(C) 2014 Stéphanie Boucher. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Boucher S, Nishida K (2014) Description and biology of two new species of Neotropical Liriomyza Mik (Diptera, Agromyzidae), mining leaves of Bocconia (Papaveraceae). ZooKeys 369: 79–97. doi: 10.3897/zookeys.369.6168
Liriomyza mystica Boucher & Nishida, sp. n., and Liriomyza prompta Boucher & Nishida, sp. n. are described from Costa Rica. Both species were reared from leaves of Bocconia frutescens L. (Papaveraceae). The latter species was also reared from B. arborea S. Watson. Larvae of L. mystica mine primary veins of large, relatively old, mature leaves, and L. prompta mine blades of small to large, mature leaves. These represent the first record of agromyzids feeding on Bocconia. Biological information is also given and illustrated.
Biocontrol of weeds, Bocconia arborea, Bocconia frutescens, leaf miner, Liriomyza mystica, Liriomyza prompta, Hawai`i, Neotropical, parasitoid wasps, systematics, taxonomy, Tree poppy
Liriomyza Mik is the second largest genus of agromyzid flies (after Phytomyza Lioy) with approximately 390 described species worldwide. Due to its high diversity, small size and mostly uniform external characters, identification at the species level is sometimes difficult for this genus. Host plant association is often an important tool for species identification especially for host-specific species. Host plants are known for 177 species (45%) of Liriomyza (
Bocconia frutescens L., commonly known as Tree poppy, Parrotweed, Plume poppy, Sea oxeye daisy or simply Bocconia is a large shrub to small tree native to tropical America occurring from Mexico to Argentina, and Bahamas (
Here we describe two new species of Liriomyza reared from Bocconia at various localities in Costa Rica.
Leaves of Bocconia frutescens and Bocconia arborea infested by the Liriomyza species have been collected at multiple localities in Costa Rica (Figs 1–3, Table 1). Most of the observation, collecting and rearing was conducted between April 2007 and July 2011. The leaves collected were taken back to a laboratory in San Isidro de Coronado (Table 1, site 15) for rearing. Approximate average temperature of the rearing chamber was 25 °C (day) and 18 °C (night). Infested parts of the leaf blade were separated from the primary vein in order to separate the two fly species, which are site-specific on the leaf. The leaf blade and the primary veins were placed either in large transparent plastic bags or translucent Tupperware for observation and rearing of the larvae, puparia and parasitoids. Most adults were obtained from rearing, except a few caught while mating on Bocconia frutescens at site 13. For some of the sites, only leaf mines were recorded via observations. Larvae, puparia, adults and parasitoids were preserved in 75% ethanol. Most adults were dried using HMDS (hexamethyldisilazane). Photographs of the life histories and live specimens of both adults and immature stages were taken with digital cameras (Nikon Coolpix 4500, 8700, and Canon PowerShot G7). The final digital images were processed using Adobe Photoshop CS4. Type specimens are deposited in the following collections (acronyms used in the text are in parentheses): Canadian National Collection of Insects, Arachnids & Nematodes, Ottawa, ON, Canada (CNC); Museo de Zoología, Universidad de Costa Rica, San José, Costa Rica (MZUCR); Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica (INBio); Lyman Entomological Museum, McGill University, Ste-Anne-de-Bellevue, QC, Canada (LEM); National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (NMNH).
Specimens of immature stages are deposited in CNC and MZUCR, and parasitoid wasps in MZUCR.
Life history of two new species of Liriomyza. 1–3 Habitats 1 Open area in a valley near Reserva Biológica Manuel Alberto Brenes in San Ramón (site 2). Arrows indicate Bocconia frutescens trees 2 Bocconia frutescens saplings (in circle) growing along the road after land slides caused by 2009 earthquake in Cinchona-Vara Blanca area (site 10) 3 Ornamental Bocconia frutescens tree (in middle) in urban area of San Isidro de Coronado (site 15) 4 Liriomyza mystica female (from site 5) 5 Liriomyza prompta female (from site 13) 6 Mating couple of Liriomyza prompta on the underside of Bocconia frutescens leaf at 7:00 am (30.v.2009, site 13) 7 Liriomyza prompta ovipositing on the upper side of Bocconia frutescens leaf blade at 3:00 pm (17.vi.2011, site 2).
Liriomyza species occurrence at different site localities in Costa Rica. All from host plant Bocconia frutescens L., unless specified otherwise.
| Sites | Province | Locality, Lat.-Long. | Elevation | Species | Imago ♂♀ | Comments/ immature stages | |
|---|---|---|---|---|---|---|---|
| 1 | Alajuela | Parque Nacional Volcán Arenal, 10°27'57"N, 084°45'18"W | 600 m | Liriomyza prompta | 0 | 0 | 1 puparium |
| Alajuela | Parque Nacional Volcán Arenal, 10°27'54"N, 084°45'15"W | 605 m | Liriomyza prompta | 0 | 0 | leaf mine | |
| 2 | Alajuela | Reserva Biológica Manuel Alberto Brenes, open valley area, (Fig. 1) 10°13'43"N, 084°34'10"W | 796 m | Liriomyza mystica | 0 | 0 | 1 puparium |
| Alajuela | Reserva Biológica Manuel Alberto Brenes, open valley area, (Fig. 1) 10°13'43"N, 084°34'10"W | 796 m | Liriomyza prompta | 2 | 2 | larvae, puparia | |
| Alajuela | Reserva Biológica Manuel Alberto Brenes, 10°13'07"N, 084°35'49"W | 850 m | Liriomyza mystica | 0 | 0 | 1 puparium | |
| Alajuela | Reserva Biológica Manuel Alberto Brenes, 10°13'07"N, 084°35'49"W | 850 m | Liriomyza prompta | 0 | 1 | oviposition, 1 puparium | |
| 3 | Alajuela | Laguna de Hule, 10°18'14"N, 084°12'26"W | 809 m | Liriomyza mystica | 0 | 0 | 1 larva |
| Alajuela | Laguna de Hule, 10°18'14"N, 084°12'26"W | 809 m | Liriomyza prompta | 0 | 0 | 1 puparium | |
| 4 | Alajuela | Bajo del Toro, 10°08'27"N, 084°19'60"W | 1380 m | Liriomyza prompta | 0 | 0 | leaf mines |
| 5 | Cartago | San Ramón de Tres Ríos, 09°56'20"N, 083°58'55"W | 1500 m | Liriomyza mystica | 1 | 5 | larvae, puparia |
| Cartago | San Ramón de Tres Ríos, 09°56'18"N, 083°58'38"W | 1600 m | Liriomyza mystica | 35 | 35 | larvae, puparia | |
| Cartago | San Ramón de Tres Ríos, 09°56'18"N, 083°58'38"W | 1600 m | Liriomyza prompta | 13 | 7 | larvae, puparia | |
| Cartago | San Ramón de Tres Ríos, 09°56'27"N, 083°58'16"W | 1670 m | Liriomyza mystica | 0 | 0 | larvae | |
| 6 | Cartago | Cervantes, 09°52'46"N, 083°49'07"W | 1500 m | Liriomyza prompta | 1 | 0 | larvae, puparia |
| Cartago | Cervantes, 09°52'46"N, 083°49'07"W | 1500m | Liriomyza mystica | 0 | 0 | larvae | |
| 7 | Cartago | Llano Grande, 09°54'41"N, 083°53'09"W | 2100 m | Liriomyza prompta | 0 | 0 | leaf mines |
| 8 | Cartago | Chicuá, Irazú Volcano, 09°56'49"N, 083°52'00"W | 2765 m | Liriomyza mystica | 0 | 0 | 1 larva |
| 9 | Heredia | Santo Domingo de Heredia, 09°58'21"N, 084°05'29"W | 1133 m | Liriomyza prompta | 0 | 1 | larvae, puparia, on Bocconia arborea |
| 10 | Heredia | Cinchona-Vara Blanca, (Fig. 2) 10°13'26"N, 084°09'47"W–10°11'02"N, 084°09'18"W | 1200–1800m | Liriomyza mystica | 0 | 0 | larvae, puparia |
| Heredia | Cinchona-Vara Blanca, (Fig. 2) 10°13'26"N, 084°09'47"W–10°11'02"N, 084°09'18"W | 1200–1800m | Liriomyza prompta | 0 | 0 | larvae, puparia | |
| 11 | Limón | Guácimo, 10°08'34"N, 083°42'27"W | 480 m | Liriomyza prompta | 0 | 0 | leaf mines |
| 12 | Puntarenas | Bosque Eterno de los Niños, Bajo del Tigre, 10°30'31"N, 084°49'12"W | 1130 m | Liriomyza prompta | 0 | 2 | larvae, puparia |
| 13 | Puntarenas | Estación Biológica Monteverde, 10°19'09"N, 084°48'32"W | 1538 m | Liriomyza prompta | 1 | 1 | mating (Fig. 6) |
| Puntarenas | Estación Biológica Monteverde, 10°19'09"N, 084°48'32"W | 1538 m | Liriomyza mystica | 1 | 1 | mating, larvae and puparia | |
| 14 | San José | San Pedro de Montes de Oca, 09°56'27"N, 084°02'36"W | 1236 m | Liriomyza prompta | 0 | 0 | larvae, puparia |
| 15 | San José | San Isidro de Coronado, (Fig. 3) 09°58'18"N, 084°00'22"W | 1420 m | Liriomyza prompta | 6 | 12 | larvae, puparia |
| San José | San Isidro de Coronado, (Fig. 3) 09°58'18"N, 084°00'22"W | 1420 m | Liriomyza mystica | 0 | 1 | larvae, puparia | |
| 16 | San José | San Gerardo de Rivas area, 09°28'13"N, 083°35'07"W–09°27'51"N, 083°34'15"W | 1457–2031 m | Liriomyza prompta | 0 | 0 | leaf mines |
| 17 | San José | Parque National Chirripó, forest fire area approx. 09°27'20"N, 083°31'59"W | ca. 2700 m | Liriomyza mystica | 0 | 0 | 1 puparium, 1 larva |
Both species of Liriomyza were present at most of the field sites, sometimes containing larvae of the two species on the same leaf. A total of 127 adult specimens representing two new species of Liriomyza were obtained from nine localities in Costa Rica (Table 1). Most of these specimens were reared from Bocconia frutescens except four specimens that were collected while mating under leaves of Bocconia frutescens, and two reared from Bocconia arborea (Table 1). No adult specimens were successfully reared from some of the sites, but species identification was still possible with larvae and/or puparia obtained. Species description and details on biology follow.
http://zoobank.org/48883C22-4ED7-438D-B6F4-1A290B51763F
http://species-id.net/wiki/Liriomyza_mystica
Figs 4, 8–27, 48, 50Holotype ♂: COSTA RICA: Cartago: San Ramón de Tres Ríos, 1600 m, (09°56'18"N, 083°58'38"W), ex. Bocconia frutescens, larva exited 5–9.vii.2010; adult emerged 21–26.vii.2010, Kenji Nishida (LEM).
Paratype: same data as holotype (9 ♂; 8 ♀: LEM); same except larva exited: 25–28.vi.2010, adult emerged: 20–22.vii.2010 (14 ♂; 15 ♀: INBio); same except larva exited 29.vi–4.vii.2010, adult emerged 15–24.vii.2010 (8 ♂; 7 ♀: NMNH); same except adult emerged 21–25.vii.2010 (4 ♂; 4 ♀: CNC); same except along main road, (09°56'20"N, 083°58'55"W), 1500 m, collected 19.xii.2008, emerged 16–20.i.2009, K. Nishida & T. Johnson (1 ♂; 5 ♀: MZUCR). San José: San Isidro de Coronado, Centro. 1420 m, (09°53'18"N, 084°00'22"W), ex. Bocconia frutescens, adult emerged 17.vi.2010, Kenji Nishida (1 ♀: LEM). Puntarenas: Monteverde. Estación Biológica Monteverde. 1538 m, (10°19'09"N, 084°48'32"W), mating on Bocconia leaf. 18.vii.2010, Kenji Nishida (1 ♂; 1 ♀: MZUCR).
This species can be distinguished from other Neotropical species of Liriomyza by its completely yellow head and anepisternum, mesonotum almost completely brown to margin of scutellum, usually 2 + 1 dc, legs completely yellow, calypter brown on apical half with margin and fringe brown, and by the shape of the male genitalia and the shape of the anterior and posterior larval spiracles.
Frons width 0.25 mm; ratio of frons width to eye width 2.3; orbit 0.23 times width of frons at midpoint; frons slightly projecting above or in front of eye in profile (Fig. 8), forming a distinct ring (cheek) below eye; 2 reclinate ors and 2 inclinate ori (Fig. 9) (lower ori sometimes reduced or missing on one side); orbital setulae reclinate, varying in number from about 4–8; first flagellomere rounded, not enlarged in males, with slight apical pubescence; arista 0.30–0.40 mm, with short but dense pubescence; gena deep, slightly extended at rear (Fig. 8); gena height at midpoint: 0.44 times maximum eye height. Eye oblique, bare. Normally 2+1 dc (except 4 specimens with 2+0 dc and 3 specimens with 3+1 dc); acrostichals in about 4 irregular rows; prescutellar acrostichal bristles absent; 2 notopleural bristles; 1 strong postpronotal bristle with 1 or 2 small setulae; anepisternum with 1 strong bristle on posterior margin at midpoint, sometimes with a few extra setulae; katepisternum with one strong bristle on posterodorsal corner, on yellow ground. Fore and mid-tibia without lateral bristle. Wing length 1.50–1.95 mm in male and 1.85–2.20 mm in females; M1+2 ending at wing tip; costa extending to M1+2; last section of CuA1: 1.5–1.9 times length of penultimate. Cross-vein r-m located at midpoint of cell dm. Stridulatory mechanism apparently absent.
External morphology of adult Liriomyza mystica. 8 Head, lateral 9 Head, dorso-frontal 10 Thorax, dorsal 11 Thorax, dorsal (teneral specimen).
Colour. Head (including frons, orbit, face, antenna, palp) entirely bright yellow. Hind margin of eye black for a small section beyond vte; both vt on yellow ground. Occiput black. Eye sometimes with a slight bluish or greenish reflection (not as pronounced as in Liriomyza prompta, Fig. 29); mesonotum almost completely dark brown except for narrow yellow margin posteriorly (Fig. 10), prescutellar area and intra-alar area sometimes slightly paler brown resulting in a weakly defined banded pattern on thorax, most visible in teneral specimen (Fig. 11); scutellum completely yellow with small brown patches laterally. Basal scutellar bristles on brown ground (but at the limit of yellow). Postpronotum, notopleuron and anepisternum completely yellow (at most with a very small pale brown patch on one or two of the sclerites). Katepisternum mostly brown except for upper margin yellow. Calypter brown on apical half, margin and fringe also brown; halter completely white. Legs completely yellow. Abdominal tergites pale brown.
Male genitalia. Distiphallus in the form of two narrow tubules, slightly diverging apically in ventral view (Fig. 13). Mesophallus widest apical section (Fig. 13a), about 1.5–2 times larger than basal narrower tubular section (Fig. 13b). Mesophallus in lateral view with small indent at midpoint (Fig. 12). Surstylus absent. Epandrium without chitinized margin and without spines. Ejaculatory apodeme (Figs 14, 15) weakly sclerotized, symmetrical or sometimes asymmetrical with blade more expanded on one side.
Male genitalia of Liriomyza mystica. 12 Phallus, lateral 13 Phallus, ventral (see text for lines ‘a’ and ‘b’) 14, 15 Ejaculatory apodeme.
Early stages. Larval length (at maturity): 3.2–4.0 mm, slightly larger than Liriomyza prompta larva. White to creamy white with an internal orange spot at head (live specimens, Figs 24, 25, 27). Anterior spiracles about 0.13–0.23 mm distance from each other; fan-shaped and each with 5 small openings in a single row (Fig. 18). Posterior spiracles divided into 3 subequal projecting bulbs (Figs 16, 17). Cephalopharyngeal skeleton with wide arms (Fig. 19), Each mandible with 2 large teeth. Puparium pale brown to transparent (Fig. 48).
Larval characters of Liriomyza mystica. 16 Posterior spiracles 17 Posterior spiracle (close-up) 18 Anterior spiracle (note angle of view is different from Fig. 38) 19 Cephalopharyngeal skeleton.
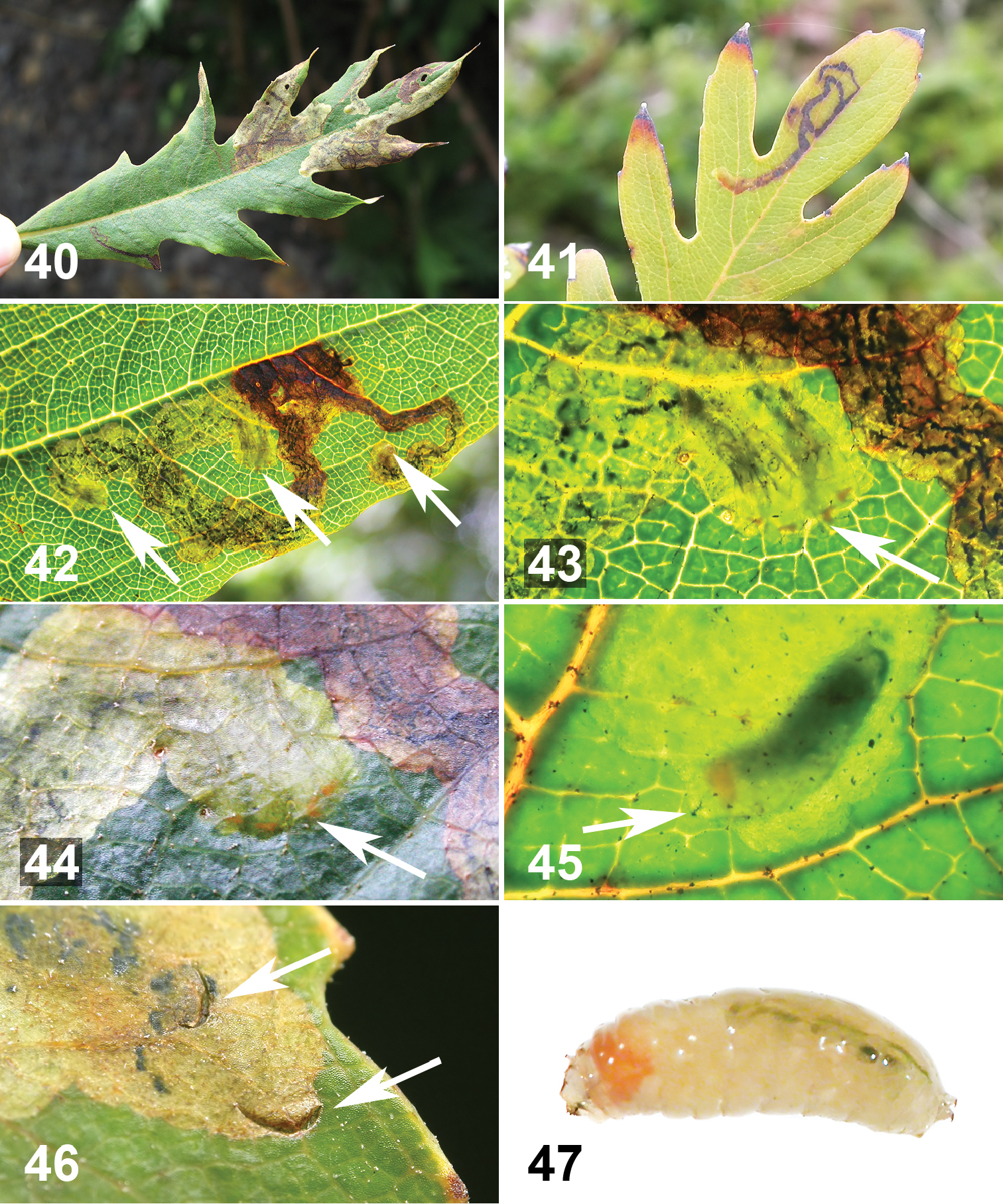
Life history of Liriomyza mystica larvae on Bocconia frutescens. 20–22 External evidence caused by internal larval feeding on vein and petiole 20 Brown to reddish brown spots (ca. 1–2 mm long) on upperside along primary vein, marked by rectangular line. Arrow indicates Liriomyza prompta mine 21 Pale brown linear spots along the primary vein seen through strong sunlight from the back. Note that lower part of vein (underside) is thicker and shown as shadow 22 Mine in pale colour zigzag, approximately 30 mm long 23 Longitudinally opened primary vein with linear mine (circle) and late instar larva (arrow) 24 Late instar larva in situ, ventral view. Cephalopharyngeal skeleton on right. Notice orange spot at head 25 Mature larva exiting from underside of vein (arrow). Close-up view, lower right. Notice orange spot at head 26 Exit hole (ca. 1 mm wide) on underside of primary vein 27 Mature larva in pre-puparial stage. Posterior on right.
Bocconia frutescens L. (Papaveraceae).
The larvae feed on spongy parenchyma and other tissues of primary veins and petioles of large, relatively old, mature leaves. Most of the larvae were in leaves of >30 cm long, with >10 mm petiole width and >10 mm thickness (n=120). The larvae were more frequently found mining in the thicker part of the primary vein including the petiole (i.e. less frequently near the leaf apex). One larva was found mining inside of a 2.2 mm width primary vein near the leaf apex. A few larvae were mining thick secondary veins. The mining appears to occur longitudinally, mostly near the upper leaf surface; the larvae left some brown to reddish brown scars along the leaf blade where the vein and blade join (Figs 20, 21, 23). These scars were more easily seen with a strong transmitted light (Fig. 21). The mines (internal tunnels) can also be distinguished by narrow pale lines (Fig. 22). When infested veins were longitudinally dissected, usually one to a few white Liriomyza prompta larvae were observed mining singly and scattered (n=12 leaves) (Figs 23, 24). Some solitary parasitoid wasp pupae were also found among the spongy parenchyma (Fig. 50). The mature fly larvae exited from either the upper side or underside of the veins (n=12 holes) (Fig. 25), each larva making a small oval-shaped hole of 1.1–1.3 mm wide (n=12) (Fig. 26). The tissue around the old exit holes was brown to reddish brown (n=5). The newly emerged larvae wiggled around in rearing plastic bags/cases for a couple of hours to a few hours before settling (Fig. 27) and starting to form a puparium. The larvae readily pupated on the plastic surfaces. In general, the puparia (Fig. 48) were more translucent (translucent pale brown) than those of Liriomyza prompta (translucent brown to dark brown) and the pupa inside was visible. Duration of the larval stage was not recorded. The larvae that exited from veins pupated between 26 to 29.vi.2010 and the adults emerged between 20 to 22.vii.2010, i.e. the pupal stage lasted approximately 25 days (n=29). A mating pair was observed on the underside of a leaf around 7:00 am (site 13). No oviposition behavior was observed for this species.
Two species of Pteromalidae: Pteromalinae: sp. 01 from sites 5, 10, 15, parasitizing late instar larva, pupating inside the leaf vein (Fig. 50); Pteromalinae sp. 02 from sites 5, 10, 13, parasitizing larva and pupating inside the host puparium; one species of Braconidae: Opiinae: Opius sp. from site 10, parasitizing larva and pupating inside the host puparium.
Liriomyza mystica is most similar to Liriomyza prompta described below and to the Neotropical species Liriomyza commelinae (Frost) and Liriomyza robustae Spencer, especially in the form of the phallus with paired tubules. But these two latter species differ from Liriomyza mystica in a number of characters, including their host plants (both known from plants in the family Commelinaceae); mesonotum with a distinctive black and yellow pattern; surstylus with a distinct spine; third antennal segment enlarged in males; shape of both anterior and posterior spiracles and pupation occurring inside the mine (
Most adult specimens of Liriomyza mystica were reared from site 5, but it was also found at other sites, up to an elevation of 2765 m (Table 1). Considering that Liriomyza mystica larvae feed inside primary veins and petiole of large, mature leaves, it made it difficult to establish Bocconia arborea as possible host due to the problems in studying large trees with large leaves. In sapling trees of ca. 1 m tall (n=2) at Santo Domingo de Heredia (site 9), no larvae or evidence of feeding was observed.
The species name is derived from the Latin mysticus (secret, mystic), referring to the hidden and inconspicuous leaf mines in primary vein and petiole.
http://zoobank.org/5933AA4E-ACDE-4A2F-8F66-4CD9AA55F1C6
http://species-id.net/wiki/Liriomyza_prompta
Figs 5–7, 20, 28–47, 49, 51Holotype ♂: COSTA RICA: Cartago: San Ramón de Tres Ríos, 1600 m (09°56'18"N, 083°58'38"W), ex. leaf mine Bocconia frutescens, larva exited 5–9.vii.2010, adult emerged 21–26.vii.2010, Kenji Nishida (LEM).
Paratype. same data as holotype (2 ♀: LEM); same except: ex. leaf mine Bocconia frutescens, emerged 1.vi.2010, Kenji Nishida (1 ♀: LEM); same except larva exited 29.vi.2010, adult emerged 20.vii.2010 (1 ♀: LEM); same except larva exited 29.vi–4.vii.2010, adult emerged 15–24.vii.2010 (7 ♂; 1♀: LEM); same except larva exited 25–28.vi.2010, adult emerged 20–22.vii.2010 (1 ♀: NMNH); same except larva exited 29.vi–4.vii.2010, adult emerged 21–25.vii.2010 (5 ♂; 1 ♀: NMNH); Cartago: Cervantes, 1500 m (09°52'46"N, 083°49'07"W), ex. Bocconia frutescens, emerged 11.vi.2010, Kenji Nishida (1 ♂: CNC); San José Province: San Isidro de Coronado, Centro 1420 m (09°58'18"N, 084°00'22"W), ex. Bocconia frutescens, adult emerged 11.vi.2010, Kenji Nishida (1 ♀: MZUCR); same except puparia formed 10–12.v.2010, adult emerged 12.vi.2010, Kenji Nishida (1 ♀: MZUCR); same except emerged 10.vi.2010 (1 ♂: INBio); same except pupation 6.vi.2010, emerged 26.vi.2010, Kenji Nishida (1 ♀: INBio); same except collected 15.v.2009, emerged 8.vi.2009, K. Nishida (4 ♂; 6 ♀: MZUCR), same except (1 ♂; 3 ♀: CNC); Puntarenas Prov., Monteverde, Estación Biológica, 1538 m (10°19'09"N, 084°48'32"W), mating on Bocconia frutescens leaf, 30.v.2009, Kenji Nishida (1♂; 1♀: INBio).
This species can be distinguished from other Neotropical species of Liriomyza by its completely yellow head and anepisternum, mesonotum almost completely brown to margin of scutellum, usually 3 + 1 dc, legs completely yellow, calypter brown on apical half with margin and fringe brown, and by the shape of the male genitalia and the shape of the anterior and posterior larval spiracles.
As in Liriomyza mystica Boucher and Nishida (described above) except as follows: arista shorter (Figs 28, 29), 0.23–0.3 mm; normally 3+1 dc (except one male specimen with 2+1 dc); generally smaller: wing length 1.4–1.7 mm in males and 1.6–2.2 mm in females; eye often with a more extensive bluish reflection (Fig. 29).
Male genitalia. Similar to Liriomyza mystica, but tubules of distiphallus more sclerotized, slightly wider, parallel sided (not diverging in ventral view). Mesophallus widest apical section (Fig. 33a), more than twice as large as narrower basal tubular section (Fig. 33b). Mesophallus in lateral view with a prominent curve near midpoint. Surstylus absent. Ejaculatory apodeme (Figs 34, 35) slightly more sclerotized than in Liriomyza mystica, and with blade slightly less expanded.
External morphology of adult Liriomyza prompta. 28 Head, lateral 29 Head, lateral (variation of eye colour) 30 Head, dorsal 31 Thorax, dorsal.
Male genitalia of Liriomyza prompta. 32 Phallus, lateral 33 Phallus, ventral (see text for lines ‘a’ and ‘b’) 34, 35 Ejaculatory apodeme.
Early stages. Larval length: 1.95–2.70 mm, slightly smaller than Liriomyza mystica larva. White to creamy white with an internal orange spot at head (Fig. 47). Anterior spiracles about 0.1 mm distance from each other. Similar to Liriomyza mystica: fan-shaped with 5 small openings. Posterior spiracles with apparently 3 bulbs, but two very small and 1 much longer, curving toward anal segment (Figs 36, 37). Cephalopharyngeal skeleton (Fig. 39) more elongated with side arms narrower than in Liriomyza mystica. Puparium translucent brown to dark brown (Fig. 49).
Larval characters of Liriomyza prompta. 36 Posterior spiracles 37 Posterior spiracle (close-up) 38 Anterior spiracle (note angle of view is different from Fig. 18) 39 Cephalopharyngeal skeleton.
Life history of Liriomyza prompta larva on Bocconia frutescens leaves 40 Leaf mines on upperside of mature, but relatively small leaf. Note that there are both single (narrow linear) and gregarious (blotch) mines 41 Active single larval mine at site 17. Note that early part of the mine becomes brown 42 Close-up of active mine with 7 larvae, seen with transmitted light (arrows indicate groups of late instar larvae): 2, 4, and 1 larvae from left to right). Early part of mine brown. Frass in dark green to black linear dots 43 Close-up of middle arrow area of Figure 42, arrow indicates four actively mining larvae. Notice four orange spots 44 Same as Figure 43, but without transmitted light 45 Close-up of actively mining late instar larva. Arrow indicates cephalopharyngeal skeleton 46 Exit holes (arrows), approximately 1.2 mm wide, near or at end of mine 47 Mature larva recently exited from mine. Posterior on right.
Life history of two new species of Liriomyza. 48 Puparium of Liriomyza mystica, in situ 49 Puparium of Liriomyza prompta, in situ 50 Pupa of Pteromaline parasitoid wasp (sp. 01) inside Bocconia frutescens leaf vein parenchyma 51 Braconid parasitoid wasp, most likely Opius sp., attempting to oviposit in mature Liriomyza prompta larva at site 13.
Bocconia frutescens L. and Bocconia arborea S. Watson (Papaveraceae)
At site 15 in late October 2012 a few males were perched on small young leaves at the apical shoot of a 2 m host tree in the morning between 6:00 and 7:00 am. The males were either sitting or flying and perching on apical young leaves. A mating couple was observed on the underside of host leaf at 7:00 am (site 13, Fig. 6). Oviposition was observed a few times by two females at site 2. At 3:00 pm (17.vi.2011), overcast with slight drizzle, the two females were walking on the upper side of leaves of a Bocconia frutescens sapling, ca. 50 cm tall. The females either oviposited in the leaf tissue at the edge of the leaf margin (n=2) or along narrow leaf veins on the upper side of the leaf blade (n=3) (Fig. 7). It was difficult to locate the eggs because of their small size and translucent colour (n=1). After oviposition, the leaf was collected and a small larva was found on 28.vi.2011. Also at site 5, three leaves were randomly collected on 24.vi.2010, and newly started mines were observed on 2–3.vii.2010 (n=6 mines). These observations suggest that the duration of the egg stage is approximately 10 days. The larvae mine mesophyll of the leaf blade either singly or gregariously (up to 7 larvae) (n=70 mines) (Figs 40–45). Mines were found on very small mature leaves of ca. 1.5 cm (n=5, at site 13) to 90 cm long (n=10, at site 10). The mature mines with a single larva were narrow and more or less linear (Figs 20, 40, 41), and mines with multiple larvae were blotch-shaped (Figs 40, 42). These mines were conspicuous on the upper side of leaves. With regard to location of mines on leaves, no patterns were noticed; mines appear to occur on any part of the leaf blade — some mines were found near the primary vein, leaf apex, or anywhere in between (uncounted). Presence of the larva in the mine can be recognized by the orange spot of the larval anterior end (Figs 43–45, 47). The early part of the mine becomes brown and the frass is seen in dark green to black linear dots (Figs 42–44). The mature larvae each made an elliptical exit hole 1.1–1.3 mm wide on the upper side of the leaves, (n=7 holes) (Fig. 46). Under rearing conditions, from the time of recognizing very early mines, the larvae completed mining within 3 to 4 days, and on the 5th day the puparia were usually formed on the plastic surface of rearing bags or containers. Young pupae were observed within a day or two after forming of the puparium (n=16). The puparia (Fig. 48) were less translucent than those of Liriomyza mystica. The pupa becomes dark 2–3 days prior to adult emergence. A cohort of larvae from site 5 which pupated on 20–23.xii.2008 emerged as adults on 16–20.i.2009 (n=6, 1 ♂, 5 ♀), other data as follows: pupation 28.vi.2011, adult emergence 24.vii.2011 (a cohort, n=4, 2 ♂, 2 ♀, site 2); pupation 12–13.ii.2012, emergence 8.ii.2012 (n=2, site 12); pupation 20.iv.2012, emergence 14.v.2012 (n=2, site 15); i.e. the pupal stage lasted nearly a month under the rearing conditions. A small population of Bocconia arborea was found at site 9; however, it was only possible to access two saplings, which were about 1 meter tall and infested by Liriomyza prompta larvae (mostly old mines). Also at sites 7, 9 and 15, populations of weedy Papaveraceae, Argemone mexicana L. were found in close proximity to Bocconia frutescens and Bocconia arborea; however, no leaf mines of Liriomyza prompta were observed.
Three species of Eulophidae: Entedoninae: sp. 01 and sp. 02 from sites 5, 6, 13, and 15, Entedoninae sp. 03 from site 15, all these were parasitizing larva and pupating inside the host puparium; Eulophinae: sp. 01 from sites 13 and 15, parasitizing late instar larva, pupating inside the host leaf mine; and two species of Pteromalinae as mentioned in Liriomyza mystica, sp. 01 from site 5 and 15, and sp. 02 from site 13 and 15. A species of Braconidae, probably an Opiinae: Opius sp. was observed parasitizing a mature Liriomyza prompta larva at site 13 (Fig. 51).
An important character differentiating larvae of this species from Liriomyza mystica described above, is that the posterior spiracles each have an elongated, somewhat hook-like bulb. These posterior spiracles are similar to those found in the Neotropical species Liriomyza commelinae and Liriomyza robustae, but the uniformly coloured mesothorax, absence of surstyli, and unforked anterior spiracles, differentiate this new species from these other Neotropical species. This species appears to be more common than Liriomyza mystica with a wider elevation range (Table 1).
The species name is derived from the Latin promptus (visible, apparent), referring to their conspicuous and common leaf mines.
We thank Costa Rican National Parks, Javier Guevara (MINAET/SINAC, San José), Marvin Hidalgo (Estación Biológica Monteverde, Monteverde), and Roberto Ledezma Carmona (of site Figure 3) for permitting the field research, William Villalobos Müller (Centro de Investigación en Biología Celular y Molecular, Universidad de Costa Rica, San José) and Josué Castro Rodríguez (El Tanque de la Fortuna, San Carlos) for help during some of the field trips, Paul Hanson (Escuela de Biología, Universidad de Costa Rica, San José) for the identification of parasitoid wasps; and Elizabeth Heffington (Lipscomb University, Nashville, Tennessee) and Terry Wheeler (McGill University) for reviewing the manuscript. Part of this study was financially supported by Tracy Johnson (USDA Forest Service, Volcano, Hawai`i), Proyecto Bocconia (Universidad de Costa Rica, San José), Pacific Southwest Research Station and Institute for Pacific Islands Forestry (Albany, California), and Hawai`i Invasive Species Council (Honolulu, Hawai`i). Part of this study was presented in the 2010 Hawai`i Conservation Conference (2010 HCC), Honolulu, Hawai`i and XIII International Symposium on Biological Control of Weeds (ISBCW 2011) in Waikoloa, Hawai`i.