(C) 2013 Jadranka Rota. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Rota J, Miller SE (2013) A new genus of metalmark moths (Lepidoptera, Choreutidae) with Afrotropical and Australasian distribution. ZooKeys 355: 29–47. doi: 10.3897/zookeys.355.6158
Niveas Rota, new genus, and its two new species, N. agassizi Rota, new species, and N. kone Rota, new species, are described and illustrated. Niveas is assigned to the subfamily Choreutinae based on morphological and molecular data. Niveas agassizi is currently known only from Kenya and only from female specimens. Niveas kone has been found on the Solomon Islands and in Papua New Guinea (PNG). In PNG, larvae of this species have been reared from several species of Ficus (Moraceae). The two species are superficially quite dissimilar from each other. However, they share features in wing pattern and venation, as well as female genitalia, and the molecular data strongly support the monophyly of Niveas.
Alpha taxonomy, DNA barcoding, Ficus spp. , Kenya, Niveas agassizi, Niveas kone, Papua New Guinea, Solomon Islands, phylogenetics
Choreutidae, commonly known as metalmark moths, are a family of micro-moths with a worldwide distribution. The family is most species-rich in the tropics, and, as is the case for numerous other small tropical invertebrates, much of its richness is still unknown to science (unpublished data). Currently, 406 species of choreutids are described (
Choreutids are medium-sized micro-moths with wingspans ranging from about one to two centimeters, often with bright colors and iridescent markings on their wings (
Through exactly such efforts over the past 20 years in Papua New Guinea (PNG), the Binatang Research Center (BRC), with a large international group of collaborators focusing on the ecology of herbivorous insects and their host plants (
Coincidentally, through separate collecting efforts by David Agassiz in Africa, a related species was discovered in Kenya. Herein these two species, as well as the genus to which they belong, are described and illustrated, and the phylogenetic position of the new genus within the family is discussed.
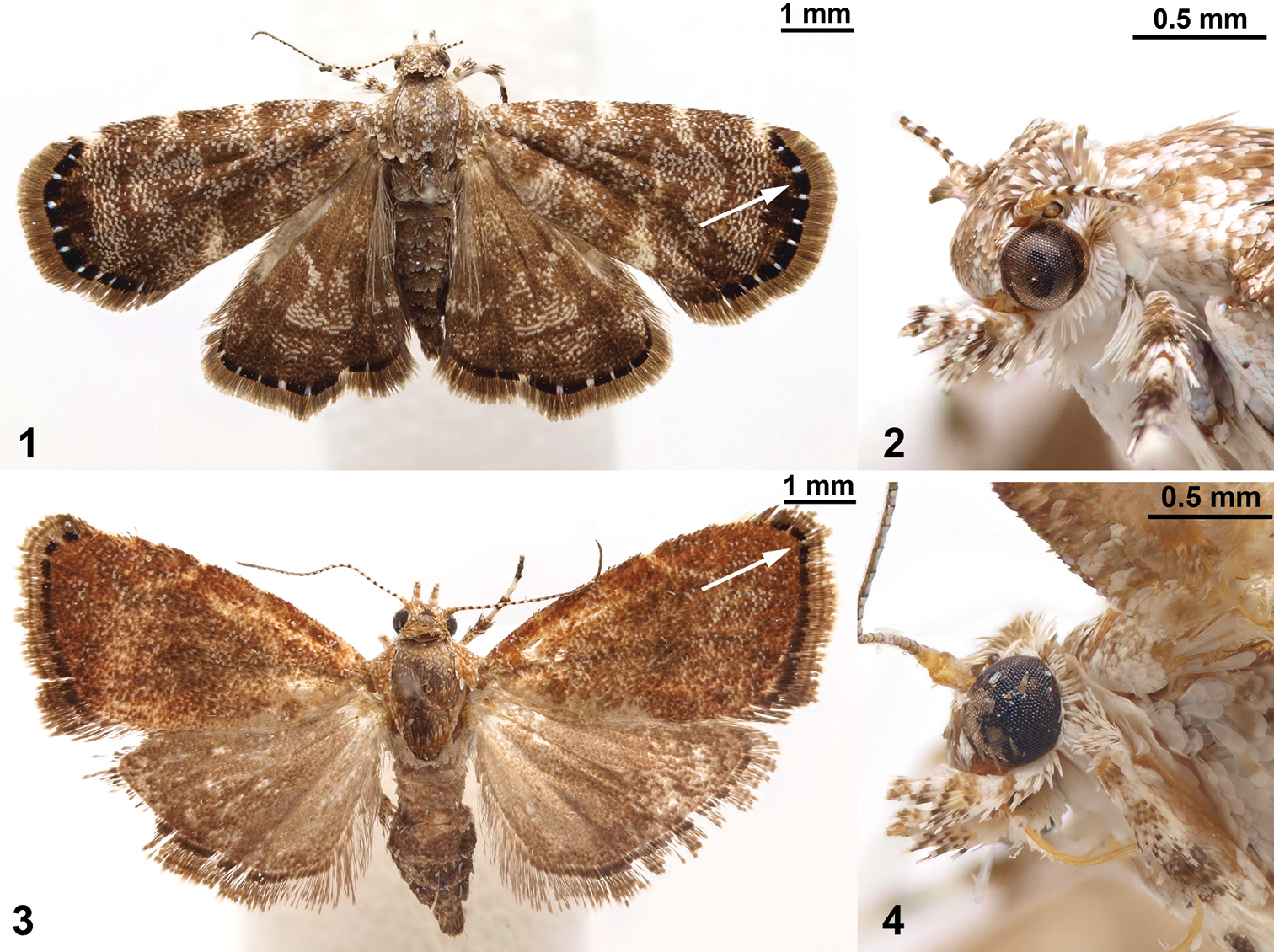
The shared presence of the terminal black band with white spots in the forewing (arrows in Figs 1, 3) was the first indication that Niveas kone Rota, sp. n. and Niveas agassizi Rota, sp. n. might be related. Initially this relationship seemed unlikely because of the disjunct geographical distribution of the two (Niveas kone being distributed in the Australasian Region and Niveas agassizi in the Afrotropical Region) and because their DNA barcodes did not suggest a close relationship. However, once the similarities in wing venation and female genitalia were noticed, and we included nuclear genes in the analysis with a more extensive choreutid molecular dataset, the results strongly supported the close relationship between Niveas kone and Niveas agassizi.
Niveas kone: 1 Habitus 2 Head. Niveas agassizi: 3 Habitus 4 Head. (In Figs 1 and 3 arrows point at the terminal black band enclosing white spots.)
All material examined is listed in Table 1. Layered photographs of specimens and slides were taken using an Olympus SZX16 microscope with motorized focus drive attached to an Olympus E520 digital camera. The photographs were then combined by using the programs Deep Focus 3.1 and Quick Photo Camera 2.3. The wing venation drawing was made digitally in Adobe Illustrator CS3 overlaid on top of a slide photograph. All images were improved in Adobe Photoshop CS3. Genitalic dissections and terminology follow
Material examined.
| Species | Type | Country | Province | Locality | Date | Collector | ID number | Host plant | Slide number | GenBank |
|---|---|---|---|---|---|---|---|---|---|---|
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 04/09/95 | BRC | USNM ENT 730507 | Ficus nodosa | ||
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 08/30/95 | BRC | USNM ENT 730558 | Ficus nodosa | ||
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 08/30/95 | BRC | USNM ENT 730572 | Ficus nodosa | HQ946542 | |
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 06/16/95 | BRC | USNM ENT 730508 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 03/19/96 | BRC | USNM ENT 730513 | Ficus variegata | HQ946551 | |
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 04/09/95 | BRC | USNM ENT 730529 | Ficus variegata | KF714836 | |
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 03/19/96 | BRC | USNM ENT 730543 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Baitabag Vill. | 03/19/96 | BRC | USNM ENT 730551 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Kamba (Mis) | 10/20/95 | BRC | USNM ENT 730576 | Ficus variegata | HQ946555 | |
| Niveas kone | Paratype | PNG | Madang | Malapau (Riwo) | 03/20/95 | BRC | USNM ENT 730498 | Ficus variegata | HQ946554 | |
| Niveas kone | Paratype | PNG | Madang | Malapau (Riwo) | 03/20/95 | BRC | USNM ENT 730519 | Ficus variegata | HQ946553 | |
| Niveas kone | Paratype | PNG | Madang | Malapau (Riwo) | 03/20/95 | BRC | USNM ENT 730535 | Ficus variegata | HQ946552 | |
| Niveas kone | Paratype | PNG | Madang | Mililat (Riwo) | 05/22/95 | BRC | USNM ENT 730604 | Ficus nodosa | HQ946544 | |
| Niveas kone | Paratype | PNG | Madang | Mis Vill. | 03/20/96 | BRC | USNM ENT 730528 | Ficus nodosa | HQ946543 | |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 04/09/95 | BRC | USNM ENT 730560 | Ficus botryocarpa | HQ946538 | |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/09/95 | BRC | USNM ENT 730602 | Ficus botryocarpa | HQ946539 | |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 12/01/96 | BRC | USNM ENT 730542 | Ficus phaeosyce | KF714835 | |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 12/02/94 | BRC | USNM ENT 730502 | Ficus pungens | HQ946546 | |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 12/09/95 | BRC | USNM ENT 730518 | Ficus variegata | female genitalia 92352 | HQ946549 |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 03/16/95 | BRC | USNM ENT 730509 | Ficus variegata | male genitalia 92355 | HQ946550 |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 03/16/95 | BRC | USNM ENT 730492 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 03/25/96 | BRC | USNM ENT 730493 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/09/95 | BRC | USNM ENT 730500 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 03/22/95 | BRC | USNM ENT 730504 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 12/13/94 | BRC | USNM ENT 730510 | Ficus variegata | ||
| Niveas kone | Holotype | PNG | Madang | Ohu Vill. | 03/13/95 | BRC | USNM ENT 730516 | Ficus variegata | HQ946548 | |
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 08/09/95 | BRC | USNM ENT 730517 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 03/16/95 | BRC | USNM ENT 730520 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/26/95 | BRC | USNM ENT 730521 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/09/95 | BRC | USNM ENT 730522 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 03/29/95 | BRC | USNM ENT 730523 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 06/16/95 | BRC | USNM ENT 730524 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/11/96 | BRC | USNM ENT 730525 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 06/27/95 | BRC | USNM ENT 730526 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 06/16/95 | BRC | USNM ENT 730531 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 12/13/94 | BRC | USNM ENT 730533 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/09/95 | BRC | USNM ENT 730553 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 12/09/95 | BRC | USNM ENT 730564 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/09/95 | BRC | USNM ENT 730588 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 05/09/95 | BRC | USNM ENT 730595 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Ohu Vill. | 09/10/95 | BRC | USNM ENT 730565 | Ficus wassa | HQ946545 | |
| Niveas kone | Paratype | PNG | Madang | Pau Vill. | 12/13/95 | BRC | USNM ENT 730515 | Ficus variegata | KF714837 | |
| Niveas kone | Paratype | PNG | Madang | Pau Vill. | 12/13/95 | BRC | USNM ENT 730547 | Ficus variegata | KF714833 | |
| Niveas kone | Paratype | PNG | Madang | Reinduk | 03/28/95 | BRC | USNM ENT 730527 | Ficus variegata | KF714834 | |
| Niveas kone | Paratype | PNG | Madang | Tab Is | 01/31/95 | BRC | USNM ENT 730506 | Ficus nodosa | ||
| Niveas kone | Paratype | PNG | Madang | Tab Is | 01/31/95 | BRC | USNM ENT 730514 | Ficus nodosa | KF714832 | |
| Niveas kone | Paratype | PNG | Madang | Tab Is | 01/31/95 | BRC | USNM ENT 730532 | Ficus nodosa | HQ946540 | |
| Niveas kone | Paratype | PNG | Madang | Tab Is | 01/31/95 | BRC | USNM ENT 730538 | Ficus nodosa | HQ946541 | |
| Niveas kone | Paratype | PNG | Madang | Wanang Vill. | 07/31/07 | BRC | USNM ENT 660733 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Wanang Vill. | 11/05/07 | BRC | USNM ENT 660794 | Ficus variegata | ||
| Niveas kone | Paratype | PNG | Madang | Wanang Vill. | 02/21/06 | BRC | USNM ENT 660722 | unknown | HQ946547 | |
| Niveas kone | Paratype | Solomon Is. | Guadalcanal | Roroni, 35 km E of Honiara; 10 m | 05/13/64 | R. Straatman | unassigned | unknown | wing 137601; female genitalia 137600 | |
| Niveas kone | Paratype | Solomon Is. | Guadalcanal | Roroni, 35 km E of Honiara; 10 m | 05/13/64 | R. Straatman | unassigned | unknown | ||
| Niveas kone | Paratype | Solomon Is. | Guadalcanal | Nini Ck., 35 km SE of Honiara | 08/05/64 | R. Straatman | unassigned | unknown | ||
| Niveas agassizi | Paratype | Kenya | County of Kwale | Mwabungu | 08/19/00 | David Agassiz | USNM ENT 730794 | unknown | HQ946715 | |
| Niveas agassizi | Holotype | Kenya | County of Kwale | Mwabungu | 08/19/00 | David Agassiz | USNM ENT 730793 | unknown | HQ946716 | |
| Niveas agassizi | Paratype | Kenya | County of Kwale | Mwabungu | 08/19/00 | David Agassiz | unassigned | unknown | female genitalia 137597 | |
| Niveas agassizi | Paratype | Kenya | County of Kwale | Mwabungu | 08/19/00 | David Agassiz | unassigned | unknown | female genitalia JR2013-02 | |
| Niveas agassizi | Paratype | Kenya | County of Kwale | Mwabungu | 08/19/00 | David Agassiz | unassigned | unknown | wing JR2013-03 | |
| Niveas agassizi | Paratype | Kenya | County of Kwale | Mwabungu | 08/20/00 | David Agassiz | unassigned | unknown | female genitalia JR2013-01 |
Field sampling and rearing protocols for the PNG material are detailed in
Locality information.
| Locality | m.a.s.l. | latitude, longitude |
|---|---|---|
| Baitabag village & Kau Wildlife Area, near Madang, Madang Province, PNG | 50 | 5°08'S, 145°46'E |
| Mis, Madang Province, PNG | 50 | 5°11'S, 145°47'E |
| Ohu Conservation Area, Ohu village near Gum river, Madang Province, PNG | 100 | 5°13'S, 145°41'E |
| Pau, Madang Province, PNG | 0 | 5°08'S, 145°46'E |
| Reinduk, Madang Province, PNG | 225 | 5°39'S, 145°24'E |
| Riwo, Madang Province, PNG | 0 | 5°09'S, 145°48'E |
| Tab Island, Madang Province, PNG | 0 | 5°10.6'S, 145°52.6'E |
| Wanang village, Madang Province, PNG | 115 | 5°13.9'S, 145°10.9'E |
| Mwabungu, County of Kwale, Kenya | 0 | 4°20.3'S, 39°37'E |
The molecular phylogeny dataset included three outgroups and 40 species of ingroup taxa, including two individuals each of Niveas kone and Niveas agassizi totaling 45 terminal units. We analyzed data from eight genes: COI (mitochondrial), CAD, EF1α, GAPDH, IDH, MDH, RpS5, and wingless (all nuclear) (
Primers.
| COI-1F | GGTCAACAAATCATAAAGATATTGG |
| COI-1R | GGwGCyCCTARtATtAaaGGWAYTA |
| EF-1F | CACATYAACATTGTCGTSATYGG |
| EF-1R | TrScgGTYTCGAAcTTCCA |
| EF-2F | GAgCGtGARCGTgGTAT |
| EF-2R | rGCtTCgAAcTCACCRGTA |
| EF-3F | TcAAgAACATGATcACyGG |
| EF-3R | GARGAyACTTCcTTcTTgA |
| EF-7F | CAAYGTtGGtTTCAACGT |
| EF-8R | ACAGCVACKGTYTGYCTCATRTC |
| GAPDH-1F | aargctggrgctgaatatgt |
| GAPDH-1R | AAGTTgTCaTGgATRACcTT |
| GAPDH-2F | gTcaTcTCyAAtGCyTCyTG |
| GAPDH-2R | TaACtTTgCCrACaGCYTT |
| GAPDH-3F | GtGCccarCARAACATcAT |
| GAPDH-3R | tcaGCgGCtTCCTTrACcT |
| IDH-1F | GGWGAYGARATGACNAGRATHATHTGG |
| IDH-1R | GGactcTTCCACATtTtYTT |
| MDH-1F | GAYATNGCNCCNATGATGGGNGT |
| MDH-1R | TCYTTrCGrGCaACYTTRTC |
| RpS5-1F | atggcngargaraaytggaayga |
| RpS5-1R | TTgTGwGCRTAcCtrCCrGC |
GenBank accession numbers and the number of base pairs for each gene fragment.
| Niveas agassizi (730793) | Niveas agassizi (Ch_JR44_1) | Niveas kone (730509) | Niveas kone (660733) | |
|---|---|---|---|---|
| COI | HQ946716 | - | HQ946550 | KF646130 |
| 609 bp | 176 bp | 658 bp | 610 bp | |
| EF1α | - | KF646128, KF646129 | - | KF646131, KF646132 |
| - | 550 bp | - | 706 bp | |
| GAPDH | - | - | - | KF646133 |
| - | 136 bp | - | 430 bp | |
| IDH | - | 135 bp | - | - |
| MDH | - | 190 bp | - | - |
| RpS5 | - | 155 bp | - | 108 bp |
Both maximum likelihood (ML) and Bayesian phylogenetic analyses were performed. ML analysis of unpartitioned data was conducted using RAxML blackbox available online (
DNA barcode sequences (COI) for Niveas kone (24 specimens) and Niveas agassizi (2 specimens) were obtained at the Biodiversity Institute of Ontario, University of Guelph, using their standard methodology (
http://zoobank.org/F352952E-0F21-464F-BD1E-278C9A0679C1
http://species-id.net/wiki/Niveas
Figs 1–9Niveas kone.
See Table 1.
Kenya, Papua New Guinea, Solomon Islands.
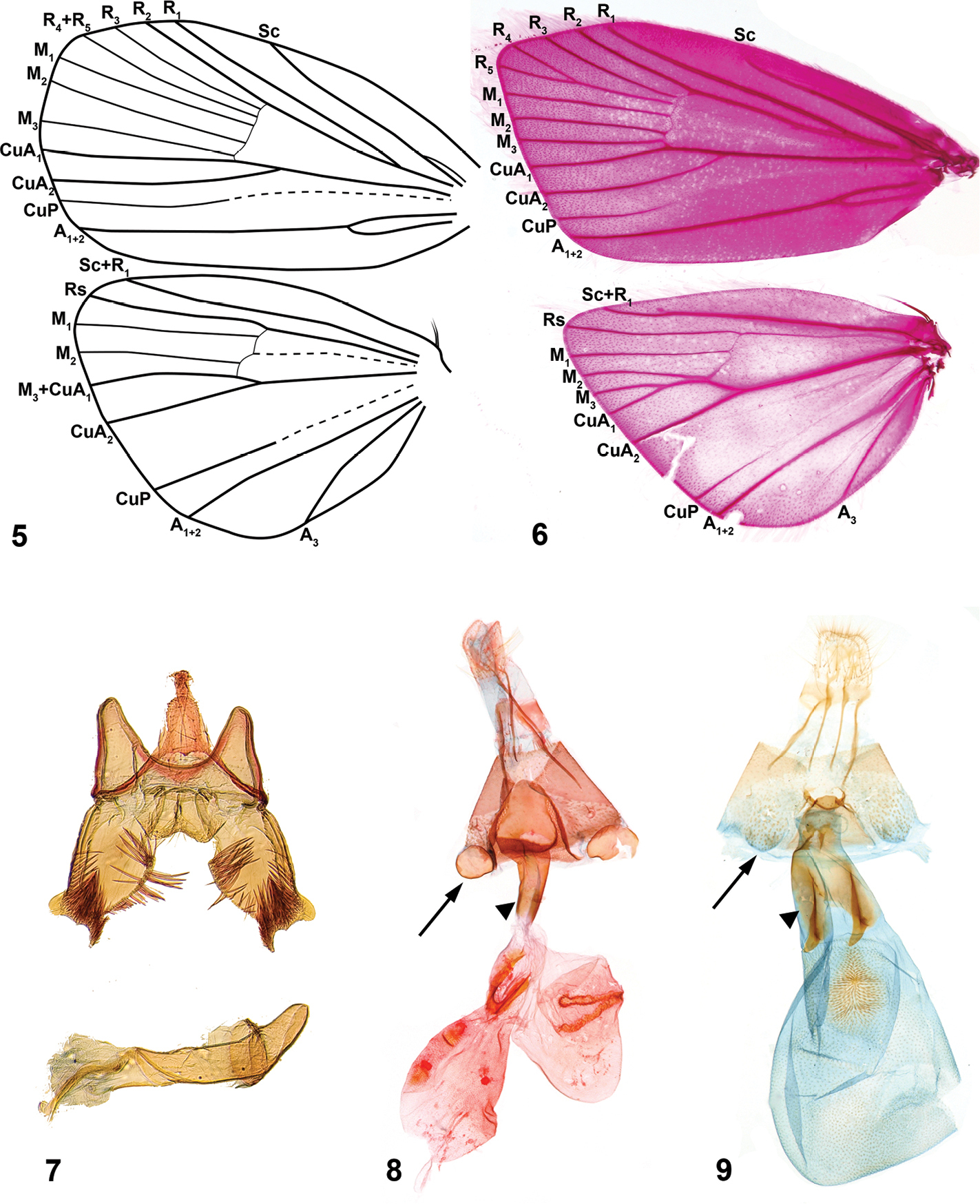
Niveas can be easily distinguished from most genera of choreutids by the wing pattern (Figs 1, 3). Superficially, species of Niveas are similar to some species of Anthophila and Choreutis, but there is no known species in either of the latter two genera with a black terminal band enclosing white spots in the forewing as in Niveas agassizi and Niveas kone. (Figs 1, 3). Forewing venation with only four radial branches or with R4 and R5 fused in the basal half is also diagnostic for the genus. Female genitalia with paired concave sclerotizations on A7 sternite are also unique to Niveas.
Head. Labial palpi with projecting ventral scale tufts (Figs 2, 4). Wings. Forewing veins R four-branched in Niveas kone (Fig. 5), five-branched in Niveas agassizi (Fig. 6), with R4 an R5 fused in basal 3/5; CuP present at termen for 1/3 to 1/5 wing length, extending as fold further towards base. Hindwing ten-veined, with M2 in close proximity to the basally fused M3 and CuA1 (Niveas agassizi) or nine-veined, apparently with M3 and CuA1 completely fused into a single vein (Figs 5, 6). Male genitalia. Tegumen rounded on top, tuba analis extending beyond tegumen; vinculum as inverted trapezoid ventrally emarginate; valva with costal margin straight, ventral margin rounded, ending with a horn-like projection; phallus twice as long as valva (Fig. 7). Female genitalia. Apophyses anteriores slightly longer than posteriores; ostium bursae on A7 with a more or less strongly sclerotized antrum; ductus bursae straight, not coiled, with strong lateral sclerotizations; corpus bursae as a single sac (Niveas agassizi) or divided into two sacs (Niveas kone) with one or more signa. A7 sternite with paired, somewhat rounded, concave sclerotizations proximally, clearly visible in Niveas kone (Fig. 8), and slightly less so in Niveas agassizi (Fig. 9).
Niveas kone: 5 Wing venation 7 Male genitalia 8 Female genitalia. Niveas agassizi: 6 Wing venation 9 Female genitalia. (In Figs 8 and 9 arrows point at the A7 sternite sclerotizations, and triangles point at the lateral sclerotizations on the ductus bursae.)
Genus Ficus (Moraceae).
The generic name is derived from Latin niveum, meaning snowy, in reference to speckles of white-tipped scales in the wings of the type species; it is not treated as a Latin word and is feminine in gender.
http://zoobank.org/9EA367B0-6B92-48FA-8075-D8D0D0BFA566
http://species-id.net/wiki/Niveas_kone
Figs 1, 2, 5, 7, 8See Table 1.
The holotype and most paratypes will be retained at USNM, with paratypes distributed to PNG National Agriculture Research Institute (Port Moresby), BMNH, Bishop Museum, Naturalis (Leiden), and CSIRO (Canberra).
Papua New Guinea, Solomon Islands.
Niveas kone can be separated from all other known choreutids based on its wing pattern (Fig. 1). Superficially, it is similar to a few species of Brenthia Clemens, 1860 and Litobrenthia Diakonoff, 1978 owing to its background color, but it lacks iridescent spots along forewing termen, which are always present in those two genera. Both male and female genitalia are very distinct from those of other choreutids (Figs 7, 8).
Head. Fig. 2. Wings. Fore- and hindwing with brown background color, speckled with white-tipped scales in an irregular pattern; a distinct black band along termen of both wings within which are more or less equidistant white spots (Fig. 1). Male genitalia. As for the genus (Fig. 7). Female genitalia. Corpus bursae split into two sacs; one sac with a V-shaped signum, the other with two round signa (Fig. 8). Immature stages. Fig. 12. See a brief note in text.
Ficus botryocarpa Miq., Ficus nodosa Teijsm. & Binn., Ficus phaeosyce K. Schum. & Lauterb., Ficus pungens Reinw. ex Blume, Ficus variegata Blume, and Ficus wassa Roxb. (Moraceae).
The species is named after the Finnish Kone Foundation (Koneen Säätiö) in appreciation of their funding of this work. The name is a noun in apposition.
http://zoobank.org/7F08322B-C0D2-450C-9DFF-ED9E4FEA5892
http://species-id.net/wiki/Niveas_agassizi
Figs 3, 4, 6, 9See Table 1.
The holotype will be deposited in National Museums of Kenya (Nairobi) (NMK), with paratypes to USNM, BMNH and NMK.
Kenya.
Niveas agassizi can be separated from other known choreutids by the wing pattern (Fig. 3). It is superficially similar to some species of Choreutis, but the latter usually have forewings with apparent patterning, and this is absent in Niveas agassizi. Female genitalia are very distinct from those of other choreutids (Fig. 9).
Male unknown. Head. Fig. 4. Wings. Forewing bronze-brown with speckled white-tipped scales over most of its surface; distinct dark brown to black band along termen with two small white spots at apex; hindwing light brown (Fig. 3). Male genitalia. Unknown. Female genitalia. Ductus bursae short and wide, opening into large corpus bursae, with one oval signum (Fig. 9). Immature stages. Unknown.
Unknown.
This species is named after David Agassiz, who collected all the known specimens and made many significant contributions to our knowledge of African micro-moths. The name is a noun in the genitive case.
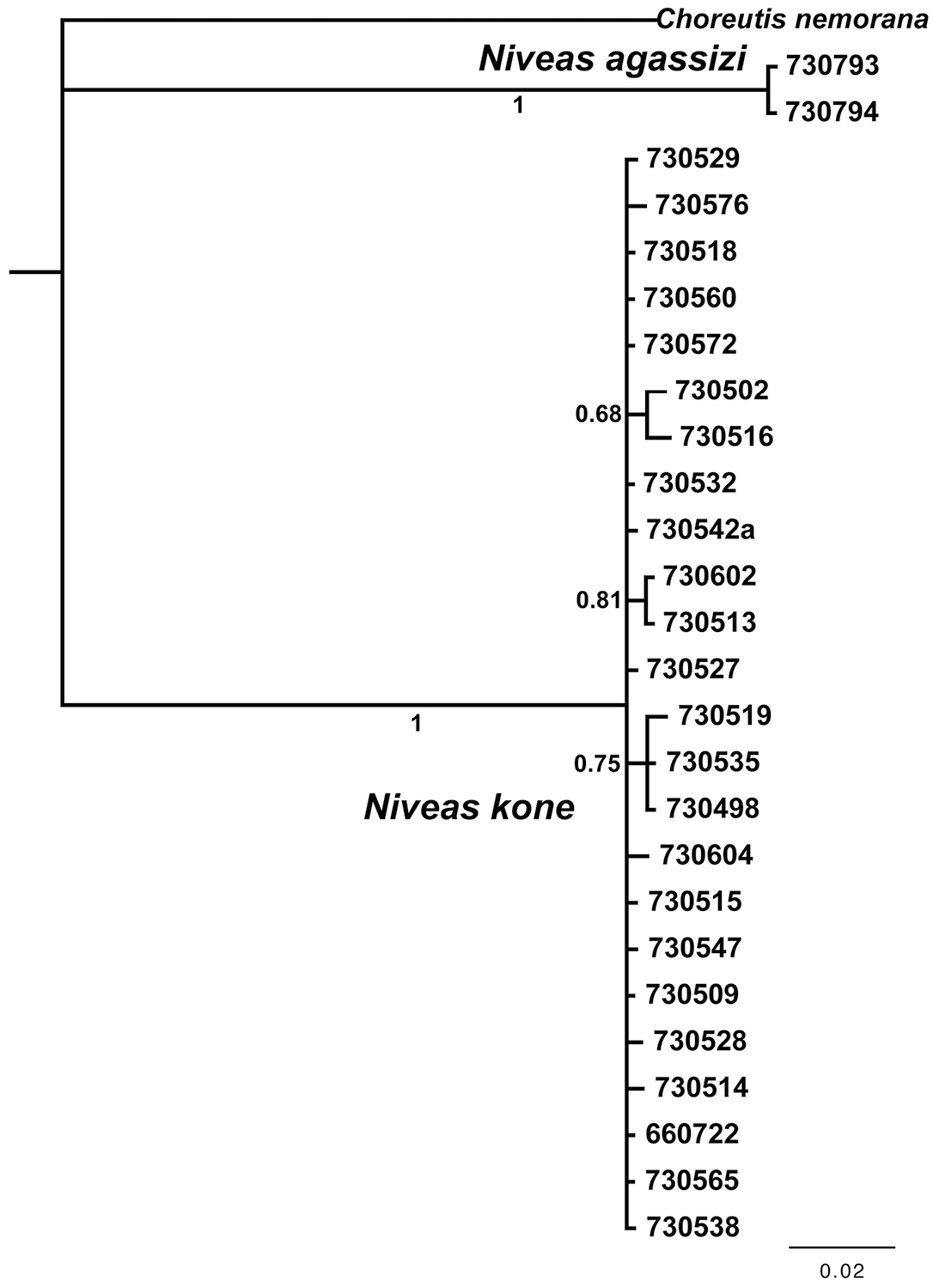
We obtained 19 full-length barcodes of Niveas kone, as well as 5 shorter fragments. These form cluster AAB7478 in the Barcode of Life Database (accessed 29 August 2013), and using the RESL algorithm as implemented there (
DNA barcode tree from a Bayesian analysis showing low divergence within species and high between species of Niveas. Numbers below or next to branches are Bayesian posterior probabilities. Specimen ID numbers are used as labels for the terminal branches.
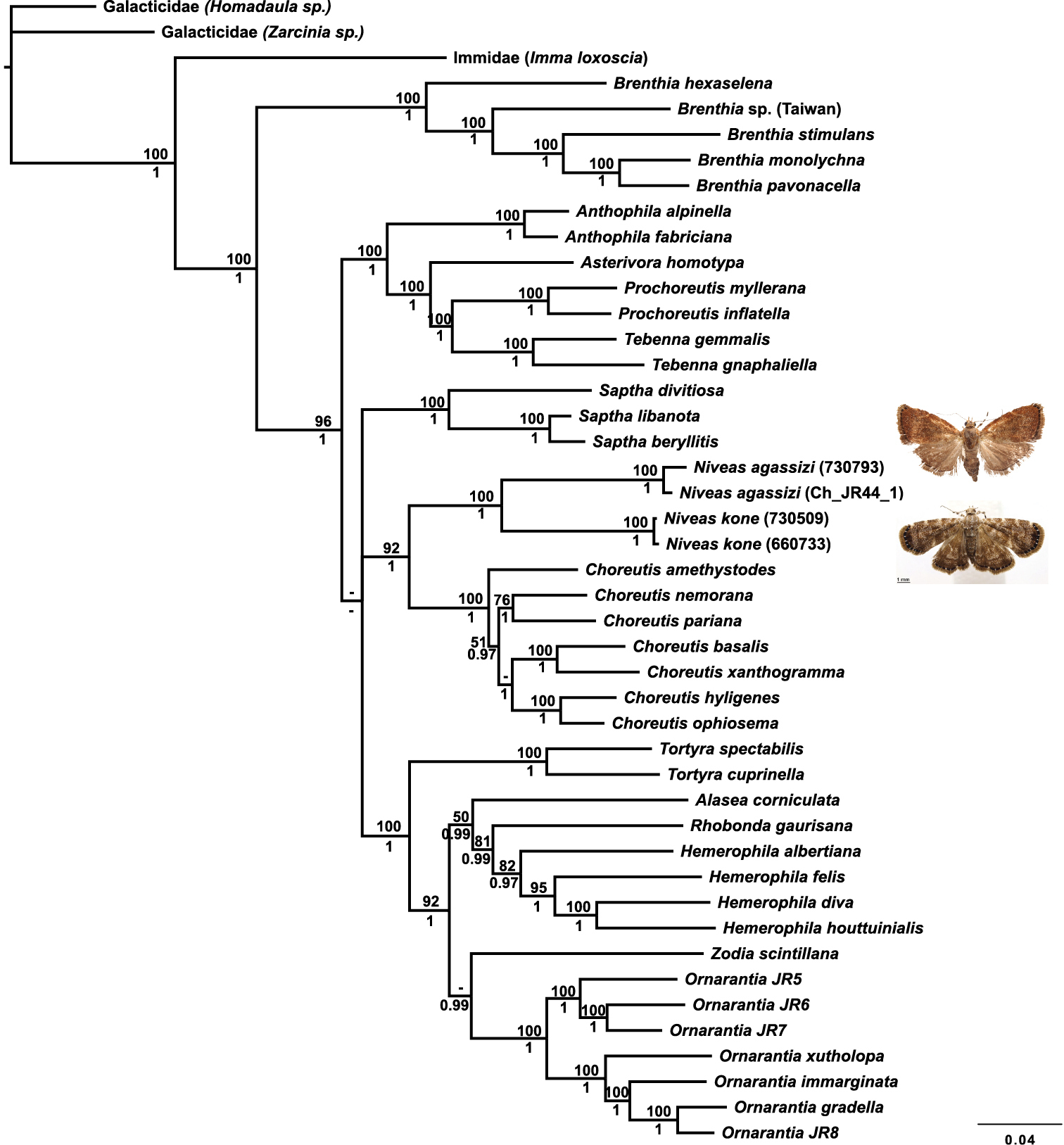
The placement of Niveas in the choreutid generic phylogeny is very strongly supported. Niveas clearly belongs within the subfamily Choreutinae (PP=1; ML BS=96), and it appears to be the sister group of Choreutis (PP=1.00; ML BS=92) (Fig. 11).
Phylogenetic tree from a Bayesian analysis showing the position of Niveas in relation to other choreutid genera. Maximum likelihood (ML) bootstraps are shown above branches, and Bayesian posterior probabilities (PP) are below branches; dashes represent ML bootstraps<50 and PP<0.95.
Further comments on the biology of Niveas kone: Over the years, BRC field teams have encountered larvae identified as Niveas kone (as project morphospecies TORT015) 118 times, of which 62 were reared to adults, usually on Ficus nodosa and Ficus variegata, but also on four other species of Ficus (see full host plant list under Niveas kone description). Larvae have been found in all months except April and November, and are described by BRC staff as being green-clear-whitish in color, with short white hairs, and one spot on the side of the head (Fig. 12). Larvae of Niveas kone share the presence of short hairs with other Choreutinae (
A photograph of the Niveas kone larvae made in the field.
Taxon descriptions are also organized in tabular format for ease of comparison (see Appendix).
The two species of Niveas described herein are superficially quite different, but upon closer examination it becomes apparent that they share a number of morphological features. We consider the following as potential autapomorphies of Niveas: fusion or reduction in R veins in the forewing (Figs 5, 6); presence of round, concave sclerotizations on the A7 sternite in females (arrows in Figs 8, 9); strong lateral sclerotizations at the base of the ductus bursae (triangles in Figs 8, 9); and the presence of a terminal black band with white spots in the forewing (arrows in Figs 1, 3). In all other Choreutinae genera there are five fully-separated radial veins in the forewing; the A7 sternite in the female, as well as the base of the ductus bursae, are evenly sclerotized; and if present, a black terminal band in the forewing lacks white spots.
The split between Niveas kone and Niveas agassizi has presumably happened a long time ago based on the large COI divergence between them and the length of branches in the phylogenetic analysis including the nuclear genes. We considered assigning each species to its own monotypic genus because of their different external appearance, as well as some of the differences in venation and some aspects of female genitalia. It is unfortunate that Niveas agassizi is known from females only as perhaps the morphology of the male genitalia would help clarify the status of this species. However, we believe that Niveas kone and Niveas agassizi being each other’s closest relatives among the currently known species of choreutids is best conveyed by assigning them to a single genus and therefore we opted for this more conservative approach. It is conceivable that other species of Niveas that might bridge this gap in both genetic and morphological variation will be discovered in the future. On the other hand, it is also possible that a new genus will need to be erected to accommodate Niveas agassizi and its currently unknown relatives.
Papua New Guinea: This paper stems from a rearing campaign led by Vojtech Novotny, George Weiblen, Yves Basset, and Scott Miller, and supported by the US National Science Foundation (grants DEB-0211591, 0515678 and others), Czech Science Foundation grant 206/09/0115 and others, and Czech Ministry of Education & European Union grant CZ.1.-07/2.3.00/20.0064. We thank the staff at the PNG Binatang Research Center for field assistance, PNG land owners for access to field sites and assistance, and PNG agencies for permits. DNA barcoding was provided by Paul Hebert through a grant from Genome Canada and the Ontario Genomics Institute in support of the iBOL project. Karolyn Darrow, Lauren Helgen and Margaret Rosati provided assistance at the Smithsonian. Kenya: We thank David Agassiz for sharing his material of African choreutids and the National Museums of Kenya for facilitating our collaboration. Phylogenetics: We thank Niklas Wahlberg for designing primers used in this project; and Carlos Peña and Eero Vesterinen for laboratory assistance. Phylogenetic analyses were conducted on the freely available Bioportal cluster (http://www.bioportal.uio.no) and RAxML blackbox (http://phylobench.vital-it.ch/raxml-bb/). We thank John Brown and an anonymous reviewer for helpful comments on an earlier version of the manuscript. SEM also thanks the Natural History Museum, London, Bishop Museum, Honolulu, and International Center for Insect Physiology and Ecology, Nairobi, for their continued support of this research program. JR was funded by the Finnish Kone Foundation experienced researcher grant during this project.
Taxon descriptions organized in tabular format for ease of comparison.
| Taxon | Niveas Rota, gen. n. | Niveas kone Rota, sp. n. | Niveas agassizi Rota, sp. n. |
|---|---|---|---|
| Type species | Niveas kone | ||
| Material examined | See Table 1. | See Table 1. | See Table 1. |
| Material deposited | The holotype and most paratypes will be retained at USNM, with paratypes distributed to PNG National Agriculture Research Institute (Port Moresby), BMNH, Bishop Museum, Naturalis (Leiden), and CSIRO (Canberra). | The holotype will be deposited in National Museums of Kenya (Nairobi) (NMK), with paratypes to USNM, BMNH and NMK. | |
| Distribution | Kenya, Papua New Guinea, Solomon Islands. | Papua New Guinea, Solomon Islands. | Kenya. |
| Diagnosis | Niveas can be easily distinguished from most genera of choreutids by the wing pattern (Figs 1, 3). Superficially, species of Niveas are similar to some species of Anthophila and Choreutis, but there is no known species in either of the latter two genera with a black terminal band enclosing white spots in the forewing as in Niveas agassizi and Niveas kone. (Figs 1, 3). Forewing venation with only four radial branches or with R4 and R5 fused in the basal half is also diagnostic for the genus. Female genitalia with paired concave sclerotizations on A7 sternite are also unique to Niveas. | Niveas kone can be separated from all other known choreutids based on its wing pattern (Fig. 1). Superficially, it is similar to a few species of Brenthia and Litobrenthia owing to its background color, but it lacks iridescent spots along forewing termen, which are always present in those two genera. Both male and female genitalia are very distinct from those of other choreutids (Figs 7, 8). | Niveas agassizi can be separated from other known choreutids by the wing pattern (Fig. 3). It is superficially similar to some species of Choreutis, but the latter usually have forewings with apparent patterning, and this is absent in Niveas agassizi. Female genitalia are very distinct from those of other choreutids (Fig. 9). |
| Description | Figs 1–9. | Figs 1, 2, 5, 7, 8. | Male unknown. Figs 3, 4, 6, 9. |
| Head | Labial palpi with projecting ventral scale tufts (Figs 2, 4). | Fig. 2. | Fig. 4. |
| Wings | Forewing veins R four-branched in Niveas kone (Fig. 5), five-branched in Niveas agassizi (Fig. 6), with R4 an R5 fused in basal 3/5; CuP present at termen for 1/3 to 1/5 wing length, extending as fold further towards base. Hindwing ten-veined, with M2 in close proximity to the basally fused M3 and CuA1 (Niveas agassizi) or nine-veined, apparently with M3 and CuA1 completely fused into a single vein (Figs 5, 6). | Fore- and hindwing with brown background color, speckled with white-tipped scales in an irregular pattern; a distinct black band along termen of both wings within which are more or less equidistant white spots (Fig. 1). | Forewing bronze-brown with speckled white-tipped scales over most of its surface; distinct dark brown to black band along termen with two small white spots at apex; hindwing light brown (Fig. 3). |
| Male genitalia | Tegumen rounded on top, tuba analis extending beyond tegumen; vinculum as inverted trapezoid ventrally emarginate; valva with costal margin straight, ventral margin rounded, ending with a horn-like projection; phallus twice as long as valva (Fig. 7). | As for the genus (Fig. 7). | Unknown. |
| Female genitalia | Apophyses anteriores slightly longer than posteriores; ostium bursae on A7 with a more or less strongly sclerotized antrum; ductus bursae straight, not coiled, with strong lateral sclerotizations; corpus bursae as a single sac (Niveas agassizi) or divided into two sacs (Niveas kone) with one or more signa. A7 sternite with paired, somewhat rounded, concave sclerotizations proximally, clearly visible in Niveas kone (Fig. 8), and slightly less so in Niveas agassizi (Fig. 9). | Corpus bursae split into two sacs; one sac with a V-shaped signum, the other with two round signa (Fig. 8). | Ductus bursae short and wide, opening into large corpus bursae, with one oval signum (Fig. 9). |
| Immature stages | Fig. 12. See a brief note in text. | Unknown. | |
| Host plants | Genus Ficus (Moraceae). | Ficus botryocarpa Miq., Ficus nodosa Teijsm. & Binn., Ficus phaeosyce K. Schum. & Lauterb., Ficus pungens Reinw. ex Blume, Ficus variegata Blume, and Ficus wassa Roxb. (Moraceae). | Unknown. |
| Etymology | The generic name is derived from Latin niveum, meaning snowy, in reference to speckles of white-tipped scales in the wings of the type species; it is not treated as a Latin word and is feminine in gender. | The species is named after the Finnish Kone Foundation (Koneen Säätiö) in appreciation of their funding of this work. The name is a noun in apposition. | This species is named after David Agassiz, who collected all the known specimens and made many significant contributions to our knowledge of African micro-moths. The name is a noun in genitive case. |