(C) 2012 Daisuke Uyeno. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of copepod, Sarcotretes umitakae sp. n., of the siphonostomatoid family Pennellidae is described based on female specimens from the rattail Coelorinchus jordani Smith and Pope (Actinopterygii: Gadiformes: Macrouridae) caught in the East China Sea. This species is characterized by exhibiting the following characters: the large proboscis projects strongly; the head bears paired lateral processes which are bulbous and taper into a slender horn; the twisting neck is significantly longer than the trunk; and the trunk bears an anterior constriction with a reduced abdomen.
Mesoparasitic copepods, Sarcotretes umitakae sp. n. , new species, East China Sea, rattail, mesopelagic fishes
Sarcotretes Jungersen, 1911, a pennellid genus, was originally established based on Sarcotretes scopeli Jungersen, 1911 from Ireland, the eastern North Atlantic (
Two specimens of the rattail Coelorinchus jordani infected with copepods were caught in the East China Sea off the Tokara Islands, Kagoshima, Japan on 8 October 2011 during the cruise (UM-11-06) of the Umitaka-maru, a training and research vessel of Tokyo University of Marine Science and Technology (TUMSAT). The fishes were collected using an otter trawl towed for 30 minutes between two sites (29°58.02'N, 127°43.79'E to 29°59.57'N, 127°44.28'E) around 309 m in depth and, immediately after capture, they were preserved in 70% ethanol with copepods attached. In the laboratory, copepods were carefully removed from the tissues of fishes, and then soaked in lactophenol for a whole day before dissection. The appendages of the copepods were observed after dissecting with the method of
urn:lsid:zoobank.org:act:879FD1B6-AC17-4DD0-9E27-AFD4EF2A4DEF
http://species-id.net/wiki/Sarcotretes_umitakae
Figures 1–4Holotype female (NSMT–Cr 22253) and 2 paratypic females (NSMT–Cr 22254), ex Coelorinchus jordani Smith and Pope (Gadiformes: Macrouridae), taken off the Tokara Islands (29°58.02'N, 127°43.79'E to 29°59.57'N, 127°44.28'E), Kagoshima, East China Sea, Japan, 308.5–309.3 m depth, 8 October 2011, reg. K. Wakabayashi and Y. Tanaka.
off the Tokara Islands (29°58.02'N, 127°43.79'E to 29°59.57'N, 127°44.28'E), Kagoshima, East China Sea, Japan.
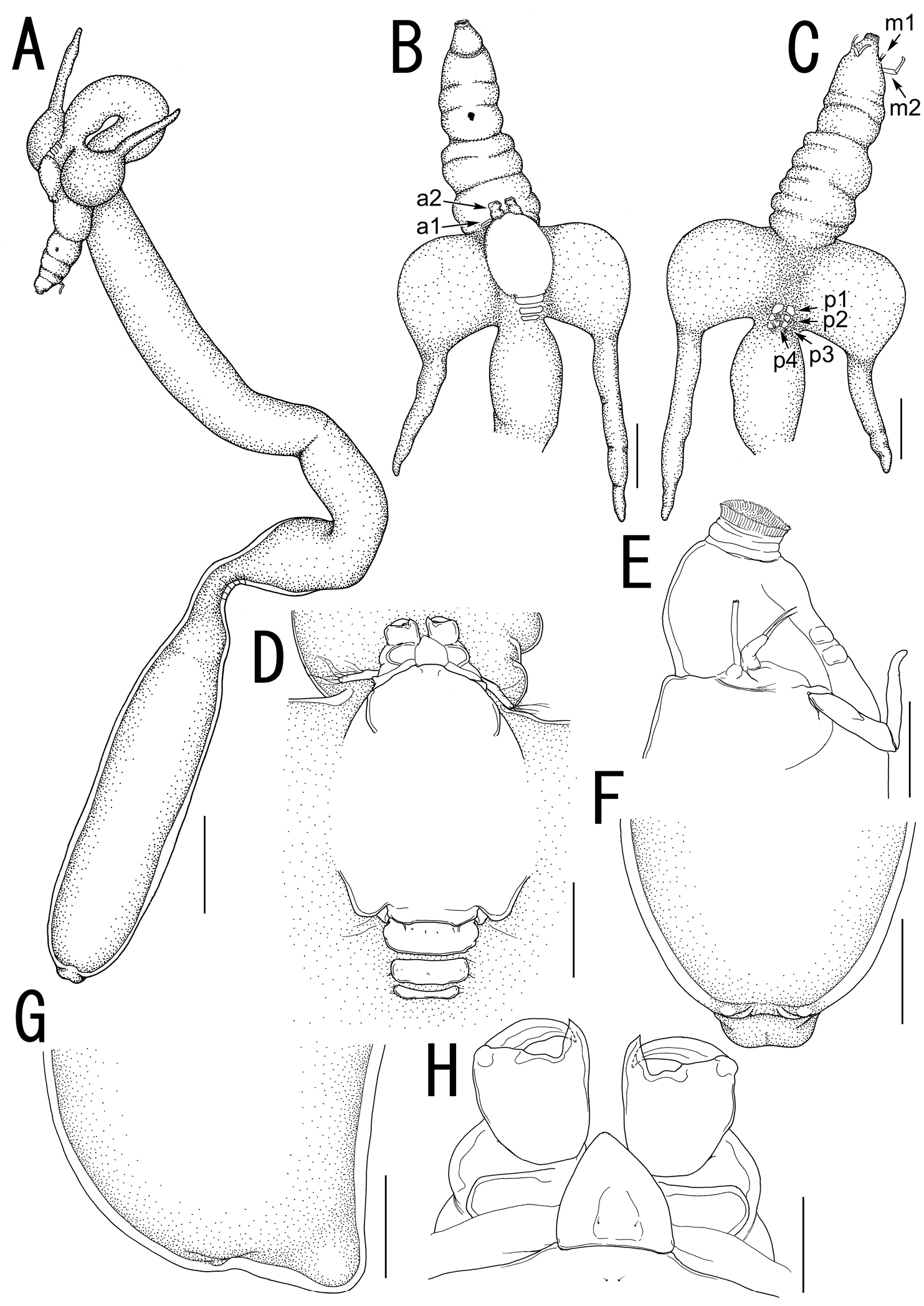
Body (Fig. 2A) elongate, comprising head, neck, and trunk. Total length 43.42 (from tip of cephalothorax to end of abdomen). Head (holdfast) composed of cephalosome to third pediger (Fig. 2A, B), bearing elongate oral area projecting forward as cylindrical proboscis with multiple constrictions and mouth tube at its tip, with paired lateral processes (Fig. 2A, B) consisting of bulbous base drawn out into highly sclerotized horn-like process. Vestige of dorsal shield of cephalothorax and tergites of first to third visible on dorsal surface of head (Fig. 2D). Two paired small sclerites on ventral surface of basal region of oral cone (Fig. 2E). Neck (Fig. 2A) slender, longer than trunk, twisting and bearing bulge and constriction at posterior portion. Cylindrical trunk (Fig. 2A) 12.23 long (from enlarged end of neck to abdomen), 3.08 wide at widest part bearing paired hemispherical protrusions and reduced abdomen (Fig. 2F, G). Caudal rami absent.
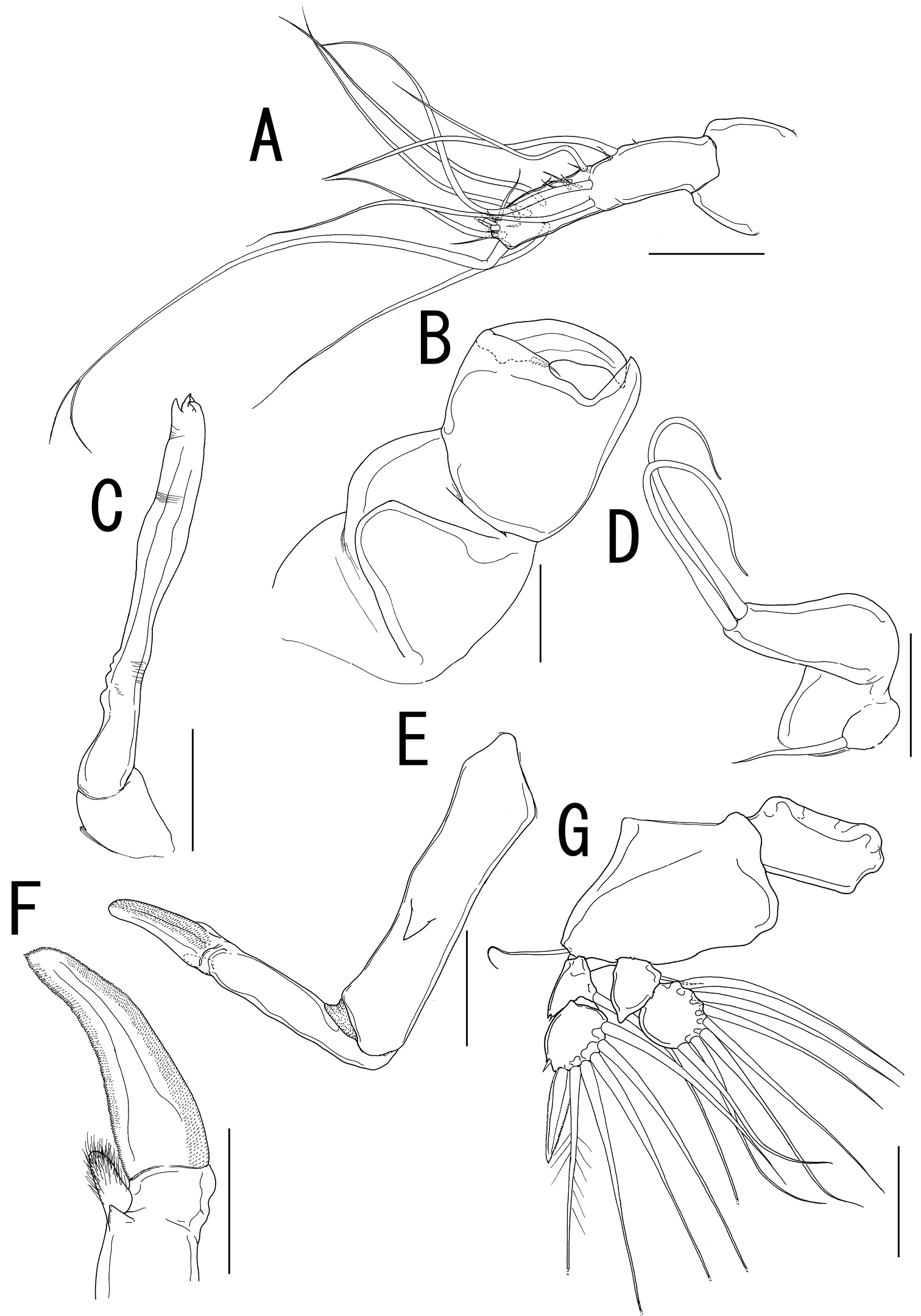
Rostral area (Fig. 2H) triangular. Antennule (Fig. 3A) not segmented, located on sclerotized protrusion, bearing 10 blunt, long elements, and at least 17 short elements; 1 long distal seta with bifurcated tip. Antenna (Fig. 3B) subchelate, 3-segmented; proximal segment, unarmed; middle segment stout with a pointed process on innerdistal corner, hollowed out to receive terminal claw; terminal segment representing terminal claw with single basal seta. Mandible (Fig. 3C) located on lateral side of base of oral cone (Fig. 2E), represented by sclerotized process with unequal processes tip. Maxillule (Fig. 3D) bilobate; large inner lobe tipped with two naked setae; small outer lobe bearing one naked seta. Maxilla (Fig. 3E) 2-segmented; proximal segment rod-like, bearing round protrusion with pointed process in middle portion; terminal segment rod-like, tipped with curved spinulose process, small pointed process, and setulous lobe (Fig. 3F). Maxilliped absent.
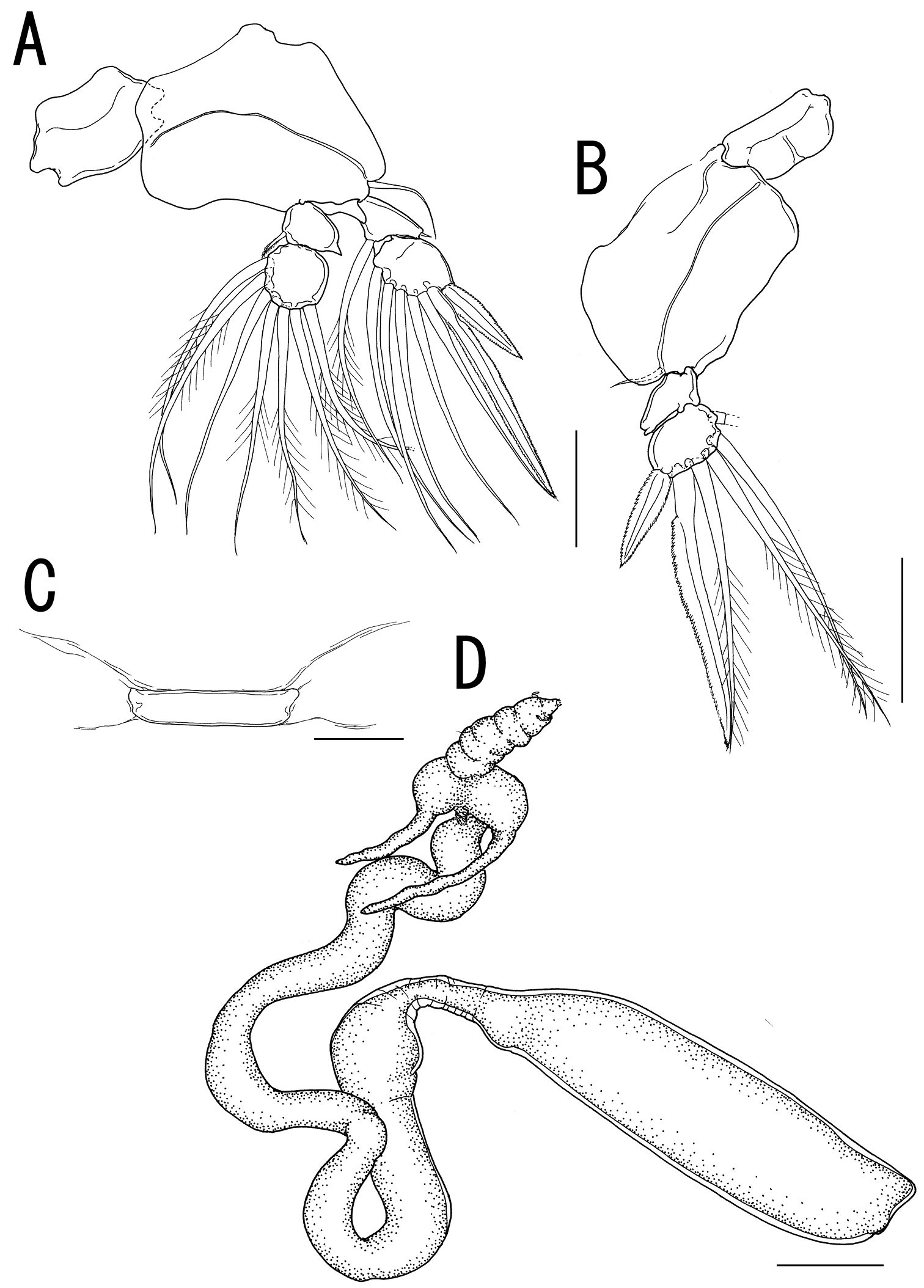
Legs 1 to 4 occurring tightly together and located between paired lateral processes of holdfast. Legs 1 and 2 (Figs. 3G, 4A) biramous, composed of inter coxal sclerites, protopods, and 2-segmented rami. Leg 3 (Fig. 4B) uniramous, without endopod; leg armature formula as follows:
Leg 4 (Fig. 4C) represented by vestigial intercoxal sclerite. Legs 5 and 6 absent.
Variability of female. The necks of all paratypes twist and turn in complex fashion (Fig. 4D). Measurements of the body parts of the specimens from the type series (n = 3) are as follows: body length (anterior margin of the head to distal end of the posterior processes on the trunk) 30.26–50.12 (41.27 ± 10.10); trunk length 11.27–13.48 (12.33 ± 1.11); trunk width 2.84–3.17 (3.03 ± 0.17).
Male. Unknown.
The copepod attaches to various parts of the body surface of the host fishes (Fig. 1A). The head to the neck of the copepod was embedded in the host’s musculature, and the trunk was protruded into the water (Fig. 1B)
The specific name “umitakae” refers to the Umitaka-maru, a training and research vessel of TUMSAT.
Currently, three species of Sacotretes: Sacotretes eristaliformis, Sacotretes longirostris, and Sarcotretes scopeli, are considered to be valid (
Sarcotretes umitakae sp. n., female on Coelorinchus jordani Smith and Pope. A two specimens of Coelorinchus jordani (181.5 mm TL and 142.8 mm TL) carrying the type series of Sarcotretes sp. n. (arrowheads) B coloration in life of paratype NSMT–Cr 22254 attached to host’s body. Scale bars: A=20 mm; B=3 mm.
Sarcotretes umitakae sp. n., female, holotype NSMT–Cr 22253. A habitus B anterior portion of body, dorsal, a1 = antennule, a2 = antenna C same, ventral, m1 = maxillule, m2 = maxilla, p1 = leg 1, p2 = leg 2, p3 = leg 3, p4 = vestige of leg 4 D vestige of dorsal cephalothoracic shield E tip of proboscis, lateral F posterior portion of body, ventral G same, lateral H rostral area and antennae, dorsal. Scale bars: A=3 mm; B, C, F, G=1 mm; D=500 μm; E=300 μm; H=150 μm.
Sarcotretes umitakae sp. n., female, holotype NSMT–Cr 22253. A left antennule, anterior B left antenna, anterior C left mandible D left maxillule E left maxilla, lateral F distal part of left maxilla G right leg 1, anterior. Scale bars: A, B, E, G=100 μm; C, D=70 μm; F=50μm.
Sarcotretes umitakae sp. n., female, holotype NSMT–Cr 22253. A left leg 2, anterior B right leg 3, anterior C vestige of leg 4. Sarcotretes umitakae sp. n., female, paratype NSMT–Cr 22254 D habitus. Scale bars: A, B=100 μm; C=30 μm; D=3 mm.
Despite the fact that some morphological characters of Sarcotretes species (e.g. the shape of the holdfast, the length and flexure of the neck, and the length of the proboscis) show variability, they have been conventionally used to distinguish the species in this genus (
The discovery of Sarcotretes umitakae sp. n. in this study shows that there are at least 2 species of the genus in Japanese waters.
| 1 | Proboscis slightly projecting; holdfast composed of broad base with or without terminal process; neck shorter than or as long as trunk | 2 |
| – | Proboscis elongate, strongly projecting; holdfast comprising slender processes; neck significantly longer than trunk | 3 |
| 2 | Body up to approximately 25 mm long; neck shorter than trunk; leg 4 absent | Sarcotretes scopeli |
| – | Body approximately 45 mm or longer (about twice length of Sarcotretes scopeli); neck about as long as trunk; vestige of leg 4 (intercoxal sclerite) present | Sarcotretes eristaliformis |
| 3 | Slender, horn-like lateral processes on head (holdfast); trunk not constricted; reduced abdomen conical, projecting posteriorly | Sarcotretes longirostris |
| – | Lateral process bulbous tapering into slender, elongate horn; trunk with anterior constriction; abdomen reduced, vestigial | Sarcotretes umitakae sp. n. |
We would like to acknowledge the captain and crew of the Umitaka-maru (TUMSAT) for their support during the cruise. We also thank Dr. Tadashi Tokai (TUMSAT), an organizer of the cruise, for his courtesy and understanding of our study on the parasitic copepod fauna of the East China Sea. We are grateful to Dr. Yuji Tanaka (TUMSAT) for his assistance in collecting the specimens onboard the vessel. Part of this work received a financial support from Grants-in-Aid for JSPS Fellows (No. 23-4311 to D.U.).