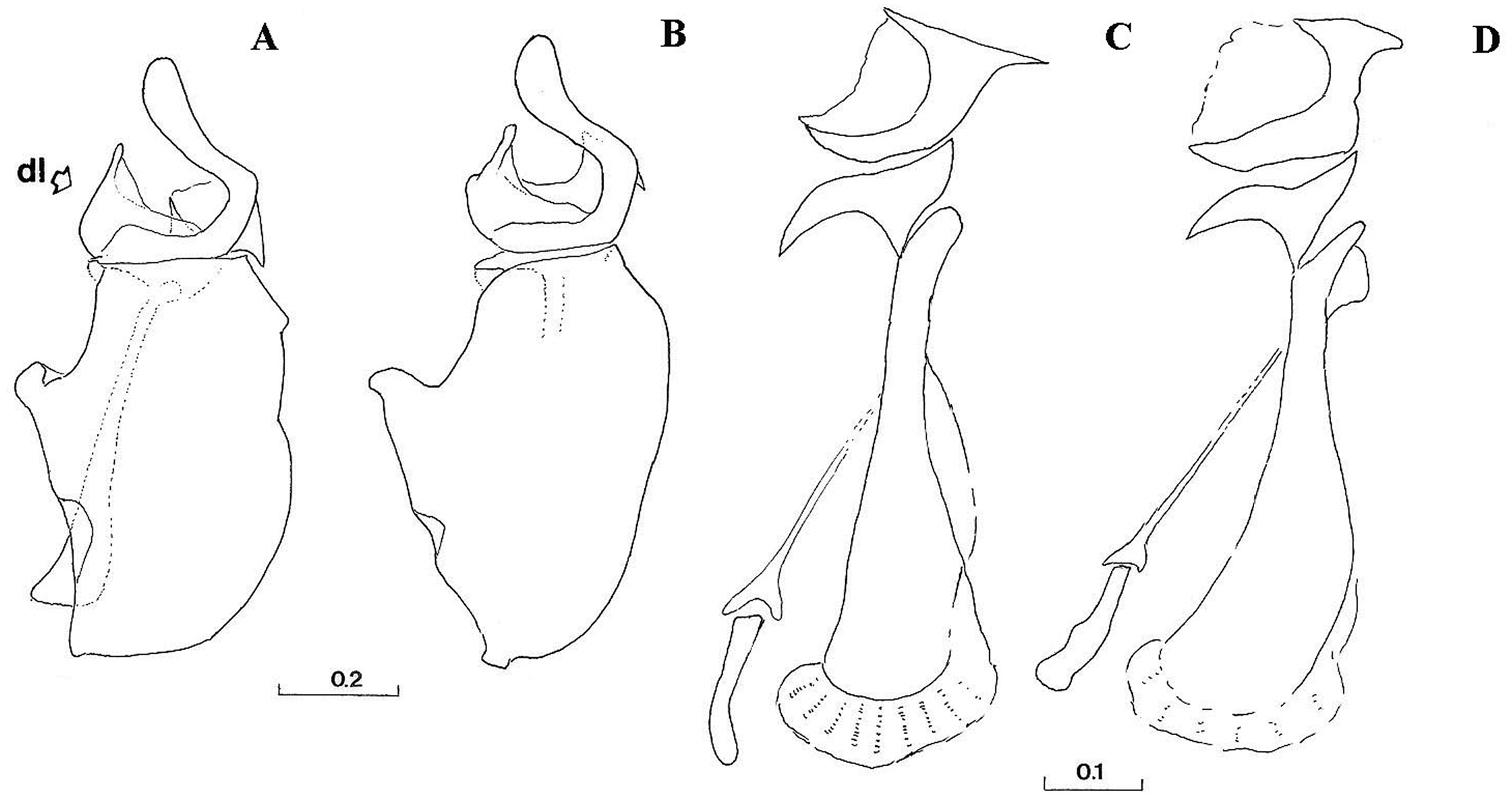
Genitalia: Dorsal lobe of gonostylus broad basally (Fig. 8A), without distinct dorsolateral extension (Figs 1A, 2A, 2B), present in Cheilosia pascuorum (Figs 1B, 1C, 2C, 2D); theca of hypandrium in ventral view with large quadrilateral excavation (Figs 4A, 4B).
FEMALE (Figs 6A, 6B, 7, 9B, 10, 11B). Similar to the male, except for normal sexual dimorphism and the following characters: pile is general shorter and more extensively pale, and legs less dark, basal 1/4 of femora, basal 1/3 and apical 1/4 of tibiae pale; frons with two lateral channels, shiny, except dusted antero-lateral corners (Fig. 6B), covered with pale hairs, except a few black hairs around ocellar triangle and above antennae; thorax pale haired, except for a few black hairs on post-alar calli and near wing base; tibiae of fore and middle legs pale, except dark central ring; basal tarsi of fore and middle legs pale; tergites covered with long and erect hairs, except adpressed hairs on central part of tergites 1–4.
Size. Male, body length: 8.9–11.1 mm; wing length: 7.9–9.2 mm (14 specimens were measured). Female, body length: 10.5 mm; wing length: 8.7 mm (1 specimen was measured).
Species related to Cheilosia pascuorum, but differs in the following characteristics: face covered with long hairs (Figs 5, 6), bare in Cheilosia pascuorum; clypeus dusted, shining in Cheilosia pascuorum; male genitalia: dorsal lobe of gonostylus without distinct dorsolateral extension (Figs 1A, 2A, 2B), present in Cheilosia pascuorum (Figs 1B, 1C, 2C, 2D).
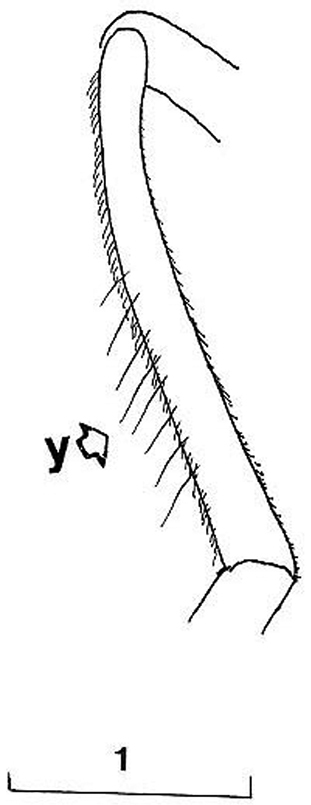
Based on the identification keys for European hoverflies, this species can be confused with four other Cheilosia species which have long facial hairs: Cheilosia barbata (Fig. 3A), Cheilosia lasiopa Kowarz, 1885 (Fig. 4D), Cheilosia melanopa (Fig. 4E) and Cheilosia vulpina (Fig. 4C). Identification of the new described species is possible based on a combination of the following characters: arista bare; central disc of scutum shining; vein M1 meeting vein R4+5 at an obtuse angle (Fig. 7: x); tergites 1-3 pale haired; sternites obviously grey dusted; male: dorsal lobe of gonostylus broader basally (Fig. 8A); female: arista about 3 times as long as third antennal segment (Fig. 11B); hairs on scutum long and erected (Fig. 9B); hind tibia on posterodorsal surface with few longer hairs (Fig. 10: y).
The specific epithet is derived from the Latin nouns (in apposition) of feminine gender in the nominative case: “barba” (beard) and “facies” (face). The name indicates the presence of long hairs on the face.
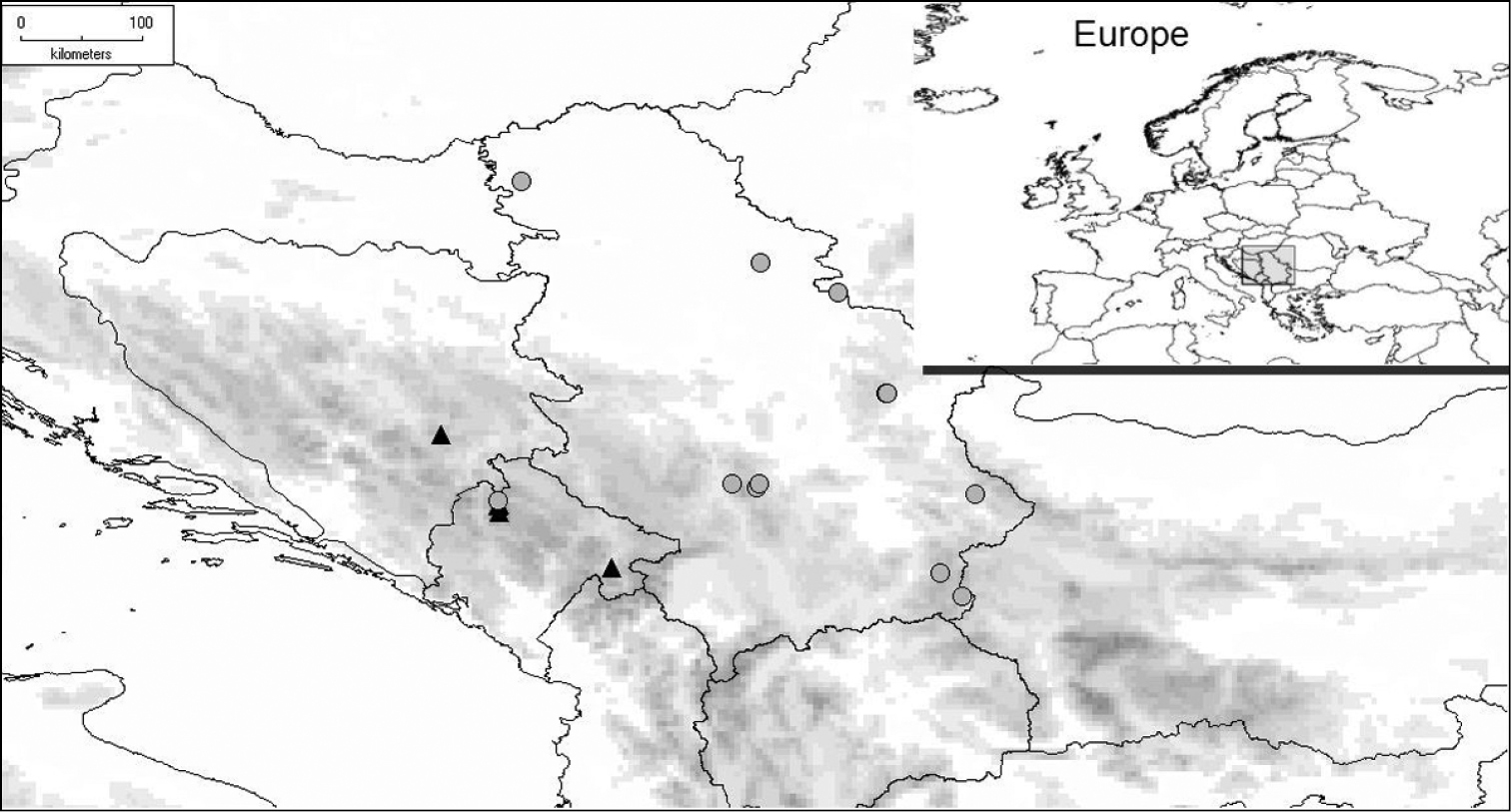
(Fig. 13). Cheilosia barbafacies sp. n. is found in two Dinaric mountains in the central part of the Balkan Peninsula, in Durmitor (Montenegro) and Jahorina (Bosnia-Herzegovina), while the related species, Cheilosia pascuorum, has a wider range extending from the Alps, across the Balkan Peninsula, to Romania and the European part of Russia (
Although Cheilosia barbafacies has long facial hairs, it is closely related to Cheilosia pascuorum with a non-hairy face. Species with long facial hairs were assigned to “group B” of
In
Some females of Cheilosia barbafacies sp. n. are similar to females of Cheilosia redi, and they can be separated by the following characters:
Cheilosia barbafacies sp. n.: vein M1 meeting vein R4+5 with an obtuse angle (Fig. 7: x); hairs on scutum and tergites long and erected (Fig. 9B); hind tibia on posterodorsal surface with few longer hairs (Fig. 10: y).
Cheilosia redi: vein M1 meeting vein R4+5 with an acute angle; hairs on scutum and tergites shorter and significantly adpressed; hind tibia on posterodorsal surface without long hairs.
Gonostylus, dorsolateral view (z indicates the dorsal margin of gonostylus): A Cheilosia barbafacies sp. n., Durmitor, Montenegro B Cheilosia pascuorum, Doroslovo, Serbia C Cheilosia pascuorum, Kopaonik, Serbia. Scale in mm.
Gonostylus (z indicates the dorsal margin of gonostylus). A–B Cheilosia barbafacies sp. n.: A left gonostylus, left lateral view B right gonostylus, right lateral view C–D Cheilosia pascuorum: C left gonostylus, left lateral view D right gonostylus, right lateral view. Scale in mm.
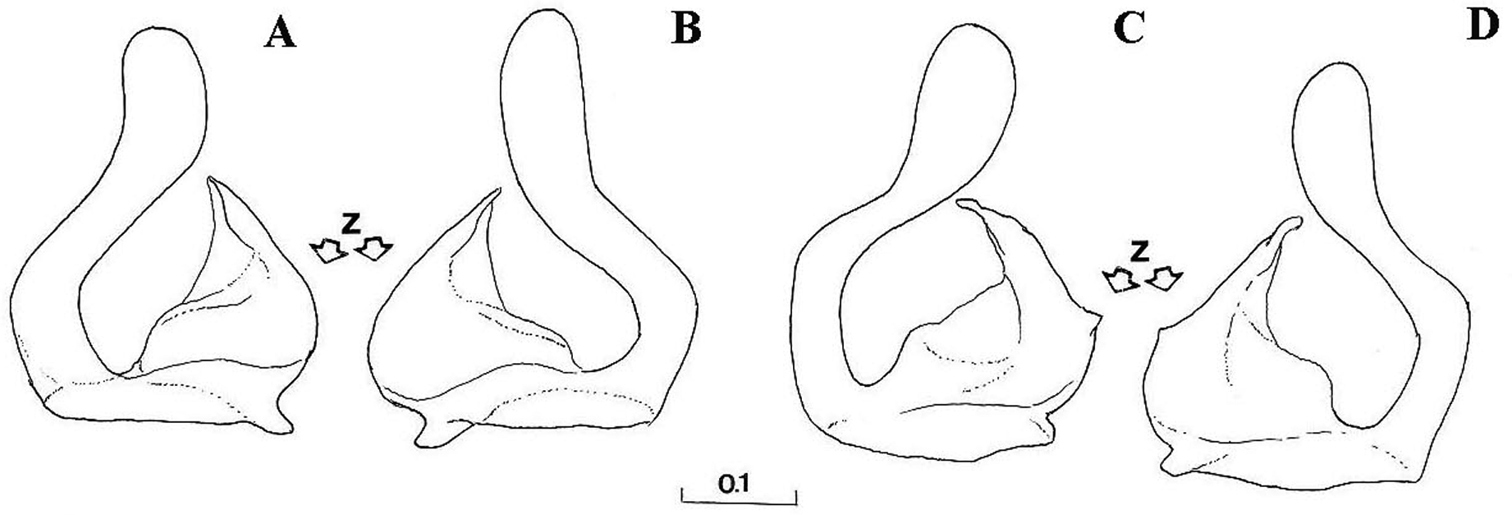
Right gonostylus, right lateral view: A Cheilosia barbata B Cheilosia ingerae C Cheilosia balkana D Cheilosia proxima E Cheilosia gigantea F Cheilosia velutina G Cheilosia rufimana. Scale in mm.
A–B Cheilosia barbafacies sp. n., theca of hypandrium, ventral view: A Montenegro, Durmitor B Bosnia-Herzegovina, Jahorina C–E right gonostylus, lateral view: C Cheilosia vulpina D Cheilosia lasiopa E Cheilosia melanopa. Scales in mm.
Cheilosia barbafacies sp. n., male, head, lateral view. Scale in mm.
Cheilosia barbafacies sp. n., head: A female, lateral view B female, dorsal view C male, dorsal view. Scale in mm.
Cheilosia barbafacies sp. n., female, tip of wing (x indicates the meeting point of vein M1 and vein R4+5). Scale in mm.
A–B Male genitalia, hypandrium, right lateral view (dl indicates the dorsal lobe of gonostylus): A Cheilosia barbafacies sp. n. B Cheilosia pascuorum. C–D Male genitalia, aedeagus and associated structures, right lateral view: C Cheilosia barbafacies sp. n. D Cheilosia pascuorum. Scales in mm.
Cheilosia barbafacies sp. n., mesonotum, lateral view: A male B female. Scale in mm.
Cheilosia barbafacies sp. n., hind tibia, posterodorsal view (y indicates the hairs on posterodorsal surface). Scale in mm.
Cheilosia barbafacies sp. n., antennae, lateral view: A male B female. Scale in mm.
A, D Male genitalia, epandrium, dorsal view: A Cheilosia barbafacies sp. n. D Cheilosia pascuorum B, C Surstylus, right lateral view B Cheilosia barbafacies sp. n. C Cheilosia pascuorum. Scale in mm.
Distribution of Cheilosia barbafacies (▲) and Cheilosia pascuorum (●).
| 1 | Face with long hairs | 2 |
| – | Face bare | 3 |
| 2 | Tergites pale haired (at least 1-3 in males); arista nearly bare. Male: central disc of scutum shining; dorsal lobe of gonostylus broad basally (Fig. 8A). Female: vein M1 meeting vein R4+5 at an obtuse angle (Fig. 7: x) | Cheilosia barbafacies sp. n. |
| – | At least tergite 3 in posterior half with black hairs in males, and in females tergites 2-4 with triangular area of adpressed black hairs; arista with short pubescence. Male: central disc of scutum dull; dorsal lobe of gonostylus of a different form (Fig. 4C). Female: vein M1 meeting vein R4+5 at right or acute angle | Cheilosia vulpina (Meigen, 1822) |
| 3 | Holoptic: males | 4 |
| – | Dichoptic: females | 10 |
| 4 | 3rd antennal segment orange to reddish-brown, at least basoventrally clear reddish | 5 |
| – | 3rd antennal segment black to blackish-brown (in some specimens paler, but not partly clear reddish) | 6 |
| 5 | Face in lateral view almost flat between central prominence and upper mouth edge; margin of upper calypter often partly with short black setulae; tergite 3 posteromedially with an area of black bristly-hairs, often also tergite 2 with such hairs near hind margin; genitalia (Fig. 3F) | Cheilosia velutina Loew, 1840 |
| – | Face in lateral view obviously concave between central prominence and upper mouth edge; margin of upper calypter with pale setulae; tergites 2 and 3 generally with pale (reddish) hairs, but single, short, black-bristly hairs maybe present posteromedially on tergite 3; genitalia (Fig. 3G) | Cheilosia rufimana Becker, 1894 |
| 6 | Margin of upper calypter with short black or dark brown setulae; frons slightly swollen; gonostylus in Fig. 3B | Cheilosia ingerae Nielsen & Claussen, 2001 |
| – | Margin of upper calypter with short pale setulae; frons not swollen | 7 |
| 7 | Abdomen (including pregenital segments) pale haired | 8 |
| – | Abdomen partly black haired, at least pregenital segments with few black hairs | 9 |
| 8 | Tergite 3 shiny (sometimes dull on anterior margin); vein M1 meeting vein R4+5 at an acute angle; arista with short pubescence; dorsal lobe of gonostylus basally narrowed (Fig. 3C) | Cheilosia balkana Vujić, 1994 |
| – | Tergite 3 dull centrally; vein M1 meeting vein R4+5 at right or obtuse angle; arista bare; dorsal lobe of gonostylus very broad basally (Fig. 8B) | Cheilosia pascuorum(Becker, 1894) |
| 9 | Basal 2/3 of hind femur with the anterodorsal hair fringe longer than the anteroventral hair fringe; genitalia with the dorsal lobe of gonostylus with a more or less distinct hook on its dorsal margin (Fig. 3D) | Cheilosia proxima (Zetterstedt, 1843) |
| – | Basal 2/3 of hind femur with the anterodorsal hair fringe as long as or shorter than the anteroventral hair fringe; genitalia with the dorsal lobe of gonostylus simple (Fig. 3E) | Cheilosia gigantea (Zetterstedt, 1838) |
| 10 | 3rd antennal segment orange to reddish-brown, at least basoventrally clear reddish | 11 |
| – | 3rd antennal segment black to blackish-brown (in some specimens paler, but not partly clear reddish) | 12 |
| 11 | Face in lateral view almost flat between central prominence and upper mouth edge; in dorsal view central prominence of face occupying the whole width of face; occiput behind the upper corners of the eyes shining; lunula generally dark or brownish; scutum coarsely punctured, partly wrinkled with short, inclined, pale hairs | Cheilosia velutina Loew, 1840 |
| – | Face in lateral view usually concave between central prominence and upper mouth edge (but not distinctly so in all specimens); in dorsal view central prominence of the face not occupying the whole width of the face; occiput behind the upper corners of the eye often completely grey dusted; lunula generally yellowish; scutum with fine punctures, at least anterior half with erect or semi-erect, predominately pale, short hairs, not longer than diameter of hind tibiae, with some longer hairs often intermixed laterally and in front of scutellum | Cheilosia rufimana Becker, 1894 |
| 12 | Frons relatively broad (ratio between length and width 1.2-1.4, average 1.3); pleura: posterior anepisternum predominately shining, at most anterior third of the sclerite thinly dusted; barrette (upper edge of meropleuron) more or less shining, contrasting with the dusting of the adjacent sclerites; basal 2/3 of hind femora with anteroventral hair fringe as long as diameter of hind femur | Cheilosia ingerae Nielsen & Claussen, 2001 |
| – | Frons relatively narrow (ratio between length and width 1.4-1.7, average 1.6); generally more than anterior third of posterior anepisternum grey dusted, often sclerite completely dusted; barrette dusted; basal 2/3 of hind femur with or without of anteroventral hair fringe | 9 |
| 13 | Vein M1 meeting vein R4+5 at an obtuse angle (as in Fig 7); arista bare (as in Fig. 4B); tergites predominately pale haired | Cheilosia pascuorum (Becker, 1894) |
| – | Vein M1 meeting vein R4+5 at an acute or right angle; arista pubescent; tergites partly black haired | 14 |
| 14 | Legs black, exceptionally knees paler | Cheilosia balkana Vujić, 1994 |
| – | On legs at least front and mid tibiae pale on both ends | 15 |
| 15 | Basal 2/3 of hind femur with the anteroventral hair fringe long, often obviously longer than diameter of hind femur; apex of hind femur ventrally with some black bristles or spines | Cheilosia gigantea (Zetterstedt, 1838) |
| – | Hind femur without anteroventral hair fringe, occasionally with single longer hairs anteroventrally which are shorter than, or rarely as long as, the diameter of the hind femur; apex of the hind femur ventrally most often without black bristles or spines… | Cheilosia proxima (Zetterstedt, 1843) |
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (projects No. 173002 and No. 43002) and the Provincial Secretariat for Science and Technological Development of the Republic of Serbia (Genetic resources of agroecosystems in Vojvodina and sustainable agriculture). We also thank Edward Petri and Mike Taylor for kindly improving the English language of this text.