(C) 2014 Zoya Yefremova. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Yefremova Z, González-Santarosa G, Lomeli-Flores JR, Bautista-Martínez N (2014) A new species of Tamarixia Mercet (Hymenoptera, Eulophidae), parasitoid of Trioza aguacate Hollis & Martin (Hemiptera, Triozidae) in Mexico. ZooKeys 368: 23–35. doi: 10.3897/zookeys.368.6468
Tamarixia aguacatensis Yefremova, sp. n. (Hymenoptera: Eulophidae: Tetrastichinae) is described from Mexico as a parasitoid of the avocado psyllid, Trioza aguacate Hollis & Martin (Hemiptera: Triozidae). Trioza aguacate is a serious pest of avocado, Persea americana Miller. A key to the species of Tamarixia Mercet in Mexico is given.
Insecta, Chalcidoidea, Tamarixia aguacatensis, Trioza aguacate, Persea americana, Mexico
The Mexican fauna of Psyllidae is poorly known, and even less known there are psyllid parasitoids. At least four Tamarixia Mercet (Eulophidae: Tetrastichinae) species have been recorded in Mexico as psyllid parasitoids: Tamarixia leucaenae Bouček from Heteropsylla cubana Crawford (Psyllidae: Ciriacreminae), Tamarixia triozae (Burks) from Bactericera cockerelli (Sulc) (Psyllidae: Triozinae) (
The most studied species is Tamarixia triozae, which was first recorded by
The avocado psyllid was discovered for the first time in Mexico in 1995, on avocado trees (Persea americana Miller) (
Periodic samples were taken in avocado groves in the town of Salvador Escalante, Michoacán, from January 2012 to January 2013; however, presence of the parasitoid was detected only in April and May 2012. To recover some of the parasitoids, buds and avocado leaves with parasitized nymphs of Trioza aguacate were collected; these are recognized by their ochre brown tone (Fig. 1). No more than 10 mummies per jar were collected.
Mummy of Trioza aguacate nymph.
The collected material was placed in glass jars covered with organza fabric to wait for the parasitoids to emerge. Overall parasitism of the nymphs was 14.6%; but when we recorded only the large nymphs the percent parasitism was 46.7%.
After the parasitoids were processed, pictures were taken of the diagnostic characteristics to compare this species with the already described species (
Morphological terminology follows that of
The following acronyms are used for the depositories of specimens:
CNIN The National Insect Collection at the Instituto de Biología, Universidad Autónoma de Mexico, Mexico City, Mexico.
FSCA Florida State Collection of Arthropods, Gainesville, Florida, USA.
USNM United States National Museum of Natural History, Washington, D.C., USA.
TAUI The National Collection of Insects, Zoological Museum, Department of Zoology, Tel Aviv University, Tel Aviv, Israel.
Tamarixia bicolor Mercet, 1924: 57 (original designation).
Tamarixia can be distinguished by the following combination of features: fore wing with a single seta on the dorsal surface of the submarginal vein, propodeum without a Y-shaped carina; plicae and paraspiracular carinae absent, midlobe of mesoscutum with 2 pairs of long adnotaular setae (three pairs setae in Tamarixia dahlsteni Zuparko, 2011) and additional 2 pairs of short setae in the upper part in a horizontal row and 1 seta near notauli in Tamarixia aguacatensis sp. n. (Fig. 7). The anterior margin of the female hypopygium is almost straight, and the males have exceptionally long genitalia. An additional diagnostic character is that the toruli are closer to eye margin than to each other. Species are generally shiny black, but may have yellow markings on the gaster and/or head. The gaster of the female subcircular to ovate; one seta of each cercus 1.5 times or more the length of the next longest seta.
Species of Tamarixia are primary ectoparasitoids of psyllids (
Tamarixia is a cosmopolitan genus, with about 50 described species (
Keys to Tamarixia species are available for Europe (
| 1 | F3 subquadrate or transverse (Figs 14, 16, 19, 21), F1 1.2–1.3 times as long as F3 | 2 |
| – | F3 1.8–2.0 times as long as broad (Fig. 6), F1 1.45–1.5 times as long as F3 | Tamarixia aguacatensis sp. n. |
| 2 | Mesoscutum with complete median line | Tamarixia radiata (Waterston) |
| – | Mesoscutum with incomplete median line (Fig. 7) | 3 |
| 3 | Propodeum steeply inclined relative to longitudinal axis of the body | Tamarixia schina Zuparko |
| – | Propodeum inclined 45 degrees from longitudinal axis of the body (Fig. 5) | 4 |
| 4 | F2 as long as F3, F1 2.2 times as long as broad, clava 1.3 times as long as funicle (Fig. 16) | Tamarixia triozae (Burks) |
| – | F2 1.4 times as long as F3, F1 1.8 times as long as broad, clava 1.5 times as long as funicle (Fig. 19) | Tamarixia leucaenae Bouček |
| 1 | Pedicel 1.5 times as long as F1 (Figs 15, 17, 20, 22) | 2 |
| – | Pedicel as long as F1 or slightly longer (1.1 times as long as F1) (Fig. 8) | Tamarixia aguacatensis sp. n. |
| 2 | Clava 5.0 times as long as broad (Fig. 22) | Tamarixia radiata (Waterston) |
| – | Clava 4.0 times as long as broad | 3 |
| 3 | F2, F3 1.3–1.4 times as long as broad (Fig. 15) | Tamarixia schina Zuparko |
| – | F2, F3 1.8–2.0 times as long as broad | 4 |
| 4 | Whorled setae of F1 reaching the top of F3, whorls of F4 reaching top of C2 (Fig. 17) | Tamarixia triozae (Burks) |
| – | Whorled setae of F1 reaching top of F4, whorls of F4 attach out apical sensillum (Fig. 20) | Tamarixia leucaenae Bouček |
http://zoobank.org/2E77279C-F3E8-4C9F-97A8-4329A33AC45D
http://species-id.net/wiki/Tamarixia_aguacatensis
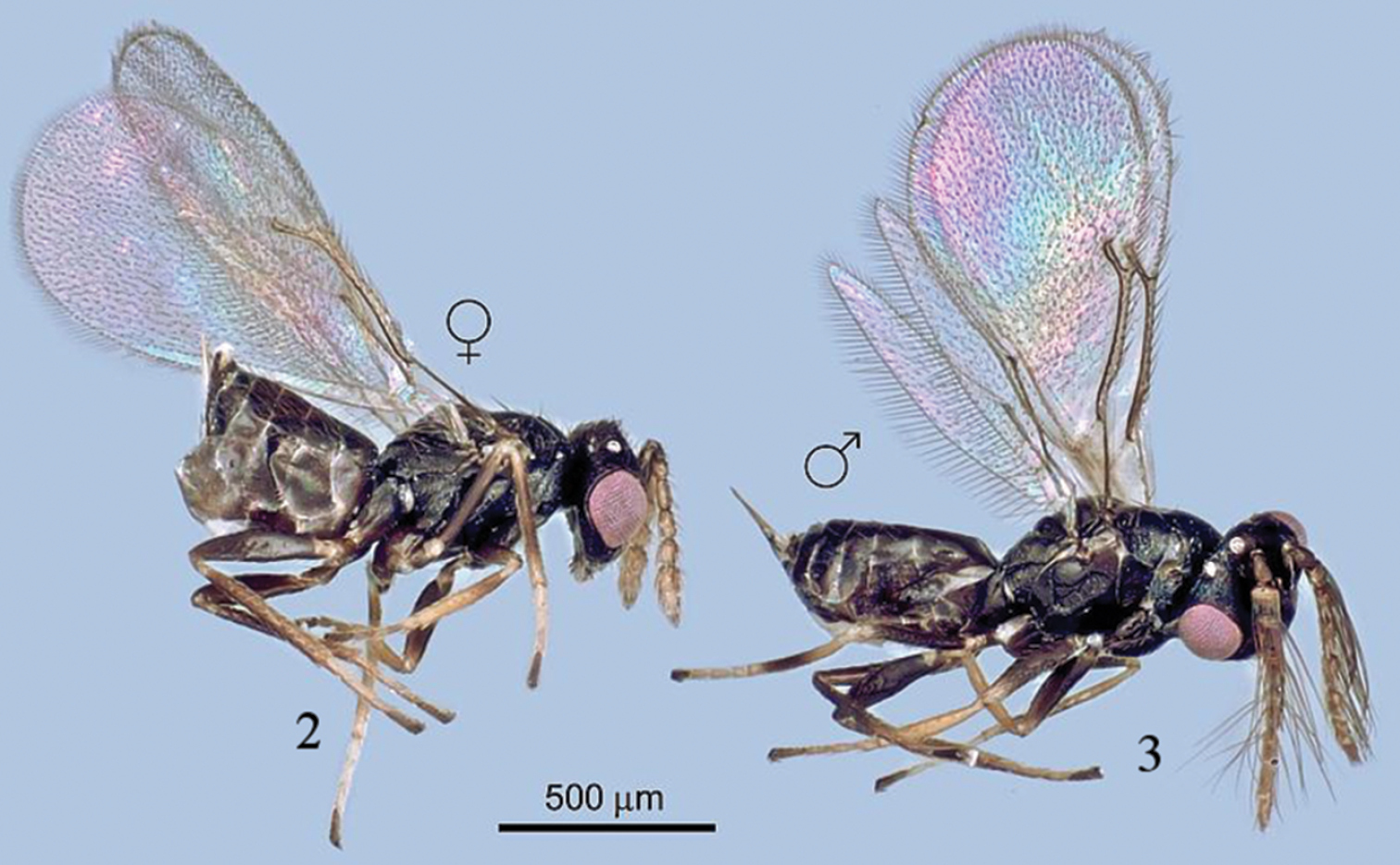
Figs 2–13(female): MEXICO, Michoacán, Salvador Escalante, Ejido El Tarascon, 19°26'29.81N, 101°49'53.03W, 1, 910 m, 2.iv.2012, G. González-Santarosa (deposited in TAUI). PARATYPES (same data): 3 ♀, 3 ♂ (CNIN); 1 ♀, 1 ♂ (USNM); 2 ♀, 4 ♂ (TAUI).
FEMALE (Fig. 2). Body length: 0.85–1.04 mm; fore wing length: 2.07–2.94 mm. Body shiny black, eye pink; antenna yellow, scape black except yellow ventrally and apically; pedicel dark dorsally and basally, yellow-brown on ventral surface; flagellar segments and clava sandy yellow; tegula yellow; legs brown dark, coxae brown, trochanters brown, trochantelli yellow, basal and distal apices of pro- and meso- femora and tibiae yellow, and metafemur and tibia brown; tarsi yellow except apical segment brown. Metanotum yellow. Gaster brown. Wings hyaline, venation brownish.
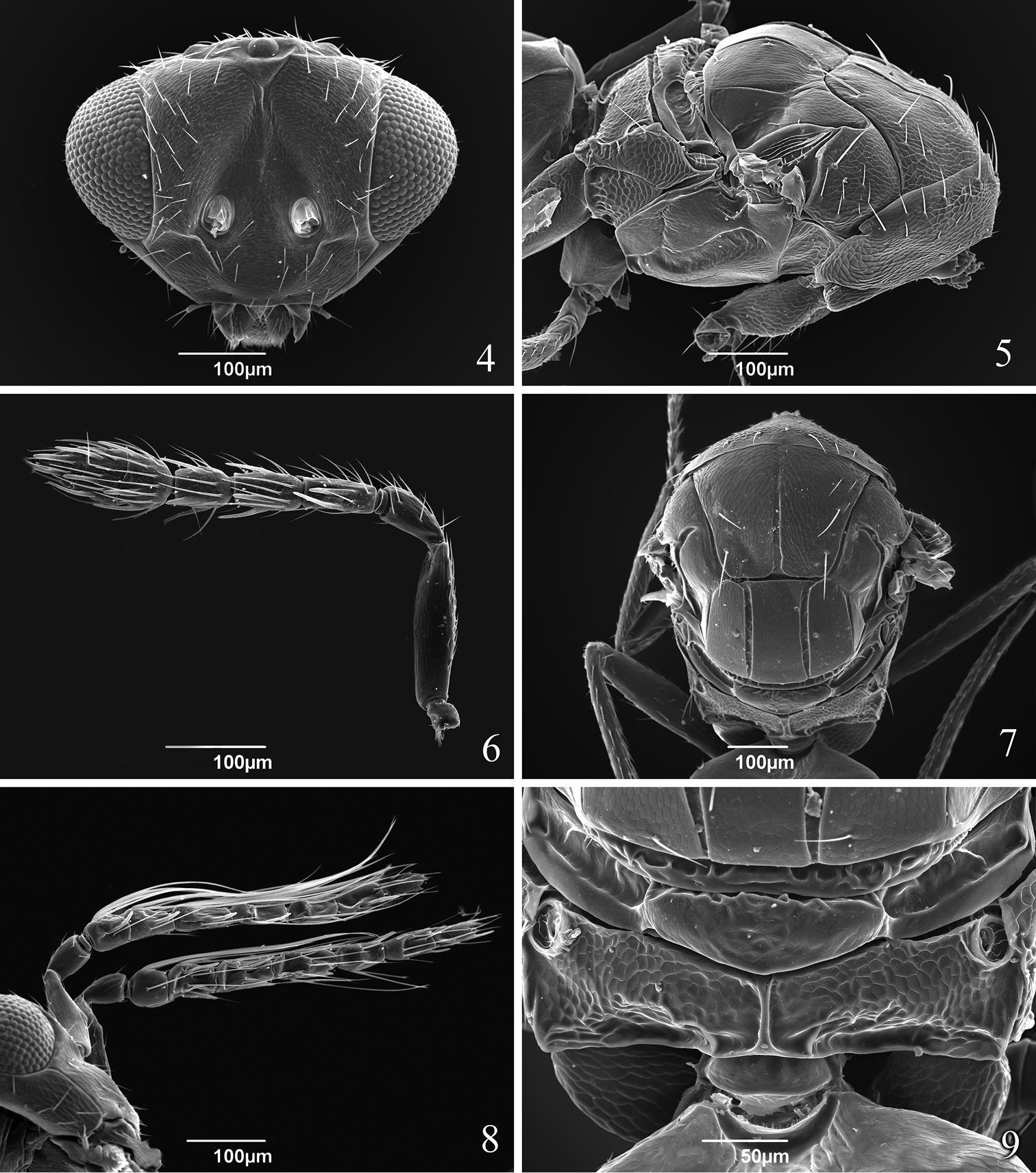
Head 2.2 times as wide as long (Fig. 4). POL 2.0–2.2 times OOL. Face smooth; vertex, frons, areas near orbits and lower face setose. Malar sulcus present. Toruli slightly above lower level of eyes. Mandible with upper long tooth and several lower short teeth. Scrobes depressed and sutured (inverted V-shaped). Eye bare. Antenna (Fig. 6) with scape 2.3 times as long as pedicel, 1 discoid anellus, pedicel as long as F1 and F2 combined, F1 2.2 times as long as broad and equal to F2, F2 2.0 times as long as broad and 1.3 times as long as F3, clava 3-segmented, 2.3–2.4 times as long as broad and 2.4–2.6 times as long as F3.
Tamarixia aguacatensis, female and male (habitus).
Tamarixia aguacatensis. Female: 4 Head, frontal view 5 Mesosoma, lateral view 6 Antenna 7 Mesosoma, dorsal view 9 Propodeum. Male 8 Both antennae on the head.
Mesosoma. Pronotum short, with 8 marginal setae (Fig. 5). Mesoscutum 1.5 times as long as broad with an incomplete median line (0.63 length of mesocutum) and with 2 pairs of long adnotaular setae (Fig. 7). Mesoscutum with additional 2 pairs of short setae in the upper part in a horizontal row and 1 seta near notauli (Figs 5, 7). Mesocutum, scutellum and dorsellum finely reticulate. Scutellum with two submedian lines closer to each other than to sublateral lines, with 2 pairs of setae; first pair of setae in the middle of scutellum. Mesosoma in lateral view higher than the plane of propodeum and inclined at an angle less than 45 degrees from the longitudinal axis of the body (Fig. 5). Propodeum (Fig. 9) strongly reticulate, with a complete simple median carina; spiracle with a rim. Callus with 2 long setae in one row (Fig. 7).
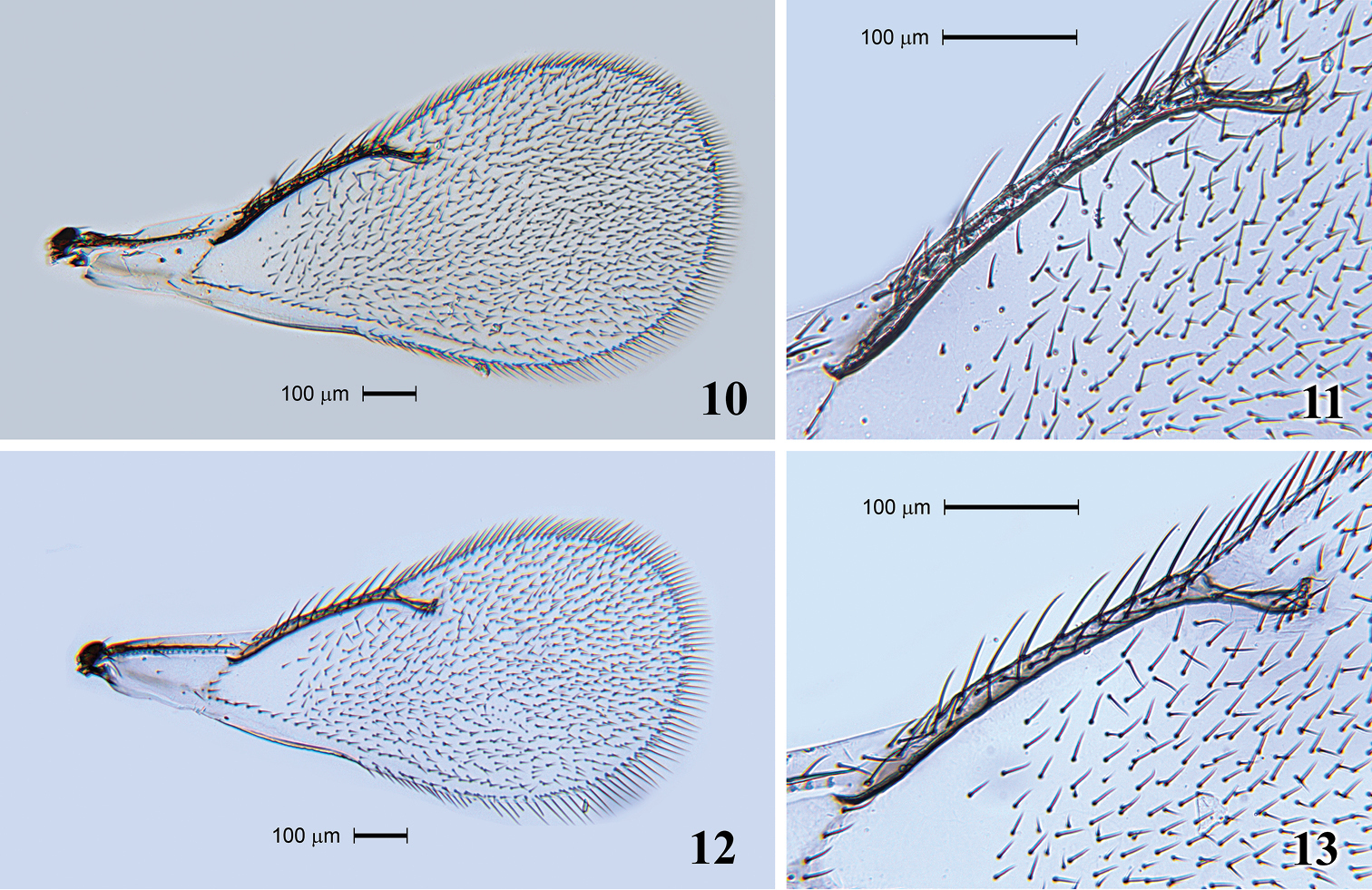
Fore wing (Fig. 10) 2.6 times as long as broad. SMV with 1 seta. Speculum extending along half length of MV and closed. SMV 1.2 times as long as MV. MV with 8 setae (Fig. 11). STV 3.4 times shorter than MV. PMV absent. Hind wing acute at apex.
Tamarixia aguacatensis. Female: 10 Fore wing 11 Marginal vein with setae. Male: 12 Fore wing 13 Marginal vein with setae.
Gaster 1.16–1.27 times as long as broad. Ovipositor sheaths slightly visible (Fig. 2).
MALE (Figs 3, 12, 13). Body length 0.8–1.00 mm. Colour of body very similar to that of female except gaster with tergite 1 completely yellow. Antennal scape dorsally dark brown; pedicel, and funicle sandy yellow. Coxae of all legs brown, trochanters brown, trochantelli yellow, pro- and meso- femora brown except yellow at apex, metafemur and tibia brown, tarsi yellow except apical segment dark brown. Tegula yellow. Eyes pink. Ocelli white.
Head. POL 1.6–1.8 times OOL. Antenna (Fig. 8). Scape with ventral plaque about 0.2 length in the basal half. Pedicel 1.0–1.2 times as long as F1, F2 1.1 times as long as F1, F3 1.18 times as long as F2 and equal to F4, C1 equal to C2 and C3 1.2 times as short as C2. Four funicle segments with whorled setae; whorls of F1 reaching middle of F3, whorls of F2 reaching base of F4, whorls of F3 reaching tip of C3, whorls of F4 reaching middle of C2, whorls of C1 reaching base of C3, whorls of C2 reaching middle of C3, whorls of C3 reaching apical placoid sensillum. Scutellum smooth between submedian lines, and submedian and sublateral lines. Fore wing 2.1 times as long as broad (Fig. 12). Speculum slightly larger than that in female and MV with 9 setae (Fig. 13). Metasoma. Gaster 1.65–1.8 times as long as broad. Genitalia with two long longitudinal digital sclerites. Aedeagus very long, 2.3 times as long as gaster (Fig. 3). Parameres triangular with one long parameral seta.
Tamarixia aguacatensis resembles Tamarixia leucaenae (examined were two female paratypes (FSCA) with the following data: Trinidad and Tobago, Trinidad Island, “UWJ Field, stn. (Lab)”, on Leucaena sp., det. by Z. Bouček, 1988) from which it differs by the colour of the female: legs dark brown except coxae and trochanters brown, trochantelli yellow (coxae yellowin Tamarixia leucaenae); in addition, the female of Tamarixia aguacatensis differs from that of Tamarixia leucaenae in having F1-F3 2.0–2.2 times as broad as long and clava 2.4 times as broad as long (F1 1.7 times as long as broad, F2 1.4 times as broad as long, F3 subquadrate and clava 2.0 times as broad as long in Tamarixia leucaenae).
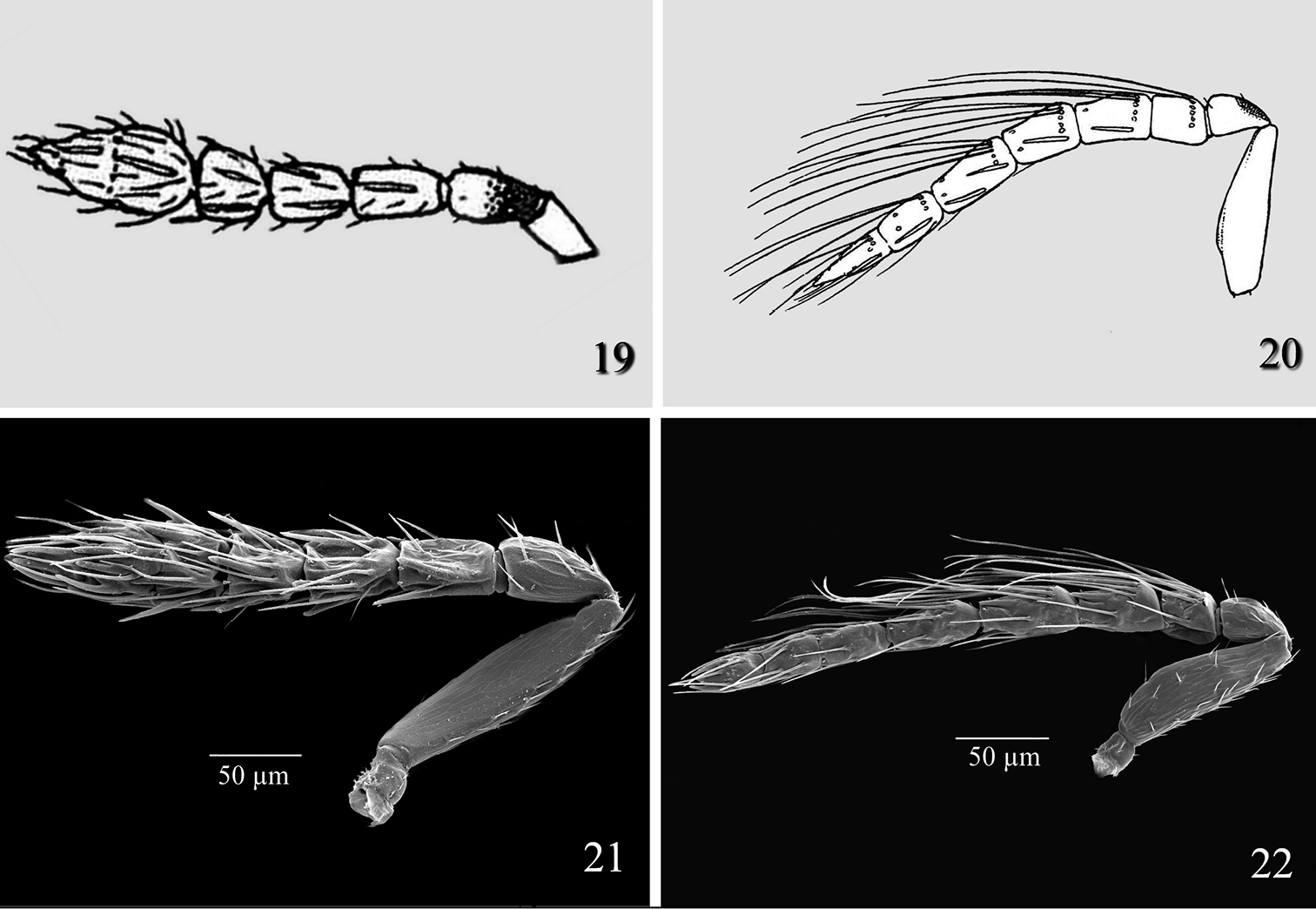
The female antenna of Tamarixia aguacatensis differs from that of Tamarixia schina (Fig. 14) as follows: F1-F3 2.0–2.2 times as broad as long and clava 2.3–2.4 times as broad as long (F1 1.8 times as broad as long, F2 1.2 times as broad as long, F3 transverse, and clava 1.8 times as broad as long in Tamarixia schina). The male antenna of Tamarixia aguacatensis differs from that of Tamarixia schina (Fig. 15, illustrated here for the first time) as follows: pedicel equal in length to F1 (1.5 times as long as F1 in Tamarixia schina), F1 and F2 equal, F2 1.2 times as long asF3 (F1, F2 and F3 equal in Tamarixia schina), clava 2.5 as long as F3 (2.0 times as long as F3 in Tamarixia schina), clava 2.0 times as long as broad (1.5 times as long as broad in Tamarixia schina). Additionally, the metanotum and propodeum are inclined much less in Tamarixia aguacatensis than in Tamarixia schina.
Tamarixia schina: 14 Female antenna 15 Male antenna. Tamarixia triozae: 16 Female antenna 17 Male antenna 18 Female fore wing.
Female of Tamarixia aguacatensis differs from that of Tamarixia triozae (Fig. 16) by in having F1-F3 2.0–2.2 times as broad as long and clava 2.3–2.4 times as broad as long (F1 2.0 times as broad as long, F2 1.7 times as broad as long, F3 subquadrate, and clava 1.6–1.7 times as broad as long in Tamarixia triozae). The male antenna of Tamarixia aguacatensis differs from that of Tamarixia triozae (Fig. 17) as follows: pedicel equal to length F1 (1.6 times as long as F1 in Tamarixia triozae), F1 and F2 equal to each other, F2 1.2 times longer than F3 (F1 subquadrate, F2 1.17 times shorter than F3 in Tamarixia triozae), clava 2.5 as long as F3 (2.2 times as long as F3 in Tamarixia triozae).
Female of Tamarixia aguacatensis differs from that of Tamarixia radiata (Fig. 19) in having F1-F3 2.0–2.2 times as broad as long, clava 2.4 times as broad as long (F1 1.6 times as broad as long, F2 1.5 times as broad as long, F3 subquadrate, and clava 2.0 times as broad as long in Tamarixia radiata). The male antenna of Tamarixia aguacatensis differs from that of Tamarixia radiata (Fig. 20) as follows: F1 and F2 equal to each other (pedicel equal in length to F1 in both species), F2 1.2 times longer than F3 (F1 1.4 times as short as F2, F2 equal to F3 in Tamarixia radiata), clava 2.5 as long as F3 (5.0 times as long as F3 in Tamarixia radiata), whorled setae of F1 reaching middle of F3 (reaching top of F4 in Tamarixia radiata), whorls of F2 reaching base of F4 (Fig. 8) (reaching middle of C2 (Fig. 22) in Tamarixia radiata).
Tamarixia leucaenae: 19 Female antenna 20 Male antenna 21 Tamarixia radiata: 21 Female antenna 22 Male antenna.
The male antenna of Tamarixia aguacatensis resembles that of Tamarixia psyllae Yefremova & Yegorenkova from Yemen that was reared from Trioza erytrea (Del Guercio) (
The male antenna of Tamarixia aguacatensis resembles that of Tamarixia dryi (Waterston), reared from Trioza citri Laing in Kenya (
Tamarixia aguacatensis also resembles Tamarixia flavigaster (Brothers & Moran), described from South Africa from Psyllidae on Calodendrum capense (L.) (
Mexico.
Known from Trioza aguacate, as a nymphal parasitoid.
The species name is derived from its host, Trioza aguacate.
Tamarixia aguacatensis is the fifth known species of Tamarixia in Mexico. It can be distinguished from other congeneric species in the country by having two pairs of short setae in the horizontal row on mesoscutum (Fig. 7).
We thank Greta Hanako Rosas Saito and Jorge Valdez Carrasco (Colegio de Postgraduados, Carretera México-Texcoco Km. 36.5, Montecillo, Texcoco, Estado de México, Mexico) for their technical assistance with scanning electron microscopy, we also thank Serguei Triapitsyn (University of California, Riverside, California, USA) and John Huber (Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Ontario, Canada) for their comments.