(C) 2014 Ning Sun. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Sun N, Marusik YM, Tu L (2014) Acanoides gen. n., a new spider genus from China with a note on the taxonomic status of Acanthoneta Eskov & Marusik, 1992 (Araneae, Linyphiidae, Micronetinae). ZooKeys 375: 75–99. doi: 10.3897/zookeys.375.6116
A new “micronetine” genus Acanoides gen. n. is erected to accommodate two species from China: Acanoides beijingensis sp. n. as the type species and Acanoides hengshanensis (Chen & Yin, 2000), comb. n., with the females described for the first time. The genitalic characters and somatic features of the new genus were studied by means of light microscopy and scanning electron microscopy (SEM). The monophyly of the new genus was tested by a phylogenetic analysis based on molecular data. Descriptions of the new genus, the new species and the new combination are presented; SEM images and microscopy pictures of somatic and genitalic characters are provided in detail. To distinguish from other genera with similar genitalic characters, we compare the new genus with the species of Acanthoneta Eskov & Marusik, 1992, Epibellowia Tanasevitch, 1996 and Wubanoides Eskov, 1986. Four putative synapomorphies for Acanoides gen. n. are suggested to support its monophyly that could be tested in the future. Furthermore, redescriptions of the epigynal morphology of Acanthoneta aggressa Chamberlin & Ivie, 1943 (Nearctic) and on the male of A. dokutchaevi Eskov & Marusik, 1993 (Far East Asia, firstly recorded from China) are provided. Based on comparison with Poeciloneta, from which Acanthoneta stat. n. was separated by Saaristo and Tanasevitch (1996), a revised diagnosis is proposed to support the generic status.
Taxonomy, new species, new genus, genitalic morphology, movable epigynum
Micronetinae Hull, 1920 is a fairly large subfamily of Linyphiidae Blackwall, 1859, including 1199 species placed in 90 genera (
Poeciloneta hengshanensis (Chen & Yin, 2000) from China, originally placed in Lepthyphantes Menge, 1866, has its male palp very similar to that of Poeciloneta (Acanthoneta) aggressa (Chamberlin & Ivie, 1943). Acanthoneta Eskov & Marusik, 1992 is one of the three genera raised from subgeneric status by
Females of Poeciloneta hengshanensis (previously unknown) were found in new material from China. However, its epigynal conformation is neither congruent with that of Poeciloneta aggressa, nor with any other species of Poeciloneta. Based on the presence of an extensible basal part, the movable epigynum accords with the diagnosis of the subfamily Ipainae Saaristo, 2007 (for example Ipa Saaristo, 2007 and Solenysa Simon, 1894). Additionally, we found another new species with genitalic morphology very similar to that of Poeciloneta hengshanensis: the male palpal morphology similar to Acanthoneta and a movable epigynum in accordance with ipaine type.
A new genus Acanoides gen. n. is erected here for these two species. To test the placement of the new genus within Linyphiidae, a phylogenetic analysis based on newly sequenced molecular data of the two species and that of other linyphiids downloaded from NCBI was conducted. In the present study, the two species and the new genus are described. Characters of copulatory organs and somatic features of both species are illustrated by means of SEM and light microscopy. To distinguish the new genus from other “micronetine” genera with similar male palpal morphology and ipaine genera with a similar movable epigynum, the new genus is compared with the genera Acanthoneta (Micronetinae), Wubanoides Eskov, 1986 and Epibellowia Tanasevitch, 1996 (Ipainae). Due to limited material available for examination, comparisons are largely based on descriptions and illustrations provided by
Specimens were examined and measured using a Leica M205A stereomicroscope. Male palps and epigyna were examined after they were dissected from the body. Left structures (e.g. palps, legs, etc.) were depicted. Embolic divisions were excised by breaking the membranous column which connects the suprategulum and radix. Male palps and epigyna were cleared in methyl salicylate. Digital images were taken with a Leica DFC 500 camera, as composites of multiple focus images assembled using the software package LEICA APPLICATION SUITE. Scanning electron microscopy (SEM) images were taken using a S-3400N scanning electron microscope at the China Agricultural University. For SEM examination the specimens were prepared following
AER anterior eye row
ALE anterior lateral eye(s)
AME anterior median eye(s)
AMEd diameter of AME
PER posterior eye row
PLE posterior lateral eye(s)
AX apex of embolus
DM distal membrane of terminal apophysis
DSA distal suprategular apophysis
EM embolic membrane
EP embolus proper
FiG Fickert’s gland
LC lamella characteristica
P paracymbium
PCA proximal cymbial apophysis
PH pit hook
R radix
SE serrated area on embolus
SPT suprategulum
TA terminal apophysis
TH thumb of embolus
CO copulatory opening
CG copulatory groove
DP dorsal plate
EA extensible area of epigynal basal part
EB epigynal basal part
FG fertilization groove
MP median plate
S spermathecae
SC scape
ST stretcher
VP ventral plate
Based on the dataset of
Five genes: cytochrome c oxidase subunit I (CO1) and 16S rRNA (16S) and three nuclear genes 18S rRNA (18S), 28S rRNA (28S) and Histone H3 (H3) were sequenced for Acanoides beijingensis sp. n. and Acanoides hengshanensis. Molecular procedures for sequencing follow that of
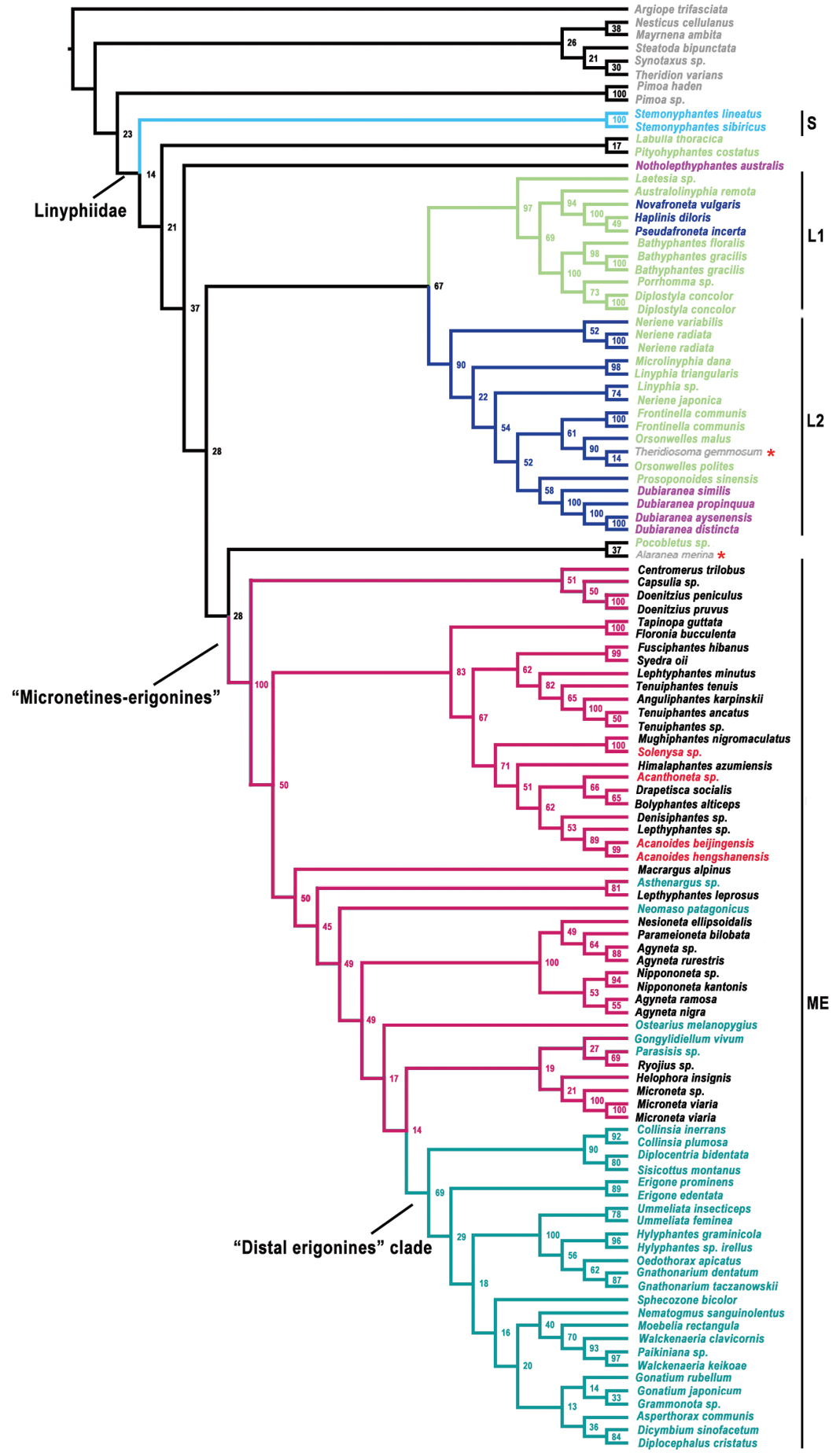
All five genes were sequenced for Acanoides beijingensis sp. n. and Acanoides hengshanensis (Appendix - Table S1). The monophyly of Linyphiidae and its sister relationship with Pimoidae were not recovered in the result of phylogenetic analysis as two outgroup taxa: cyatholipid Alaranea and theridiosomatid Theridiosoma are embedded within Linyphiidae (Appendix - Fig. S1). Besides some weakly supported deeper branches, four robustly supported clades are recognized: Stemonyphantes clade (clade S), “micronetines-erigonines” clade (clade ME), “linyphiines”-1 clade (clade L1) and “linyphiines”-2 (clade L2). For the seven subfamilies currently proposed, only Stemonyphantinae Wunderlich, 1986 (the Stemonyphantes clade) and Mynogleninae Lehtinen, 1967 are monophyletic, while the mynoglenines clade and the Dubiaranea clade fall into clades L1 and L2 respectively that make Linyphiinae Blackwall, 1859 become a paraphyletic group; taxa of Micronetinae form a paraphyletic group, nested with taxa of Ipainae and Erigoninae within clade ME. The two Acanoides species form a robustly supported monophyly, distantly related to Acanthoneta and Solenysa.
The result of the phylogenetic analysis based on molecular data suggests that the new species from Beijing is the sister taxon of Poeciloneta hengshanensis which had ever been transferred to Acanthoneta by
Our results suggest an unknown Lepthyphantes species as a sister group to the Acanoides clade. Lepthyphantes Menge, 1866, which includes almost 500 species, is not a natural group (
The genitalic characters of Acanoides make its subfamily placement problematic due to the epigynal character in accordance with Ipainae type, but the male palpal morphology of the “micronetine” type. Redelimitation of Mironetinae (
With greatly increased ingroup sampling, the result of the present study produce a similar topology with that of
Although with ingroup sampling about two times increased, the sampling size of the current dataset seems not to be enough to resolve the placements of Acanoides and Acanthoneta, as well as Poeciloneta, from which Acanthoneta were separated (
Acanoides beijingensis sp. n.
Two species, Acanoides beijingensis sp. n. and Acanoides hengshanensis (Chen & Yin, 2000) comb. n.
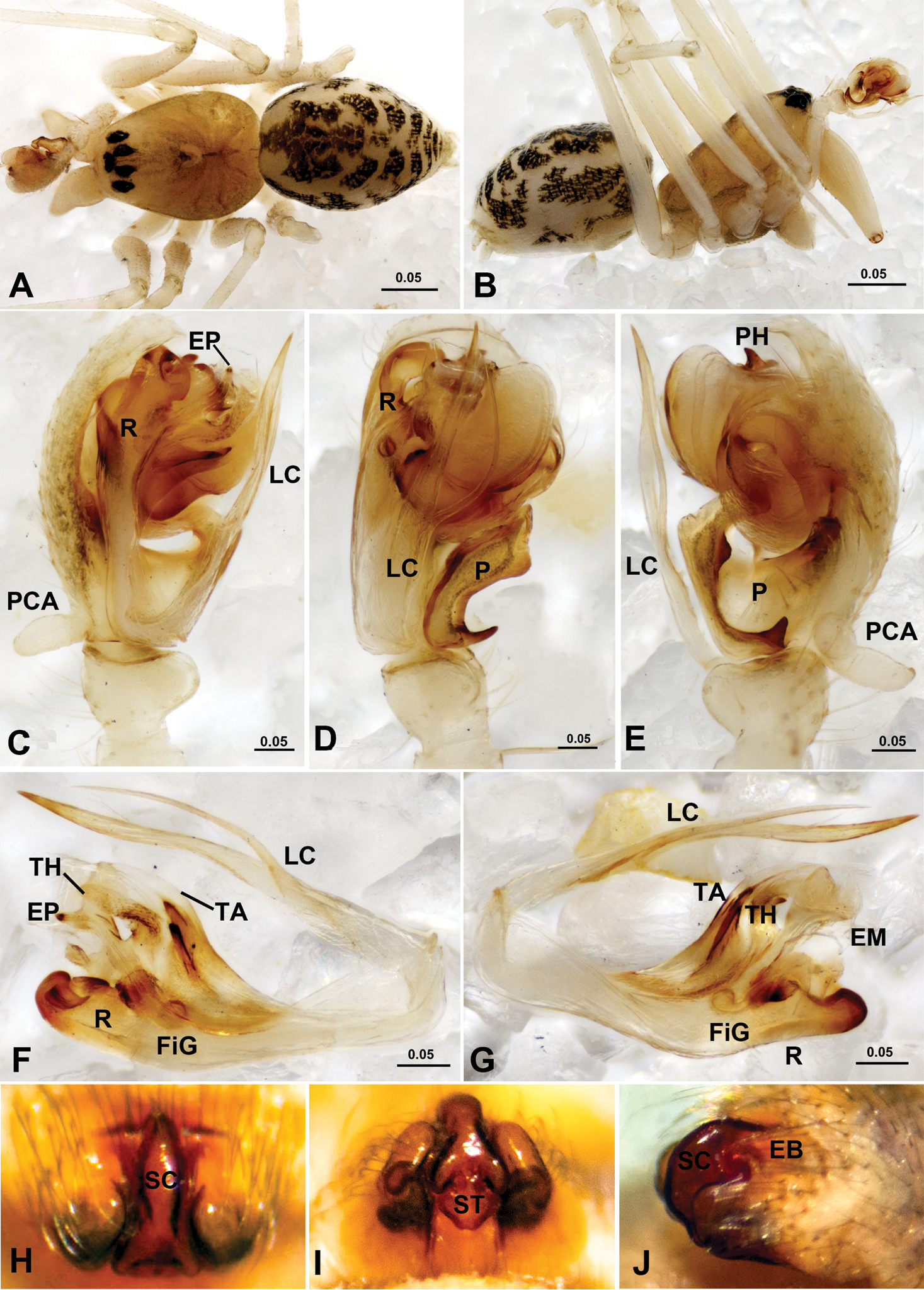
The males of Acanoides gen. n. can be distinct from Acanthoneta by the sharp embolus proper, the slender lamella characteristica unbranched, and by the Fickert’s gland located in the membranous area outside the radix (Figs 2D, 3D). The females can be distinguished by the ventrally folded extensible epigynal basal part (Figs 2F, 3F).
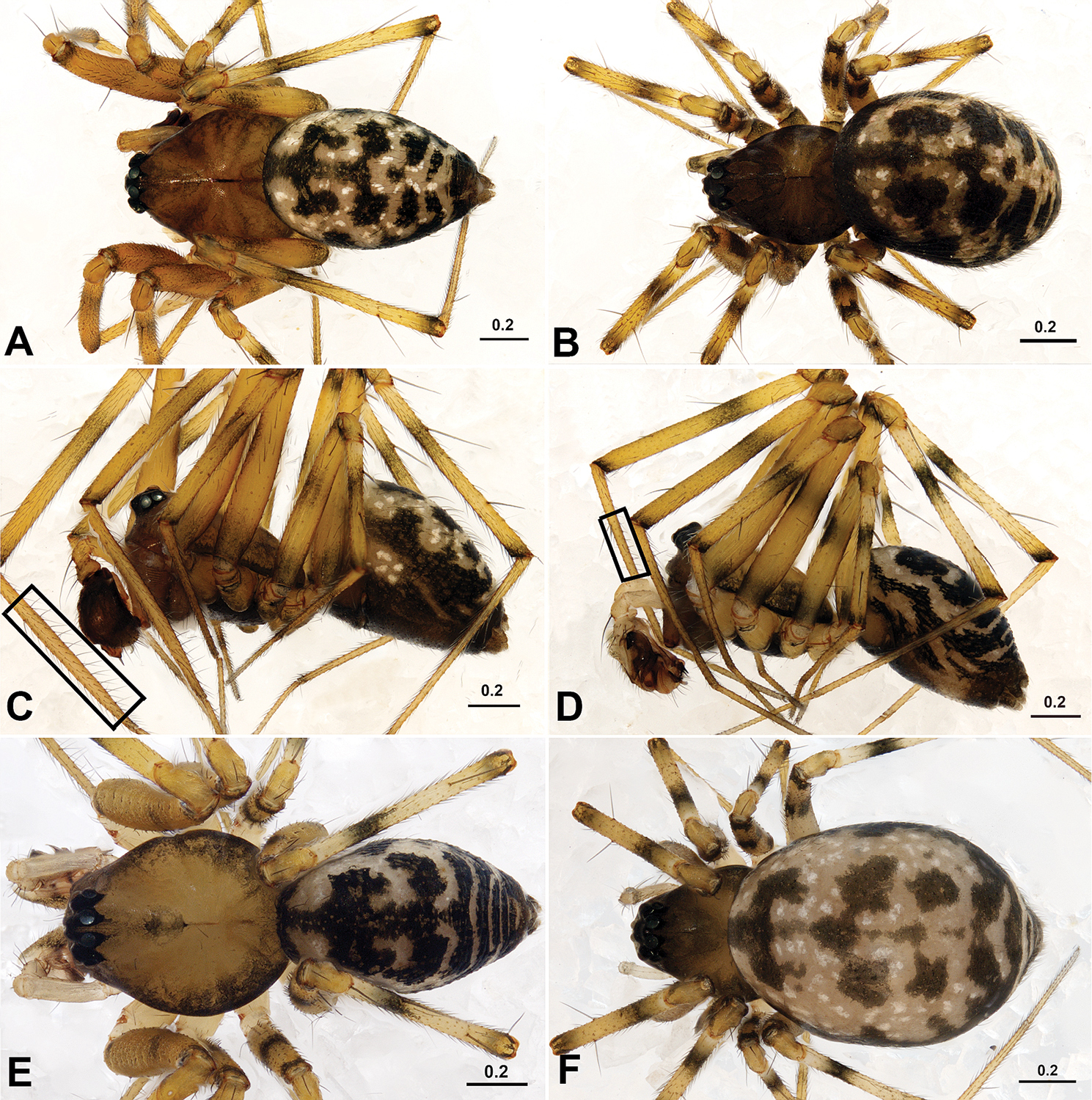
Male total length 2.34–2.73; female total length 2.10–2.42. Carapace yellowish-brown. Male carapace unmodified. AMEs smallest, others subequal; from the dorsal view AER recurved, PER straight, eyes separated by AMEd, ALE and PLE juxtaposed. Chelicerae medium-sized, with strong stridulatory ridges, female fang groove with three promarginal and three retromarginal teeth in Acanoides beijingensis sp. n., and two promarginal and two retromarginal teeth in Acanoides hengshanensis. Chaetotaxy: Ti I–IV: 2-2-2-2; Mt I–IV: 1-1-1-1; Mt I of males with two rows of ventral bristles, one prolateral, one retrolateral (Fig. 1C, 1D); Tm I about 0.25, Tm IV absent. Both species have a haplotracheate system.
Acanoides beijingensis sp. n. (A–C) and Acanoides hengshanensis (D–F). A male, dorsal B female, dorsal C male, lateral, rectangle indicates ventrolateral rows of bristles on Mt I D male, lateral, rectangle indicates ventrolateral rows of bristles on Mt I E male, dorsal F female, dorsal. [Scale bars: mm].
Male palp (Figs 2A–E, 3A–E, 4A–B, 5A–B). Cymbium with proximal apophysis. Paracymbium medium to large-sized, with one tooth on lateral margin. Distal suprategular apophysis not modified as pit hook, or absent. Embolic division: radix long and narrow, Fickert’s gland located in the membranous area connecting radix and embolus; embolus wide and strongly sclerotized with serrated area, embolus proper sharp with a thumb and an apex at each side; lamella characteristica unbranched, long and narrow with sharp sclerotized apex, almost parallel to radix; terminal apophysis with distal membrane.
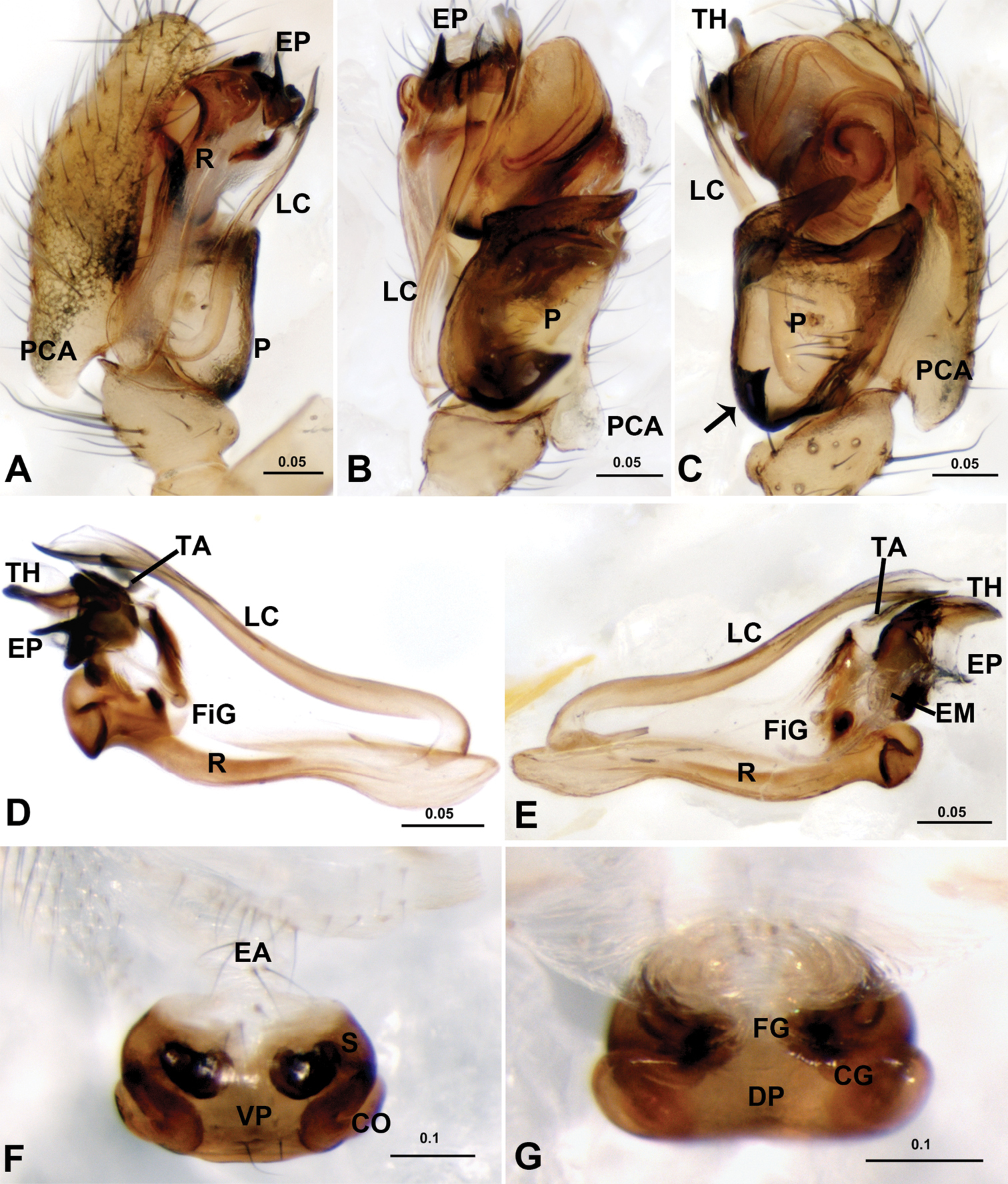
Acanoides beijingensis sp. n. A male palp, prolateral B male palp, prolateral, with embolic division removed C male palp, retrolateral D embolic division, ventral E embolic division, dorsal F epigynum, ventral G epigynum, dorsal H epigynum, lateral. CG copulatory groove; CO copulatory opening; DP dorsal plate; EA extensible area of epigynal basal part; EM embolic membrane; EP embolus proper; FG fertilization groove; FiG Fickert’s gland; LC lamella characteristica; MP median plate; P paracymbium; PCA proximal cymbial apophysis; R radix; S spermathecae; TA terminal apophysis; TH thumb of embolus; VP ventral plate. [Scale bars: mm].
Acanoides hengshanensis. A male palp, prolateral B male palp, ventral C male palp, retrolateral, arrow indicates pointed tooth on posterolateral margin D embolic division, ventral E embolic division, dorsal F epigynum, ventral G epigynum, dorsal. CG copulatory groove; CO copulatory opening; DP dorsal plate; EA extensible area of epigynal basal part; EM embolic membrane; EP embolus proper; FG fertilization groove; FiG Fickert’s gland; LC lamella characteristica; P paracymbium; PCA proximal cymbial apophysis; R radix; S spermatheca; TA terminal apophysis; TH thumb of embolus; VP ventral plate. [Scale bars: mm].
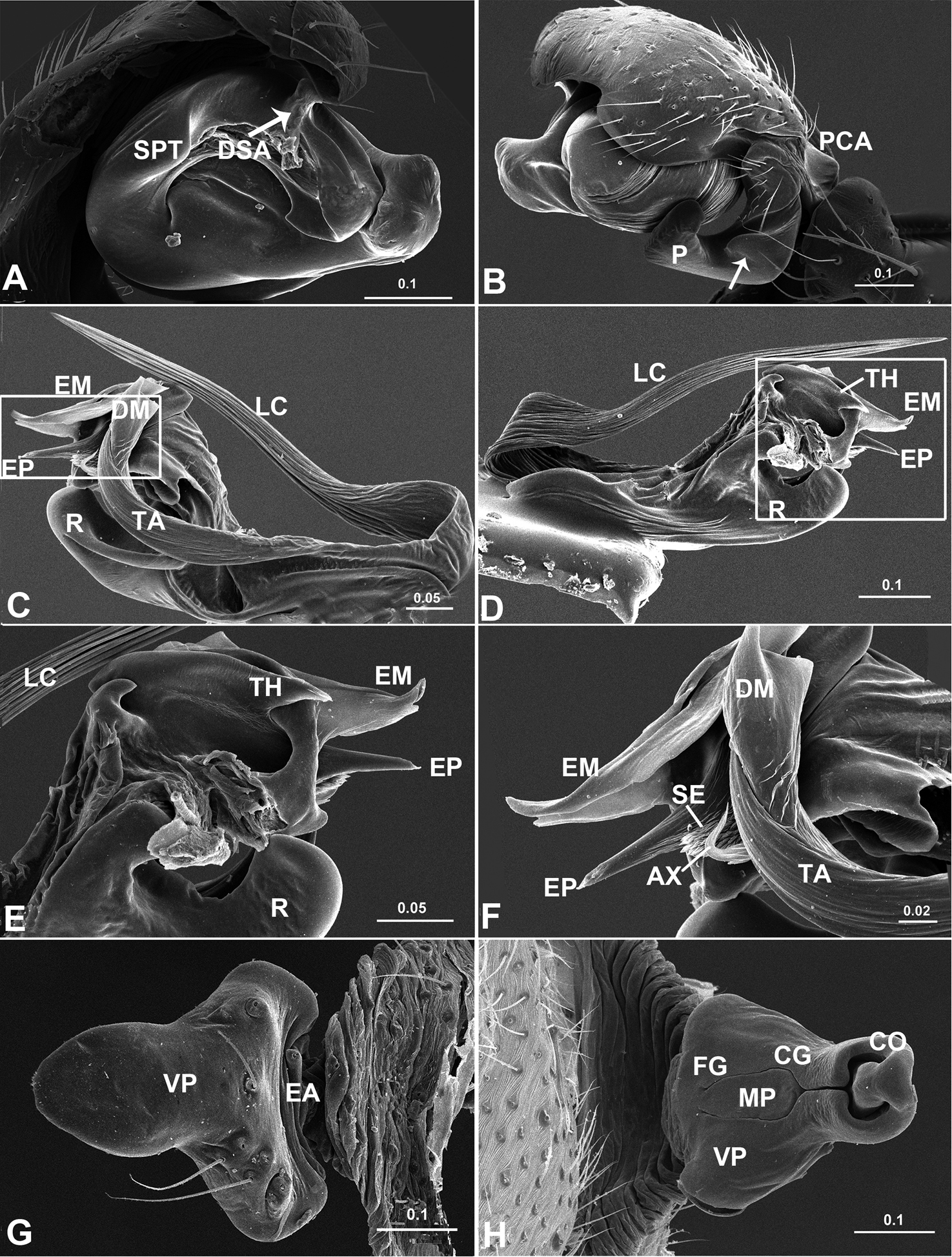
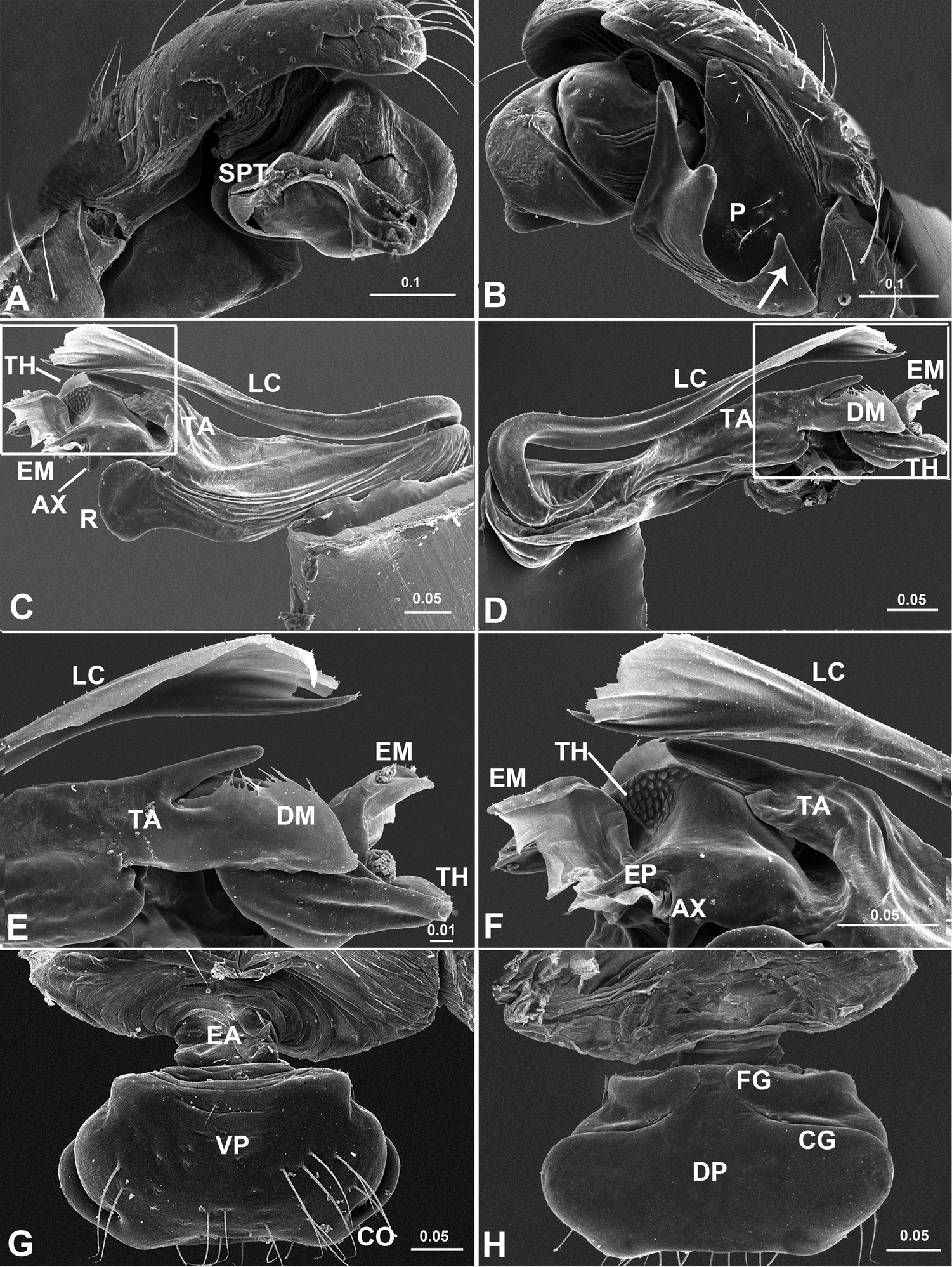
Acanoides beijingensis sp. n. A palp (embolic division removed), prolateral B palp, retrolateral, arrow indicates half rounded lateral tooth on paracymbium C embolic division, ventral D embolic division, dorsal E detail of D F detail of C G epigynum, ventral H epigynum, dorsal. AX apex of embolus; CG copulatory groove; CO copulatory opening; DM distal membrane of terminal apophysis; DSA distal suprategular apophysis; EA extensible area of epigynal basal part; EM embolic membrane; EP embolus proper; FG fertilization groove; LC lamella characteristica; MP median plate; P paracymbium; PCA proximal cymbial apophysis; R radix; S spermatheca; SE serrated area on embolus; SPT suprategulum; TA terminal apophysis; TH thumb of embolus; VP ventral plate. [Scale bars: mm].
Acanoides hengshanensis. A palp (embolic division removed), prolateral B palp, retrolateral, arrow indicates pointed tooth on posterolateral margin C embolic division, ventral D embolic division, dorsal E detail of D F detail of C G epigynum, ventral H epigynum, dorsal. AX apex of embolus; CG copulatory groove; CO copulatory opening; DM distal membrane of terminal apophysis; EA extensible area of epigynal basal part; EM embolic membrane; EP embolus proper; FG fertilization groove; LC lamella characteristica; P paracymbium; PCA proximal cymbial apophysis; R radix; S spermatheca; SPT suprategulum; TA terminal apophysis; TH thumb of embolus; VP ventral plate. [Scale bars: mm].
Epigynum (Figs 2F–H, 3F–G, 4G–H, 5G–H). Protruding, with deeply wrinkled basal part, extensible and ventrally folded in constricted state. Epigynum well sclerotized, epigynal cavity present (in Acanoides beijingensis sp. n.) or absent (in Acanoides hengshanensis), both scape and stretcher absent.
The genus name, Acanoides, is a combination of the first four letters of “Acanthoneta” and the last five letters of “Wubanoides”. “-oides” itself in Latin means “similar to”, masculine in gender.
Due to limitations of the current dataset the monophyly of Acanoides could not be tested explicitly in our phylogenetic analyses, however it is supported by the following four putative synapomorphies: sharp embolus proper, slender and unbranched lamella characteristica, outside radix located Fickert’s gland and ventrally folded extensible epigynal basal part.
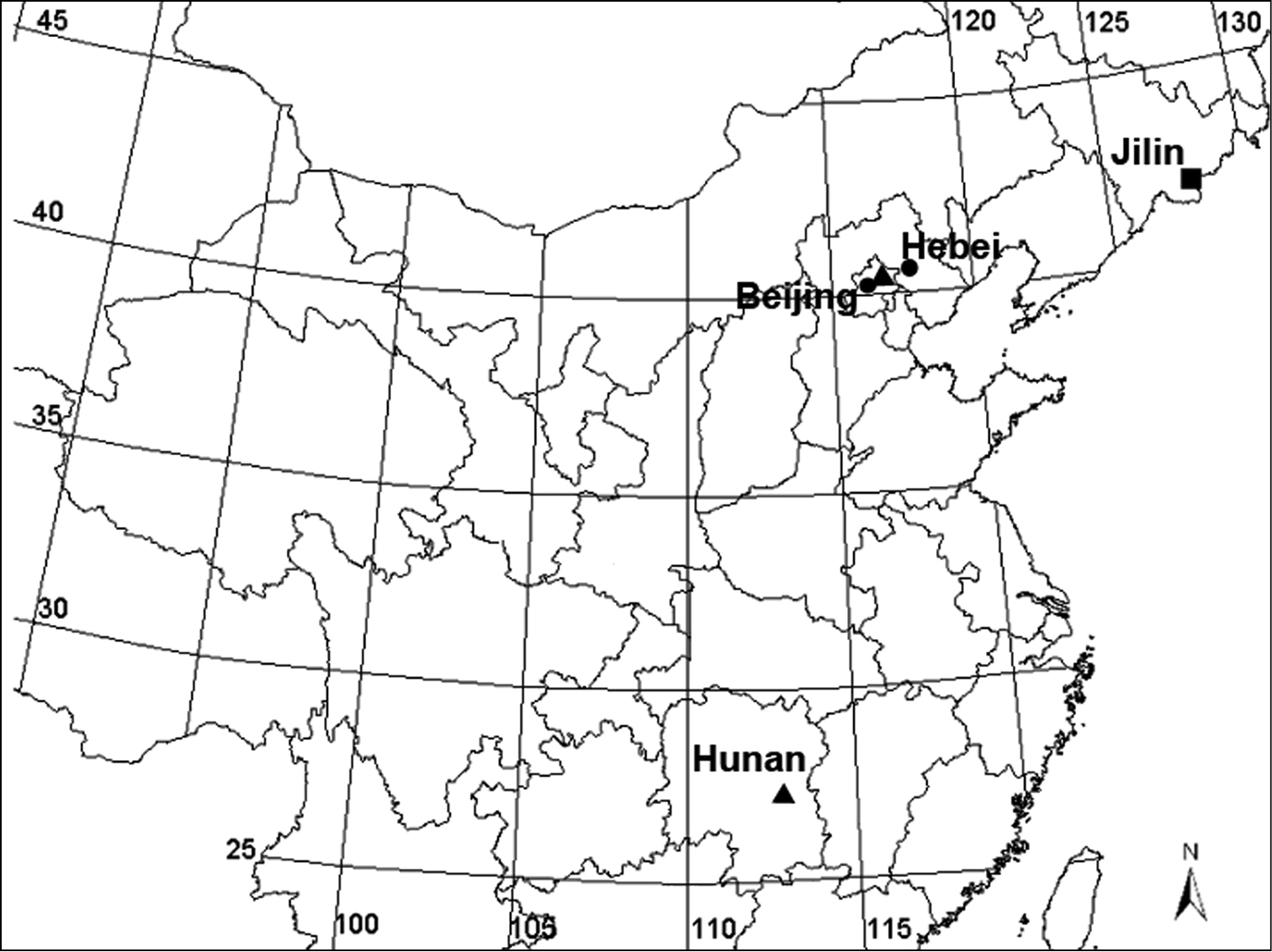
China (Beijing, Hunan, Hebei) (Fig. 7).
The males of Acanoides gen. n. have the palp of a “micronetine” type: presence of the Fickert’s gland, the boat-shaped radix, the trunk-like embolus with a pointed proper and a thumb, as well as the well developed terminal apophysis and lamella characteristica (
Acanthoneta dokutchaevi (A–G) and Acanthoneta aggressa (H–J). A male, dorsal B male, lateral C male palp, prolateral D male palp, ventral E male palp, retrolateral F embolic division, ventral G embolic division, dorsal H epigynum, ventral I epigynum, posterior J epigynum, lateral (H–J photos provided by Don Buckle). EB epigynal basal part; EM embolic membrane; EP embolus proper; FiG Fickert’s gland; LC lamella characteristica; P paracymbium; PCA proximal cymbial apophysis; PH pit hook; R radix; SC scape; ST stretcher; TA terminal apophysis; TH thumb of embolus. [Scale bars: mm].
Collecting localities of Acanoides species and Acanthoneta aggressa, Acanoides beijingensis sp. n. (Beijing, Hebei); Acanoides hengshanensis (Hunan, Beijing); Acanthoneta dokutchaevi (Jilin).
The result of phylogenetic analysis based on molecular data indicates that Ipainae is not a monophyletic group as the movable epigynum independently evolved in Acanoides and Solenysa (Appendix - Fig. S1). This is also supported by the tracheal characters: haplotracheate type in Acanoides, but intermediate type in Solenysa, with the median pair extending into the prosoma (
http://zoobank.org/CE596A12-9C21-4B8F-97FC-F31CBC61CD7E
http://species-id.net/wiki/Acanoides_beijingensis
Figs 1A–C, 2, 4China, Beijing: Mt. Yangtaishan, 39°20.15'N, 115°34.52'E, alt. ca 320m, 15 Oct. 2007, L. Tu leg.
Holotype, ♂ (CNU), China, Beijing, Mt. Yangtaishan, 39°20.15'N, 115°34.52'E, alt. ca 320 m, 15 Oct. 2007, L. Tu leg. Paratypes, 2 ♂♂ and 3 ♀♀ (CNU), same data as holotype.
1 ♂ and 2 ♀♀ (CNU), China, Hebei Province, Mt. Wulingshan, 40°33.61'N, 117°29.69'E, alt. ca 1100 m, 12 Aug. 2009, L. Tu leg.
The male of Acanoides beijingensis sp. n. can be distinguished from Acanoides hengshanensis by the spine-shaped lamella characteristica (Figs 2D, 4C), ribbon-like in the latter (Figs 3D, 5C); by the hook-shaped terminal apophysis (Fig. 4C), straight in the latter (Fig. 5D); and by the presence of a distal suprategular apophysis (Fig. 4A), absent in the latter. The female is distinct by having the epigynum two times longer than wide (Fig. 2F), shorter than wide in Acanoides hengshanensis (Fig. 3F); and by the presence of a remnant epigynal cavity (Fig. 2G), totally absent in Acanoides hengshanensis (Fig. 3G).
Male holotype (Fig. 1A, C): Total length 2.69. Carapace 1.22 long, 1.01 wide. Abdomen 1.39 long, 0.88 wide. Lengths of legs: I 3.88 (1.05 + 1.18 + 0.99 + 0.66); II 3.02 (1.03 + 0.73 + 0.69 + 0.57); III 2.66 (0.87 + 0.88 + 0.51 + 0.40); IV 3.78 (1.12 + 1.09 + 0.93 + 0.64). Female (Fig. 1B): Total length 2.12. Carapace 0.93 long, 0.78 wide. Abdomen 1.25 long, 0.83 wide. Lengths of legs: I 6.10 (1.68 + 2.04 + 1.43 + 0.95); II 5.43 (1.56 + 1.74 + 1.24 + 0.89); III 4.39 (1.24 + 1.13 + 1.10 + 0.75); IV 5.88 (1.79 + 1.78 + 1.46 + 0.83). Tm I: 0.20. For other somatic features see description of the genus.
Male palp (Figs 2A–C, 4A–B). Cymbium with proximal apophysis. Paracymbium narrow, half rounded lateral tooth strongly sclerotized. Distal suprategular apophysis blunt, not modified as pit hook. Embolic division: radix long and narrow; Fickert’s gland located in the membranous area connecting radix and embolus; embolus main body short and wide, strongly sclerotized, with serrated area on ventral surface; embolus proper sharp with pointed thumb and tail-like apex at each side; unbranched lamella characteristica long and slender, with sharp and strongly sclerotized apex; terminal apophysis hook-shaped with distal membrane.
Epigynum (Figs 2F–H, 4G–H). Two times longer than wide, wrinkled basal part extensible and ventrally folded in constricted state. Median plate and epigynal cavity present, without scape and stretcher. Copulatory openings opened dorsally.
The species name refers to the type locality.
Males (n = 3). Total length 2.61–2.73. Carapace: 1.13–1.27 long, 0.95–1.05 wide. Abdomen 1.34–1.45 long, 0.71–0.99 wide.
Females (n = 3). Total length 2.10–2.23. Carapace: 0.90–0.96 long, 0.74–0.78 wide. Abdomen: 1.10–1.38 long, 0.79–0.88 wide.
China (Beijing, Hebei) (Fig. 7).
Although Acanoides beijingensis sp. n. looks quite different from Acanoides hengshanensis in the shape of the male paracymbium and in terms of female epigynal morphology, the strongly sclerotized embolus main body and the sharp embolus proper, the location of Fickert’s gland, the presence of a ventrally folded extensive area of the epigynal basal part and the absence of a scape and stretcher, shared by the two species suggest they are closely related. A close relationship between the two species is additionally supported by the phylogenetic analysis (Appendix - Fig. S1).
http://species-id.net/wiki/Acanoides_hengshanensis
Figs 1D–F, 3, 5Holotype of Lepthyphantes hengshanensis Chen & Yin, 2000, ♂ (HNU), China, Hunan Province, Mt. Hengshan, 27°18'N, 112°42'E, 1–7 Aug. 1995, C. Yin leg. (examined).
3 ♂♂ and 4 ♀♀, China, Beijing, Mt. Yangtaishan, Dajue Temple, 40°03.06'N, 116°05.97'E, alt. 50 m, 15 Oct. 2007, L. Tu leg.
See diagnosis for Acanoides beijingensis sp. n.
Male (Fig. 1D–E): Total length 2.39. Carapace 1.02 long, 0.78 wide. Abdomen 1.37 long, 0.78 wide. Lengths of legs: I 5.03 (1.37 + 1.56 + 1.32 + 0.78), II 3.33 (0.98 + 0.98 + 0.83 + 0.54), III 3.47 (0.98 + 1.07 + 0.88 + 0.54), IV 4.63 (1.27 + 1.41 + 1.22 + 0.73). Tm I: 0.24. Female (Fig. 1F): Total length 2.42. Carapace 0.96 long, 0.78 wide. Abdomen 1.80 long, 1.25 wide. Lengths of legs: I 4.21 (1.18+ 1.42 + 0.96 + 0.65), II 3.19 (0.98 + 1.06 + 0.66 + 0.49), III 2.81 (0.84 + 0.85 + 0.68 + 0.44), IV 3.70 (1.08 + 1.19 + 0.89 + 0.54). Tm I: 0.23. For other somatic characters see description of the genus.
Male palp (Figs 3A–C; 5A–B). Cymbium with distinct proximal apophysis pointing backwards. Paracymbium wide and U-shaped, with triangular tooth on posterolateral margin. Distal suprategular apophysis absent. Embolic division: radix long and narrow; Fickert’s gland located in the membranous area connecting radix and embolus; embolus main body large and strongly sclerotized with serrated area; embolus proper sharp with large thumb and pointed apex; lamella characteristica long and slender with bifurcated ends, one sharp and sclerotized, one membranous; terminal apophysis straight, with distal membrane.
Epigynum (Figs 3F–G, 5G–H). Short and wide, box-shaped, strongly sclerotized; wrinkled basal part extensible and ventrally folded in constricted state. Neither median plate nor epigynal cavity present. Copulatory openings located on ventral surface, slits of epigynal grooves extending laterally, passing from ventral to dorsal surface, then convergent mesally. No scape, no stretcher.
Males (n = 3). Total length 2.34–2.41. Carapace: 1.09–1.12 long, 0.72–0.93 wide. Abdomen 1.14–1.42 long, 0.68–0.83 wide.
Females (n = 4). Total length 2.32–2.42. Carapace: 0.87–1.01 long, 0.75–0.81 wide. Abdomen: 1.63–1.82 long, 0.76–1.22 wide.
China (Beijing, Hunan) (Fig. 7).
Poeciloneta aggressus (Chamberlin & Ivie, 1943).
Three species: Acanthoneta aggressa Chamberlin & Ivie, 1943 (Nearctic), Acanthoneta dokutchaevi Eskov & Marusik, 1993 (Far East Asia) and Acanthoneta furcata Emerton, 1913 (Nearctic).
Originally Acanthoneta was described as a subgenus of Poeciloneta, including two species: Poeciloneta (Acanthoneta) aggressa and Poeciloneta (Acanthoneta) furcata. One additional species Acanthoneta dokutchaevi was assigned to the subgenus by
Males of Acanthoneta differ from Poeciloneta by the long radix almost parallel with the long lamella characteristica (Fig. 6F), in the latter the radix is normal boat-shaped, lamella characteristica large and ribbon-like (
http://species-id.net/wiki/Acanthoneta_aggressa
Fig. 6H–JNo material examined, epigynum pictures were provided by Don Buckle (Saskatoon, Canada): 1 ♀, Canada, Alberta, Chinook Lake, under log in spruce or fir woods, 49°40'N, 114°30'W, 25 Jul. 1988, D. J. Buckle leg.
Epigynum (Fig. 6H–J). Slightly protruding, without extensible area at basal part. Epigynal cavity, with posteriorly extended lateral wall, surrounding sigmoid folded scape; scape long and narrow, with well developed lateral lobes hosting copulatory openings and distal stretcher.
Across North America from Washington State to Québec (
http://species-id.net/wiki/Acanthoneta_dokutchaevi
Fig. 6A–G1 ♂, China, Jilin Province, Mt. Changbaishan, Ski. 42°01.54'N, 128°04.25'E, alt. ca 1260 m, 31 July 1971.
Male (Fig. 6A–B). Chelicera long, with strong stridulatory ridges. Chaetotaxy: Ti I–IV: 2-2-2-2; Mt I–IV: 1-0-0-1; Tm I about 0.80, Tm IV present. For other somatic characters see description by
Male palp (Fig. 6C–E). Cymbium with proximal apophysis erected. Paracymbium wide, with two pointed teeth on lateral margin. Distal suprategular apophysis modified as pit hook. Embolic division: radix long and narrow; Fickert’s gland located within radix; embolus main body trunk-like with serrated area, pointed embolus proper and well developed thumb; lamella characteristica fork-like branched, long and slender, almost parallel to radix; terminal apophysis with distal membrane and two strongly sclerotized teeth on ventral side.
Female. Unknown.
The male of this species is similar to the type species Acanthoneta aggressa. It differs only by the shape of the paracymbium. For a detailed comparison see
Far East Asia: Magadan Area (
We would like to thank Lyubomir Penev, Dimitar Dimitrov, Nikolaj Scharff, Gustavo Hormiga and Jeremy Miller for their comments on an earlier version of this paper. We would like to thank Don Buckle for providing the epigynum pictures of Acanthoneta aggressa, Fang Wang for helping molecular analysis and Chen Wei for field assistance. English of the final draft was kindly checked by David Penney. The study was supported by Natural Sciences Foundation, China (NSFC-30670244, NSFC-30970314, and NSFC-30911120070) and Russian Foundation for Basic Research grants (No.11-0401716 and 12-04-01548).
Linyphiid phylogeny resulting from Maximum Likelihood analysis based on molecular data. Numbers at the nodes are bootstrap value. Branches in color indicate the four robustly supported clades within linyphiids: S Stemonyphantes clade (blue) L1 “linyphiines”-1 clade (pale green) L2 “linyphiines-2” clade (dark blue) ME “micronetines-erigonines” clade (red, with “Distal erigonines” clade in green). Taxa in different colors sampled from different groups: grey, outgroup; blue, Stemonyphantinae; pale green, Linyphiinae; dark blue, Mynogleninae; pink, Dubiaraneinae; black, Micronetinae; red, Ipainae, Acanthoneta and Acanoides gen. n.; green, Erigoninae. Red stars indicate the two out-group taxa: cyatholipid Alaranea and theridiosomatid Theridiosoma embedded within Linyphiidae.
GenBank accession numbers. Data of the taxa labeled with “#” are newly sequenced; the taxa labeled with “*” come from