Citation: Hastriter MW, Meyer MD, Sherwin RE, Dittmar K (2014) New distribution and host records for Hectopsylla pulex Haller (Siphonaptera, Tungidae) with notes on biology and morphology. ZooKeys 389: 1–7. doi: 10.3897/zookeys.389.7042
Hectopsylla pulex Haller is documented for the first time from Cochise County, Arizona on the Lesser Long-nosed bat, Leptonycteris yerbabuenae Martinez and Villa (Phyllostomidae). This represents the first record of this flea on this Chiropteran Family. The most favorable site of attachment of H. pulex appears to be the head, particularly the ears and tragus. The potential interference of echolocation caused by flea attachment at or near the tragus is discussed in addition to host preferences and specialized morphological features. It is demonstrated that expansion of abdominal segments during egg development is a function of mechanical design and not neosomy such as occurs in Tunga monositus Barnes and Radovsky, Neotunga euloidea Smit and some vermipsyllid fleas.
Arizona, bats, fleas, Leptonycteris, neosomy, Phyllostomidae
Members of the flea genus Hectopsylla Barrera parasitize birds, small non-volant mammals and bats. A comprehensive review of the genus Hectopsylla was provided by
As part of a long-term ecological study of insectivorous bats in the southwestern United States between 23 June and 4 September 2013, 23 Lesser Long nosed bats, Leptonycteris yerbabuenae Martinez and Villa (Phyllostomidae), were captured using a mist net placed adjacent to a hummingbird feeder. Bats were weighed, measured, and examined and fleas were removed with forceps and preserved in 70% ethanol pending processing. A total of three female fleas were collected from the ears of a young adult male Leptonycteris yerbabuenae. One flea is deposited in the Brigham Young University DNA flea voucher collection and the other two in the collection of Christopher Newport University. Images were prepared using an Olympus BX61 Compound Microscope, Olympus CC12 digital camera accompanied with an Olympus Microsuite™ B3SV program and Adobe Photoshop, CS4.
A single attached replete female flea was observed anterior to the tragus of Leptonycteris yerbabuenae captured at Paradise, Cochise County, Arizona on 23 June 2013. This specimen was not collected but a cellular phone photo was taken to document what probably represents a replete female Hectopsylla pulex. Although tentative, this identification is supported by the facts that Hectopsylla pulex is: 1) the only representative of the genus that occurs on bats 2) it is the only member of the genus previously reported in the United States, 3) it was present in the same locality, and 4) that it was present in simultaneous collections of Hectopsylla pulex on the same host species (Leptonycteris yerbabuenae). On 27 July 2013, three additional female Hectopsylla pulex specimens were observed and removed from the anterior base of the tragus of Leptonycteris yerbabuenae captured in White Tail Canyon, Chiracahua Range, Cochise County, Arizona. A photograph was taken of the one flea prior to its removal from the left ear (Fig. 1), while the other two specimens attached at the same site on the right ear were collected but not photographed. No additional fleas were noted on the other 21 Leptonycteris yerbabuenae specimens examined.
Young male Leptonycteris yerbabuenae with Hectopsylla pulex attached near tragus. Insert is enlargement of attached Hectopsylla pulex (arrows indicate previous flea attachment sites).
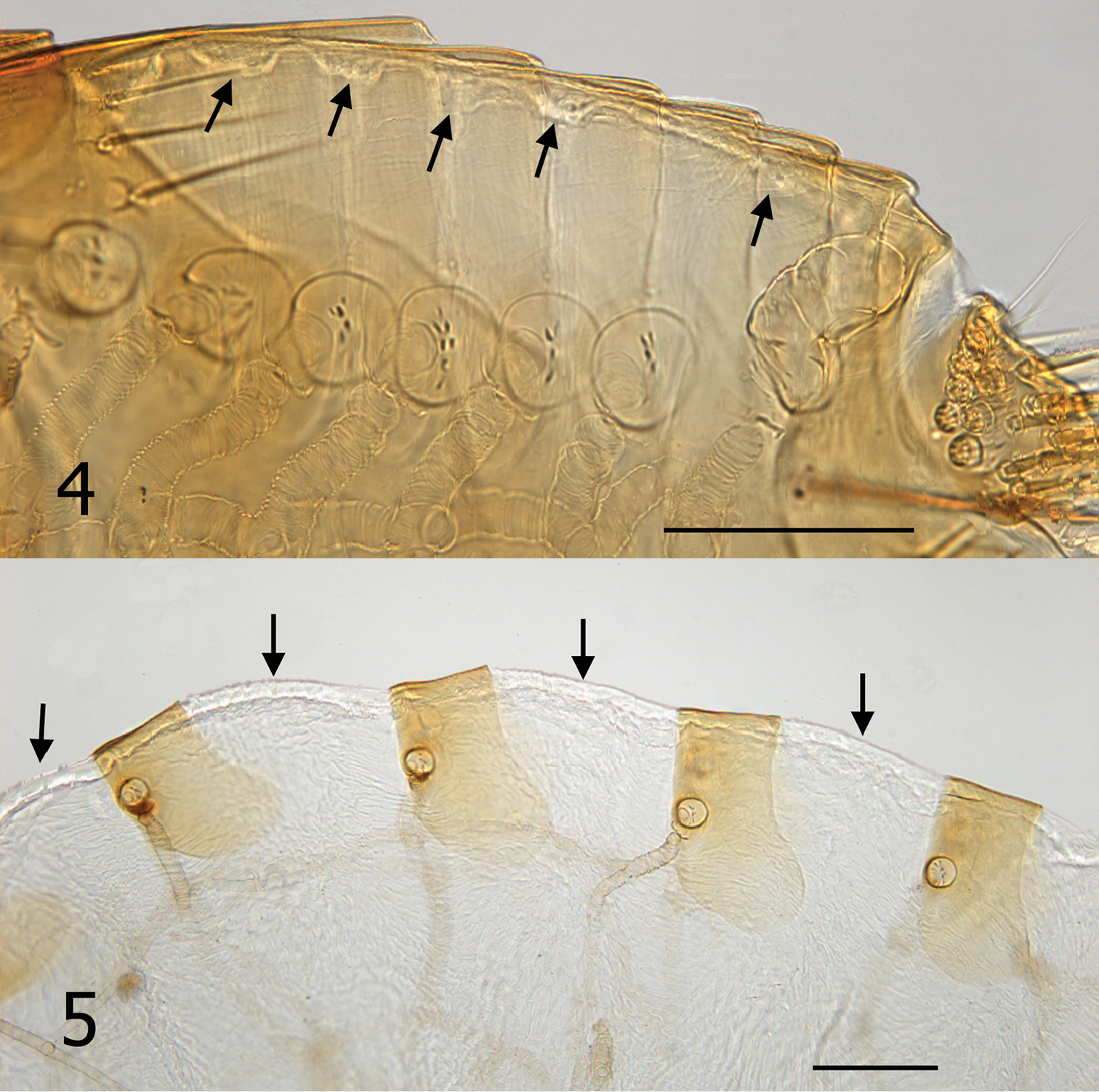
Modest swelling of the skin of the bat adjacent to the base of the attached flea (Fig. 1, arrows) possibly indicates residual scarring from previously attached stick-tight fleas. These fleas attach and feed for extended periods. Little is known about their biology; however, other stick-tight fleas, e.g., Echidnophaga gallinacea (Westwood), Juxtapulex echidnophagoides Wagner and Tunga penetrans (Linnaeus), attach to their host with similar lacinae that are serrated along their margin to enable attachment (Fig. 2, arrow). This group of fleas represents two types of stick-tight fleas: 1) those that attach, feed, and drop off, and 2) those that attach, feed to repletion, become gravid, and ultimately die in situ. The first type attaches, retain their legs, and eventually detach and fall off of the host. These may feed multiple times. The second type that includes Tunga penetrans and Hectopsylla pulex remain attached and autosever their legs as often noted by black scarring of the apices of each severed leg. Severing usually occurs at the apices of the coxae or femora.
Hectopsylla pulex. 2 Overview of an unfed female, Pacora, Panama collected from bat guano. Arrow indicates lacinae 3 Overview of a replete gravid female, host: Leptonycteris yerbabuenae, White Tail Canyon, Chiracahua Range, Cochise County, Arizona.
It is unknown whether males of Hectopsylla pulex copulate with females on or off the host. Males of Hectopsylla pulex have never been found attached to a host and males have been collected only from bat guano of molossid bats (
Tergites of Hectopsylla pulex. 4 Unfed non-gravid female from Pacora, Panama; arrows indicate membrane prior to expansion 5 Replete gravid female, arrows indicate expanded membrane to accommodate expansion from feeding and egg development.
Hectopsylla pulex was reported by
According to
Only 11 of the total 69 fleas taken from these two bat species had fleas attached on areas other than the ear and tragus.
Comparison of Hectopsylla pulex from Panama (
This work was conducted under CNU IACUC approved protocol, USFWS Permit TE71473A-0, and Arizona DGF SCP Permit 621260. We thank the U.S. Customs and Border Protection for financial support, USFW Service and AMEC for logistical and collaborative support, and Ambre Delpopolo for providing the original bat image published herein. The senior author acknowledges with gratitude the continued support of Michael F. Whiting, Shawn Clark, and the staff of the Monte L. Bean Life Science Museum, Brigham Young University for providing space, materials and equipment to carry on studies of Siphonaptera.