(C) 2013 Eduardo Suárez-Morales. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Suárez-Morales E, Jarquín-González J (2013) A new species of Peltidium Philippi, 1839 (Crustacea, Copepoda, Harpacticoida) from the Pacific coast of Mexico. ZooKeys 325: 21–32. doi: 10.3897/zookeys.325.5726
During the analysis of phytal meiobenthic samples collected from a rocky-sandy beach in the state of Nayarit, in the Mexican Pacific, several specimens of harpacticoid copepods were obtained and taxonomically examined. These specimens were found to represent an undescribed species of the peltidiid genus Peltidium Philippi, 1839. The new species, Peltidium nayarit sp. n. is described herein. It resembles Peltidium nichollsi Geddes and Peltidium lerneri Geddes from Bahamas but also the widespread Peltidium speciosum Thompson & Scott and Peltidium purpureum Philippi. The new species from the Mexican Pacific differs from its known congeners by its possession of a unique combination of characters, including a modified pectinate seta on the antennary exopod, three terminal setae on the second endopodal segment of leg 1, third exopodal segment of leg 1 with three elements, inner terminal claw twice as long as outer claw, female fifth leg with 5 exopodal setae, exopodal setae I-III stout, spinulose and seta IV being as long as seta V. This is the second species of the family known to be distributed in the Eastern Tropical Pacific and in Mexico. Pending additional data, the distribution of this species appears to be restricted to this area of the Mexican Pacific.
Crustacean fauna, marine copepods, phytal meiobenthos, associated copepods, taxonomy
Research on phytal meiobenthos has been advancing in many regions, but there are large areas in which this important community has received little attention (
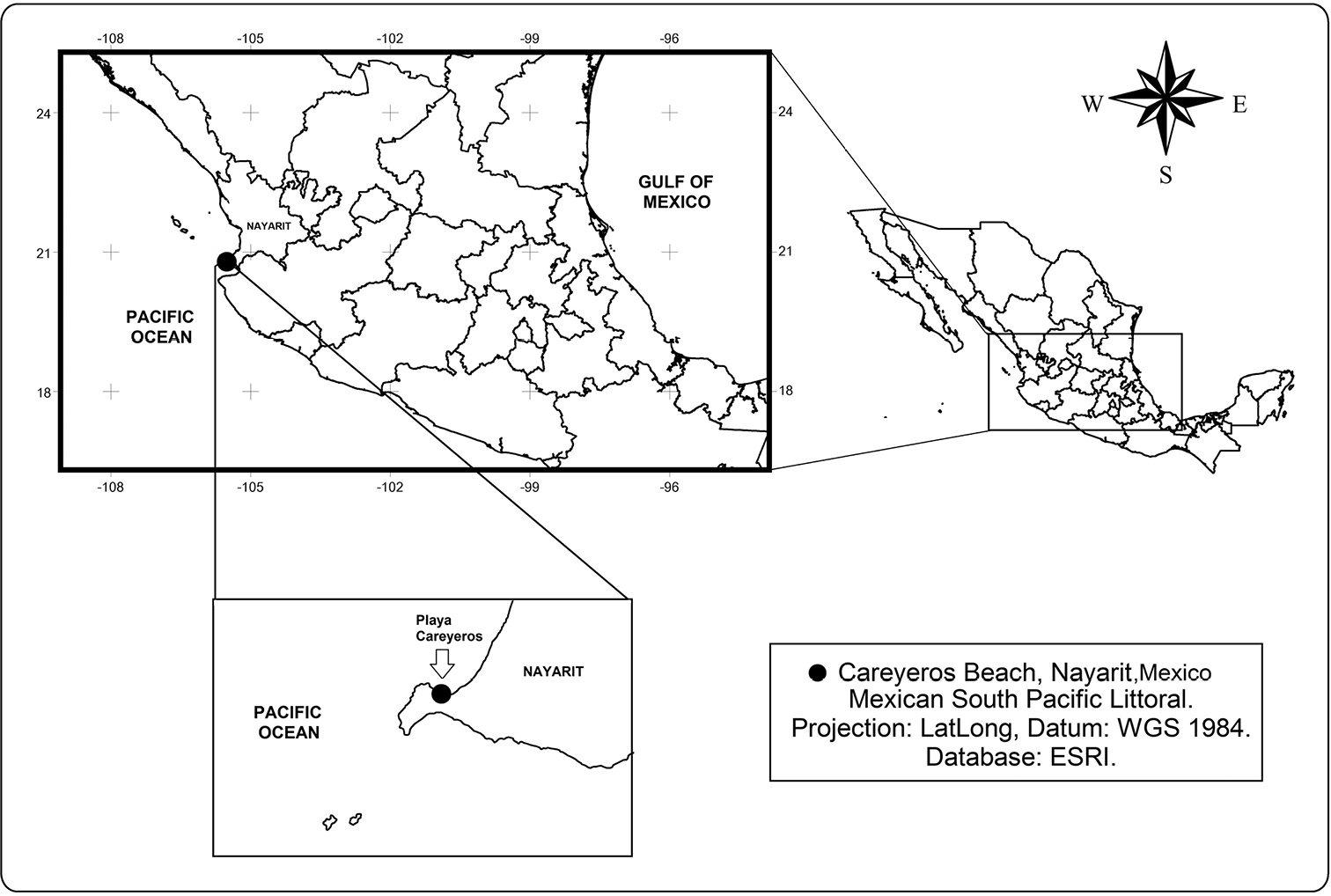
Playa Careyeros (Fig. 1) is a rocky-sandy area on the southern coast of Nayarit, on the Pacific coast of Mexico. It is influenced by the California Current and the North Equatorial current, with high salinity, temperature gradients and local patterns of coastal circulation (
Location of Playa Careyeros, in the State of Nayarit, on the Pacific coast of Mexico, the type locality of Peltidium nayarit sp. n.
A biological survey of the littoral habitats of Playa Careyeros, Nayarit was performed in March 2013. Qualitative samples of algae were taken manually during free diving samplings in the littoral environments of the surveyed area, mainly of algae associated with coral rock at depths not exceeding 3 m. Harpacticoid copepods were extracted by washing the sample through a set of 1.1 and 0.59 mm sieves. Copepods were fixed in 96% ethanol and were then sorted from the original samples and transferred to 70% ethanol with glycerine for long-term preservation. Selected specimens were then placed in glycerol for taxonomical examination and dissection. The dissected appendages were mounted on slides using glycerol as mounting medium and sealed with Entellan®, a fast-drying sealant. Figures were drawn with the aid of a camera lucida. Observations were made with an Olympus BX51 with Nomarski DIC microscope. Morphological terminology follows
http://zoobank.org/A29D34BE-E213-4840-828D-E39DEEC78E22
http://species-id.net/wiki/Peltidium_nayarit
Figs 2 – 4Adult female holotype (ECO-CHZ-08979) partially dissected, mounted on glycerine sealed with Entellan®, Playa Careyeros, Nayarit, Mexico, coll. Jani Jarquín-González, Patricia Salazar and Ramiro Gallardo, March 23, 2013, depth=2–3 m, algal patch from coral rock. Paratypes: three adult females, partially dissected, slides, mounted in glycerine sealed with EntellanÒ, same site, date, and collector (ECO-CHZ-08980), seven undissected adult female specimens preserved in ethanol, vial (ECO-CHZ-08981), same site, date and collector. Two undissected adult females (USNM-1221050), same sampling data.
Playa Careyeros (20°46'59.46"N, 105°30'35.48"W), state of Nayarit, central part of the Pacific coast of Mexico.
The species is named after the Mexican state of Nayarit, where this species was originally collected. The name of the species is a noun used in apposition.
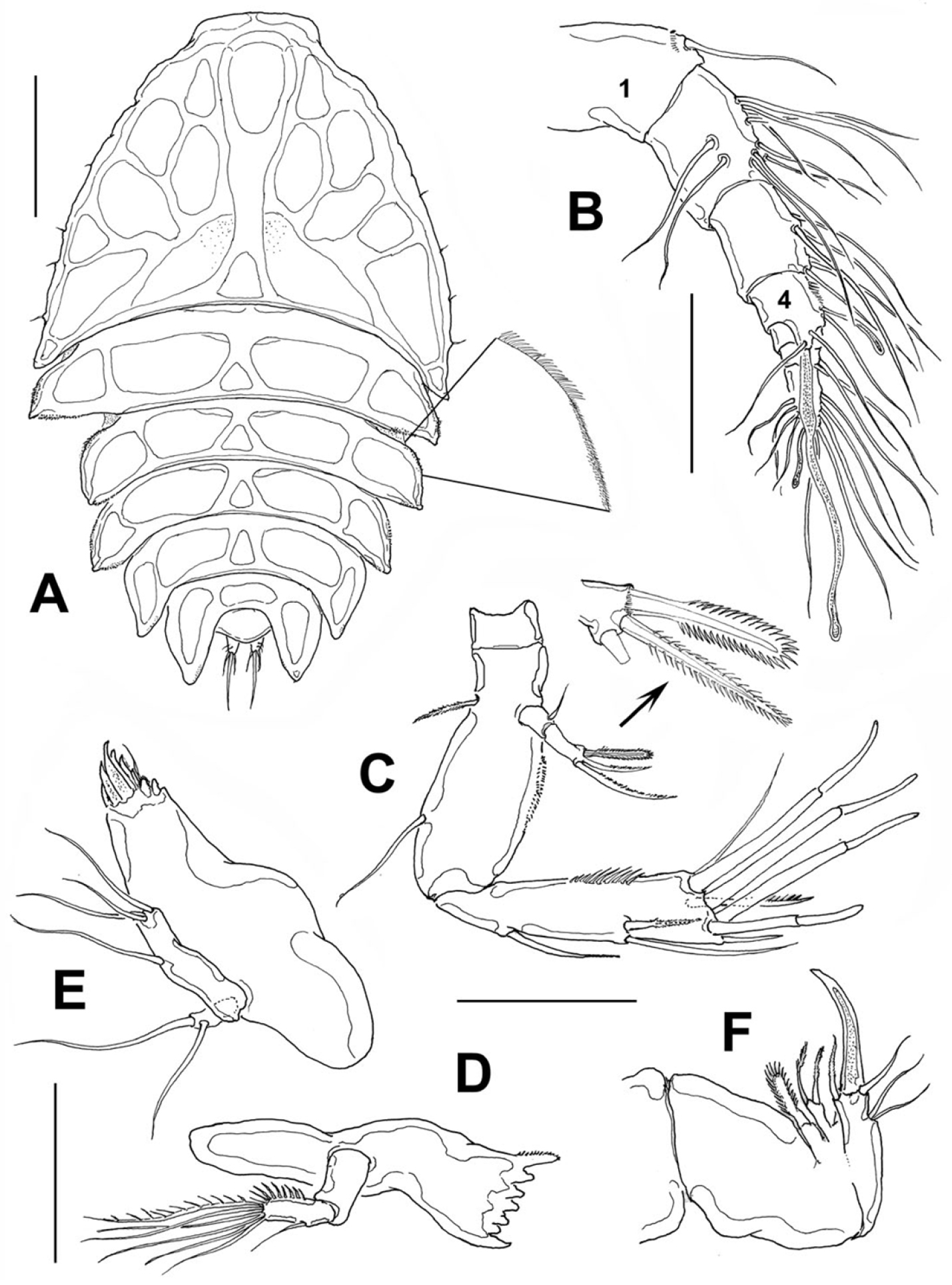
Female: Body (Fig. 2A) broad, dorsoventrally flattened, arched along longitudinal axis. Cephalosome accounting for about half the body length, with its greatest width at first pedigerous somite, behind which the body gradually tapers posteriorly. Epimera of genital somite and preceding somite pointed and backwardly directed. Length of holotype: 1.03 mm measured from tip of rostrum to posterior margin of anal somite. Length range of type females from 0.93 mm to 1.05 mm, average length 0.96 mm, n=16. Rostrum fused to cephalosome, broad, downwardly directed.
Peltidium nayarit sp. n., from Playa Careyeros, Nayarit, Mexican Pacific. A adult female, habitus, dorsal view, showing detail of ornamentation of epimeral processes of cephalothorax B antennule C antenna D mandible E maxillule F maxilla. Scales bars: A= 250 μm, B–F=100 μm.
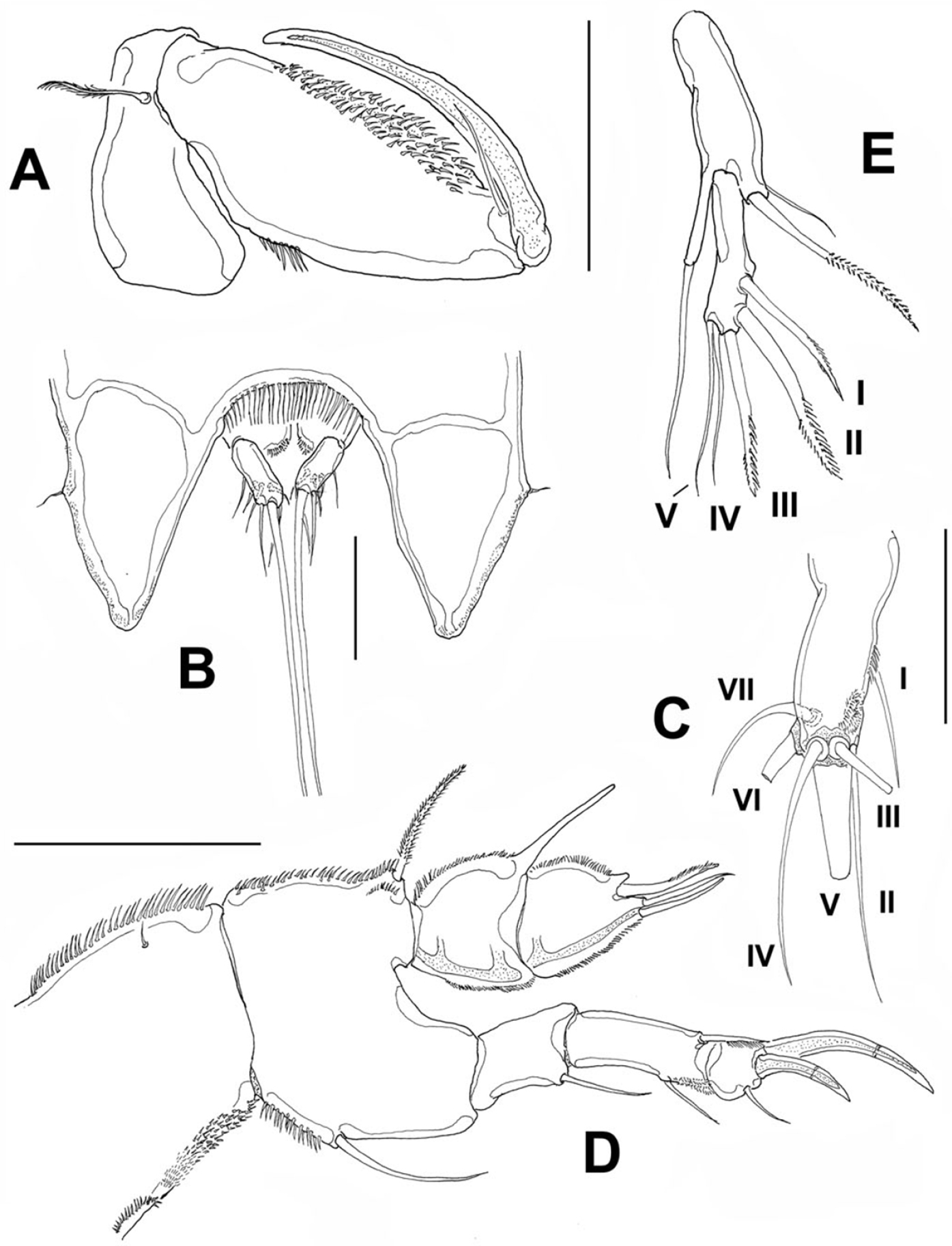
Cephalosome with a few small, sparsely distributed sensilla on lateral margins (Fig. 2A), posterior margin smooth. Succeeding prosomites, bearing legs 2-4, with flat, laterally expanded subtriangular margins ornamented with a mixed pattern of long and minute spinules (detail in Fig. 2A). First urosomite, bearing leg 5, slightly longer than succeeding genital double-somite; posterior margin of genital double-somite smooth. Anal somite with rounded posterior margin; somite naked in dorsal view, but with a row of long setae along ventral margin (Fig. 3B). Anal area moderately deep, with inner rows of short setules along margin of anal operculum. Caudal rami cylindrical, about twice as long as wide, with 7 setae. Middle caudal apical seta (V) longest. Dorsal seta (VII) about half as long as ramus (Fig. 3C).
Peltidium nayarit sp. n., adult female from Playa Careyeros, Nayarit, Mexican Pacific. A maxilliped B urosome showing anal somite and caudal rami, ventral view C right caudal ramus, ventral view showing setation following nomenclature by
Antennule (Fig. 2B) 7-segmented; first segment slightly longer than second, ornamented with distal row of spinules. Armature of antennulary segments (s=setae, ae=aesthetasc) as: 1(1), 2(10s), 3(6s), 4(3s+2ae), 5(1s), 6(2s), 7(9s+1ae). Aesthetasc on fourth segment long, about 70% of antennule length.
Antenna (Fig. 2C). Coxa small, Allobasis with short abexopodal seta and longitudinal patch of spinules on outer margin. Exopod two-segmented, elongated, first segment with short slender seta, second segment bearing three setae distally, distal margin with row of short spinules. One exopodal seta modified, with regular pectinate ornamentation along both margins (see detail in Fig. 2C). Free endopodal segment with outer row of long spinules, armed with one spine and two lateral setae plus seven distal setal elements, four of them being articulated stout setae.
Mandible (Fig. 2D) short, tapering distally. Gnathobasis with narrow diastema, armed with 5-6 monocuspidate teeth, plus stout dorsal seta fused to gnathobasis, ornamented with row of short setules. Mandibular palp small, represented by a subrectangular coxa-basis segment and a single-segmented endopod armed with an inner row of spinules, six terminal setae plus a single outer seta.
Maxillule (Fig. 2E). Praecoxal arthrite armed with eight teeth and one distal short seta. Coxa and basis fused, indistinguishable, with three terminal setae. Endopodite represented by one seta. Exopodite one-segmented, with two setae.
Maxilla (Fig. 2F). Syncoxa robust, short, with two endites, the proximal small and bearing one broad seta, distal endite cylindrical, armed with two subequal, pinnate setae; allobasis forming a strong terminal claw and with one inner basal seta, one outer basal seta and two subequal outer endopodal setae.
Maxilliped (Fig. 3A) subchelate. Coxa and basis elongate. Basis with smooth surface, bearing a well-developed seta, as depicted. First endopodal segment robust, ornamented with single row of spinules and large patch of spinules, as shown. Endopodal claw slender, slightly curved, about 1.3 times as long as basis, with single accompanying seta.
Leg 1 (Fig. 3D). Coxa elongate, ornamented with single row of small spinules on inner and outer margins, plus single short seta on inner middle part of segment. Basis wide, inner margin and part of outer margin ornamented with spinules. Inner distal basipodal seta reaching distal margin of first endopodal segment. Outer basipodal seta reaching distal end of basis. Exopod three-segmented, second exopodal segment longest, about 1.5 times as long as first segment, with patch of minute spinules on outer distal margin. Two exopodal claws on distal position of third exopodal segment; outer claw half as long as inner claw. Endopod two-segmented, shorter than exopod. Endopodal segments wide, globose (sensu
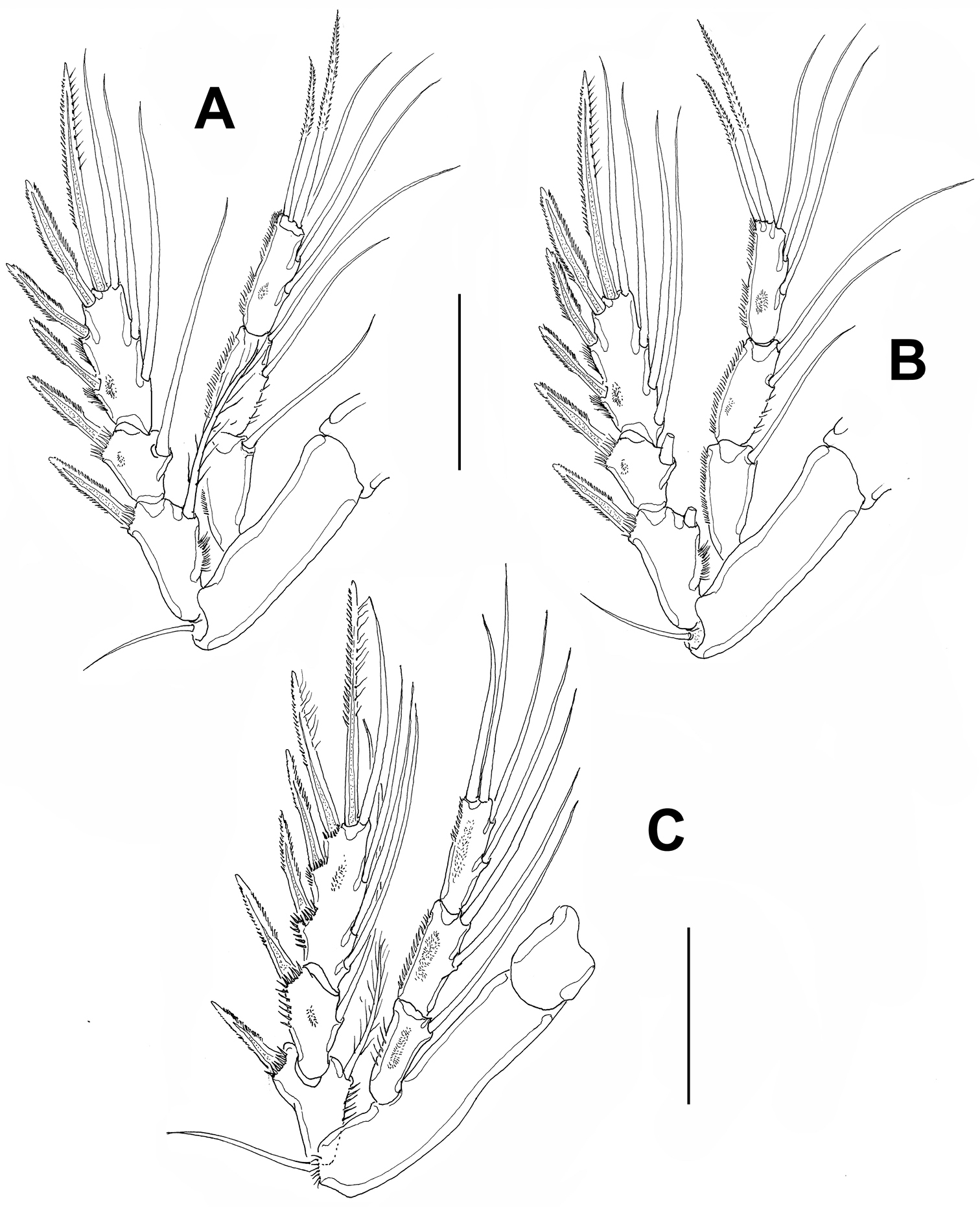
Leg 2 (Fig. 4A). Coxa small, basis transversely elongated. Basis with outer seta. Endopod three-segmented, longer than exopod, exopod reaching midlength of third endopodal segment. Exopod three-segmented, with spinules on outer margins of third segment and spinules at insertion of spines on first and second segments.
Leg 3 (Fig. 4B). Coxa, basis, and relative length of endopodal and exopodal rami as in leg 2. Endopod and exopod three-segmented.
Leg 4 (Fig. 4C). Coxa and basis as in leg 3. Exopod three-segmented, third segment with 8 setal elements. Insertion points of exopodal spines and outer margins of second and third exopodal segments with rows of spinules. Endopod three-segmented, slightly longer than exopod, outer margins of segments ornamented with rows of short spinules.
Armature of swimming legs 1-4 as in Table 1.
Leg 5 (Fig. 3E) exopod and baseoendopod separated. Baseoendopod bearing single inner seta. External seta long, borne on elongate cylindrical lobe of baseoendopod reaching half the length of exopodal lobe. Exopodite slender, with 5 setal elements (I-V) (sensu
Male: Unknown.
Peltidium nayarit sp. n., adult female from Playa Careyeros, Nayarit, Mexican Pacific. A leg 2 B leg 3 C leg 4. Scales bars: A–C=100 μm.
Armature of swimming legs 1-4 (spines in Roman numerals, setae in Arabic) of Peltidium nayarit sp. n. Sequence follows external to internal positions.
| basis | endopod | exopod | |
|---|---|---|---|
| leg 1 | 1-1 | 0-I; 2, I | 1-0;1-1; 1, II |
| leg 2 | 1-0 | 0-1;0-2;II, 1, 2 | I-0;I-1;III, I, 1, 2 |
| leg 3 | 1-0 | 0-1;0-2;II, 1, 2 | I-0;I-1;III, 1, 2 |
| leg 4 | 1-0 | 0-1;0, 2;2, 2 | I-1; I-1; III, I, 1, 3 |
The available keys to the species of Peltidium include those by
The new species can be distinguished from Peltidium lerneri by the armature of leg 1. In Peltidium lerneri the exopodal claws are subequally long, whereas the inner is twice as long as the outer one in Peltidium nayarit sp. n. In addition, the inner spiniform seta of the second endopodal segment is clearly shorter than the other two setae vs. an equal length of the three elements in the new species. Also, as indicated by
The new species shares also some important characters with Peltidium speciosum Thompson & Scott, 1903 (see
Peltidium is a very widely distributed genus with records from different regions of the world but it is not very diverse in a given area, for instance only three species are known from the Mediterranean: Peltidium gracile (Claus, 1889), Peltidium purpureum Philippi, 1839, and Peltidium robustum (Claus, 1889) (
Patricia Salazar Silva (Instituto Tecnológico de Bahía Banderas (ITBB), Nayarit) and Ramiro Gallardo Hernández (ITBB) kindly helped in the sampling of the local meiobenthic fauna and Cristian Kraker Castañeda (ECOSUR, San Cristóbal de las Casas) prepared the map of the surveyed area. We thank El Colegio de la Frontera Sur (ECOSUR-Chetumal) for logistic support during the taxonomic analysis of these samples. Linda Ward (NMNH-SI) kindly processed the assignment of the NMNH catalog number of this species. Rosa Ma. Hernández (ECOSUR) deposited the type specimens in the collection of Zooplankton at ECOSUR-Chetumal. Two anonymous reviewers provided positive, constructive comments that contributed to improve the quality of this work.