(C) 2013 Francesca Benzoni. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Benzoni F (2013) Echinophyllia tarae sp. n. (Cnidaria, Anthozoa, Scleractinia), a new reef coral species from the Gambier Islands, French Polynesia. ZooKeys 318: 59–79. doi: 10.3897/zookeys.318.5351
A new shallow water scleractinian coral species, Echinophyllia tarae sp. n., is described from the Gambier Islands, French Polynesia. It is characterized by an encrusting corallum, a few large and highly variable corallites with protruding walls, and distinctive costosepta. This coral was observed in muddy environments where several colonies showed partial mortality and re-growth. The new species has morphological affinities with both Echinophyllia echinata and with Echinomorpha nishihirai, from which it can be distinguished on the basis of the diameter and the protrusion of the largest corallite, the thickness of the septa, and the development of the size of the crown of paliform lobes.
Lobophylliidae, Echinophyllia echinata, Echinomorpha nishihirai, Tara Oceans Expedition
At present, Echinophyllia Klunzinger, 1879 is known to include eight extant zooxanthellate species (
Presently, Echinophyllia is known to occur in the Indo-Pacific, from the seas around Arabia (
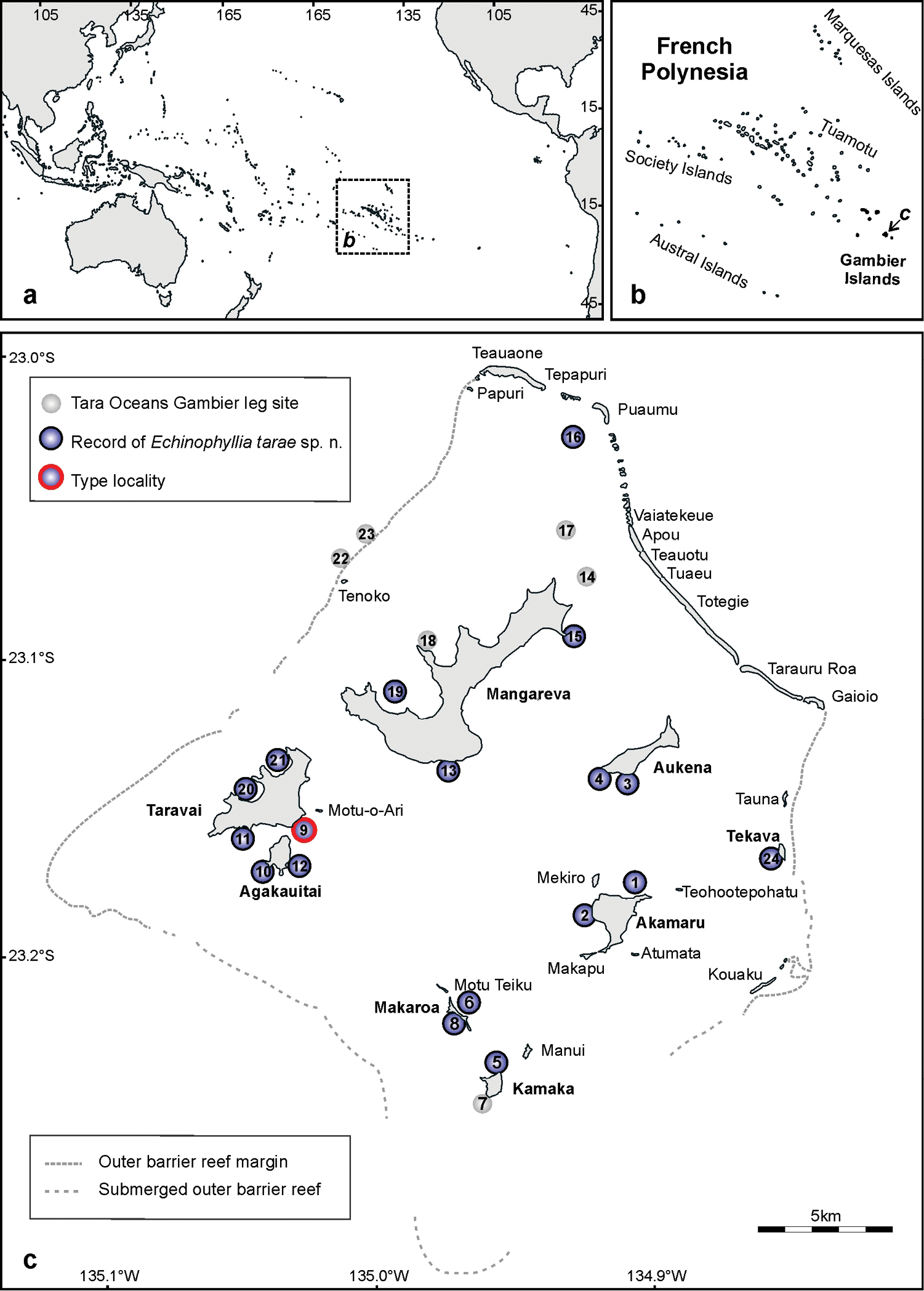
The remote and relatively poorly studied Gambier Islands are found at the southeast end of the vast French Polynesian territory (Figure 1a, b). The actual islands were once all part of the same volcano, which in time has almost completely drowned (
Map of a the Pacific Ocean, showing the position of French Polynesia b the island groups belonging to French Polynesia, and c the study area in the Gambier Archipelago showing the 24 sampling sites of the Tara Oceans leg and the sites where Echinophyllia tarae sp. n. was found. Stippled rectangle in a shows the position of the map shown in b. Arrow in b shows the position of the Gambier Archipelago shown in c.
With regard to Echinophyllia species,
A reference collection of Scleractinia was sampled in the Gambier, including coral skeletons and tissues fixed for DNA, after in situ photographs were taken. The collection contained five specimens of Echinophyllia tarae sp. n. Sampling took place during SCUBA diving at different sites around the islands of Taravai, Akamaru, and Makaroa (Figure 1). Digital images of living corals in the field were taken with a Canon G9 in an Ikelite underwater housing system. Coral specimens were collected, tagged, and for each specimen a fragment of 1 cm2 was broken off the colony and preserved in CHAOS solution for further molecular analysis. The remaining corallum was placed for 48 hours in sodium hypochlorite to remove all soft parts, rinsed in freshwater and dried for microscopic studies. Images of coral skeletons were taken with a Canon G9 digital camera and through a Leica M80 microscope equipped with a Leica IC80HD camera. For high resolution and deep field close ups of three-dimensional details of corallites and septa, a series on images of the same subject at different focus intervals were taken (approximately 10) and the images were fused using the Helicon Focus 5.3 software (
A total of 24 sites was surveyed (Table 2). Each site position was recorded with a Garmin eTrex GPS. At each site all coral species encountered in a 1 hour SCUBA dive were recorded and data were included into a geo-referenced database. At least two images per specimen were taken underwater, one of the complete colony and one close-up. Digital images were then analyzed to verify underwater preliminary records and species presence records were used to produce a species per site matrix. Data on the occurrence of Echinophyllia tarae sp. n. in the field in the different sites was extracted from this species per site database.
The holotype was deposited at Museum National d’Histoire Naturelle (MNHN) in Paris, the other four specimens are at the University of Milano-Bicocca (UNIMIB) coral facility together with the rest of the Tara Oceans Expedition collection (186 specimens), which will ultimately be housed at the MNHN once their study is completed. Specimens of other Echinophyllia species were examined at the Museum of Tropical Queensland (MTQ), Townsville, Australia, and at the Institut de Recherche pour le Développement (IRD), Nouméa, New Caledonia.
Type species (by monotypy). Madrepora aspera Ellis & Solander, 1786.
urn:lsid:zoobank.org:act:9C41F53B-3FEE-47BA-8017-48C252F65F5E
http://species-id.net/wiki/Echinophyllia_tarae
Figures 2–8, 9a, b, 10b, dHolotype: MNHN-IK.2012-8000 (Figures 2–4). Type Locality: Taravai Island, Gambier, French Polynesia (MV Tara, Tara Oceans Expedition, Site 9), 23°9.404'N, 135°1.769'E, 10 m, 30 June 2011, coll. F. Benzoni.
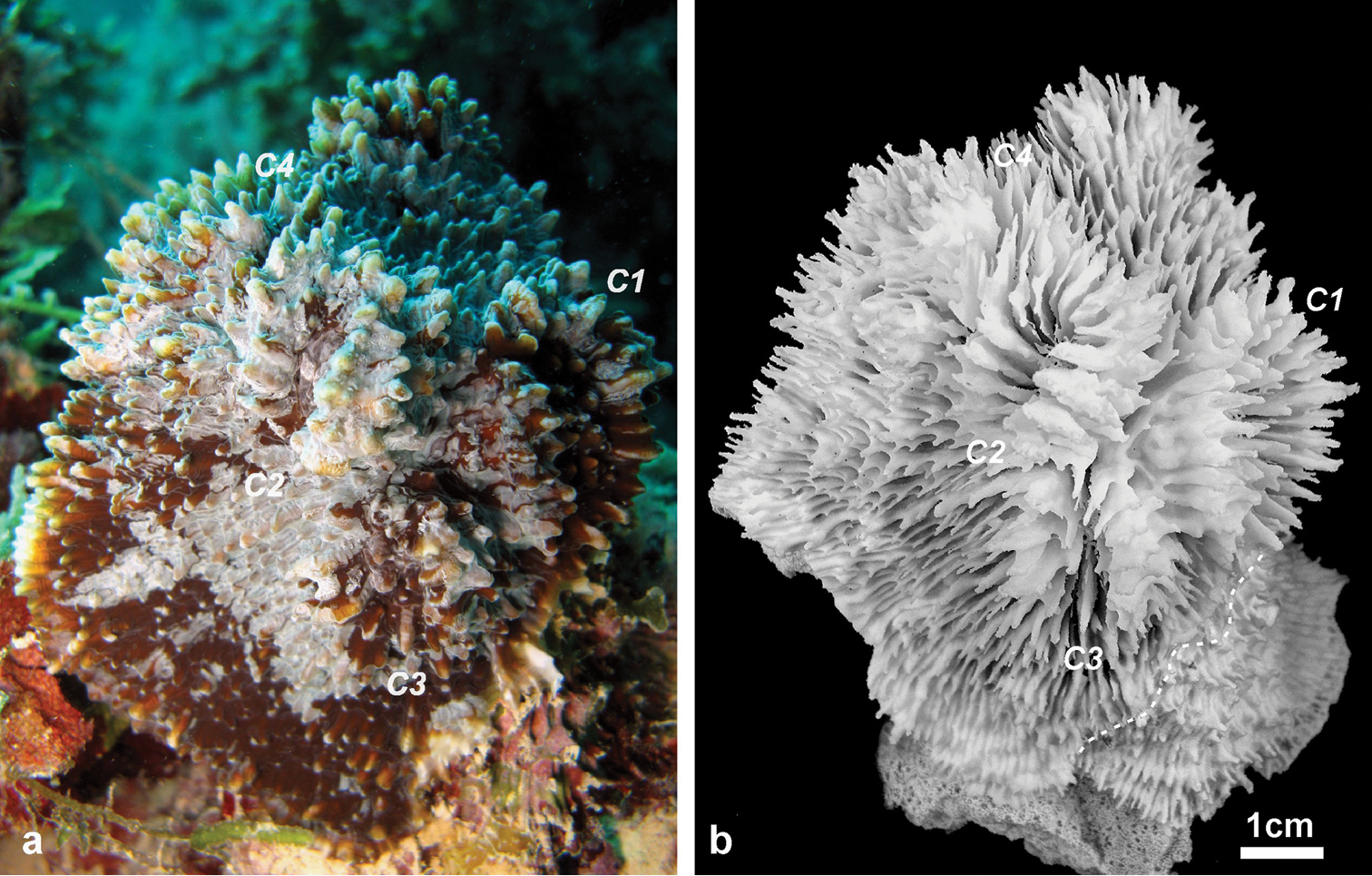
Holotype of Echinophyllia tarae sp. n. (MNHN-IK.2012–8000) a the colony in situ before collection, and b the corallum after removal of the animal tissues. C1 to 4 indicate the position of same corallite (C) in the two images. Numbers are assigned in decreasing order of corallite size, C1 being the largest. Stippled line on the specimen in b shows the boundary of living tissue at the time of collection.
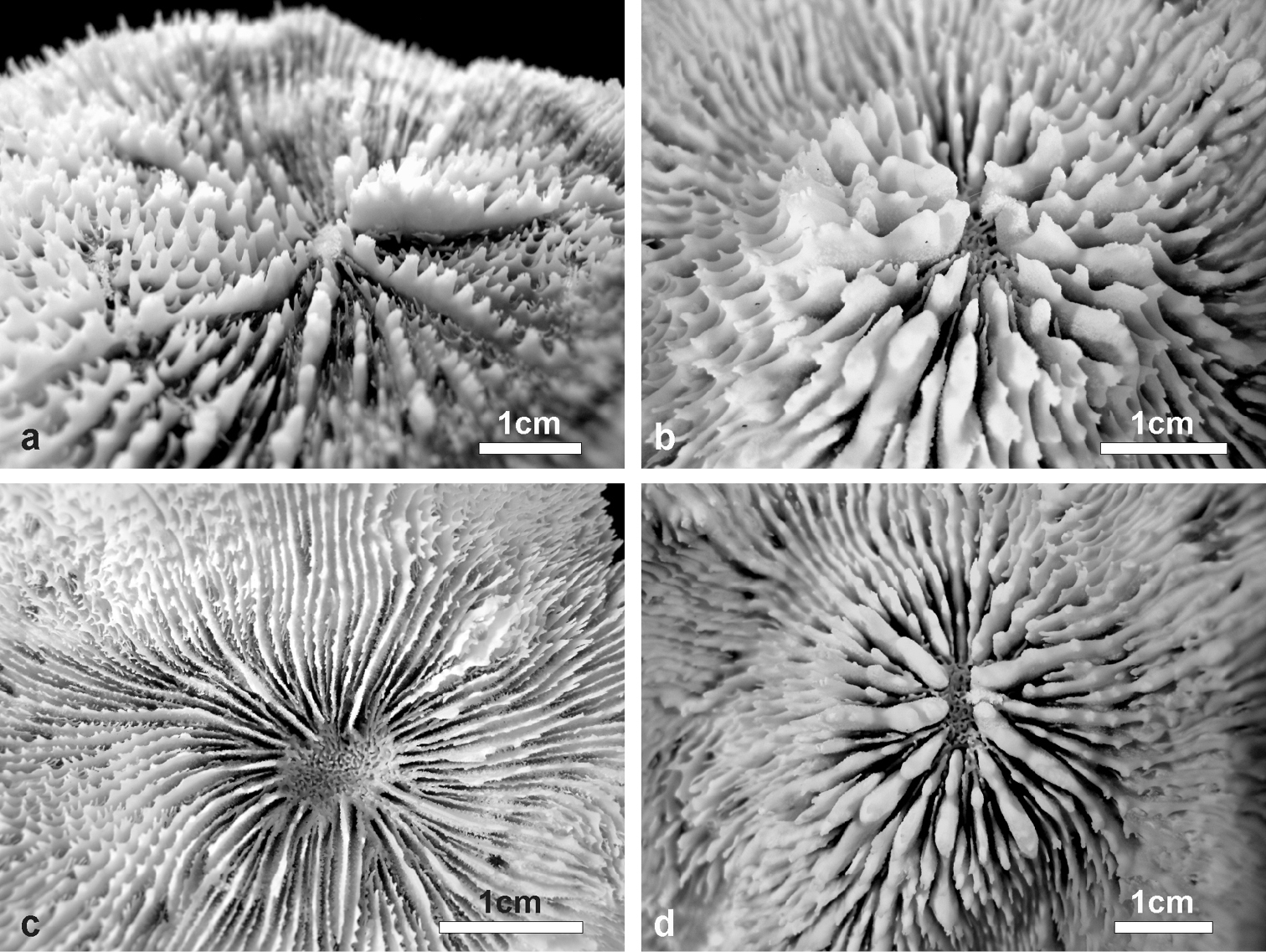
Details of corallites, septa, and costae in the holotype of Echinophyllia tarae sp. n. (MNHN-IK.2012–8000) a top view of the largest corallite in the colony, b lateral view of the same corallite shown in a, c top view of the second largest corallite, and d of the third e top and f side view of the costae. C1 to 3 indicate the corallites as shown in Figure 2. White arrows in e indicate the position of exothecal alveoli at the insertion of costae.
Specimens of Echinophyllia tarae sp. n. collected for this study a side view of the holotype (MNHN-IK.2012–8000) b specimen UNIMIB TO-GA028 c specimen UNIMIB TO-GA186 d specimen UNIMIB TO-GA099 e specimen UNIMIB TO-GA071. C1 to 3 indicate the holotype corallites as shown in Figures 1 and 2.
Corallum: The holotype is a knob-shaped, plocoid, encrusting colony attached to a fragment of a dead tabular Acropora coral (Figure 2). The specimen is 9.2 cm high, and 8.5 x 5 cm wide at the base in its original growth position.
Corallites: The 12 corallites are oval in shape and variable in size (Figure 2, 3), ranging from 3.3 cm in diameter (C1 in Figure 2, 3a) to 1.0 cm. Corallites are organically united (see terminology in
Costosepta: Variable in number depending on the size of the corallite (Figure 3a–d), exsert and thickened over the theca. The largest corallite contains 26 septa arranged in five orders (Figure 3a). Septa of the first three orders are thicker than the others. Septal teeth are elliptical in outline, large and high (> 0.6 mm) according to the parameters defined by
Columella: Well developed, deep in the fossa (Figure 3b) made by a mesh of twisted intermingled processes derived from the inner end of the higher order septa: the first two in smaller corallites (Figure 3a–d), and up to the fourth order in larger corallites (Figure 10b–d).
Colour: The living colony was mottled brown. Tips of septa and costae ornamentation varied from light beige to white.
(Gambier, French Polynesia, Tara Oceans Expedition): UNIMIB TO-GA028, Akamaru Island (Site 2), 23°11.082'N, 134°54.331'E, 26 June 2011, coll. F. Benzoni; UNIMIB TO-GA071 Makaroa Island (Site 6), 23°12.960'N, 134°57.991'E, 28 June 2011, coll. F. Benzoni; UNIMIB TO-GA099 Taravai Island (Site 11), 23°9.540'N, 135°3.055'E, 1 July 2011, coll. F. Benzoni; UNIMIB TO-GA186 Taravai Island (Site 9), 23°9.404'N, 135°1.769'E, 30 June 2011, coll F. Benzoni.
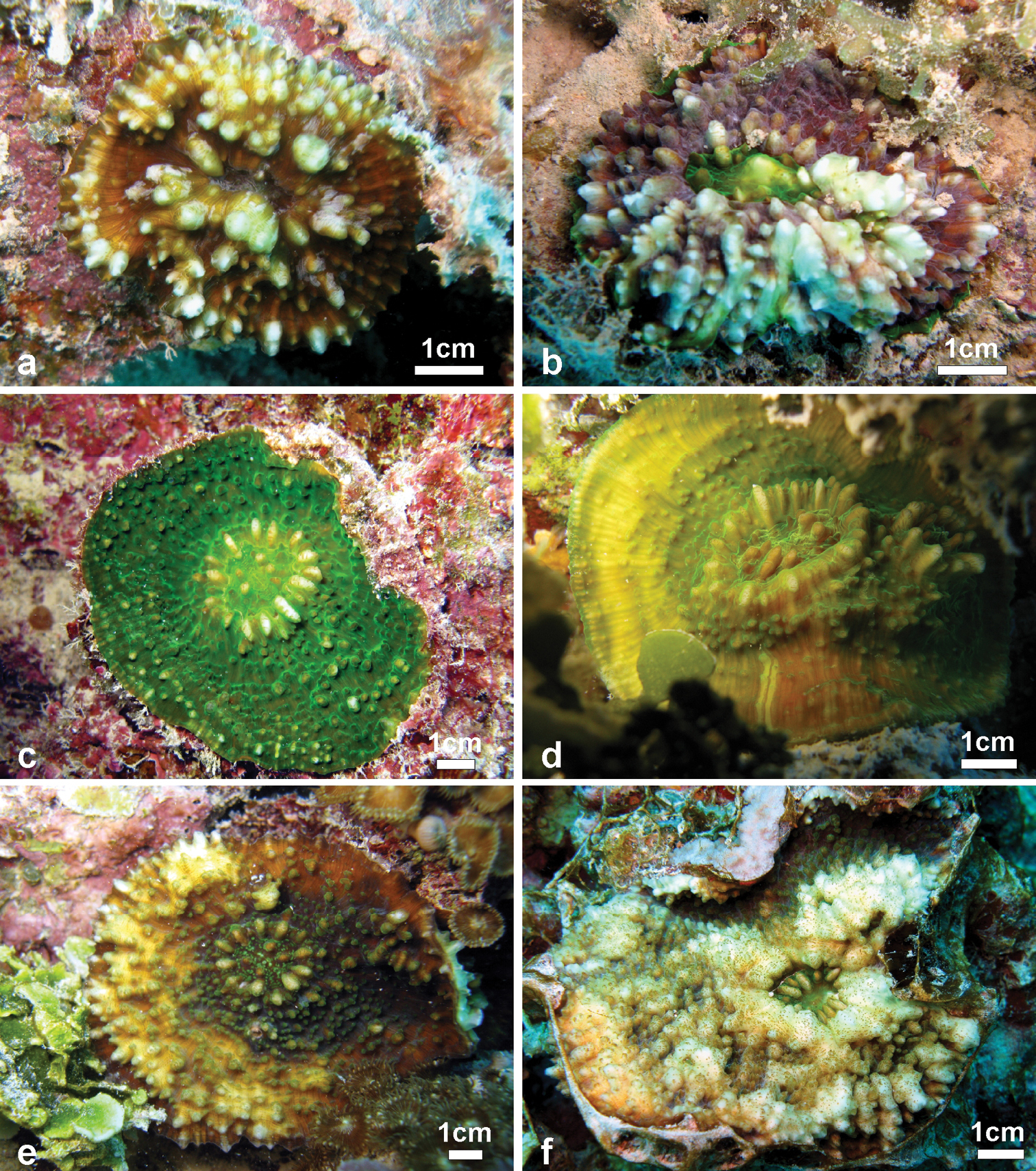
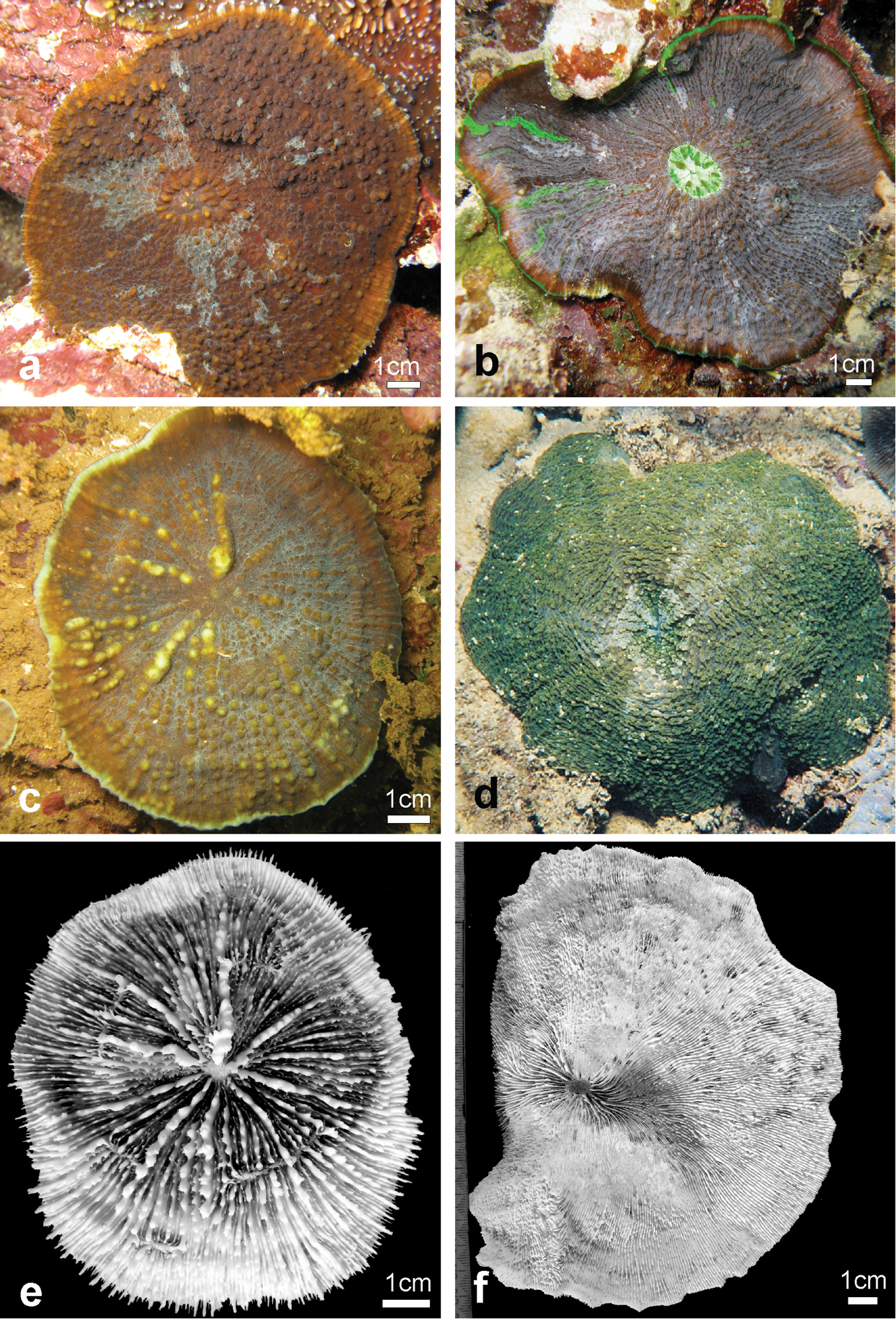
Variation of skeletal structures: Colony size is relatively small (Figures 2–8), the largest colony is 20 cm wide (Figure 6f). Corallum generally encrusting, its plane following the surface of the underlying substrate (Figures 4b, d, e) but also knob-like (Figures 4a, c) with foliose margins where they become detached from the substrate. The number of corallites per colony is low, ranging from 1 to 15. A large, central primary corallite is always present (Figures 2, 6a, b). Secondary corallites are often inclined in various directions and show variable diameter sizes within the same colony (Figures 5a–e, Table 1). The largest corallite observed (specimen UNIMIB TO-GA099, Figure 4d) is 3.5 cm in diameter, the smallest, in the same specimen, 0.9 mm (Figure 5e). The numbers of septa, orders of septa, and paliform lobes vary between and within colonies. First order septa always thicker than the others and in some cases up to 4 mm thick (Figure 5f). Columella always present, large and oval in the largest corallite, less developed is smaller corallites. Costae typically thick, alternating in size (Figure 5c, d) and strongly dentate, although variably so between specimens.
Variation in shape and size of the corallites of Echinophyllia tarae sp. n. a and b two lateral views of specimen UNIMIB TO-GA186 c specimen UNIMIB TO-GA071 d UNIMIB TO-GA028 e specimen UNIMIB TO-GA099 f close up-of a corallite of the same specimen as in a and b. White arrows in e indicate the position of secondary corallites.
Variation of corallum shape, costosepta ornamentation, and colouration in smaller colonies of Echinophyllia tarae sp. n. observed in situ a young one polyp brown coloured specimen settled on crustose coralline algae, Mangareva Island (Site 15) b a dark brown two polyp colony with green oral discs, Taravai Island (Site 21) c one polyp bright green specimen with a large raised central corallite in which the crown of pali is clearly visible and spiky and well developed costae, Makaroa Island (Site 6) d a light green colony with two raised polyps in central position, well developed crown of pali and costae ornamentation smoothening towards the colony periphery, northern lagoon pinnacles (Site 16) e brown colony with one raised polyps in central position and markedly teethed costae, Mangareva Island (Site 13) f in situ image of specimen UNIMIB TO-GA028 (Figures 4b, 5d) showing the irregularly shaped colony with a very rugged and almost inflated appearance, Akamaru Island (Site 2). Sites are indicated in Figure 1.
Dimensions of the examined specimens of Echinophyllia tarae sp. n. For each specimen the total number (n.) of corallites, the maximum and minimum diameter of the largest and smallest corallite in the colony, the number of septa, and of paliform lobes is listed. LC = largest corallite; SC smallest corallite.
| Specimen code | n. of corallites | Diam. LC max-min: (cm) | Diam. SC max-min: (cm) | n. of septa LC | n. of paliform lobes LC |
|---|---|---|---|---|---|
| MNHN-IK.2012–8000 | 12 | 3.3 – 2.0 | 1.0 – 0.9 | 26 | 11 |
| UNIMIB TO-GA028 | 4 | 3.1 – 2.4 | 1.1 – 0.9 | 39 | 14 |
| UNIMIB TO-GA071 | 4 | 2.3 – 2.1 | 1.0 – 0.9 | 34 | 8 |
| UNIMIB TO-GA084 | 4 | 3.2 – 2.4 | 1.9 – 1.8 | 29 | 11 |
| UNIMIB TO-GA099 | 3 | 3.5 – 3.0 | 1.2 – 1.0 | 52 | 24 |
Field characteristics and colouration: The colouration is showing much variation, ranging from dark brown (Figures 7a–c, e), to mottled brown (Figures 2a, 7f), beige (Figures 6e, f), and bright green (Figures 6c, 7d). The tissue on the tips of septal teeth and costal spines teeth can be lighter in colour (Figure 6c) or white (Figure 7c), possibly as a result of tissue being less thick above these structures. In very mottled colonies (Figure 7f) or with lighter colouration of the tissue over costoseptal teeth (Figure 7b) the size and shape of the corallites may be hard to detect. The crown of paliform lobes is always prominent (Figure 5) and often obvious, especially in the largest corallite (Figures 6, 7b).
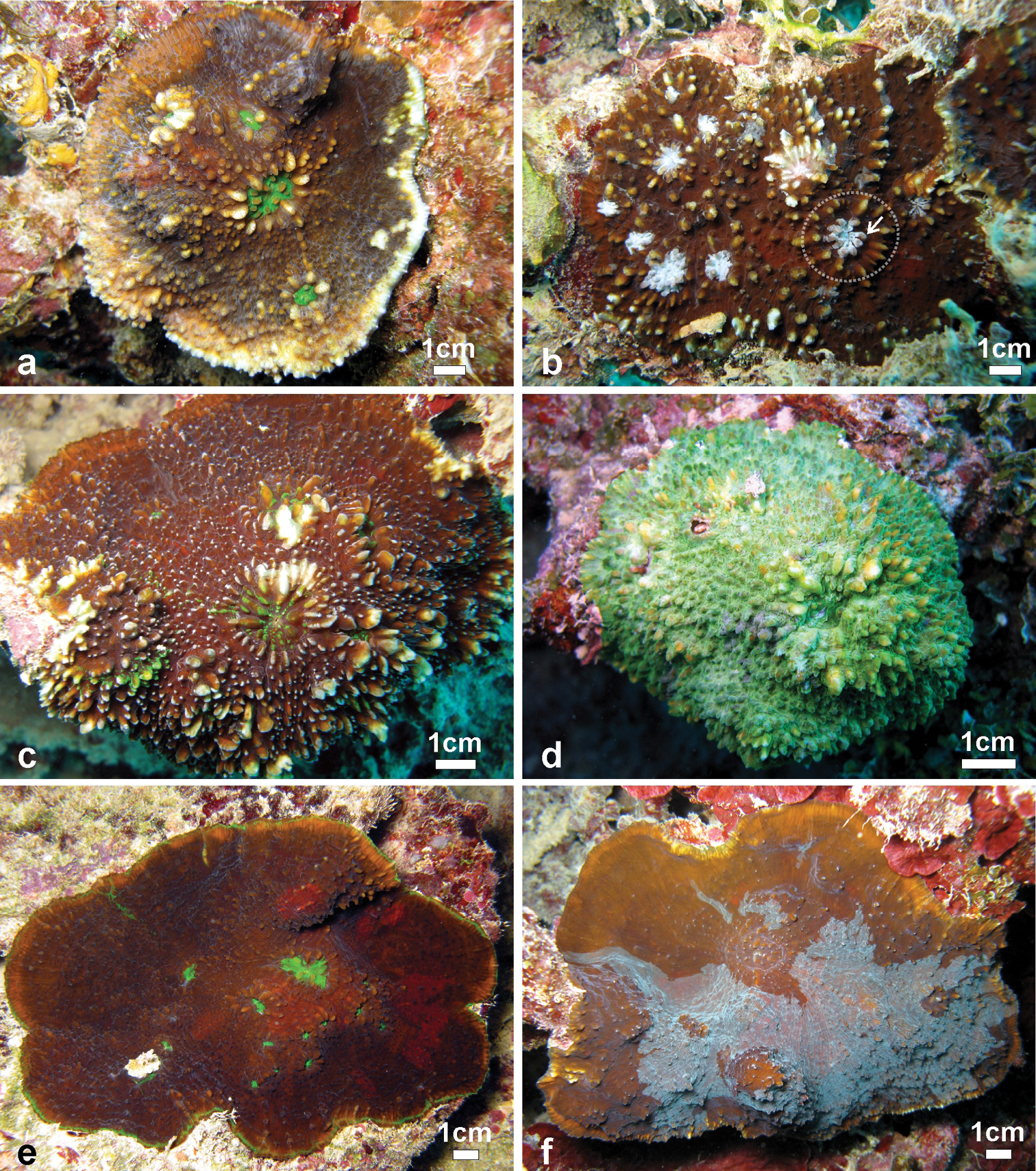
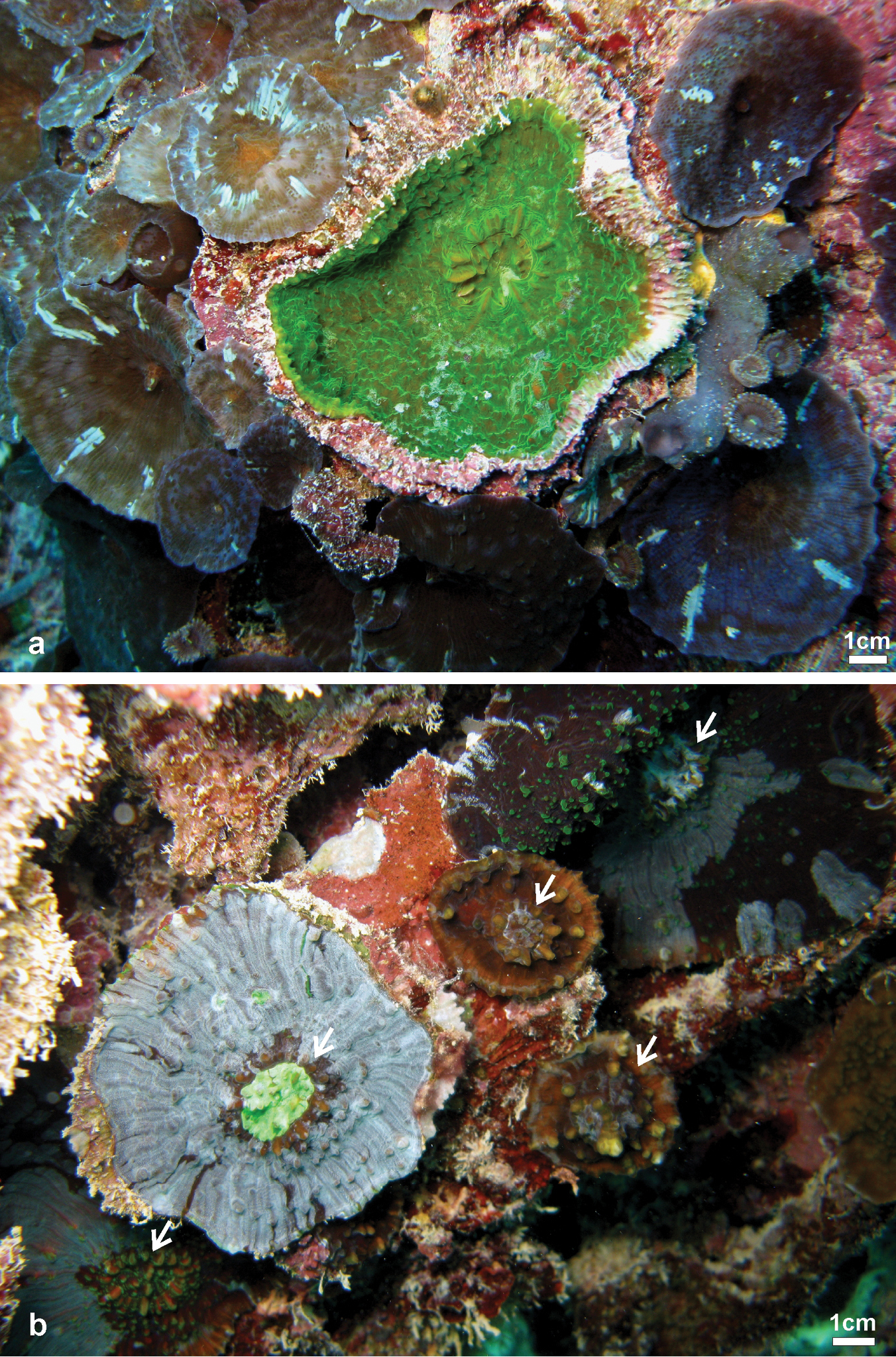
Variation of shape, spikiness of septa and costae, and colouration oflarge colonies Echinophyllia tarae sp. n. observed in situ a brown encrusting colony with free margins, bright green oral discs and raised corallites, Akamaru Island (Site 2) b brown encrusting colony with white oral discs, raised corallites (larger one in the stippled circle), and very spiky costae, Taravai Island (Site 9 – type locality), the prominent crown of paliform lobes of the largest corallite is indicated by the white arrow c brown knob shaped colony with bright green oral discs and raised corallites, note the white colouration of the tips of costae teeth, Taravai Island (Site 9 – type locality) d a bright green knob shaped colony, Taravai Island (Site 9 – type locality) e brown encrusting colony with bright green oral discs and relatively low-lying corallites, note the uniform colouration of the costae, Taravai Island (Site 9 – type locality) f mottled brown encrusting colony with free margins and relatively low-lying corallites, note the uniform colouration of the costae, Taravai Island (Site 9 – type locality). Sites are indicated in Figure 1.
Frequently observed patterns of partial death and re-growth of Echinophyllia tarae sp. n. in the fielda concave colony with a large central corallite showing a peripheral rim of skeleton encrusted by pink coralline algae and surrounded by zoanthids and corallimorpharians, Mangareva Island (Site 19) b a similar situation as in a but with re-growth occurring over previously dead colonies, note the variation of colouration in adjacent corals, Taravai Island (Site 11). White arrows in b indicate the position of larger central corallites. Sites are indicated in Figure 1.
Ecology: Echinophyllia tarae sp. n. inhabits protected reef habitats and was observed between 5 and 20 m depth. It commonly grows on dead coral fragments, usually parts of branching or tabular Acropora colonies, which are covered by crustose coralline algae and fleshy macroalgae (Figures 6–7). This species can grow on well-illuminated surfaces but also encrusts shaded underhangs. In well-lit conditions the appearance is typically corrugated (Figures 6a–b, e–f, 7a–d). However, in some cases a certain degree of inflation of the soft tissues was observed (Figures 6a–b, f), although this generally depends on the very developed ornamentation of the underlying costosepta, which is most obvious when a live colony (Figure 6f) is compared with the clean skeleton (Figures 4b, 5d). In poorly lit conditions the overall appearance is smoother and the colouration more uniform (e.g. Figures 6d, 7e–f) although the oral discs remain generally brightly coloured and different from the rest of the tissues. Re-growth of partially dead colonies, especially at the margins, is common (Figure 8). Such patters of partial death and recovery could result from competition with other benthic invertebrates, like soft-bodied corallimorpharians and zoanthids which can co-occurr with this species (Figure 8a). The observed patterns of partial death may also be caused by deposition of sediment on the living corals. In fact, Echinophyllia tarae sp. n. is most commonly found at sheltered sites characterized by calm water conditions and muddy sediment which could be stirred up and deposit on benthic organisms suffocating them (
Occurrence:This species was commonly encountered on the fringing reefs off Mangareva, Aukena, Tekava, Akamaru, Kamaka, Makaroa, Agakauitai, and Taravai islands as well as at the base of the lagoon pinnacles found in the lagoon north of Mangareva Island (Figure 1, Table 2). Its distribution outside the Gambier archipelago is unknown although it could also occur in the Austral Islands (see Discussion section). No record is known from other localities.
Affinities:In its encrusting growth form, and in the presence of a central larger and protruding corallite this species is similar to Echinophyllia echinata and Echinomorpha nishihirai (see Discussion). Ongoing molecular analyses will reveal the phylogenetic relationships of this species with its congeners.
Occurrence of Echinophyllia tarae sp. n. encountered during the Tara Oceans Expedition in the Gambier Islands. For each site the site code (as in Figure 1), island name and reef type, coordinates, sampling date, and recorded presence or absence of the species are listed.
| Site | Island, reef type | Latitude, Longitude | Date | Echinophyllia tarae sp. n. |
|---|---|---|---|---|
| 1 | Akamaru, fringing reef | 23°10.61'S, 134°54.37'W | 26/06/11 | recorded |
| 2 | Akamaru, fringing reef | 23°11.08'S, 134°55.33'W | 26/06/11 | recorded |
| 3 | Aukena, fringing reef | 23°08.44'S, 134°55.18'W | 27/06/11 | recorded |
| 4 | Aukena, fringing reef | 23°08.56'S, 134°54.74'W | 27/06/11 | recorded |
| 5 | Kamaka, fringing reef | 23°14.19'S, 134°57.47'W | 28/06/11 | recorded |
| 6 | Makaroa, fringing reef | 23°12.96'S, 134°57.99'W | 28/06/11 | recorded |
| 7 | Kamaka, fringing reef | 23°14.98'S, 134°57.80'W | 29/06/11 | not recorded |
| 8 | Makaroa, fringing reef | 23°13.32'S, 134°58.34'W | 29/06/11 | recorded |
| 9 | Taravai, fringing reef | 23°09.40'S, 135°01.77'W | 30/06/11 | recorded |
| 10 | Agakauitai, fringing reef | 23°10.17'S, 135°02.52'W | 30/06/11 | recorded |
| 11 | Taravai, fringing reef | 23°09.54'S, 135°03.05'W | 01/07/11 | recorded |
| 12 | Agakauitai, fringing reef | 23°10.35'S, 135°01.99'W | 01/07/11 | recorded |
| 13 | Mangareva, fringing reef | 23°08.25'S, 134°57.11'W | 02/07/11 | recorded |
| 14 | Mangareva/Totegie | 23°04.78'S, 134°54.99'W | 04/07/11 | not recorded |
| 15 | Mangareva, fringing reef | 23°05.45'S, 134°55.69'W | 04/07/11 | recorded |
| 16 | Lagoon pinnacles | 23°01.55'S, 134°55.69'W | 05/07/11 | recorded |
| 17 | Lagoon pinnacles | 23°04.12'S, 134°55.83'W | 05/07/11 | not recorded |
| 18 | Mangareva, fringing reef | 23°05.57'S, 134°59.16'W | 07/07/11 | not recorded |
| 19 | Mangareva, fringing reef | 23°06.14'S, 134°59.23'W | 07/07/11 | recorded |
| 20 | Taravai, fringing reef | 23°08.72'S, 135°03.09'W | 08/07/11 | recorded |
| 21 | Taravai, fringing reef | 23°08.03'S, 135°02.14'W | 08/07/11 | recorded |
| 22 | outer barrier north | 23°04.21'S, 135°00.85'W | 09/07/11 | not recorded |
| 23 | outer barrier north | 23°03.79'S, 135°00.49'W | 09/07/11 | not recorded |
| 24 | Tekava, fringing reef | 23°10.13'S, 134°51.51'W | 10/07/11 | recorded |
This species is named after MV Tara, which allowed the exploration of coral reefs in Gambier. Moreover, the name “tara” in the Polynesian language may refer to a spiny, pointed object, which applies well to the new species typically featuring pointed skeletal structures. In the same language, Tara is also the name of a sea goddess.
The study of the Echinophyllia tarae sp. n. material and the in situ observations indicated a remarkable phenotypic variation within and between specimens regarding calice size, shape, and inclination, and the number of septa and paliform lobes in the corallite. Thus, Echinophyllia tarae sp. n. is distinct from the other Echinophyllia species by the presence of a larger central corallite with a raised wall, thicker primary costosepta, and a very pronounced crown of paliform lobes. In addition, Echinophyllia tarae sp. n. forms relatively small colonies with few corallites.
The type species of Echinophyllia, Echinophyllia aspera, has overall smaller and more evenly sized corallites than Echinophyllia tarae sp. n. Although a central corallite can be recognized in small colonies of Echinophyllia aspera (
Among the remainder of the Echinophyllia species, Echinophyllia echinoporoides and Echinophyllia costata, have smaller and more numerous corallites with less prominent costosepta ornamentation and a more poorly developed crown of paliform lobes, whereas a central larger corallite is not distinguishable (
Echinophyllia orpheensis has larger corallites than any of the aforementioned Echinophyllia species. A larger central corallite can be observed in some specimens like in one of the paratypes (
Live specimens of Echinophyllia tarae sp. n. can bear strong resemblance with Echinophyllia echinata (confront Figure 9a and Figure 9c) and Echinomorpha nishihirai (confront Figure 9b and Figure 9d). However, the skeletal morphology (Figures 9e-f, 10) helps to distinguish the new species from these two. Echinophyllia echinata forms thin flat to vase shaped colonies, with a conspicuous central corallite and widely spaced radials (
Comparison of in situ morphology between specimens of Echinophyllia tarae sp. n., Echinophyllia echinata, and Echinomorpha nishihirai and corallum morphology of the latter two a Echinophyllia tarae sp. n. resembling Echinophyllia echinata, Agakauitai Island (Site 10) b another colony of the new species resembling Echinomorpha nishihirai, Taravai Island (Site 11) c Echinophyllia echinata from Cap Bocage, New Caledonia (IRD HS 3171) d Echinomorpha nishihirai, Ryukyu Islands, Japan, picture by K. Yanagiya e same specimen as in c, f holotype of Echinomorpha nishihirai (MTQ G 32483), Okinawa Island, Japan. Images c and e from the IRD LagPlon database (http://lagplon.ird.nc/consultv2_5/rechSimple.faces). Sites are indicated in Figure 1.
Comparison ofcentralcorallitemorphology between a Echinophyllia echinata b and d Echinophyllia tarae sp. n., and c Echinomorpha nishihirai: a side view of the central corallite of thesame Echinophyllia echinata specimen as in Figure 8c (IRD HS 3171) b side view of the central protocorallite of Echinophyllia tarae sp. n. (UNIMIB TO-GA099), c top view of the central corallite of Echinomorpha nishihirai holotype (MTQ G 32483) d top view of the same corallite as in b.
Echinomorpha nishihirai initially described by
In his report on the diversity and distribution of scleractinian corals of the Gambier Islands
In their compilation of zooxanthellate scleractinian coral species at 19 localities in the Eastern, South-eastern, and Central Pacific Ocean
Echinophyllia tarae sp. n. is described from the Gambier Islands, French Polynesia. The species is characterized by a high intraspecific variation of several morphological traits. It also shows typical features that distinguish it from the other Echinophyllia species and from Echinomorpha nishihirai, such as the dimensions and the protrusion of the largest corallite (centrally located in flat colonies), the thickness of the septa, and the development of the crown of paliform lobes. Although the new species is common in the Gambier Islands, its occurrence elsewhere is unknown. The sampling of coral tissue from the type specimens of Echinophyllia tarae sp. n. will allow molecular analyses in order to examine its phylogenetic relationships with its congeners and other species in the Lobophylliidae.
I am keen to thank the OCEANS Consortium and of the following people and sponsors whose committment made the Tara Oceans Expedition possible: CNRS, EMBL, Genoscope/CEA, VIB, Stazione Zoologica Anton Dohrn, UNIMIB, ANR (projects POSEIDON, PROMETHEUS, and TARA-GIRUS), FWO, BIO5, Biosphere 2, Agnés b., the Veolia Environment Foundation, Region Bretagne, World Courier, Illumina, Cap L’Orient, the EDF Foundation EDF Diversiterre, FRB, the Prince Albert II de Monaco Foundation, and Etienne Bourgois. I am deeply grateful to the Tara schooner and its captain Hervé Bouround and crew, and M. Oriot in particular, and to my colleagues during the Gambier leg. Grazie to S. Kandels-Lewis (EMBL), R. Troublé (Tara Expéditions), R. Friederich (World Courier) for making the logistics of the expedition and of the collection shipment possible. I am grateful to the Papeete authorities for the sampling permits, to Mr. P. Mery of the Haut Commissariat de la République in French Polynesia, Mrs Frogier from the Délégation à la Recherche de Polynésie française, Mrs Tatarata from the Direction de l’Environnement, and to A. Moua and F. Seguin for their help with the CITES export permits. I wish to thanks to B.W. Hoeksema (Naturalis) who retrieved the original illustration of the holotype of Echinophyllia echinata and for his comments and suggestions on this manuscripts, to S. Petek (IRD Tahiti) who has kindly provided the references in the Cahiers du Pacifique, to Pascale Joannot (MNHN) for assistance in registering the holotype, to J.E.N. Veron for permission to use the in situ image of Echinomorpha nishihirai, and to C. Payri (IRD Nouméa) and Sylvie Fiat for the use of images of Echinophyllia echinata from the LagPlon database. I am grateful to C Wallace and B. Done for access to the MTQ collections which I could examine the holotype of this species in 2012. M. Pichon (MTQ) is acknowledged for advising that the Tara Ocean coral reef leg took place in the Gambier. A special acknowledgement goes to La Commune des Gambiers, the people of Mangareva, and Marie, Michel, Gabriel, and Françoise for their kind hospitality. This is contribution number 0007 based on material collected during the Tara Oceans Expedition 2009–2012, and the first new coral species discovered during the Expedition. I am grateful to three anonymous reviewers for their helpful comments and suggestions, which helped to improve the manuscript.