(C) 2012 José Albertino Rafael. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Protosilvius gurupi sp. n. (Tabanidae, Pangoniinae) is described and illustrated based on seven female and 53 male specimens collected in the Amazonian region at Reserva Biológica Gurupi, Centro Novo do Maranhão municipality, northwest Maranhão, Brazil. This is the first record of Protosilvius in northern Brazil and in the Amazon Basin. An illustrated key to all Protosilvius species is also presented.
Amazon Basin, horseflies, neotropics, taxonomy
Currently, Protosilvius Enderlein, 1922 has been recorded only in Brazil. The genus was originally described to include Protosilvius termitiformis Enderlein, 1922.
This study is based on the examination of 60 specimens collected at Reserva Biológica do Gurupi (Rebio Gurupi) (03°14'05"S, 46°41'83"W) of the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), in northwestern Maranhão state, Brazil. Rebio Gurupi is in an Amazonian region composed mainly of primary terra firme rainforest. Specimens were collected using a "mobile" light-trap, which consisted of a white sheet (1.2 × 1.2 m) hung vertically and lit by two mercury vapour lamp (160 watts) set in the storage trunk of a pick-up truck (Fig. 28). The truck was moved slowly and continuously (4 km/h) along an unpaved road surrounded by forest, stopping each 200 meters for 30 minutes. Collecting took place from 08:00 pm to 04:00 am by two persons on each side of the light trap (Fig. 28). Specimens that landed on the sheet were collected using a vial with ethyl acetate and brought to the laboratory for sorting, mounting and species identification.
The morphological terminology and figure abbreviations are based on
The material collected was deposited in the following institutions: Coleção Zoológica do Maranhão (CZMA), Universidade Estadual do Maranhão, Caxias, Maranhão, Brazil; Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Amazonas, Brazil; Museu Paraense Emílio Goeldi (MPEG), Belém, Pará, Brazil; and Museu de Zoologia da Universidade de São Paulo (MZSP), São Paulo, São Paulo, Brazil.
The new species description was based solely on the holotype specimen. The opposite sex, based on paratype specimens, and the variations between individuals are discussed separately. The specimen length was based on the straight distance measured from the frons at antenna level (antenna excluded) to the apex of the abdomen. Wing length is the straight distance measured from the base of the costal vein to the wing apex. Label data are cited in full, including original spelling, enclosed in quotation marks (“”), with punctuation and date transcribed from the top downward. Square brackets ([ ]) are used to indicate information that is not included in the original label. The terminology used follows
The apex of the abdomen was removed and then macerated in heated 85% lactic acid (
urn:lsid:zoobank.org:act:3A1AA834-5114-4918-BC20-514836F16539
http://species-id.net/wiki/Protosilvius_gurupi
Figs 1–11HOLOTYPE female. “Brasil, MA[ranhão] [Centro Novo do Maranhão] REBIO – Res[erva] Biol[ógica do] Gurupi 03°14'05"S, 46°41'83"W “Arm[adilha] Luminosa móvel 07–15.I[Jan.].2011, F. Limeira-de-Oliveira & M. M. Abreu, cols.” (CZMA). Paratypes: same data as holotype (5 females, 22 males, CZMA; 2 females, 20 males, INPA; 5 males, MPEG; 5 males, MZSP).
Mostly light yellow, slender, and soft-bodied specimens. Thorax and abdomen with yellow bristles. Antenna with three flagellomeres after postpedicel. Wing unusually long; usually with cup cell open, without petiole if cell is closed. Abdomen unicolorous. Female tergite 9 distinctly narrower medially; tergite 10 sub-rectangular.
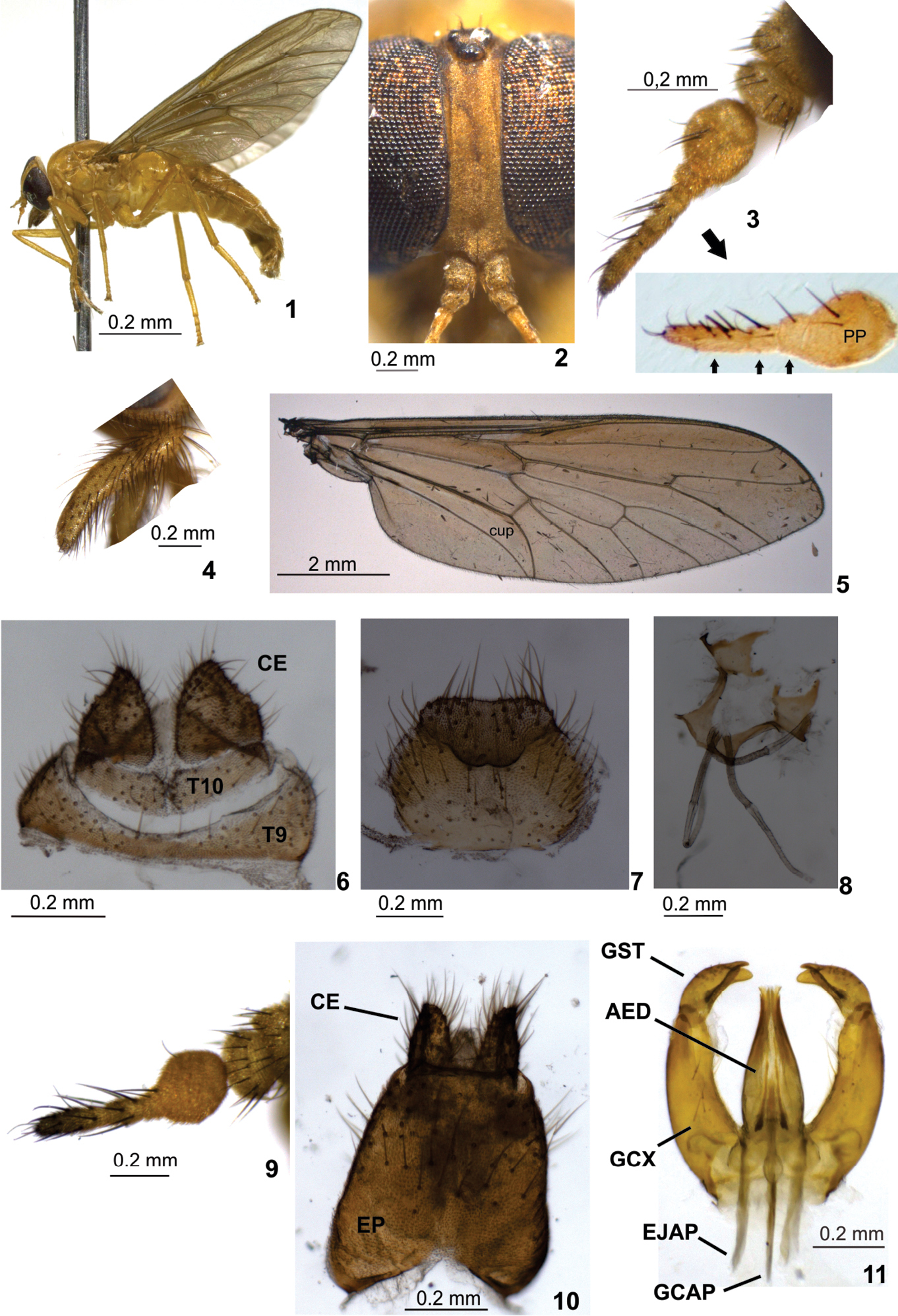
Holotype female. Body length: 8.9 mm. Specimen mostly light yellow. Head (Fig. 1) with eyes black (green in life, ) more or less suboval in profile, rounded laterally in frontal view, with very short yellowish bristles which are barely visible under higher magnification. Frons (Fig. 2) narrow, somewhat parallel sided, slightly divergent dorsally and ventrally, frontal index about 2.7, smoothly tomentose, with a median inconspicuous groove, and short, inconspicuous brown bristles. Ocellar tubercle (Fig. 2) somewhat prominent, as high as ocellus. Subcallus (Fig. 2) very small, tomentose, separation from frons indistinct. Parafacial narrow, tomentose, with long black bristles. Face convex laterally, deeply sunken medially, tomentose, without bristles, separate from parafacial by deep groove. Antenna (Fig. 3) with scape and pedicel short, plump, yellow to brown, and with robust black bristles; flagellum light yellow with robust black bristles, apparently with six flagellomeres; postpedicel swollen when observed in lateral view, with three distal flagellomeres, the first flagellomere almost totally fused to postpedicel based on a distinct incomplete suture on medial side (see Fig. 3 from a clarified antenna of a different paratype specimen); second flagellomere as long as first and with an indistinct suture; third flagellomere, the distalmost, longer than two preceding flagellomeres. Palpus (Fig. 4) with first segment somewhat swollen, second slightly narrower and slightly curved, distinctly bristled. Proboscis short, as long as palpi, membranous, with long, narrow, soft and bristled labellum.
Thorax with scutum and scutellum light brown to dark yellow, sparsely yellow bristled, with yellow pruinescence. Pleuron slightly clearer than scutum, yellow with light grey to yellow pruinescence.
Legs (Fig. 1) entirely yellow except distal half of tarsomeres 5 brown; most legs with yellow bristles, except fore tibia black bristled. All tarsomeres 1 of equal length. Hind tibial spurs slightly shorter than mid ones.
Wing (Fig. 5) 9.1 mm long, 2.9 mm wide, narrower than usual for tabanids, diffusely brownish, with costal margin slightly darker; pterostigma ill defined. Vein Sc bare dorsally and ventrally; vein R4 with short appendix; vein CuA1 with even row of small setulae; cell cup open. Halteres with stem yellow and capitulum brown and white.
Abdomen (Fig. 1) long, narrow, entirely yellow, with short golden bristles dorsally and ventrally. Terminalia: Tergite 9 (Fig. 6) narrow medially, expanded laterally; tergite 10 subrectangular in dorsal view, divided medially; cercus subtriangular. Sternite 8 (Fig. 7) wider than long, with somewhat distinct gonapophysis. Genital fork as in figure 8.
Male. Body length: 9.0 mm; wing length: 9.1 mm. Habitus similar to female specimens except head holoptic, antenna (Fig. 9) slightly weaker, cell cup narrowly open (sometimes narrowly closed, without petiole), abdomen slender and of a lighter tone, first 3–4 abdominal segments light yellow, somewhat translucent, remaining brown. Terminalia (Fig. 10): epandrium with concavity basally; cercus subquadrate in lateral view; gonocoxite slightly arched; gonostylus bifid (Fig. 11); ejaculatory apodeme and gonocoxal apodeme similar in length.
The specific epithet is a noun in apposition and refers to Reserva Biológica do Gurupi, where the specimens were collected.
Brazil, Maranhão.
Pinned, not dissected, in good condition except for a damaged left wing. We chose the best preserved specimen, among the few females collected, as holotype because in most tabanids species the primary types are females.
One female specimen without short appendix on vein R4. Female size varying from 8.6–9.6, mean 9.0 mm (n = 3). Male size varying from 8.0–10 mm, mean 9.1 cm (n = 10).
Protosilvius gurupi sp. n. is smaller than other Protosilvius species, as the biggest specimens (9.8 mm) are slightly shorter than the smallest species, Protosilvius priscus (10 mm); these differ by three flagellomeres after the postpedicel in the former and four flagellomeres in the latter. Female specimens would key out to Protosilvius termitiformis in couplet 3 of
Bionomics. Light traps are a common method for collecting many male and some female tabanids. All specimens of both sexes of Protosilvius gurupi sp. n. were collected in light traps, not one in the Malaise traps mounted nearby. The specimens were constantly collected in the light trap, either while the car was slowly moving or not. We believe the specimens are not nocturnal but they were attracted to trap when the light reached the specimens bedding in the vegetation. The collection was made in the Amazonian Region, in the state of Maranhão, in the rainy season, far from any drier area for at least 300 kilometers.
| 1 | Frons distinctly widening dorsally and ventrally (Fig. 12), over 4× as high as narrowest width. Flagellum with rather elongate postpedicel and distal portion 4-segmented, distal flagellomere longer than three preceding ones (Fig. 13). Abdominal tergites banded with silvery-white bristles on posterior margin | Protosilvius longipalpis |
| – | Frons narrow, nearly parallel sided, divergent ventrally or slightly wider dorsally and ventrally. Flagellum not as above. Abdominal tergites with only black bristles or banded with yellowish bristles on posterior margin | 2 |
| 2 | Frons divergent ventrally (Figs 21, 24) | 3 |
| – | Frons parallel sided (Fig. 14) or slightly divergent dorsally and ventrally (Figs 2, 18) | 4 |
| 3 | Frons around 3× as high as narrowest width just below ocelli (Fig. 21). Distal flagellomere longer than three preceding flagellomeres (Fig. 22) | Protosilvius priscus |
| – | Frons over 4× as high as narrowest width just below ocelli (Fig. 24). Distal flagellomere of similar length to preceding flagellomeres (Fig. 25) | Protosilvius mackerrasi |
| 4 | Frons parallel sided (Fig. 14), less than 3× as high as dorsal width, just below ocelli. Postpedicel divided into 3 flagellomeres with another partial division, so that the flagellum may seem incompletely 8–segmented (Fig. 15). Scutum and scutellum black bristled. Abdominal tergites with band on posterior margin formed by yellow bristles | Protosilvius phoeniculus |
| – | Frons somewhat parallel sided to slightly divergent dorsally and ventrally (Figs 2, 18), more than 3× as high as dorsal width, just below ocelli. Postpedicel with flagellomeres somewhat fused (Figs 3, 19). Scutum and scutellum yellow bristled. Abdominal tergites without band on posterior margin, bristles unicolorous | 5 |
| 5 | Cell cup closed, with short petiole. Tergite 9 uniformly wide medially and laterally, and tergite 10 wider medially in dorsal view (Fig. 20) | Protosilvius termitiformis |
| – | Cell cup open (Fig. 5), if closed then without petiole. Tergite 9 distinctly narrower medially and tergite 10 somewhat rectangular (Fig. 6) in dorsal view | Protosilvius gurupi sp. n. |
| 1 | Distal flagellomere widened (Fig. 16). Abdominal tergites with band on posterior margin formed by yellow bristles. Gonostylus with swollen base and bifid appendages ventrally directed (Fig. 17) | Protosilvius phoeniculus |
| – | Distal flagellomere not widened (Figs 9, 26). Abdominal tergites without band on posterior margin, bristles unicolorous. Gonostylus base not swollen and bifid appendages medially directed (Figs 11, 23, 27) | 2 |
| 2 | Upper eye facets enlarged. Medial margin of gonocoxite nearly straight and phallus ends at level of gonocoxite apex (Fig. 23) | Protosilvius priscus |
| – | Upper eye facets not enlarged. Medial margin of gonocoxite slightly curved and phallus apex ends after gonocoxite apex (Figs 11, 27) | 3 |
| 3 | Blackish specimens with blackish wings | Protosilvius mackerrasi |
| – | Yellowish specimens with diffusely brownish wings (as in figure 5) | Protosilvius gurupi sp. n. |
Protosilvius gurupi, sp. n., paratype female. 1 habitus 2 frons 3 antenna; below detail of clarified antenna of a different paratype showing sutures between distal flagellomeres (distal flagellomeres indicated by smaller seta) 4 palpus 5 wing 6 tergite 9, tergite 10 and cercus 7 sternite 8 and gonapophysis 8 genital fork and spermathecal ducts 9–11 paratype male 9 antenna 10 epandrium and cercus 11 gonostylus and aedeagus. Figs 1, 3, 4, 9 in lateral view; 2 in frontal view; 5, 6, 8 10 in dorsal view; 7, 11 in ventral view. Abbreviations: AED = aedeagus, CE = cercus, EJAP = ejaculatory apodeme, EP = epandrium, GCAP = gonocoxal apodeme, GCX = gonocoxite, GST = gonostylus, PP = postpedicel, T = tergite.
Protosilvius figures from
“mobile”light trap placed on a pick-up truck.
We would like to thank: Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for allowing the use of Reserva Biológica do Gurupi (authorization number 22809–1); Programa de Pesquisas em Biodiversidade (PPBio), and Núcleo Regional do Maranhão (Process number 558287/2009–3) for financial support; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 300305/2007–9) for JAR's fellowship. We also thank M. Sc. Rodrigo M. Vieira for help with imaging of specimens and the following students that helped with field work: Mariana M. Abreu, Jocifran A. Silva and Ernesto Augusto S. Barbosa (Centro de Estudos Superiores de Caxias/Universidade Estadual do Maranhão (CESC/UEMA)).