(C) 2013 Phuripong Meksuwan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Meksuwan P, Pholpunthin P, Segers H (2013) The Collothecidae (Rotifera, Collothecacea) of Thailand, with the description of a new species and an illustrated key to the Southeast Asian fauna. ZooKeys 315: 1–16. doi: 10.3897/zookeys.315.5330
Following previous reports indicating a remarkable high diversity of sessile rotifers in Southeast Asian freshwaters, we report on an extensive study of the diversity of Collothecidae rotifers from fifteen freshwater habitats in Thailand. A total of 13 species, including two additional infraspecific variants, of Collothecidae are recorded, one of which is described as a new species of Collotheca. We further add taxonomic remarks on some of the taxa on record and illustrate the uncinate trophi of several representatives by scanning electron microscopic images. Finally, we provide illustrated identification keys to the Collothecidae recorded to date from Southeast Asia.
Diversity, identification key, sessile rotifers, Southeast Asia, uncinate trophi
Family Collothecidae is one of two families of the rotifer Order Collothecacea. The order is diagnosed by the presence of uncinate trophi (
To date, comparatively little is known on the distribution and diversity of sessile rotifers in general and of Collothecidae in particular, which is due to the fact that these animals require life observation for identification and study. This knowledge gap is especially evident regarding sessile rotifers from tropical regions. These animals are mostly dealt with on an ad hoc basis, and much of what little information that exists is contained in more general inventories of rotifers, in which the sessile taxa are represented as chance occurrences (e.g.,
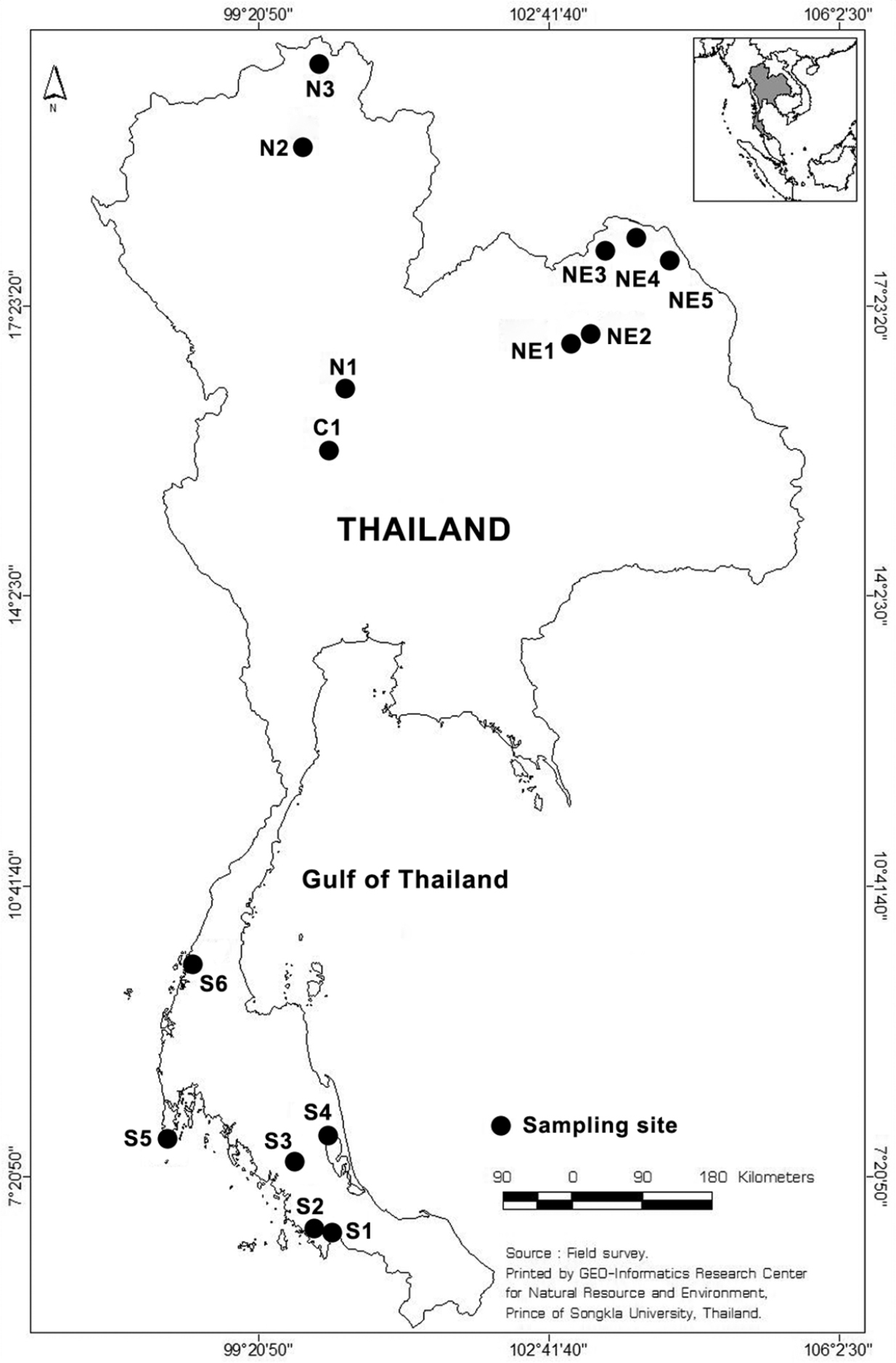
We explored 15 freshwater habitats in 12 provinces of Thailand for Collothecidae during the present study (Fig. 1). Submerged parts of different species of aquatic plant were collected qualitatively to search for sessile rotifers. Collecting and observation methods are detailed in
Sampling sites in Thailand. S, C, N and NE represent sampling sites in the Southern, Central, North and Northeast part of Thailand, respectively. Map from GIS center, PSU.
The samples examined contained 13 species and two infraspecific variants of Collothecidae (Table 1). This corresponds with ca. 28% of the world fauna of Collotheca species and all Stephanoceros species known to date (
List of Collothecidae species recorded from Thailand
| Family Collothecidae Harring, 1913 |
| Genus Collotheca Harring, 1913 |
| Collotheca algicola (Hudson, 1886) |
| Collotheca ambigua (Hudson, 1883) |
| Collotheca campanulata (Dobie, 1849) (incl. f. longicaudata (Hudson, 1883) |
| Collotheca edentata (Collins, 1872) |
| Collotheca ferox (Penard, 1914) |
| Collotheca heptabrachiata (Schoch, 1869) |
| Collotheca orchidacea sp. n. |
| Collotheca ornata (Ehrenberg, 1832) (incl. f. cornuta (Dobie, 1849) |
| Collotheca stephanochaeta Edmondson, 1936 |
| Collotheca tenuilobata (Anderson, 1889) |
| Collotheca trilobata (Collins, 1872) |
| Collotheca sp. |
| Genus Stephanoceros Ehrenberg, 1832 |
| Stephanoceros fimbriatus (Goldfusz, 1820) |
| Stephanoceros millsii (Kellicott, 1885) |
* = new to Oriental region and Thailand;
** = new to Thailand
1recorded by
The morphological characters of our specimens agree closely with the description of the species by
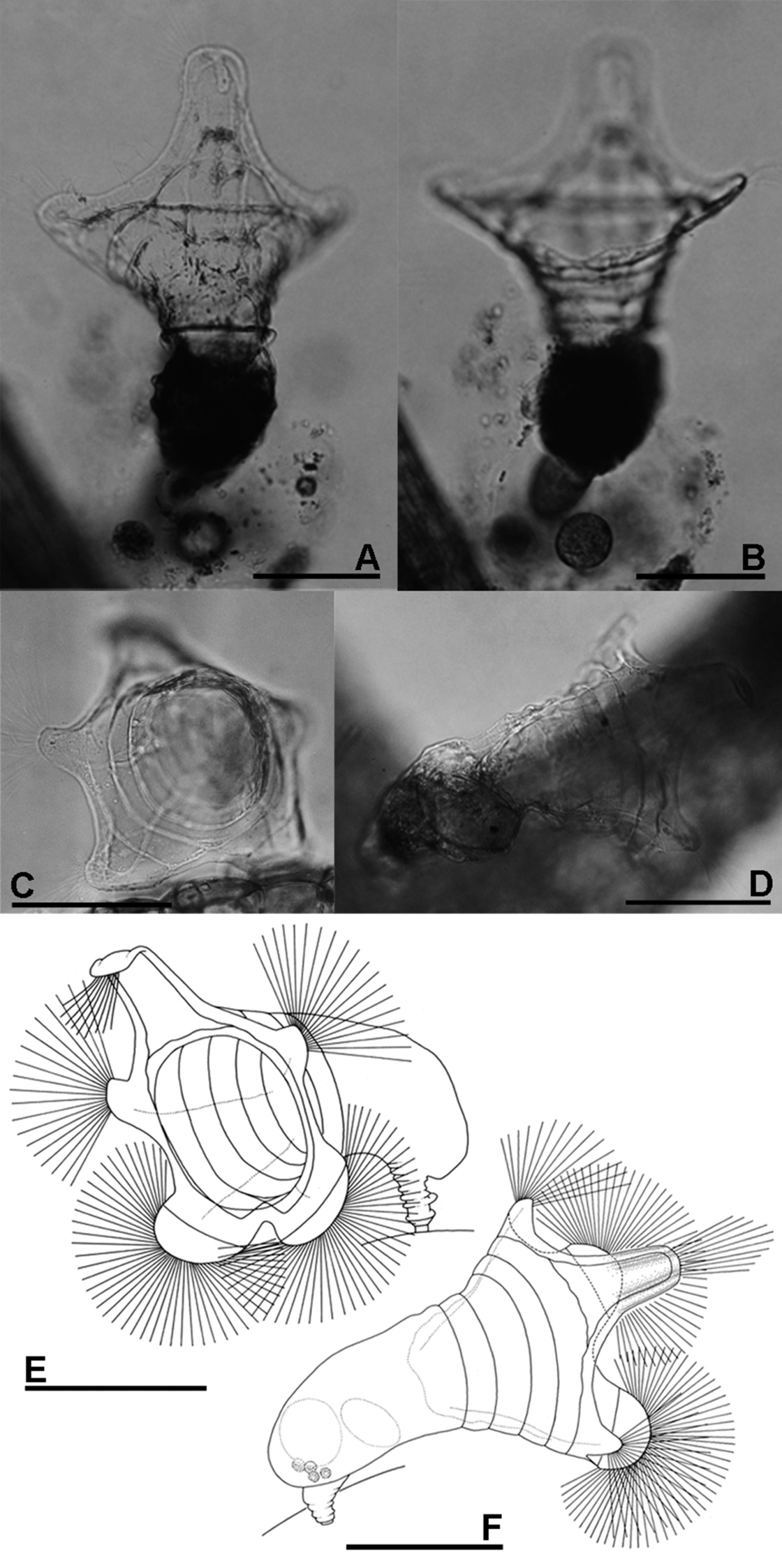
A, B Collotheca ferox (A dorsal view B ventral view) C–F Collotheca orchidacea sp. n. (C, E frontal D, F dorsal). Scale bars: A–F = 100 µm (A, B by Rapeepan Jaturapruek).
urn:lsid:zoobank.org:act:E7CA6ECF-175D-4E46-BCA8-970FA4F5C9CC
http://species-id.net/wiki/Collotheca_orchidacea
Figs 2C–F, 5EThale Noi Lake, Phatthalung Province, Thailand: 7°47.378'N, 100°8.969'E, on Utricularia sp., mostly on the surface of the bladder traps, 18 March 2012, P. Meksuwan leg.
Holotype female mounted in permanent microscope slide, in Princess Maha Chakri Sirindhorn NaturalHistory Museum, Prince of Songkla University, Songkhla, Thailand, PSUZC-PK5PM2-1. Original label: “Rotifera, Family Collothecidae, Collotheca orchidacea Meksuwan & Segers, Locality: Thale Noi Lake, Phattalung Province, Thailand, Collected by P. Meksuwan 18-3-2012, Holotype”; two paratype females in permanent microscope slides, inRoyal Belgian Institute of Natural Sciences, Brussels, Belgium, IG 32158 RIR 204-205. Original label: “Rotifera, Family Collothecidae, Collotheca orchidacea Meksuwan & Segers, Locality: Thale Noi Lake, Phattalung Province, Thailand, Collected by P. Meksuwan 18-3-2012, Paratype”.
The presence of a five-lobed corona separates the new species from most of the known members of genus Collotheca. In comparison with other Collotheca species having a five-lobed corona (Collotheca algicola (Hudson), Collotheca ambigua (Hudson), Collotheca annulata (Hood), Collotheca bilfingeri Bērziņš, Collotheca ferox and Collotheca campanulata (Dobie)), Collotheca orchidacea sp. n. can be distinguished by its uniquely well-developed thumb-shaped lateral and semi-circular ventral corona lobes. It has a relatively broad infundibulum, and short foot and trunk, similar only to Collotheca ambigua and Collotheca ferox. In addition, Collotheca orchidacea sp. n. and Collotheca ferox hold their infundibulum and corona towards the substratum, whereas most other species including Collotheca ambigua and Collotheca campanulata normally hold their body and corona upright.
Habitus (Fig. 2C–F): infundibulum funnel-shaped, trunk and corona opening held horizontally. Infundibulum and proventriculus about twice as long as the trunk. Infundibulum large, more than twice as wide as trunk. Foot short, length about half of trunk, contractile, with a short peduncle. Corona five-lobed: single dorsal, and a pair of well-developed lateral and of ventral lobes. Infundibulum with a weak line running parallel to the edge of the corona, and at least four ring-shaped structures (circular muscles?). Dorsal lobe large, elongate, basally with straight and converging lateral margins; parallel-sided medially, with smoothly curved antero-lateral corners. Tip of dorsal lobe transversally sinuate. Lateral lobes relatively the smallest, thumb-shaped, about half as wide as the dorsal lobe. Ventral lobes broadest, smoothly rounded, separated by a large and deep sinus. A group of setae present on the tip of all corona lobes.
Trophi (Fig. 5E) uncinate. Two pairs of subequal unci teeth relatively equal in length. All arrow head unci with middle groove.
Females total length ca. 340 µm. Length of infundibulum plus proventriculus ca. 190 µm, trunk ca. 100 µm, foot ca. 50 µm. Trunk width ca. 70 µm. Infundibulum width ca. 180; dorsal lobe length ca. 75 µm, width ca. 30 µm; ventral lobe width ca. 120 µm, ventral sinus depth ca. 30 µm.
The species name – orchidacea is a noun in apposition, and refers to the shape of the new species’ corona, which is reminiscent of the flower of certain orchid species. As such, the name of the species also refers to the biodiversity of Thailand, characterized by an abundance of orchid species.
The species is known from its type locality only.
This taxon (Figs 4G, H) is differentiated from the nominal form by the corona bearing an elongate projection dorsally to the dorsal lobe. The presence/absence of this projection has classically been interpreted as of infrasubspecific relevance only (see
Specimens were found in Khlong Lam Chan Non-Hunting Area, Trang province (Fig. 1: S3); the present is the first Thai record of the taxon.
We found a single specimen of a species that we could not identify (Fig. 3D). Its corona consists of two lobes, one large dorsal lobe and one minute ventral lobe, which is similar to Collotheca calva (Hudson). The specimen, however, exhibits a unique cluster of long setae dorsally on the tip of the dorsal lobe and, in addition, shows two ring-shaped structures in the infundibulum. The presence of an egg in its gelatinous case indicates that the specimen was mature and not some incompletely developed juvenile. We believe that it represents an undescribed species but refrain from describing and naming it due to the lack of a sufficient number of specimens. The animal occurred in Khlong Lam Chan Non-Hunting Area, Trang province (Fig. 1: S3).
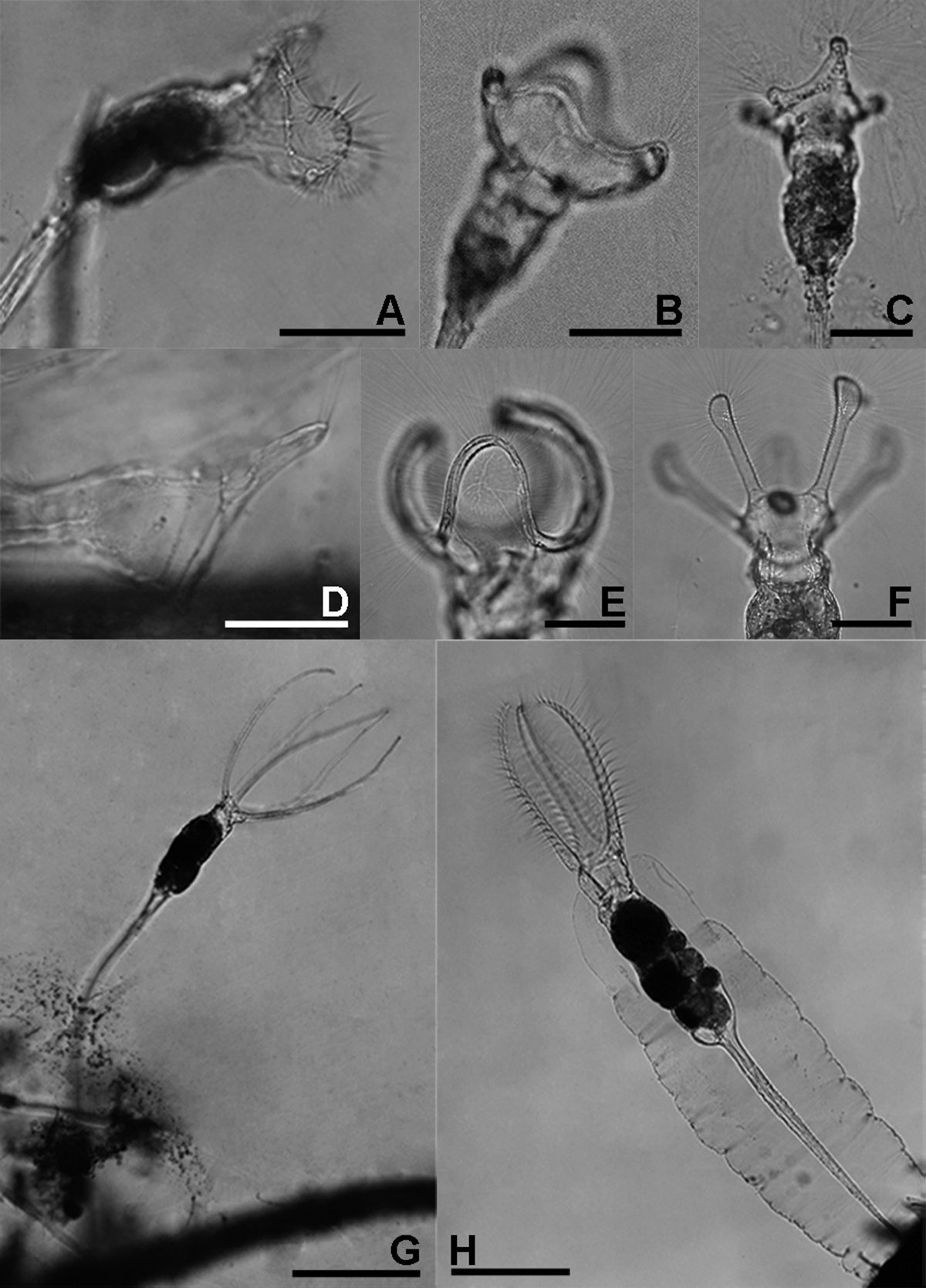
Collotheca and Stephanoceros species. A Collotheca stephanochaeta, lateral B Collotheca campanulata f. longicaudata, ventral C Collotheca ornata, dorsal D Collotheca spec., lateral E Collotheca trilobata, lateral F Collotheca tenuilobata, ventral G Stephanoceros millsii, lateral H Stephanoceros fimbriatus, lateral. Scale bars: B–D = 50 µm, A, E, F = 100 µm, G, H = 250 µm.
A Collotheca heptabrachiata, lateral B Collotheca ornata, ventral C Stephanoceros fimbriatus, lateral D Collotheca stephanochaeta, lateral E Collotheca ambigua, ventral F Collotheca algicola, ventral G, H Collotheca ornata f. cornuta (G dorsal H lateral) I Collotheca campanulata f. longicaudata, attachment stalk J Collotheca ferox, ventral corona margin. Scale bars: A, B, D–H, J = 50 µm, C, I = 100 µm.
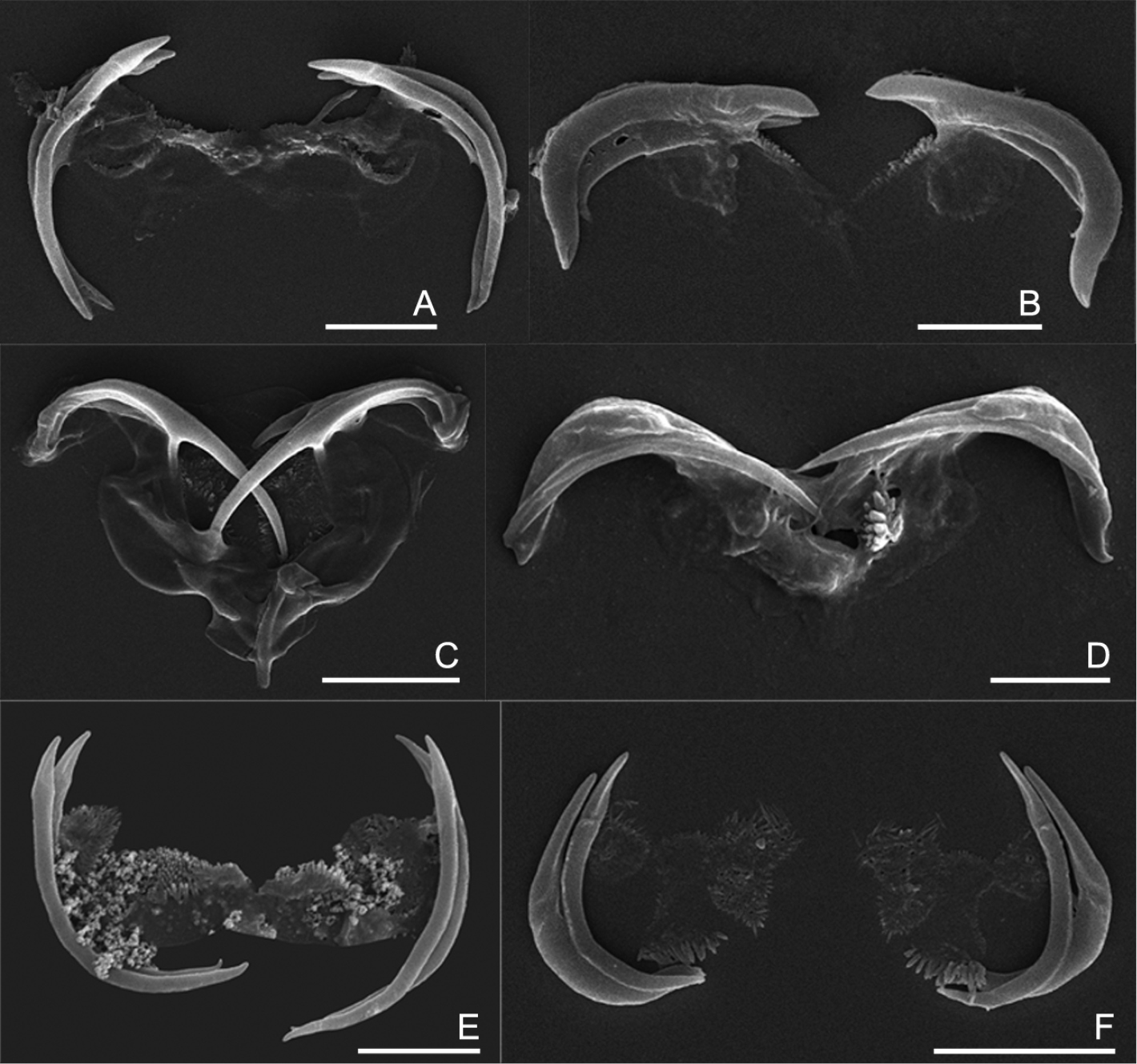
The uncinate trophi of Collothecidae species. A Collotheca ferox B Collotheca campanulata C Collotheca trilobata D Collotheca tenuilobata E Collotheca orchidacea sp. n. F Stephanoceros millsii. Scale bars: A, B, D, E = 5 µm, C, F = 10 µm.
Genus Stephanoceros is diagnosed (
We believe that the diagnosis of Stephanoceros is questionable, considering that neither the presence of long corona lobes (also in Collotheca judayi Edmondson and Collotheca tenuilobata (Anderson)) nor the presence of transverse rows of cilia on the corona lobes (present in Collotheca stephanochaeta, absent in Stephanoceros millsii, see below) can serve as synapomorphic diagnostic feature for the genus. To the contrary, we hypothesize that the two species now attributed to Stephanoceros are merely species in which the prolongation of corona lobes already present in many species of Collotheca has evolved to its greatest extent. We look forward to a more complete phylogenetic analysis of the taxa, knowing that a molecular phylogenetic study of the group is on-going. A synonymy between Collotheca and Stephanoceros would have to result in the reallocation of all taxa of the junior Collotheca to the senior Stephanoceros.
We found specimens matching the descriptions of two taxa in Stephanoceros, Stephanoceros fimbriatus (Fig. 3H) and Stephanoceros millsii (Fig. 3G, 5F) (see
Because the morphological characters of these two taxa enable a reliable diagnosis and because the two have wide and overlapping distribution ranges, we argue that these two taxa are distinct species, in contrast to
Stephanoceros millsii is common in Thailand whereas Stephanoceros fimbriatus is quite rare in our survey. Both species are cosmopolitan (
The uncinate trophi type is one of nine trophi types recognized in phylum Rotifera (
We found that, in all species examined, the uncinate trophi are composed of two pairs of large and sturdy unci teeth, whereas manubria, rami and fulcrum are less developed components (Figs 5A–F). Of the unci, the distal tips can be gradually sharpened (5C–D), stout (5B), or with set-off tips (5A), and the tips may carry a terminal, median groove (e.g., 5E–F). The unci are mostly strongly curved, either more or less evenly (e.g., 5A, E) or in their proximal third (5B), or terminally (5C), and the terminal tips may be slightly incurved (5A), straight (5E), or outcurved (5B). The unci pairs can be relatively equal (5A, D–F) or strongly unequal (5B, C) in length. The unci teeth are quite sturdy, as they are not easily dissolved by low concentration of commercial bleach (lower than 5% final concentration). The manubria, rami and fulcrum, on the other hand, are very weak and dissolve easily in bleach making it particularly hard to reliably compare their morphology. Nevertheless, the rami scleropilli usually remain after treatment (5E–F).
As illustrated here, the uncinate trophi, in particular the unci, do exhibit features that might be useful for taxonomic analysis. We suggest that 1) shape of the head of the unci; 2) shape of the unci teeth; and 3) relative size of the two pairs of unci teeth might be registered in future studies of Collotheca rotifers. Of course, the inclusion of these features in taxonomic analysis requires addition of information on more species of Collotheca, and evaluation of the intraspecific variability by comparing different populations of Collotheca species.
As mentioned above, Collothecidae species are essentially ambush predators. They remain immobile until a prey organism, guided by their long cilia and infundibulum that forms a fyke, and water current created by the beating of short cilia, comes in range of a sensory organ situated dorsally on the inner side of the infundibulum. When this organ is triggered, the cilia, corona lobes and infundibulum contract which restrains the prey organism within the infundibulum, and the prey is finally ingested whole. We observed that some species of Collotheca, and these appear to be species that have an enlarged funnel-shaped infundibulum, arrange their corona near the surface of the substrate they are attached to (e.g., Collotheca sp., Figs 3D; Collotheca ferox, Fig. 2A, B - note that the specimen in Figs 2A, B was not in normal position; Collotheca orchidacea sp. n., Figs 2C, D). Other species, mostly those that have a relatively smaller infundibulum but well-developed bands of cilia along the corona or on knobs, and a long foot, expose their expanded corona in the water column (e.g., Collotheca campanulata f. longicaudata, Fig. 3B; Collotheca ornata, Fig. 3C; Collotheca tenuilobata, Fig. 3F). We hypothesize that the two groups may have different diets. The latter group probably feeds on free-swimming, planktonic/periphytic organisms, while species of the former group may target browsing animals, in a way that is strikingly similar to Cupelopagis vorax (Leidy, 1857) (
The keys presented here include all species recorded hitherto from Southeast Asia (Brunei, Cambodia, Indonesia, Malaysia, Myanmar, Philippines, Singapore, Thailand, Timor Leste, Vietnam), as included in
Dichotomous key
| 1 | Length of corona lobe(s) shorter than trunk (Figs 3A–F) | (genus Collotheca), 2 |
| – | Length of corona lobes in adult specimens as long as, or longer than trunk (Figs 3G, H) | (genus Stephanoceros), 16 |
| 2(1) | Animals free-living (planktonic) | 3 |
| – | Animals fixosessile, permanently attached to a substratum | 5 |
| 3(2) | Corona edge circular, smooth; inner side of infundibulum with five rudimentary lobes | Collotheca pelagica |
| – | Corona with projections bearing groups of cilia | 4 |
| 4(3) | Corona with a single dorsal lobe carrying one group of long cilia | Collotheca libera |
| – | Corona with five knob-shaped projections, the dorsal one on a triangular lobe; all bearing a group of long cilia (Fig. 3C, 4B) | Collotheca ornata f. natans |
| 5(2) | Corona with well-defined, rounded or club-shaped knobs (Figs 3C, F) | 6 |
| – | Corona circular or with broad lobes, no knob(s) (Figs 3B, D, E) | 8 |
| 6(5) | Corona with seven knobs, the dorsal on a small, triangular lobe (Fig. 4A) | Collotheca heptabrachiata |
| – | Corona five projections (Fig. 4B) | 7 |
| 7(6) | Corona with equal, elongated lobes terminating in club-shaped knobs (Fig. 3F) | Collotheca tenuilobata |
| – | Corona lobes unequal and/or less than three times their width (Figs 3C, 4B) | Collotheca ornata |
| Within this species two infrasubspecific variants have been recorded from Southeast Asia. One (Collotheca ornata f. natans) is pelagic (see (3)), while Collotheca ornata f. cornuta is diagnosed by the presence of an elongate projection on the dorsal corona lobe (Figs 4G, H). | ||
| 8(5) | Corona circular, smooth, bearing only short cilia | Collotheca edentata |
| – | Corona with broad lobes | 9 |
| 9(8) | Corona with one large dorsal and one smaller ventral lobe, dorsal lobe with a group of elongate, parallel cilia (Fig. 3D) | Collotheca sp. |
| – | Corona with a dorsal lobe and a ventral sinus (Fig. 3B) | 10 |
| 10(9) | Corona with three lobes separated by clear, smoothly concave sinuses between the dorsal and ventral lobes (Fig. 3E) | 11 |
| – | Corona with five lobes, the lateral ones may be only indicated (Figs 3B, 4F) | 12 |
| 11(10) | Corona consisting of homogeneous rows of cilia (Fig. 3E) | Collotheca trilobata |
| – | Corona consisting of transversal sets of short, stiff cilia (Fig. 4D) | Collotheca stephanochaeta |
| 12(9) | Lateral corona lobes larger than ventral lobes, these set close together and separated by a shallow and narrow V-shaped sinus (Figs 2A, B, 4J) | Collotheca ferox |
| – | Lateral corona lobes smaller than ventral lobes (Figs 3B, 4F) | 13 |
| 13(12) | Lateral corona lobes well-develloped, thumb-shaped; ventral lobes large, rounded (Figs 2C–F) | Collotheca orchidacea sp. n. |
| – | Lateral corona lobes lower than wide or only indicated (Figs 3B, 4F) | 14 |
| 14(13) | Ventral sinus deep, broadly U-shaped, wider than the width of the ventral lobes (Fig. 4E) | Collotheca ambigua |
| – | Ventral sinus shallow (Fig. 4F) | 15 |
| 15(14) | Ventral lobes triangular with rounded tip, dorsal lobe relatively narrow (Fig. 4F) | Collotheca algicola |
| – | Ventral lobes rounded, dorsal lobe broad (Fig. 3B) | Collotheca campanulata |
| Within this species one infrasubspecific variant has been recorded from Southeast Asia. Collotheca campanulata f. longicaudata is characterised by the presence of an extraordinary long peduncle (secreted attachment stalk: Fig. 4I). | ||
| 16(1) | Corona lobes stout and robust, carrying parallel, transversal sets of robust cilia (Figs 3H, 4C) | Stephanoceros fimbriatus |
| – | Corona lobes slender, carrying dense, longitudinal rows of fine, cilia (Fig. 3G) | Stephanoceros millsii |
Characters
Species (a) free-living (pelagic); (b) living attached to a substratum (fixosessile)
Corona edge: (a) circular, smooth; (b) with well-defined knobs (Figs 3C, F); (c) with lobes (Figs 3B, E)
Number of corona projections: (a) one dorsal; (b) two: one dorsal, one ventral (Fig. 3D); (c) three: one dorsal, two lateral (Fig. 3E); (d) five: one dorsal, two lateral, two ventral (Figs 3B, 4F); (f) seven (Fig. 4A)
Length of corona projections: (a) much shorter than trunk (Figs 3C, D); (b) strongly elongated and parallel sided (Figs 3G, H)
Diversification of corona projections: (a) none, all projections more or less equal (Figs 3A, F–H); (b) differentiated (Figs 3B–E)
Lateral corona lobes: (a) absent (3E); (b) indicated (sinus between dorsal and ventral lobe is not smoothly concave or indicated by presence of a distinct group of particularly long cilae: Figs 4E, F); (c) well-developed (Figs 2A–F)
Ventral corona projections: (a) with one midventral lobe, (b) with two knobs (Fig. 4B); (c) two rounded triangular lobes (Fig. 4F); (d) two semicircular lobes (Figs 3B, E)
Ventral corona sinus: (a) shallow, narrow (Fig. 4J); (b) shallow, broad (Figs 3B, 4F); (c) deep, broad, U-shaped (Fig. 4E)
Special features: (a) elongate projection dorsally on dorsal corona lobe (Figs 4G, H); (b) peduncle (attachment stalk) longer three times diameter of foot than foot (Fig. 4I); (c) cilia inserted in parallel sets of transverse rows (Figs 4C, D); (e) group of elongate cilia dorsally on dorsal corona lobe (Fig. 3D)
Collotheca algicola: 1b, 2c, 3d, 4a, 5b, 6b, 7c, 8b
Collotheca ambigua: 1b, 2c, 3d, 4a, 5b, 6b, 7d, 8c
Collotheca campanulata: 1b, 2c, 3d, 4a, 5b, 6b, 7d, 8b (+9b: f. longicaudata)
Collotheca edentata: 1b, 2a
Collotheca ferox: 1b, 2c, 3d, 4a, 5b, 6c, 7c, 8a
Collotheca heptabrachiata: 1b, 2b, 2c, 3f, 4a, 5b, 6a, 7b
Collotheca libera: 1a, 2c, 3a, 4a
Collotheca orchidacea sp. n.: 1b, 2c, 3d, 4a, 5b, 6c, 7d, 8c
Collotheca ornata: (1b), 2b, 2c, 3d, 4a, 5b, 6b, 7b (+9a: f. cornuta; 1a: f. natans)
Collotheca pelagica: 1a, 2a
Collotheca stephanochaeta: 1b, 2c, 3c, 4a, 5a, (6b), 7d, 8b, 9c
Collotheca tenuilobata: 1b, 2b, 2c, 3d, 4b, 5a
Collotheca trilobata: 1b, 2c, 3c, 4a, 5b, 6a, 7d, 8c
Collotheca sp.: 1b, 2c, 3b, 4a, 5b, 6a, 7a, 9e
Stephanoceros fimbriatus: 1b, 2c, 3d, 4b, 5a, 9c
Stephanoceros millsii: 1b, 2c, 3d, 4b, 5a
This research was supported by Ph.D. thesis supporting grant, Graduate school, Prince of Songkla University to PM. Part of this work was done during a visit of HS to Prince of Songkla University, supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission. We further wish to acknowledge Prof. Dr WH De Smet and two anonymous reviewers for their suggestions and corrections on the manuscript of this paper.