(C) 2013 Werner E. Holzinger. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Holzinger WE, Schlosser L (2013) The Auchenorrhyncha fauna of peat bogs in the Austrian part of the Bohemian Forest (Insecta, Hemiptera). In: Popov A, Grozeva S, Simov N, Tasheva E (Eds) Advances in Hemipterology. ZooKeys 319: 153–167. doi: 10.3897/zookeys.319.4324
The first overview on the Auchenorrhyncha fauna of peat bogs of the Austrian Bohemian Forest is presented. Seven oligotrophic peat bog sites were studied in 2011 by suction sampler (“G-Vac”) and 93 Auchenorrhyncha species (with 7465 adult specimens) were recorded. Eleven species (about 18 % of the individuals) are tyrphobiontic or tyrphophilous. The relative species abundance plot is not very steep; the six most abundant species represent 50 % of the individuals. The most common species is Conomelus anceps (17 % of the individuals). Compared to the whole Austrian Auchenorrhyncha fauna, the fauna of peat bogs comprises distinctly more univoltine species and more species hibernating in nymphal stage. Densities of adult Auchenorrhyncha in peat bogs are low in spring (about 10–60 individuals per m²) and high in July, with up to 180 (±50) individuals per m². Disturbed peat bogs have higher species numbers and higher Auchenorrhyncha densities in total, but lower numbers and densities in peat bog specialists.
Diese Studie gibt erstmals einen Überblick über die Zikadenfauna der Moore des österreichischen Anteils des Böhmerwaldes. Sieben Moorflächen wurden 2011 mittels Saugfang quantitativ untersucht. Insgesamt konnten 93 Zikadenarten (in 7465 adulten Individuen) festgestellt werden. Elf Arten (ca. 18 % der Individuen) sind tyrphobiont oder tyrphophil. Die Gesamtdominanzkurve ist nicht sehr steil; die sechs häufigsten Arten repräsentieren 50 % aller Individuen, die häufigste Art ist Conomelus anceps (17 % der Individuen). Vergleicht man die Zikadenfauna der Moore mit der Zikadenfauna Österreichs, so weist erstere deutlich mehr univoltine Arten und mehr Larvalüberwinterer auf. Phänologisch betrachtet sind die Dichten adulter Zikaden in Mooren im Frühling niedrig (ca. 10–60 Individuen pro m²) und im Juli hoch (bis 180 ± 50 Individuen pro m²). Gestörte Moorlebensräume haben höhere Artenzahlen und höhere Zikadendichten, aber die Artenzahl und Individuendichte der Moorspezialisten ist deutlich geringer als in ungestörten Mooren.
Auchenorrhyncha, Fulgoromorpha, Cicadomorpha, peat bogs, wetland, species composition, Bohemian Forest, Austria
Peat bogs are characterized by very wet, acidic and oligotrophic conditions, and their soil is of organic origin. They are among the most threatened habitats in Central Europe, due to dewatering, peat mineralization, land reclamation, afforestation, nutrient contamination and recently by climate change. Within the last century, over 90 % of all peat bogs in Austria were devastated or completely destroyed (
Auchenorrhyncha are among the most abundant animal groups in peatlands. The majority of species is stenoecious, specialized on both habitat conditions and host plants (
Seven typical oligotrophic peat bog sites of the Bohemian Forest were studied in 2011. Quantitative samples were taken monthly (from May until September) by a suction sampler (“G-Vac”, see
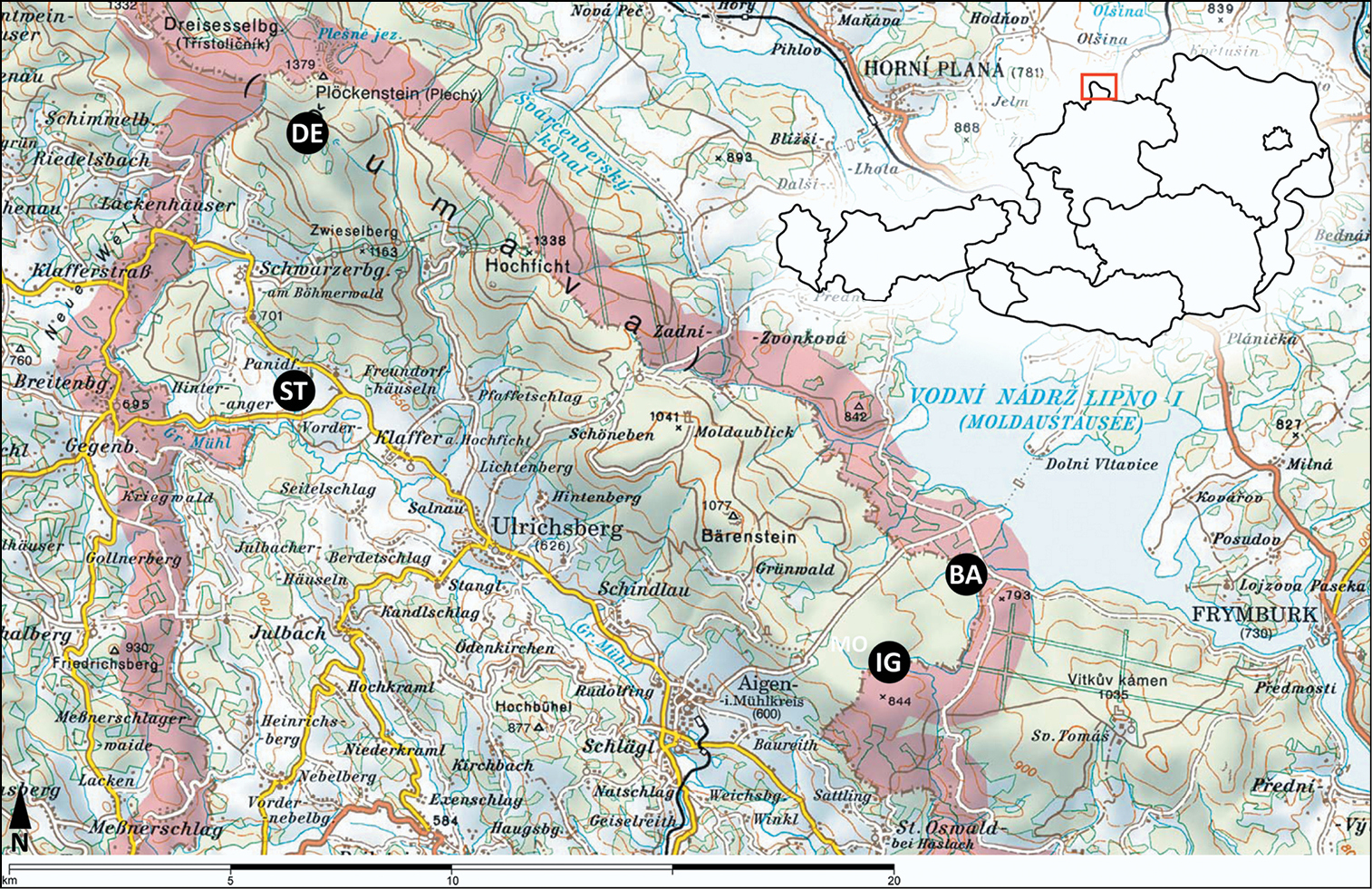
The study sites are located in the very north of Upper Austria, close to the German and Czech border. They are shown in Fig. 1 and characterized in Table 1 and 2.
Location of the sampling sites in the Bohemian Forest (Upper Austria), overview. Samling sites: DE = Deutsches Haidl, ST = Stadlau, BA = Bayrische Au, IG = Moor am Iglbach. Map source: AMAP 3D.
Study sites, coordinates and sampling dates on these sites (according to the Upper Austrian environmental lawyer, unpublished).
| Code | Site name | Coordinates | Altitude | Area (ha) | Site description | Sampling dates |
|---|---|---|---|---|---|---|
| BA | Bayrische Au | 48°40'49"N, 14°03'32"E | 720 m | 33.8 | oligotrophic peat bog of national importance ( |
13.5., 22.6., 27.7., 22.8., 24.9.2011 |
| ST-1 | Stadlau 1 | 48°42'29"N, 13°51'12"E | 610 m | 7.1 | dewatered, partially eroded and heavily nutrient contaminated remnants of a formerly large peat bog area | 17.5., 20.6., 25.7., 21.8., 23.9.2011 |
| ST-2 | Stadlau 2 | 48°42'23"N, 13°51'18"E | 610 m | |||
| ST-3 | Stadlau 3 | 48°42'32"N, 13°51'15"E | 610 m | |||
| IG-1 | Iglbach-Moor 1 | 48°39'10"N, 14°01'44"E | 800 m | 3.9 | drained peat bog complex, oligotrophic to mesotrophic, partially still very wet | 19.5., 22.6., 26.7., 20.8., 24.9.2011 |
| IG-2 | Iglbach-Moor 2 | 48°39'09"N, 14°01'30"E | 800 m | |||
| DE | Deutsches Haidl | 48°45'43"N, 13°51'13"E | 1242 m | 2.8 | acidic oligotrophic peat bog of international importance ( |
18.5., 25.6., 27.7., 22.8., 25.9.2011 |
Vegetation and management of the study sites (according to the Upper Austrian environmental lawyer, unpublished).
| Code | Site name | Vegetation | Site management |
|---|---|---|---|
| BA | Bayrische Au | Patchy mixture of Phalaridetum arundinaceae, Caricetum rostratae, Caricetum gracilis, Caricetum nigrae, Sphagnetum magellanici | parts of the peat bog formerly used for peat-ditching; no management today |
| ST-1 | Stadlau 1 | Molinion, Sphagnetum magellanici | some years grazed (cattle) but not in 2011 |
| ST-2 | Stadlau 2 | Caricetum nigrae, Caricetum rostratae, Junco-Molinietum | some years grazed (cattle) but not in 2011 |
| ST-3 | Stadlau 3 | Junco-Molinietum | mowing once a year (July), grazed (cattle) |
| IG-1 | Iglbach-Moor 1 | Caricetum rostratae | no management |
| IG-2 | Iglbach-Moor 2 | Caricetum rostratae | no management |
| DE | Deutsches Haidl | Caricetum limosae, Sphagnetum magellanici, Sphagno girgensohnii-Piceetum | no management |
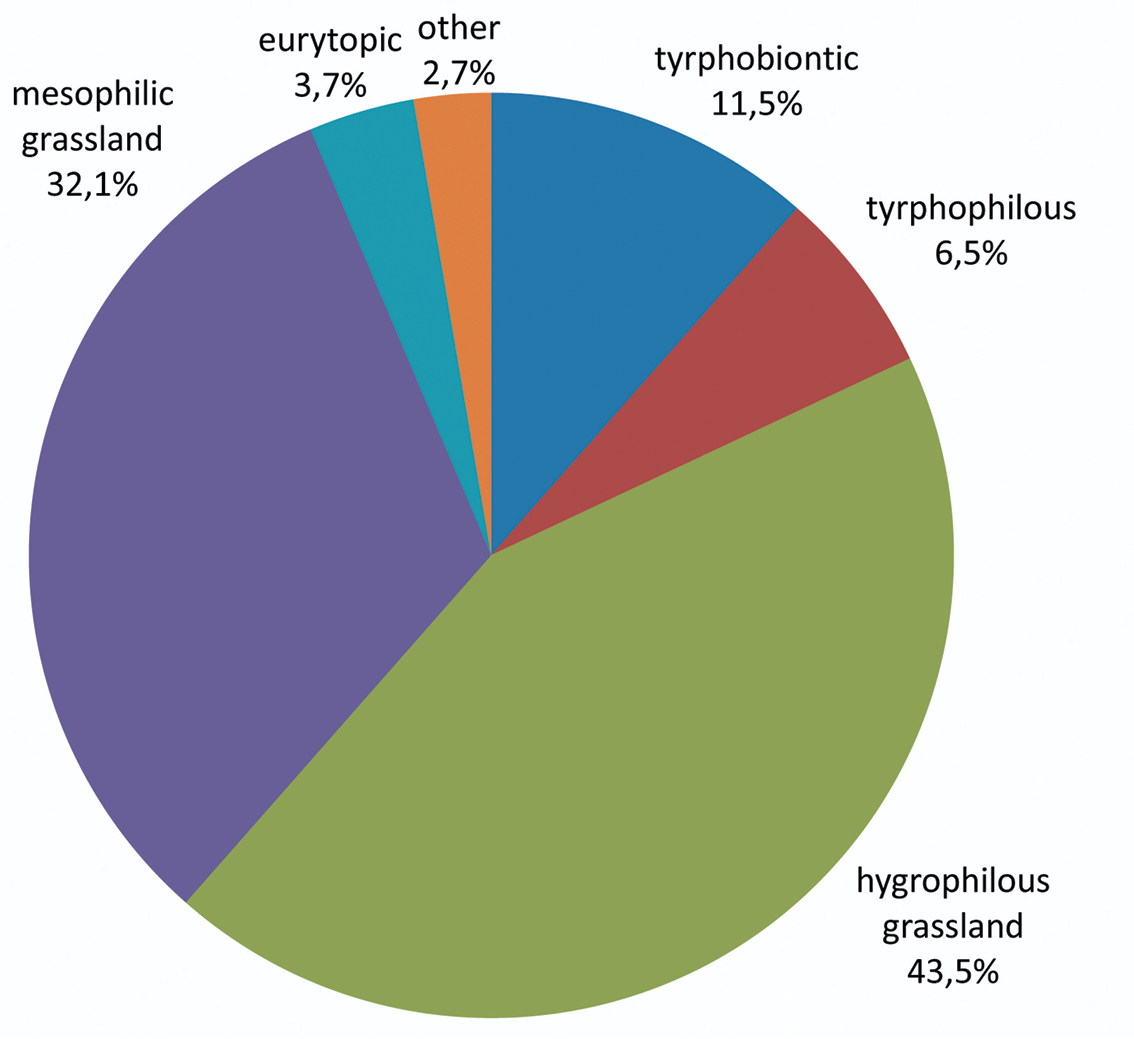
A total number of 93 Auchenorrhyncha species (7465 adult specimens) were collected and identified (Tables 3 and 4). The most abundant species is Conomelus anceps representing almost 17 per cent of the total number of specimens, followed by Jassargus pseudocellaris (5.5 %), Muellerianella extrusa (9.2 %), Sorhoanus xanthoneurus (7.6 %) and Macustus grisescens (5.2 %). The relative species abundance plot (Fig. 2) is not very steep; the six most abundant species represent only 50 % of the total individuals, and the 75 % mark is reached at species number 14.
Overview on Auchenorrhyncha collected in seven peat bogs in the Austrian part of the Bohemian Forest. Abbreviations: BA = Bayrische Au, ST = Stadlau, IG = Moor am Iglbach, DE = Deutsches Haidl.
| BA | ST-1 | ST-2 | ST-3 | IG-1 | IG-2 | DE | Total | |
|---|---|---|---|---|---|---|---|---|
| Total number of adult specimens | 1389 | 735 | 667 | 1724 | 891 | 1446 | 613 | 7465 |
| Total number of taxa | 31 | 29 | 30 | 50 | 44 | 47 | 19 | 93 |
| Number of tyrphobiontic and tyrphophilous individuals | 50 | 80 | 155 | 26 | 154 | 290 | 580 | 1333 |
| Number of tyrphobiontic and tyrphophilous species | 5 | 5 | 4 | 5 | 7 | 5 | 3 | 11 |
| Percentage of peat bog specialists (individuals) | 3.6 | 10.8 | 23.2 | 1.5 | 17.2 | 20.0 | 94.6 | 17.9 |
| Percentage of peat bog specialists (species) | 16.1 | 17.2 | 13.3 | 10.0 | 15.9 | 10.6 | 15.8 | 11.8 |
Auchenorrhyncha species of peat bogs in the Austrian part of the Bohemian Forest. The species are grouped into ecological types after
| No. | Species | Total number of specimens/percentage of total abundance of sampling site | Total ind. | rel. abd. (%) | RL A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | ST-1 | ST-2 | ST-3 | IG-1 | IG-2 | DE | |||||
| Tyrphobiontic species | |||||||||||
| 1 | Sorhoanus xanthoneurus (Fieber, 1869) | 1 0, 1 | 567 92, 5 | 568 | 7.6 | CR | |||||
| 2 | Kelisia vittipennis (J. Sahlberg, 1868) | 1 0, 1 | 18 2, 4 | 87 13 | 24 2, 7 | 150 10, 4 | 280 | 3.8 | VU | ||
| 3 | Stroggylocephalus livens (Zetterstedt, 1840) | 6 0, 4 | 6 | 0.1 | CR | ||||||
| 4 | Cixius similis Kirschbaum, 1868 | 1 0, 2 | 1 | <0.1 | VU | ||||||
| Tyrphophilous species | |||||||||||
| 5 | Sorhoanus assimilis (Fallén, 1806) | 16 1, 2 | 48 6, 5 | 55 8, 2 | 1 0, 1 | 33 3, 7 | 25 1, 7 | 12 2 | 190 | 2.5 | VU |
| 6 | Paradelphacodes paludosa (Flor, 1861) | 15 1, 1 | 1 0, 1 | 6 0, 9 | 2 0, 1 | 61 6, 8 | 74 5, 1 | 159 | 2.1 | EN | |
| 7 | Oncodelphax pullula (Boheman, 1852) | 12 0, 9 | 9 1, 2 | 7 1 | 17 1, 9 | 15 1 | 60 | 0.8 | EN | ||
| 8 | Cicadula saturata (Edwards, 1915) | 4 0, 5 | 11 1, 2 | 26 1, 8 | 40 | 0.5 | |||||
| 9 | Macrosteles ossiannilssoni Lindberg, 1954 | 15 0, 9 | 15 | 0.2 | NT | ||||||
| 10 | Kelisia ribauti Wagner, 1938 „boreomontan“ | 1 0, 1 | 7 0, 8 | 8 | 0.1 | EN | |||||
| 11 | Kelisia ribauti Wagner, 1938 „mediterran“ | 7 0, 4 | 7 | 0.1 | EN | ||||||
| Hygrophilous grassland species | |||||||||||
| 12 | Conomelus anceps (Germar, 1821) | 847 61 | 16 2, 2 | 177 26, 5 | 22 1, 3 | 17 1, 9 | 156 10, 8 | 2 0, 3 | 1237 | 16.6 | LC |
| 13 | Muellerianella extrusa (Scott, 1871) | 115 8, 3 | 193 26, 3 | 71 10, 6 | 40 2, 3 | 35 3, 9 | 233 16, 1 | 1 0, 2 | 688 | 9.2 | DD |
| 14 | Macustus grisescens (Zetterstedt, 1828) | 62 4, 5 | 113 15, 4 | 27 4 | 1 0, 1 | 89 10 | 88 6, 1 | 8 1, 3 | 388 | 5.2 | LC |
| 15 | Forcipata citrinella (Zetterstedt, 1828) | 206 11, 9 | 206 | 2.8 | NT | ||||||
| 16 | Megamelus notula (Germar, 1830) | 15 1, 1 | 12 1, 6 | 1 0, 1 | 33 1, 9 | 31 3, 5 | 98 6, 8 | 190 | 2.5 | NT | |
| 17 | Kelisia praecox Haupt, 1935 | 7 1 | 8 1, 2 | 134 15 | 4 0, 3 | 153 | 2 | VU | |||
| 18 | Cicadula quadrinotata (Fabricius, 1794) | 7 0, 5 | 4 0, 6 | 48 2, 8 | 19 2, 1 | 22 1, 5 | 101 | 1.3 | LC | ||
| 19 | Kelisia pallidula (Boheman, 1847) | 24 3, 6 | 58 4 | 82 | 1.1 | EN | |||||
| 20 | Jassargus sursumflexus (Then, 1902) | 26 1, 9 | 33 4, 5 | 1 0, 1 | 2 0, 1 | 1 0, 1 | 63 | 0.8 | LC | ||
| 21 | Muellerianella brevipennis (Boheman, 1847) | 5 0, 4 | 2 0, 3 | 2 0, 3 | 15 0, 9 | 12 1, 3 | 21 1, 5 | 1 0, 2 | 58 | 0.8 | LC |
| 22 | Xanthodelphax straminea (Stål, 1858) | 6 0, 4 | 1 0, 1 | 11 1, 2 | 12 0, 8 | 30 | 0.4 | VU | |||
| 23 | Macrosteles viridigriseus (Edwards, 1922) | 15 0, 9 | 15 | 0.2 | LC | ||||||
| 24 | Stenocranus major (Kirschbaum, 1868) | 9 0, 6 | 9 | 0.1 | LC | ||||||
| 25 | Cicadula albingensis Wagner, 1940 | 7 1 | 7 | 0.1 | LC | ||||||
| 26 | Erzaleus metrius (Flor, 1861) | 5 0, 4 | 5 | 0.1 | LC | ||||||
| 27 | Athysanus quadrum Boheman, 1845 | 2 0, 1 | 2 | <0.1 | EN | ||||||
| 28 | Streptanus sordidus (Zetterstedt, 1828) | 2 0, 2 | 2 | <0.1 | LC | ||||||
| 29 | Struebingianella lugubrina (Boheman, 1847) | 1 0, 1 | 1 | <0.1 | VU | ||||||
| Mesophilic grassland species | |||||||||||
| 30 | Jassargus pseudocellaris (Flor, 1861) | 410 23, 8 | 1 0, 1 | 411 | 5.5 | LC | |||||
| 31 | Cicadella viridis (Linnaeus, 1758) | 44 3, 2 | 65 8, 8 | 37 5, 5 | 3 0, 2 | 93 10, 4 | 155 10, 7 | 1 0, 2 | 398 | 5.3 | LC |
| 32 | Neophilaenus lineatus (Linnaeus, 1758) | 45 3, 2 | 70 9, 5 | 90 13, 5 | 91 10, 2 | 26 1, 8 | 322 | 4.3 | LC | ||
| 33 | Delphacodes venosus (Germar, 1830) | 59 4, 2 | 34 4, 6 | 38 5, 7 | 1 0, 1 | 40 4, 5 | 110 7, 6 | 1 0, 2 | 283 | 3.8 | NT |
| 34 | Arthaldeus pascuellus (Fallén, 1826) | 52 3, 7 | 97 5, 6 | 53 5, 9 | 41 2, 8 | 243 | 3.3 | LC | |||
| 35 | Dicranotropis divergens Kirschbaum, 1868 | 110 6, 4 | 110 | 1.5 | LC | ||||||
| 36 | Psammotettix confinis (Dahlbom, 1850) | 79 4, 6 | 3 0, 3 | 1 0, 1 | 1 0, 2 | 84 | 1.1 | LC | |||
| 37 | Acanthodelphax spinosa (Fieber, 1866) | 1 0, 1 | 2 0, 3 | 58 3, 4 | 12 1, 3 | 6 0, 4 | 1 0, 2 | 80 | 1.1 | LC | |
| 38 | Anaceratagallia ribauti (Ossiannilsson, 1938) | 64 3, 7 | 64 | 0.9 | LC | ||||||
| 39 | Anoscopus albifrons (Linnaeus, 1758) | 4 0, 5 | 44 2, 6 | 4 0, 4 | 5 0, 3 | 57 | 0.8 | LC | |||
| 40 | Criomorphus albomarginatus Curtis, 1833 | 12 1, 6 | 3 0, 4 | 18 2 | 11 0, 8 | 44 | 0.6 | LC | |||
| 41 | Anoscopus flavostriatus (Donovan, 1799) | 2 0, 1 | 9 0, 5 | 13 1, 5 | 15 1 | 39 | 0.5 | LC | |||
| 42 | Aphrodes diminuta Ribaut, 1952 | 1 0, 1 | 1 0, 1 | 9 0, 5 | 5 0, 6 | 23 1, 6 | 39 | 0.5 | DD | ||
| 43 | Psammotettix cephalotes (Herrich-Schäffer, 1834) | 27 1, 6 | 1 0, 2 | 28 | 0.4 | NT | |||||
| 44 | Javesella forcipata (Boheman, 1847) | 27 3, 7 | 1 0, 1 | 28 | 0.4 | LC | |||||
| 45 | Javesella dubia (Kirschbaum, 1868) | 26 1, 5 | 26 | 0.4 | LC | ||||||
| 46 | Errastunus ocellaris (Fallén, 1806) | 19 1, 4 | 5 0, 3 | 24 | 0.3 | LC | |||||
| 47 | Agallia brachyptera (Boheman, 1847) | 3 0, 4 | 6 0, 9 | 12 0, 7 | 21 | 0.3 | LC | ||||
| 48 | Eupteryx notata Curtis, 1937 | 18 1 | 18 | 0.2 | LC | ||||||
| 49 | Athysanus argentarius Metcalf, 1955 | 5 0, 4 | 3 0, 4 | 3 0, 4 | 1 0, 1 | 1 0, 1 | 13 | 0.2 | LC | ||
| 50 | Graphocraerus ventralis (Fallén, 1806) | 13 0, 8 | 13 | 0.2 | LC | ||||||
| 51 | Rhopalopyx adumbrata (C. Sahlberg, 1842) | 2 0, 3 | 10 1, 1 | 1 0, 1 | 13 | 0.2 | LC | ||||
| 52 | Anoscopus serratulae (Fabricius, 1775) | 7 0, 4 | 7 | 0.1 | LC | ||||||
| 53 | Elymana sulphurella (Zetterstedt, 1828) | 4 0, 2 | 4 | 0.1 | LC | ||||||
| 54 | Euscelis incisus (Kirschbaum, 1858) | 4 0, 2 | 4 | 0.1 | LC | ||||||
| 55 | Xanthodelphax flaveola (Flor, 1861) | 4 0, 4 | 4 | <0.1 | EN | ||||||
| 56 | Cicadula persimilis (Edwards, 1920) | 3 0, 2 | 3 | <0.1 | LC | ||||||
| 57 | Megophthalmus scanicus (Fallén, 1806) | 3 0, 3 | 3 | <0.1 | LC | ||||||
| 58 | Cercopis vulnerata Rossi, 1807 | 1 0, 1 | 1 0, 1 | 2 | <0.1 | LC | |||||
| 59 | Dicranotropis hamata (Boheman, 1847) | 1 0, 1 | 1 0, 1 | 2 | <0.1 | LC | |||||
| 60 | Diplocolenus bohemani (Zetterstedt, 1840) | 2 0, 1 | 2 | <0.1 | LC | ||||||
| 61 | Philaenus spumarius (Linnaeus, 1758) | 1 0, 1 | 1 0, 1 | 2 | <0.1 | LC | |||||
| Eurytopic species | |||||||||||
| 62 | Deltocephalus pulicaris (Fallén, 1806) | 1 0, 1 | 1 0, 1 | 194 11, 3 | 4 0, 4 | 5 0, 3 | 205 | 2.7 | LC | ||
| 63 | Macrosteles laevis (Ribaut, 1927) | 39 2, 3 | 39 | 0.5 | LC | ||||||
| 64 | Javesella pellucida (Fabricius, 1794) | 1 0, 1 | 23 1, 3 | 1 0, 1 | 2 0, 1 | 27 | 0.4 | LC | |||
| 65 | Laodelphax striatella (Fallén, 1826) | 3 0, 2 | 1 0, 1 | 1 0, 2 | 5 | 0.1 | LC | ||||
| Mesophilic boundary species | |||||||||||
| 66 | Macrosteles septemnotatus (Fallén, 1806) | 37 5 | 4 0, 6 | 41 | 0.5 | LC | |||||
| 67 | Stiroma bicarinata (Herrich-Schäffer, 1835) | 2 0, 1 | 1 0, 1 | 19 1, 3 | 22 | 0.3 | LC | ||||
| 68 | Endria nebulosa (Ball, 1900) | 3 0, 3 | 5 0, 3 | 8 | 0.1 | ||||||
| 69 | Hardya tenuis (Germar, 1821) | 1 0, 1 | 4 0, 4 | 1 0, 1 | 6 | 0.1 | LC | ||||
| 70 | Balclutha calamagrostis Ossiannilsson, 1961 | 1 0, 1 | 1 0, 1 | 2 | <0.1 | LC | |||||
| 71 | Aphrophora alni (Fallén, 1805) | 1 0, 1 | 1 | <0.1 | LC | ||||||
| 72 | Evacanthus interruptus (Linnaeus, 1758) | 1 0, 1 | 1 | <0.1 | LC | ||||||
| 73 | Hyledelphax elegantula (Boheman, 1847) | 1 0, 1 | 1 | <0.1 | LC | ||||||
| 74 | Javesella discolor (Boheman, 1847) | 1 0, 1 | 1 | <0.1 | LC | ||||||
| Montane grassland species | |||||||||||
| 75 | Verdanus abdominalis (Fabricius, 1803) | 27 1, 6 | 2 0, 2 | 6 0, 4 | 35 | 0.5 | LC | ||||
| 76 | Planaphrodes bifasciata (Linnaeus, 1758) | 12 0, 7 | 12 | 0.2 | LC | ||||||
| 77 | Jassargus alpinus (Then, 1896) | 4 0, 7 | 4 | 0.1 | LC | ||||||
| 78 | Erythria manderstjernii (Kirschbaum, 1868) | 3 0, 5 | 3 | <0.1 | LC | ||||||
| 79 | Neophilaenus exclamationis (Thunberg, 1784) | 1 0, 1 | 1 | <0.1 | LC | ||||||
| Silting zone species | |||||||||||
| 80 | Stroggylocephalus agrestis (Fallén, 1806) | 3 0, 4 | 6 0, 7 | 12 0, 8 | 21 | 0.3 | EN | ||||
| 81 | Cosmotettix costalis (Fallén, 1826) | 3 0, 4 | 3 0, 4 | 6 | 0.1 | EN | |||||
| 82 | Limotettix striola (Fallén, 1806) | 1 0, 1 | 1 | <0.1 | VU | ||||||
| Xerothermophilic grassland species | |||||||||||
| 83 | Eupelix cuspidata (Fabricius, 1775) | 1 0, 1 | 1 0, 1 | 4 0, 4 | 6 | 0.1 | NT | ||||
| 84 | Doratura stylata (Boheman, 1847) | 2 0, 1 | 2 | <0.1 | LC | ||||||
| 85 | Delphacinus mesomelas (Boheman, 1850) | 1 0, 1 | 1 | <0.1 | VU | ||||||
| 86 | Streptanus marginatus (Kirschbaum, 1858) | 1 0, 1 | 1 | <0.1 | DD | ||||||
| Hygrophilous forest species | |||||||||||
| 87 | Planaphrodes nigrita (Kirschbaum, 1868) | 5 0, 6 | 5 | 0.1 | LC | ||||||
| 88 | Macropsis cerea (Germar, 1837) | 2 0, 1 | 2 | <0.1 | LC | ||||||
| 89 | Doliotettix lunulatus (Zetterstedt, 1840) | 1 0, 1 | 1 | <0.1 | |||||||
| Mesophilic forest species | |||||||||||
| 90 | Fagocyba cruenta (Herrich-Schäffer, 1838) | 4 0, 7 | 4 | 0.1 | LC | ||||||
| 91 | Hesium domino (Reuter, 1880) | 3 0, 2 | 3 | <0.1 | LC | ||||||
| 92 | Ulopa carneae Wagner, 1955 | 1 0, 2 | 1 | <0.1 | EN | ||||||
| Riparian species | |||||||||||
| 93 | Paraliburnia adela (Flor, 1861) | 8 0, 6 | 8 | 0.1 | EN | ||||||
Species abundance ranking. The species are ordered by their relative abundance (descending).
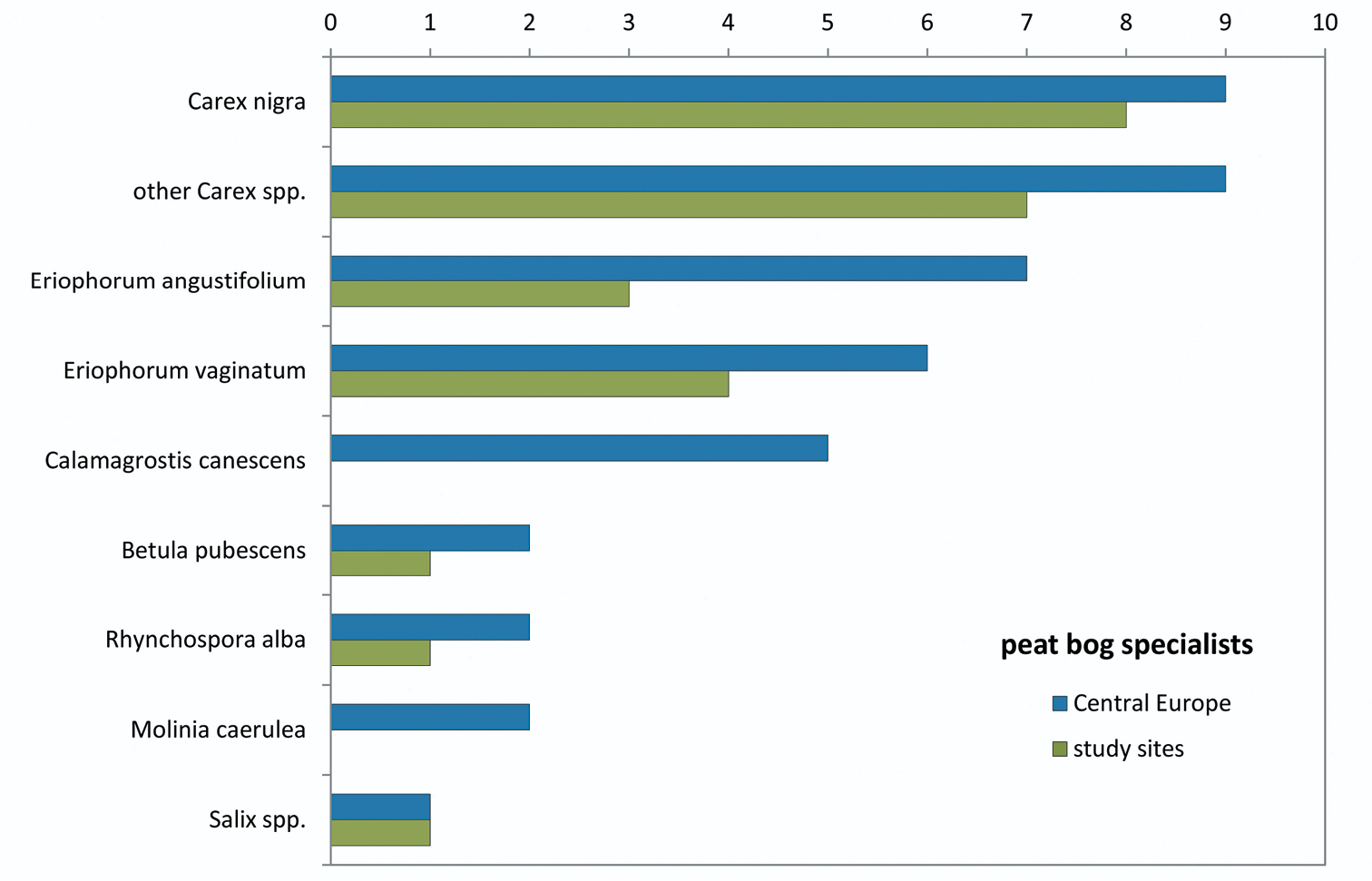
Eleven species are peat bog specialists, i.e. tyrphobiontic or tyrphophilous, according to
Auchenorrhyncha communities of the Austrian Bohemian Forest peat bogs: Presence of the ecological types (after
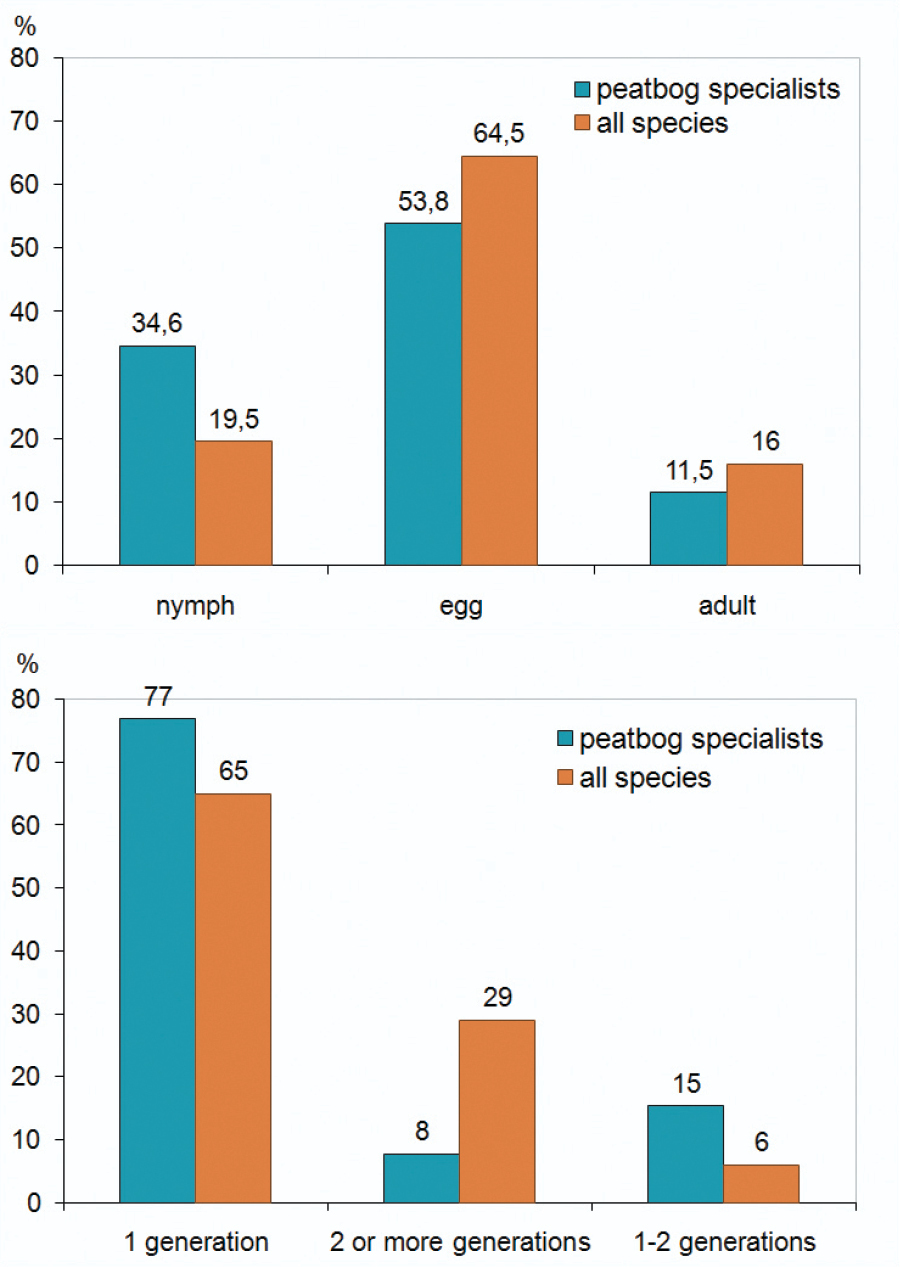
Percentage of the hibernation stages (left) and of generation numbers (right) of Auchenorrhyncha species recorded in Bohemian Forest peat bogs compared to those of the whole Austrian Auchenorrhyncha fauna (data from
Seven species could be found on all sites, among them one peat bog specialist (Sorhoanus assimilis). 18 species occur in at least five of the seven sites, among them three more peat bog specialists (Kelisia vittipennis, Paradelphacodes paludosa, Oncodelphax pullula). Almost half of the species (44) were recorded only on one site (among them four peat bog specialists).
The Auchenorrhyncha communities of the peat bogs show higher proportions of univoltine species than the total fauna of Austria. The number of species hibernating in nymphal stages is also higher in peat bogs than in the total fauna of Austria (Fig. 4). This might be caused by comparatively unsuitable conditions (low temperature, high humidity) for Auchenorrhyncha development in these habitats.
The vast majority of the Central European Auchenorrhyncha species is mono- or oligophagous, specialised on one or few host plant species or genera (see
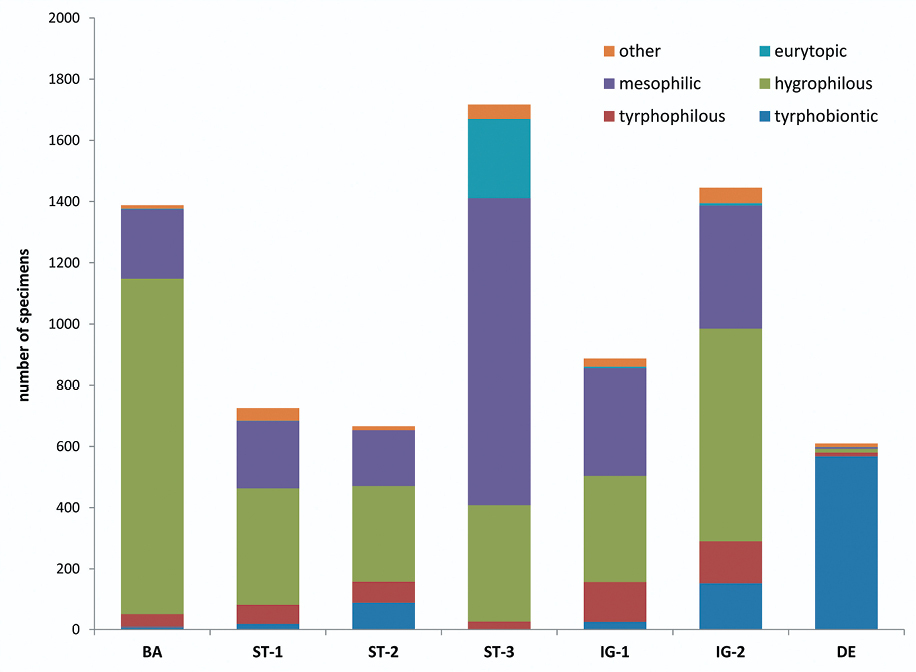
Total number of Auchenorrhyncha collected at the seven peat bog sites. Colours = ecological types (after
Number of tyrphobiontic and tyrphophilous species specialised on wetland plant species (data from
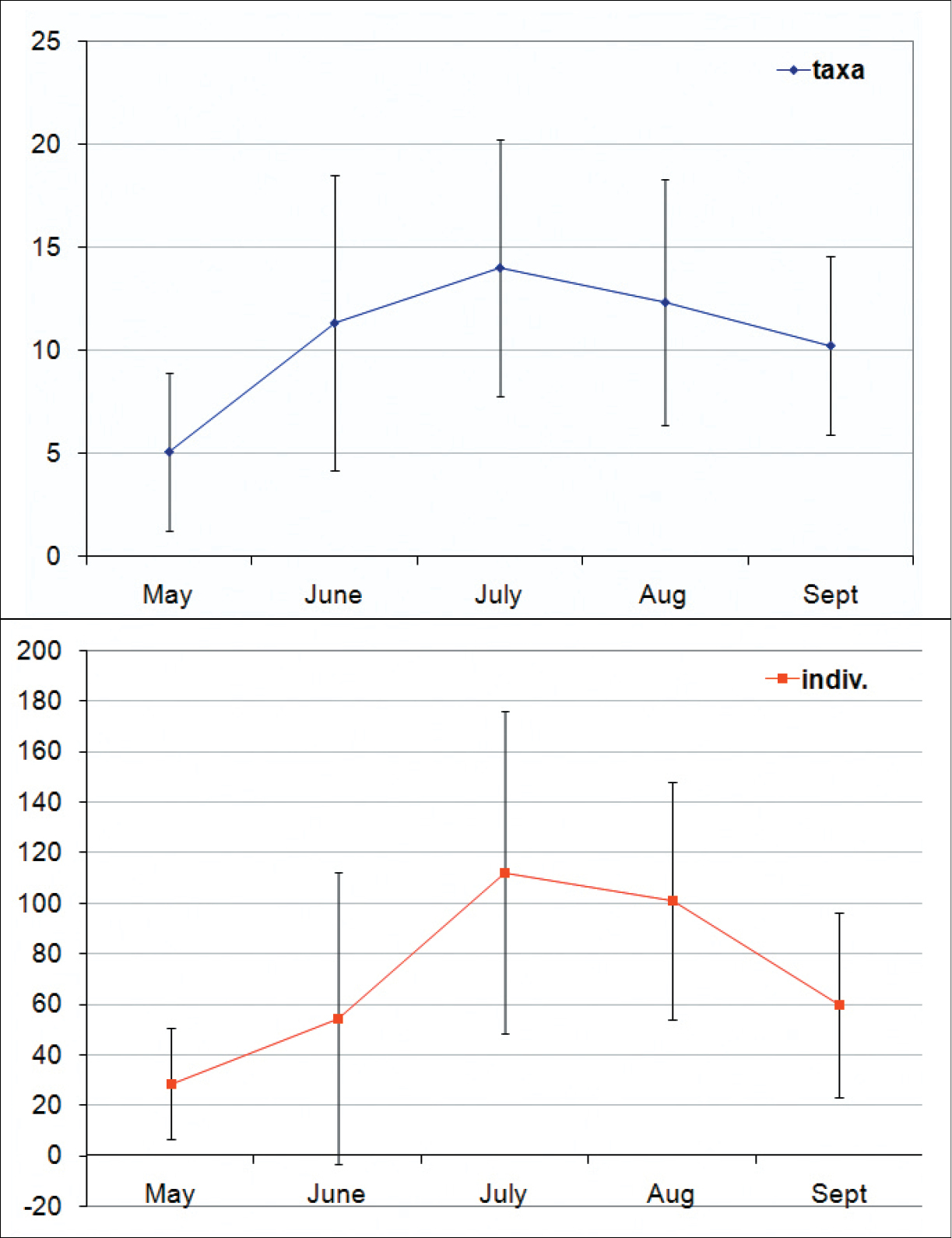
The densities of adult Auchenorrhyncha in peat bogs are low in spring (about 10–60 individuals per m²), increase towards July up to 180 (±50) individuals per m² and slowly decreases afterwards (Fig. 7). Disturbed sites have higher species numbers and higher Auchenorrhyncha densities in total but lower numbers and densities in peat bog specialists. The highest proportion of peat bog specialists (almost 95 %) was found in the undisturbed site „Deutsches Haidl“ (Fig. 5).
These Auchenorrhyncha densities of peat bogs are similar to those of other Central European grassland habitats (pastures and meadows: about 50–200 adult specimens/m²; alpine meadows: about 50–100 specimens/m²; ÖKOTEAM unpublished data).
Seasonal mean numbers of Auchenorrhyncha species (left) and adult hopper specimens (=individuals/m²; right) in the peat bogs of the Austrian part of the Bohemian Forest.
We are grateful to F. Gusenleitner (Biologiezentrum Linz), S. Guttmann and M. Hageneder (Government of Upper Austria), D. Hammerschmid, M. Pöstinger, K. Zimmerhackl (ÖNJ Haslach) and the Forstverwaltung Stift Schlägl for their support. The project was partially funded by the “Klima und Energiefonds” of the Austrian Government (Project No A760674).