(C) 2013 Molly M. McDonough. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: McDonough MM, Sotero-Caio CG, Ferguson AW, Lewis PJ, Tswiio M,Thies ML (2013) Mitochondrial DNA and karyotypic data confirm the presence of Mus indutus and Mus minutoides (Mammalia, Rodentia, Muridae, Nannomys) in Botswana. ZooKeys 359: 35–51. doi: 10.3897/zookeys.359.6247
We use a combination of cytochrome b sequence data and karyological evidence to confirm the presence of Mus indutus and Mus minutoides in Botswana. Our data include sampling from five localities from across the country, including one site in northwestern Botswana where both species were captured in syntopy. Additionally, we find evidence for two mitochondrial lineages of M. minutoides in northwestern Botswana that differ by 5% in sequence variation. Also, we report that M. minutoides in Botswana have the 2n=34 karyotype with the presence of a (X.1) sex-autosome translocation.
Africa, rodent, distribution, karyotype, sex-autosome translocation, cytochrome b
Delineating geographic distributions of African Mus (subgenus Nannomys Peters, 1876) in Sub-Saharan Africa has been especially challenging due to a combination of incomplete taxon sampling throughout the region as well as uncertainties in species identification resulting from their highly conserved morphology. Despite morphological similarities, African pygmy mice (Nannomys) are characterized by a high degree of chromosomal variation, including chromosomal rearrangements such as Robertsonian translocations, pericentric inversions, heterochromatin additions, and tandem fusions (see summary in
Within the southern African country of Botswana, the taxonomy of Mus has never fully been resolved. Early assessments of the regional mammalian fauna (
Distributions for three species of Nannomys in southern Africa. Dark grey indicates distribution for Mus minutoides, light grey for Mus indutus, and stippled pattern for Mus setzeri, adapted from
Regarding chromosomal rearrangement in southern Africa, Mus minutoides from South Africa exhibit Robertsonian fusions with two major monophyletic groups showing either a diploid number of 2n=18 – where all of the acrocentric chromosomes are fused to produce metacentric elements, or a 2n=34 – where sex-chromosome translocations have been reported (
Our objective was to utilize material from recent collecting efforts and molecular techniques to accurately delimit which species of Nannomys occur within Botswana. Further, we describe karyotypes for individuals from this region and make comparisons with previously published data from South Africa.
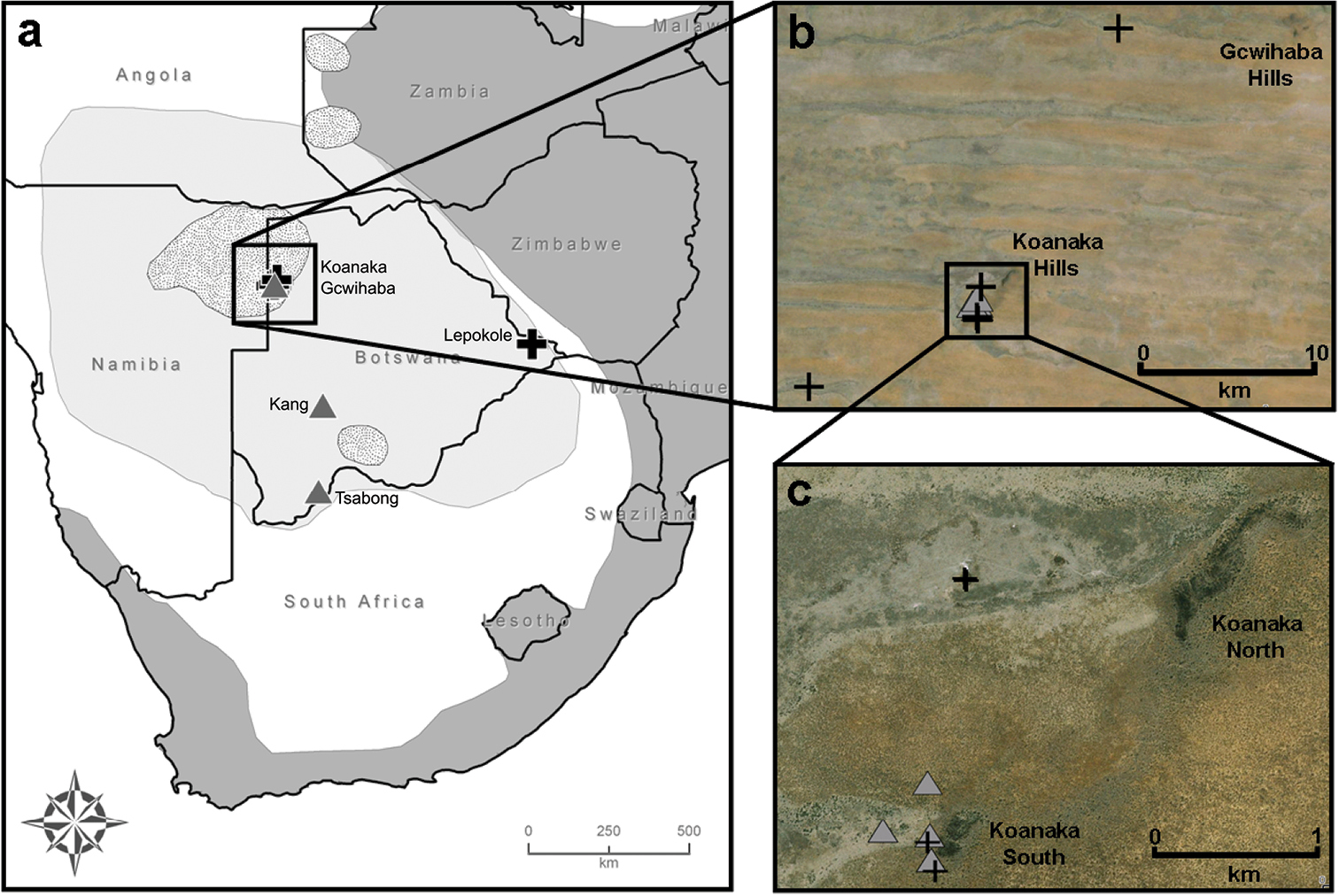
Our mitochondrial phylogeny was generated from combining previously published sequences deposited on GenBank (Appendix) with those derived from sequencing new specimens collected during field trips to Botswana conducted in 2008, 2009, and 2011 (Table 1, Appendix). We collected 16 specimens of Mus from five localities in Botswana including: Gcwihaba Caves (20°00.99'S, 21°15.89'E); Kang (23°32.10'S, 22°32.76'E); Koanaka Hills (20°09.60'S, 21°11.61'E); Lepokole Hills (21°49.59'S, 28°23.94'E); and Tsabong (25°56.57'S, 22°25.44'E) (Fig. 1a–c). Specimens were collected using Sherman live traps, pitfall traps, or Museum Special snap traps. Standard external measurements were recorded in the field (Table 1). Specimens were preserved as skins with complete skeletons (SSPS), skulls only, or as whole bodies in alcohol (alc.) and deposited at the at the Natural Science Research Laboratory (NSRL) at the Museum of Texas Tech University, Lubbock, Texas, USA or the Botswana National Museum, Gaborone, Botswana. Tissue samples were preserved in 95% ethanol, lysis buffer, or flash frozen in liquid nitrogen for future genomic analyses (2011 material) and deposited in the NSRL. Field collecting methods followed taxon specific guidelines for wild mammals (
Locality information for 16 specimens of Mus (Nannomys) collected in Botswana during June 2008, July 2009, and August 2011. Verbatim coordinates were recorded in the field using a handheld Garmin GPS Rino 120 unit using the datum WGS84. Elevations given in meters.
| Tissue No. | Genbank No. | Species | District | Specific Locality | Verbatim Coordinates | Verbatim Coordinate System | Verbatim SRS | Verbatim Elevation | Latitude, Longitude | Elev. | Coordinate Uncertainty |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TK170604 | KF184321 | Mus indutus | Kgalagadi | Berry Bush Farm, 8 km N, 2 km E Tsabong (Tshabong) | -25.94283, 22.42405 | Decimal degrees | WGS84 | 971 | 25°56.57'S, 22°25.44'E | 970 | 31.5 m |
| TK172845 | KF184320 | Mus indutus | Kgalagadi | Berry Bush Farms, 8 km N, 2 km E Tsabong (Tshabong) | -25.94283, 22.42405 | Decimal degrees | WGS84 | 971 | 25°56.57'S, 22°25.44'E | 970 | 31.5 m |
| TK172826 | KF184322 | Mus indutus | Kgalagadi | Kalahari Rest, 16 km N, 25 km W Kang | -23.53498, 22.54607 | Decimal degrees | WGS84 | 1158 | 23°32.10'S, 22°32.76'E | 1160 | 31.5 m |
| TK172785 | KF184310 | Mus minutoides | Central | Lepokole Hills, 3.6 km S, 4.9 km E Lepokole Village | -21.82653, 28.39898 | Decimal degrees | WGS84 | 784 | 21°49.59'S, 28°23.94'E | 780 | 31.5 m |
| TK164851 | KF184309 | Mus minutoides | Ngamiland | Koanaka Hills (Ncqumtsa Hills), 150 km W Tsao (Tsau), water hole | 34K 0511309 7767149 | UTM | WGS84 | 1019 | 20°11.58'S, 21°06.49'E | 1020 | 31.5 m |
| TK154612 | KF184311 | Mus minutoides | Ngamiland | Koanaka Hills (Ncqumtsa Hills), 150 km W Tsao (Tsau), main camp | 34K 0520241 7770802 | UTM | WGS84 | 1024 | 20°09.60'S, 21°11.62'E | 1020 | 31.5 m |
| TK164817 | KF184316 | Mus indutus | Ngamiland | " | 34K 0520219 7770803 | UTM | WGS84 | 1021 | 20°09.60'S, 21°11.61'E | 1020 | 31.5 m |
| TK164820 | KF184318 | Mus indutus | Ngamiland | " | 34K 0520219 7770803 | UTM | WGS84 | 1021 | 20°09.60'S, 21°11.61'E | 1020 | 31.5 m |
| TK164753 | KF184319 | Mus indutus | Ngamiland | " | 34K 0520210 7770958 | UTM | WGS84 | 1020 | 20°09.51'S, 21°11.60'E | 1020 | 31.5 m |
| TK164752 | KF184312 | Mus minutoides | Ngamiland | " | 34K 0520198 7770976 | UTM | WGS84 | 1020 | 20°09.51'S, 21°11.60'E | 1020 | 31.5 m |
| TK164751 | KF184315 | Mus indutus | Ngamiland | " | 34K 0519948 7770988 | UTM | WGS84 | 1022 | 20°09.50'S, 21°11.45'E | 1020 | 31.5 m |
| TK164757 | KF184323 | Mus indutus | Ngamiland | " | 34K 0519948 7770988 | UTM | WGS84 | 1022 | 20°09.50'S, 21°11.45'E | 1020 | 31.5 m |
| TK164939 | KF184317 | Mus indutus | Ngamiland | " | 34K 0520201 7771287 | UTM | WGS84 | 1027 | 20°09.34'S, 21°11.60'E | 1030 | 31.5 m |
| TK164768 | KF184313 | Mus minutoides | Ngamiland | " | 34K 0520416 7772600 | UTM | WGS84 | 1026 | 20°08.62'S, 21°11.72'E | 1030 | 31.5 m |
| TK164769 | KF184308 | Mus minutoides | Ngamiland | " | 34K 0520408 7772612 | UTM | WGS84 | 1020 | 20°08.62'S, 21°11.72'E | 1020 | 31.5 m |
| TK164967 | KF184314 | Mus minutoides | Ngamiland | Gcwihaba Caves, 18.8 km N, 114.2 km W Tsao (Tsau) | 34K 0527701 7786660 | UTM | WGS84 | 986 | 20°00.99'S, 21°15.89'E | 990 | 31.5 m |
Genomic DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen Inc., Chatsworth, California). The complete cytochrome b gene (cytb, 1140 nucleotides) was amplified following methods outlined in
Appropriate models of evolution were examined using MEGA version 5 (
Specimens were karyotyped in the field using bone marrow after 1 h of in vivo incubation with Velban (Sigma-Aldrich, St. Louis, Missouri), following the methods described in
In order to assess the nature of the X-autosome translocation of the specimens that exhibited the translocation, we compared the X-chromosome of our specimens with those from South Africa using images of inverted DAPI-banding, and G-banding (
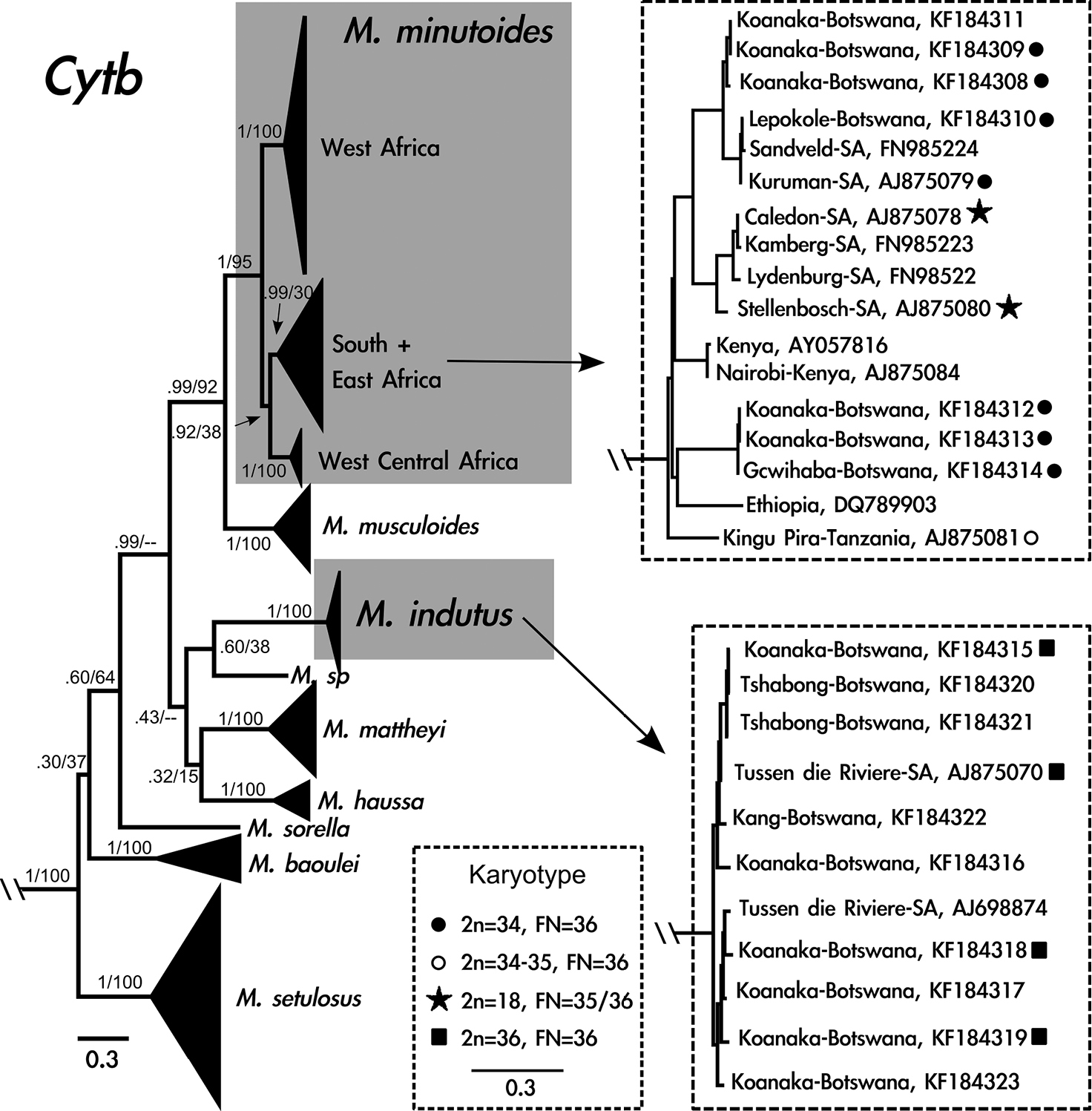
The model with the lowest AICc (Akaike Information Criterion, corrected) and BIC (Bayesian Information Criterion) scores was the General Time Reversible (GTR) model using a discrete gamma distribution (+G) and a fraction of invariable sites (+I). Overall, the two methods of phylogenetic analysis resulted in similar tree topologies, except that the Maximum-likelihood analysis recovered weak support for the south + east Mus minutoides clade (Fig. 2). Additionally, the relationship between Mus indutus, Mus sp., Mus mattheyi, Mus haussa, and the portion of the phylogeny that includes Mus minutoides and Mus musculoides was unresolved in the Maximum-likelihood analysis, though it was well-supported using Bayesian inference.
Cytochrome b gene tree generated from 741 base pairs including 125 taxa using Bayesian inference. Grey boxes indicate species of interest: Mus minutoides and Mus indutus. Clades that include Mus from Botswana are enlarged to the right of the phylogeny. Diploid and fundamental numbers are shown for individuals sampled in this study and
Sixteen cytb sequences were generated from specimens from Botswana, corresponding to two species. Seven individuals are phylogenetically related to Mus minutoides from South Africa and nine individuals cluster with Mus indutus. Five individuals, captured from the same locality in the Koanaka Hills region of northwestern Botswana, represent two clades within Mus minutoides that are 5% different in cytb sequence variation (Fig. 2). Six of the individuals of Mus indutus were collected in the Koanaka Hills alongside both of these lineages of Mus minutoides (Fig. 1c).
Karyotypes for individuals in the Mus minutoides clade exhibited a diploid number of 34 and fundamental number (as defined by
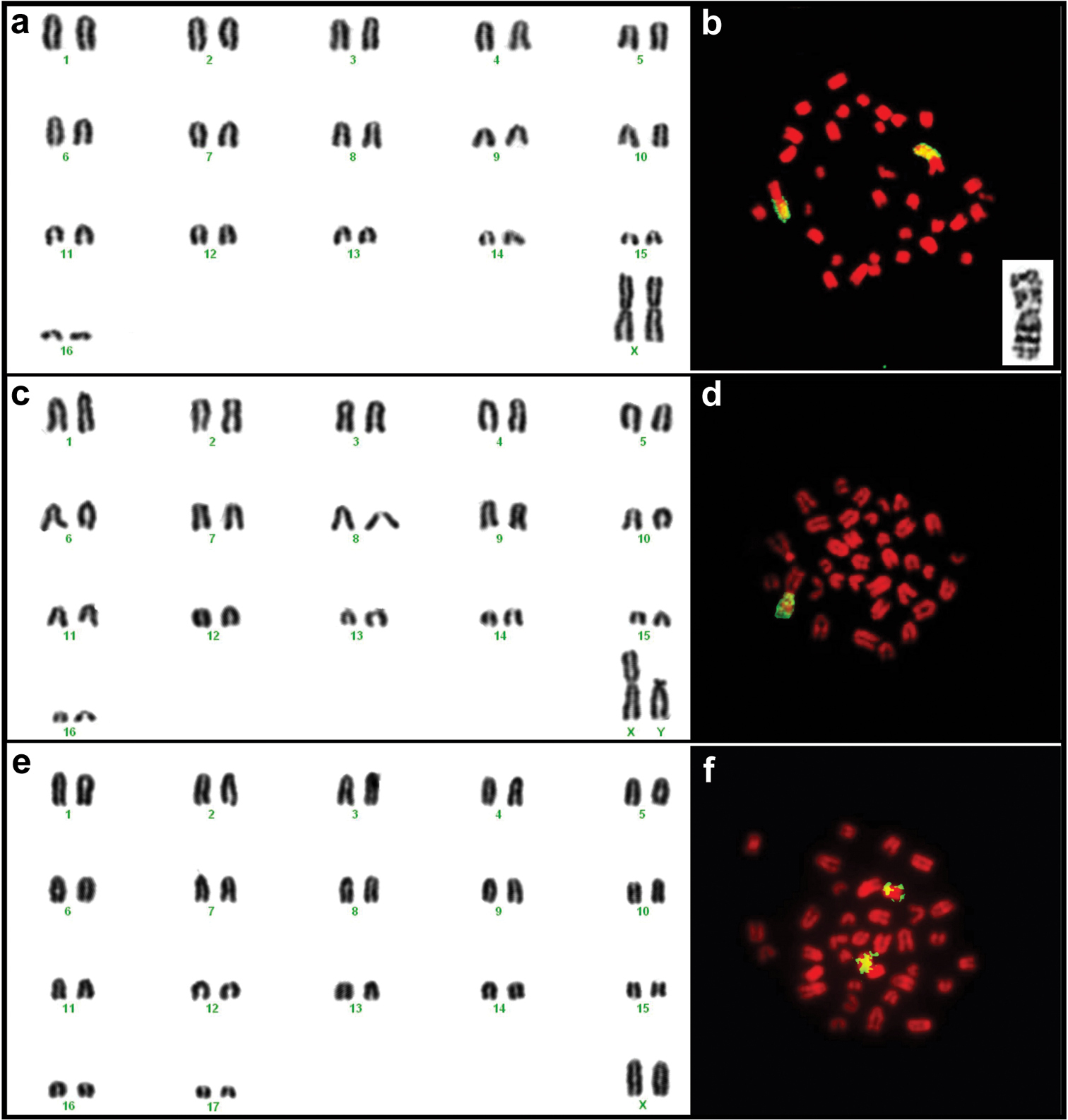
Karyotypes of female TK164752 (a) and male TK164768 (c) Mus minutoides and female TK164753 Mus indutus (e) from Botswana. The chromosome arms identified in yellow on the images to the right of each karyogram correspond to regions of homology to the X chromosome of Mus musculus detected by FISH for female TK164752 (b) and male TK164768 (d) Mus minutoides and female TK164820 Mus indutus (f). Note that in Mus minutoides, a single chromosome arm shows homology to the X chromosome of the house mouse, indicating the presence of an X-autosome translocation, whereas a whole acrocentric chromosome corresponds to the X of Mus indutus. The insert on (b) represents the (1.X) translocation of individual TK164752 Mus minutoides, with the long arm corresponding to the X chromosome.
Individuals of Mus (Nannomys) collected in Botswana including GenBank number, final species identification, gender determined in the field, museum preparation type (Alcoholic=alc; skin, skull, postcranial skeleton=SSPS; or Skull only), collection date, total length (TL), tail length (T), hind foot (HF), ear (E), weight in grams, karyotype, and sex-chromosome.
| Genbank No. | Species | Gender “Field” | Prep. Type | Coll. Date | TL | T | HF | E | Weight (g) | Karyotype | Gender “Lab” |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KF184315 | Mus indutus | Female | SSPS | 16-Jul-09 | 95 | 42 | 13 | 13 | 4, 5 | 2n=36, FN=36 | XX |
| KF184316 | Mus indutus | Female | SSPS | 22-Jul-09 | 85 | 40 | 10 | 10 | 2, 9 | none | - |
| KF184317 | Mus indutus | Female | SSPS | 27-Jul-09 | 101 | 43 | 14 | 11 | 5, 1 | none | - |
| KF184318 | Mus indutus | Female | SSPS | 22-Jul-09 | 14 | 45 | 12 | 10 | 6, 3 | 2n=36, FN=36 | XX |
| KF184319 | Mus indutus | Female | SSPS | 15-Jul-09 | 110 | 45 | 13 | 11 | 6, 75 | 2n=36, FN=36 | XX |
| KF184320 | Mus indutus | Male | SSPS | 18-Aug-11 | 98 | 45 | 15 | 10 | 4 | none | - |
| KF184321 | Mus indutus | Female | Alc | 25-Aug-11 | 75 | [23] | 14 | 10 | 3 | none | - |
| KF184322 | Mus indutus | Female | Skull Only | 17-Aug-11 | 109 | 40 | 15 | 11 | 5 | none | - |
| KF184323 | Mus indutus | Male | SSPS | 20-Jul-09 | 86 | 42 | 14 | 12 | 4 | none | - |
| KF184308 | Mus minutoides | Female | SSPS | 20-Jul-09 | 107 | 43 | 14 | 12 | 5, 5 | 2n=34, FN=36 | XX |
| KF184309 | Mus minutoides | Male | SSPS | 24-Jul-09 | [80] | [23] | 13 | 11 | 4, 6 | 2n=34, FN=36 | XY |
| KF184310 | Mus minutoides | Female | SSPS | 16-Aug-11 | 93 | 45 | 13 | 10 | 3, 5 | 2n=34, FN=36 | XY |
| KF184311 | Mus minutoides | Male | SSPS | 26-Jun-08 | 102 | 47 | 14 | 12 | 5, 8 | none | - |
| KF184312 | Mus minutoides | Female | SSPS | 15-Jul-09 | 111 | 52 | 15 | 11 | 5, 5 | 2n=34, FN=36 | XX |
| KF184313 | Mus minutoides | Female | SSPS | 20-Jul-09 | 99 | 44 | 12 | 9 | 4 | 2n=34, FN=36 | XY |
| KF184314 | Mus minutoides | Female | SSPS | 26-Jul-09 | 96 | 47 | 14 | 10 | 3, 7 | 2n=34, FN=36 | XY |
Efforts to resolve the geographic distributions of African pygmy mice remain in a state of flux. Regional studies involving DNA sequence data and karyotypes, such as presented here, contribute to a broader understanding of this complex genus. Historical (see
Mitochondrial sequence and cytogenetic data confirm the presence of both Mus minutoides and Mus indutus in Botswana. These specimens represent the first DNA sequences for these two species in Botswana, which we also made available for use in a recent paper by
Also of interest is the fact that no Mus setzeri were collected from either the Koanaka Hills or Gcwihaba Caves although their current range – as delimited by
Mus minutoides in Botswana exhibit the 2n=34 karyotype with the diagnostic (X.1) and (Y.1) sex-autosome translocations that have also been documented in specimensfrom South Africa (
We found that three of our gender identifications made in the field (Table 2, “Gender Field”) did not match the identifications made from karyotype assessments (Table 2, “Gender Lab”) indicating the potential for X*Y females. Therefore, we attempted to examine these specimens for the possibility of sex reversal in Mus minutoides, which has been documented in other countries (
Our data presented here agree with previous molecular phylogenies of Nannomys, with well-defined clades representing Mus minutoides and Mus indutus exhibiting diploid and fundamental numbers consistent with those reported in the literature.
We thank R. Baker for providing travel funds, laboratory funds, and facilities to conduct the molecular research. For access to specimens and assistance during museum visits, we thank K. Helgen and D. Lunde of the National Museum of Natural History, Smithsonian Institution and for loan of tissues H. Garner and K. MacDonald of the NSRL, Museum of Texas Tech University Museum. For assisting with trapping locations, we thank J. and D. Thomas of Berry Bush Farms, B. and M. Sieberhagen of Kalahari Rest, and the village development committee chairperson at Lepokole Village. We also thank the Ministry of Youth, Sport, and Culture for assistance with 2008-2009 permits [permit number: CYSC 1/17/21(81)]. For assistance with permits in 2011 we thank Tuelo Nkwane with the Botswana Ministry of Environment, Wildlife, and Tourism [permit number: EWT 8/36/4 XVI (46)]. Specimens were collected under SHSU IACUC issued to MLT (permit number: 08-04-03-1005-3-01). For assistance with field support and logistics during the 2008 and 2009 trips we thank M. Gabadirwe, J. Marenga, B. Mokotedi and for 2011 we thank the Botswana National Museum, including K. Lemson and Jaime Castro Navarro of the Universidad Nacional Autónoma de México. For assistance with logistics, field support, and field supplies for all years we thank J. Marais. We would especially like to thank Mr. N. E. Mosesane of the Botswana National Museum for his support of this work. A RAPID grant award 0910365 from the United States National Science Foundation as well as Faculty Research Grants and Exploratory Grants for Research from Sam Houston State University to MLT and PJL were used to fund 2008–2009 expeditions. An Explorers Club-Exploration Fund Research Grant and Texas Academy of Science Research Grant to MMM provided partial funding for 2011 fieldwork.
Individuals included in the molecular phylogeny representing eleven species with the country of origin, GenBank number and the original citation for the original description. RCA = Central African Republic.
| Mus (Nannomys) | Country | Genbank No. | Reference |
|---|---|---|---|
| Mus baoulei | Benin | EU603991-92 | |
| Guinea | EU603995 | ||
| Ivory Coast | EU603993-94, 98 | ||
| Mus bufo | Burundi | DQ789905 | |
| Mus haussa | Chad | AJ875071 | |
| Mali | AJ698877 | ||
| Niger | AJ875072-73 | ||
| Senegal | AJ875074 | ||
| Mus indutus | Botswana | KF184315-23 | This paper |
| South Africa | AJ698874 | ||
| South Africa | AJ875070 | ||
| Mus mattheyi | Burkina Faso | AJ877114 | |
| Guinea | EU603970-73 | ||
| Mali | AJ698876 | ||
| Mali | AJ875066-67 | ||
| Senegal | AB125781 | ||
| Senegal | AJ875068 | ||
| Togo | AJ875069 | ||
| Mus minutoides | Botswana | KF184308-14 | This paper |
| Congo | DQ789929 | ||
| Gabon | DQ789911, 20, 26 | ||
| Guinea | AJ875076-77 | ||
| Guinea | EU603936-37, 60-61, 64-65 | ||
| Ivory Coast | EU603925-28, 30-33, 35, 45, 47, 49, 54-56, 58, 999, 001-02, 005 | ||
| Kenya | AJ875084 | ||
| Kenya | AY057816 | ||
| RCA | DQ789938-39 | ||
| South Africa | AJ875078-80 | ||
| South Africa | FN985222-24 | ||
| Tanzania | AJ875081 | ||
| Mus musculoides | Cameroon | HM635855-56 | |
| Guinea | EU603968-69 | ||
| Guinea | DQ789902 | ||
| Ivory Coast | EU603967 | ||
| Ivory Coast | DQ789901 | ||
| Mali | Z96069 | ||
| Mali | AJ698875 | ||
| Mali | AJ875075 | ||
| Mali | JX292892-93 | ||
| Mus setulosus | Cameroon | EU603989 | |
| Cameroon | DQ789900 | ||
| Gabon | AJ698873 | ||
| Guinea | AJ875083 | ||
| Guinea | EU603976, 78, 82-83, 86 | ||
| Ivory Coast | EU603974-75, 77, 79-81, 84-85, 88, 97 | ||
| Ivory Coast | GU830865, 67, 69 | ||
| RCA | AJ875082 | ||
| RCA | EU603990 | ||
| Mus sorella | RCA | DQ789904 | |
| Mus sp. | Chad | AJ875085 | |
| Mus tenellus | Ethiopia | DQ789903 |