(C) 2014 Pei Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Wang P, Xiao Q, Zhou W-C, Hwang C-C (2014) Revision of three camaenid and one bradybaenid species (Gastropoda, Stylommatophora) from China based on morphological and molecular data, with description of a new bradybaenid subspecies from Inner Mongolia, China. ZooKeys 372: 1–16. doi: 10.3897/zookeys.372.6581
We have revised the taxonomy of three camaenid and one bradybaenid species from China and described one new subspecies of the genus Bradybaena (Family Bradybaenidae) from Inner Mongolia, China. The genitalia of three Satsuma (Family Camaenidae) species S. mellea stenozona (Moellendorff, 1884), S. meridionalis (Moellendorff, 1884), comb. n. and S. uncopila (Heude, 1882), comb. n. assigned to the genus Bradybaena previously, lack a dart sac and mucous glands. Moreover, the molecular phylogeny has revealed close relationships between the three species and the genus Satsuma. Two species, S. stenozona (Moellendorff, 1884) from Fuzhou and Ganesella citrina Zilch, 1940 from Wuyi Mountain, are considered as synonymous and should be a subspecies of S. mellea mellea (Pfeiffer, 1866) because of the morphological and molecular similarities. Meanwhile, the other two are placed in the genus Satsuma: S. meridionalis (Moellendorff, 1884), comb. n. and S. uncopila (Heude, 1882), comb. n. G. virgo Pilsbry, 1927 differs from species of the genera Ganesella and Satsuma not only in its shell, but also in anatomical characters, such as having a dart sac and mucous gland, and lacking a flagellum. Additionally, phylogenetic analyses highly support the sister relationship with other Bradybaena species. Thus, placement of G. virgo Pilsbry, 1927 in the genus Bradybaena issuggested.
Satsuma, Ganesella, Bradybaena, revision, new subspecies
The land snail families Camaenidae and Bradybaenidae are extremely specious, and both families are difficult groups to deal with in terms of taxonomy. The camaenids occur across a wide geographical area from the northern to southern hemisphere, such as China, Japan, Taiwan, Philippines, Indonesia, New Guinea, Australasia, America (
The genus Ganesella Blanford, 1863 (sensu
The traditional classification of Ganesella relies predominantly on shell features. Purportedly characteristic features are, for instance, a thin, high, lustrous and conical shell, white to pale brown shell color, and a slightly descending body whorl (
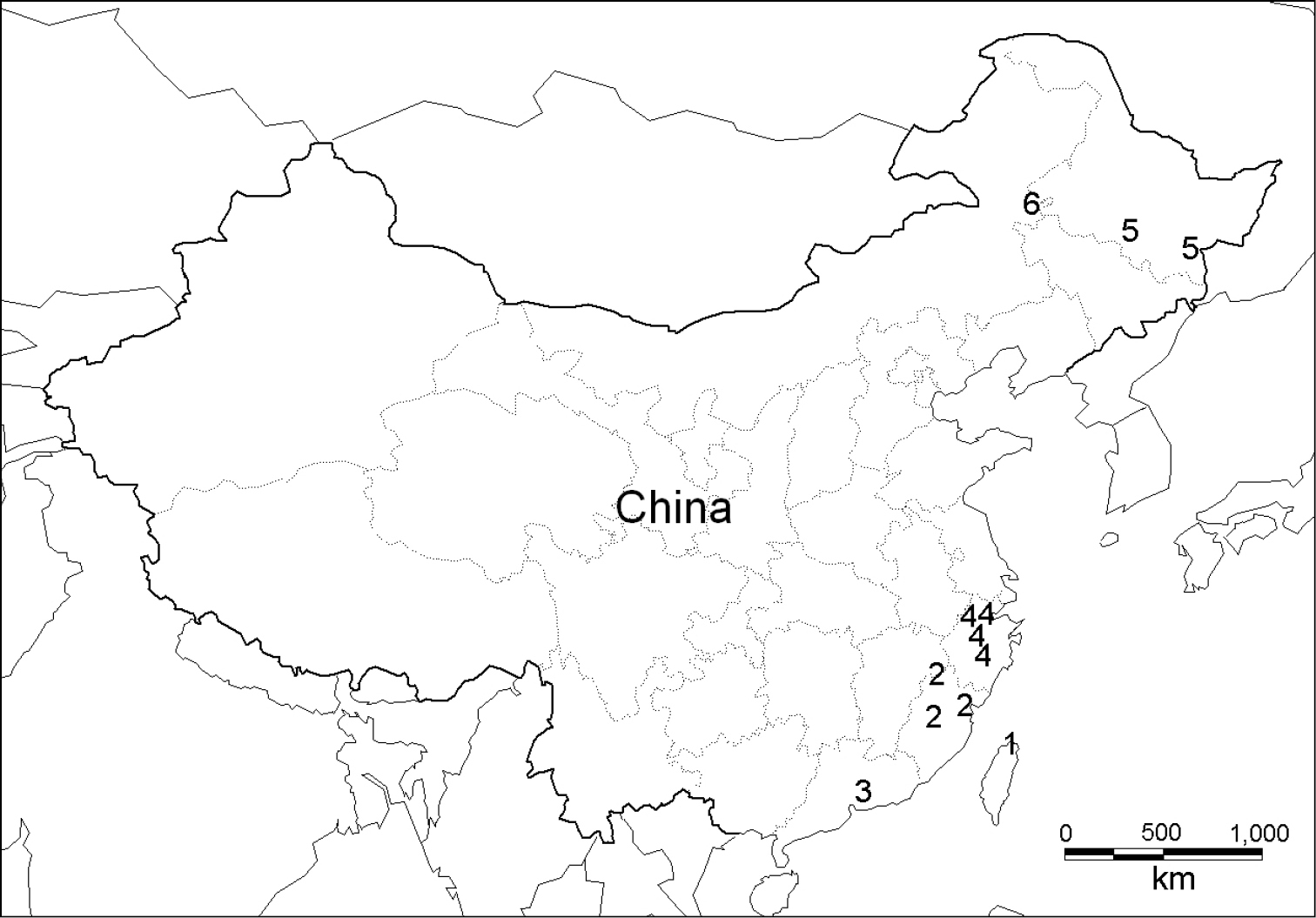
Material. This study is based on material collected by the authors from several sites in China (Fig. 1). Live adults were drowned in water for 12–24 hours, then killed in hot water, preserved in 75% or 95% ethanol, and stored at -20°C. Samples have been deposited in the State Key Laboratory of Molluscan Quarantine and Identification, Fujian Entry-Exit Inspection & Quarantine Bureau, Fuzhou, China (FJIQBC).
Map of sampling sites. 1 Satsuma mellea mellea (Pfeiffer, 1866) 2 Satsuma mellea stenozona (Moellendorff, 1884) 3 Satsuma meridionalis (Moellendorff, 1884) 4 Satsuma uncopila (Heude, 1882) 5 Bradybaena virgo virgo (Pilsbry, 1927) 6 Bradybaena virgo mongolia subsp. n.
Abbreviations used: IZCAS, Institute of Zoology, Chinese Academy of Science Museum, Beijing, China; SMF, Senckenberg Natural History Museum, Frankfurt am Main, Germany.
Methods. Shells were measured to 0.1 mm using electronic calipers. Standard shell parameters were taken following
Genitalia of adult snails were dissected under a dissecting microscope (ZEISS Discovery V20). All drawings were traced with the aid of a Canon 550D digital camera. Terminology for reproductive system follows
Total genomic DNA was extracted from muscle tissue of foot using Qiagen DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) was performed to amplify a fragment (615 bp) of the mitochondrial cytochrome c oxidase subunit I gene (COI) using a pair of universal primers (LCO1490: 5’-ggtcaacaaatcataaagatattgg-3’; HCO2198: 5’-taaacttcagggtgaccaaaaaatca-3’) (
Sample information.
| Family | Sampling | Locality | Collection date | Coordinates | Accession number |
|---|---|---|---|---|---|
| Camaenidae | Satsuma mellea stenozona (n=2) | Gushan, Fuzhou, Fujian | 2010.10 | 26°03'26"N, 119°24'02"E | KF765745/KF765746 |
| Satsuma mellea stenozona | Wuyi Mountain, Fujian | 2010.10 | 27°39'02"N, 117°58'01"E | KF765744 | |
| Satsuma mellea mellea | Ilan, Taiwan | 1997.06 | 24°45'05"N, 121°36'43"E | KF765743 | |
| Satsuma meridionalis | Luofushan, Guangdong | 2010.11 | 23°16'03"N, 114°03'37"E | KF765756 | |
| Satsuma uncopila | Hangzhou, Zhejiang | 2011.10 | 30°07'04"N, 120°02'26"E | KF765758 | |
| Satsuma largillierti |
Japan | AB242499 | |||
| Satsuma pekanensis |
Taiwan | EF204833 | |||
| Satsuma nux |
Taiwan | EF057347 | |||
| Satsuma batanica pancala |
Taiwan | AB480901 | |||
| Satsuma nux paiwanis |
Taiwan | EF204824 | |||
| Satsuma succincta |
Taiwan | EF204839 | |||
| Bradybaenidae | Cathaica fasciola fasciola | Beijing | 2008.10 | 39°59'51"N, 116°10'50"E | KF765749 |
| Plectotropis yonganensis | Yongan, Fujian | 2011.03 | 26°03'32"N, 117°19'44"E | KF765747 | |
| Plectotropis brevibarbis | Tianmu Mountain, Zhejiang | 2011.05 | 30°20'21"N, 119°23'58"E | KF765748 | |
| Aegista permellita | Leshan, Sichuan | 2011.05 | 29°32'45"N, 103°46'16"E | KF765759 | |
| Bradybaena ravida | Xiaoshan, Zhejiang | 2011.05 | 30°10'19"N, 120°16'20"E | KF765753 | |
| Bradybaena similaris | Fuzhou, Fujian | 2008.08 | 26°09'50"N, 119°16'55"E | KF765752 | |
| Bradybaena sequiniana | Badong, Hubei | 2011.06 | 31°02'46"N, 110°22'18"E | KF765750 | |
| Bradybaena brevispira | Emei Mountain, Sichuan | 2011.05 | 29°35'28"N, 103°22'47"E | KF765755 | |
| Bradybaena magnaciana | Chongqin | 2011.06 | 29°46'21"N, 106°27'53"E | KF765754 | |
| Bradybaena virgo virgo | Haerbin, Heilongjiang | 2008.08 | 45°42'31"N, 126°38'38"E | KF765751 | |
| Helicidae | Cornu aspersum | France | 2010.08 | KF765757 |
†: sequence from Genbank.
Type species. Satsuma japonica Pfeiffer, 1847, original designation.
http://species-id.net/wiki/Satsuma_mellea_stenozona
Figs 2A; 3A; 4AFuzhou (26°5'N, 119°18'E), China.
Bradybaena stenozona: Fuzhou, Fujian, Lectotype (SMF 8833), paralectotype (SMF 8832); National Forest Park of Fuzhou, Fujian (May 6, 2007, 26°09'50.36"N, 119°16'55"E; FJIQBC 18220–18237); Drum Mountain of Fuzhou, Fujian (Oct. 16, 2010, 26°03'26"N, 119°24'2"E; FJIQBC 18238–18245); YuHua Hole of Jiangle, Fujian (Jun. 1, 2007, 26°41'59"N, 117°30'55"E, FJIQBC 18146–18250). Ganesella citrina: Guadun, Wuyi Mountain, Fujian, Holotype (SMF 47228), paratypes (SMF 47229); Wuyi Mountain, Fujian (Oct. 12, 2010, 27°39'2"N, 117°58'01"E, FJIQBC 18251–18255).
Dextral, medium sized, about 14.5 mm in height, 21.0 mm in width, thin but solid, straw colored, glossy; 5 1/2 whorls. Apex obtuse. Suture deep. Spire low conical, slowly increasing, slightly convex. Body whorl fast expanding, convex, with weakly angulated margin. Periphery bluntly angulated with red-brown peripheral band, extending from apex to columellar lip. Whorls slightly descending at the front. Surface with oblique, curved growth lines, and staggered, delicate spiral lines. Aperture diagonal and round to lunate. Peristome white, slightly expanded and reflected. Inner lip with thin callus only. Basal lip curved. Columellar lip margin slightly expanded. Umbilicus open, small.
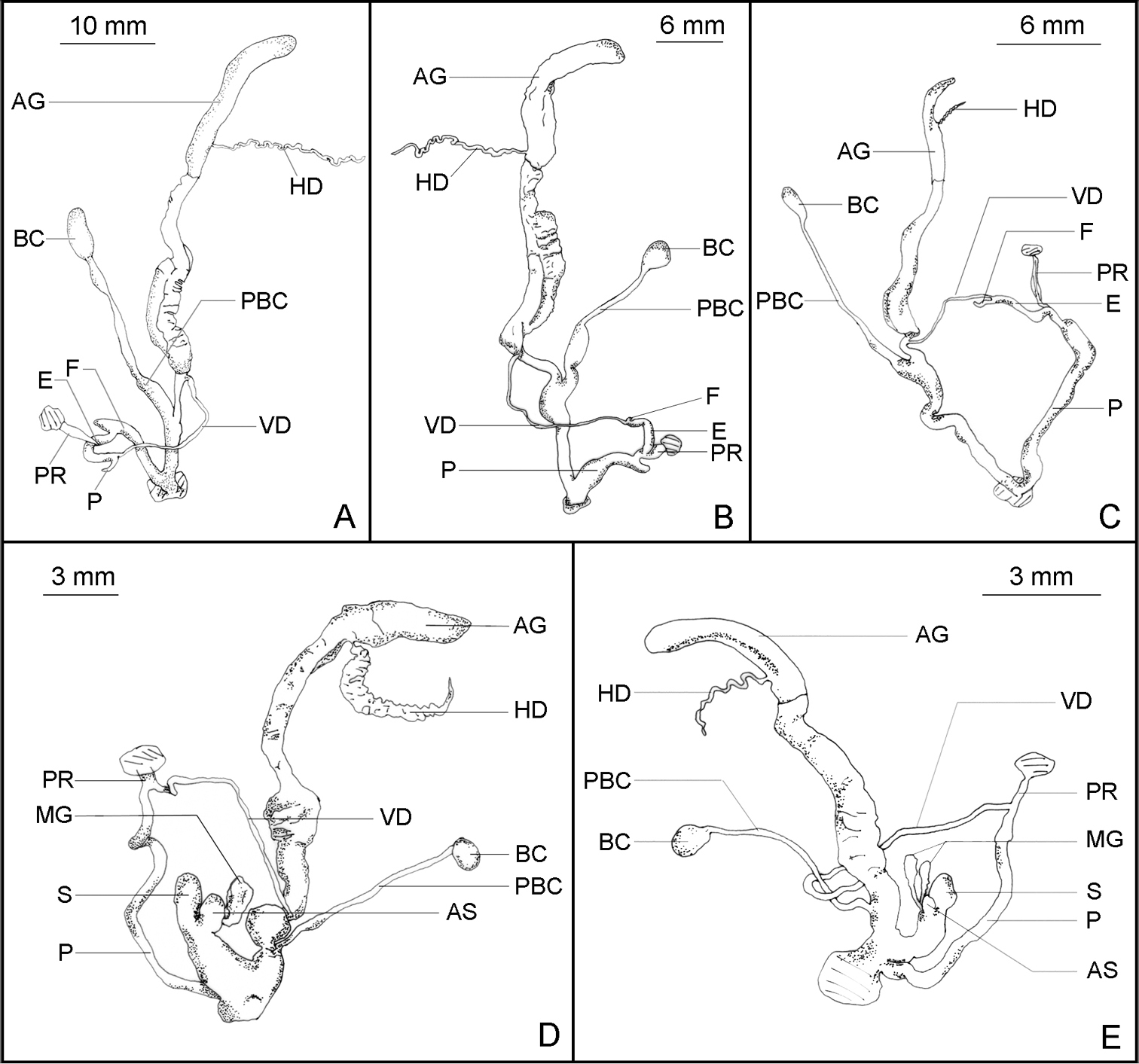
Penis slender, with a short penial caecum near the penis retractor. Epiphallus as wide as penis, half as long as penis. Flagellum short, about 1/5 of length of epiphallus. Penis retractor muscle thin and long. Vas deferens short. Free oviduct moderately long, slightly inflated. Vagina short. Pedunculus of bursa copulatrix inflated at base, fusiform. Bursa copulatrix oval.
One of the collected sites, Yuhua Hole, Jiangle, Fujian belongs to a Karst land form (limestone), all others are on Danxia land forms (acidic soil). Snails generally live under rotten branches and fallen leaves in forests, and actively crawl on trees during rainy seasons. Population density is generally not high in these locations. In Fuzhou, snails become active in early April, brisk in May and June, lie dormant in the soil by the end of October; juveniles and eggs aestivate during winter. Newly hatched snails will grow into adult in 7–8 months, then mate and spawn, about 100–200 eggs at once. Eggs are large, 1.5–2.0 mm in diameter.
This species has been placed in Bradybaena for a long time. Based on a study of the types,
In the present study, the phylogenetic analyses based on COI showed close phylogenetic relationships and short genetic distances between specimens identified as Satsuma stenozona, Ganesella citrina and Satsuma mellea (Fig. 5). The shell features of Satsuma stenozona from Fuzhou and Ganesella citrina from Wuyi Mountain do not reveal obvious differences. The differences mentioned by
Photographs of shells. A Satsuma mellea stenozona (Moellendorff, 1884) (FJIQBC 18221, Fuzhou, China) B Satsuma meridionalis (Moellendorff, 1884) (FJIQBC 18415, Guangdong, China) C Satsuma uncopila (Heude, 1882) (FJIQBC 18417, Hangzhou, China) D Bradybaena virgo virgo (Pilsbry, 1927) (FJIQBC 18432, Haerbin, China) E Bradybaena virgo mongolia subsp. n. (Holotype, FJIQBC 18466, Inner Mongolia, China).
Ecological photographs of snails. A Satsuma mellea stenozona (Moellendorff, 1884) (National Forest Park, Fuzhou, Fujian) B Satsuma meridionalis (Moellendorff, 1884) (Luofu Mountain, Guangdong) C Satsuma uncopila (Heude, 1882) (Lingshan Hole, Hangzhou, Zhejiang).
Reproductive system. A Satsuma mellea stenozona (Moellendorff, 1884) (FJIQBC 18237, Fuzhou, China) B Satsuma meridionalis (Moellendorff, 1884) (FJIQBC 18416, Guangdong, China) C Satsuma uncopila (Heude, 1882) (FJIQBC 18423, Hangzhou, China) D Bradybaena virgo virgo (Pilsbry, 1927) (FJIQBC 18462, Haerbin, China) E Bradybaena virgo mongolia subsp. n. (Paratype, FJIQBC 18471, Inner Mongolia, China).
Phylogenetic tree inferred by maximum likelihood (ML) method based on COI gene. The tree is rooted with Cornu aspersum (Müller, 1774). Numbers near the nodes represent bootstrap values.
http://species-id.net/wiki/Satsuma_meridionalis
Figs 2B; 3B; 4BLuofu Mountain, Guangdong (23°16'03"N, 114°03'37"E), China.
Luofu Mountain, Lectotype (SMF 9155), paralectotype (SMF 9156); Luofu Mountain, Guangdong (Nov. 3, 2010, 23°16'03"N, 114°03'37"E; FJIQBC 18407–18416).
Sinistral, medium sized; about 11.0 mm in height, 15.2 mm in width, thin but solid, yellowish-brown in color, depressed conic; 5 1/2 whorls. Surface with dense growth lines and weak spiral lines. Spire slightly low conical, slowly increasing, slightly convex. Body whorl fast expanding, quite convex. With slight, slender and dull red band on periphery of body whorl for most specimens. Periphery bluntly angulated. Aperture descending and elliptical. Peristome thin, sharp, slightly reflected. Inner lip with thin callus. Columellar lip short, reflected, slightly covering umbilicus. Umbilicus deep, round, and about 1/5 of width of shell.
Penis thick and short, with an expanded base. Penial caecum short. Epiphallus slender, about 2/3 of length of penis. Flagellum short and small, about 1/10 of length of epiphallus. Penis retractor muscle thick and wide. Vas deferens long and slender. Oviduct thin. Vagina longer than penis, expanding at posterior end. Pedunculus of bursa copulatrix expanding at base. Bursa copulatrix oval.
The species usually lives in the wet bushes and grass near farmland, especially on limestone cliffs and in cracks with more humus, or under rotten branches and fallen leaves; occasionally within human settlements. This snail is sensitive to low temperature, aestivates from November to March. Animals often feed on all kinds of crops, especially tender shoot and leaf.
Originallyit was described as variety of Helix fortunei (Pfeiffer, 1850) for its uniformly yellowish-corneous color and globularly conic shell shape. Subsequently,
http://species-id.net/wiki/Satsuma_uncopila
Figs 2C; 3C; 4CThe Yangtze valley, China.
Lingshan Hole, Hangzhou, Zhejiang (Oct. 5, 2011, 30°07'04"N, 120°02'26"E; FJIQBC 18417–18423); Tianmu Mountain, Zhejiang (May 6, 2011, 30°20'21"N, 119°23'58"E; FJIQBC 18424–18245); Yaolin fairyland, Tonglu, Zhejiang (May 25, 2008, 29°53'08"N, 119°37'09"E, FJIQBC 18426–18428); Shuanglong Hole, Jinhua, Zhejing (May 2, 2009, 29°12'23"N, 119°37'09"E, FJIQBC 18429–18431).
Sinistral, medium sized, about 11.5 mm in height, 16.8 mm in width, thin, fawn colored, conical. Whorls 5. Surface with short and diagonal growth lines, and weak spiral lines. Spire higher. Body whorl fast increasing, expanding but not descending at the front. Periphery smooth, not convex. Apex obtuse. Suture deep. Aperture elliptical. Peristome slightly thickened, reflected, white, occasionally reddish-brown. Columellar lip reflected, slightly covering umbilicus. Umbilicus narrow and small.
Penis long and thicker. Epiphallus slender, about 1/4 of length of penis. Flagellum short, thin, about 1/3 of length of epiphallus. Penis retractor muscle thin, moderately long. Vas deferens short, slender. Oviduct thin, short. Vagina long, gradually expanding towards posterior end. Pedunculus of bursa copulatrix slender, expanding at base. Bursa copulatrix oval.
The snail ordinarily lives in the wet bushes and grass on hills, especially in places that are rich in humus, under rotten branches and fallen leaves; also frequently found on limestone cliffs and in cracks.
This species has previously been placed in the family Bradybaenidae, but it is here transferred to the Camaenidae for the lack of dart sac and mucous gland. Following our phylogenetic analyses, we assign it to the genus Satsuma (Fig. 5).
http://species-id.net/wiki/Bradybaena_virgo_virgo
Figs 2D; 4DUiju, North Pyongan, North Korea.
Plant Park of Haerbin, Heilongjiang (Aug. 26, 2008, 45°42'31"N, 126°38'38"E; FJIQBC 18432–18462); Suburb of Jidong, Heilongjiang (Aug. 29, 2008, 45°14'57”, 131°09'01"E; FJIQBC 18463–18465).
Dextral, medium sized, about 12.0 mm in height, 13.5 mm in width, thin but solid, semitranslucent, glossy, spherical. Whorls 6–6 1/2. Apex sharp. Suture deep. Spire conical, slowly increasing, convex. Body whorl fast expanding, convex, about 3/4 of height of shell. Surface pale white or yellow, with dense and clear growth lines, and unambiguous spiral lines. Aperture descending at the front, elliptical. Peristome reflected. Columellar lip reflected, partly covering umbilicus. Umbilicus small.
Penis long, slender, moderately wide. Flagellum absent. Penis retractor muscle thin, wide and short. Vas deferens short and slender. Oviduct short and inflated. Dart sac large, oval, with one smaller accessory sac. One mucus gland, kinkled. Pedunculus of bursa copulatrix slender, short. Bursa copulatrix oval.
The snail often lives on damp pastures, especially near ditch, or in grass.
This species is the first intermediate host of Eurytrema pancreaticum, a parasite of humans and livestock (
http://zoobank.org/58D99BDE-0764-49DE-9954-B3C5EE7A024C
http://species-id.net/wiki/Bradybaena_virgo_mongolia
Fig. 2E; 4EFor the type locality, adjective.
(FJIQBC 18466) Shell height 6.5 mm, width 7.0 mm, height of aperture 3.5 mm, width of aperture 3.6 mm, October 5, 1982, collected from the type locality.
(FJIQBC 18467–18471) and (IZCAS TM 126010–126018) Shell height 5.5–7.0 (6.4±0.40) mm, width 6.4–7.5 (7.1±0.25) mm, height of aperture 3.2–3.6 (3.4±0.13) mm, width of aperture 3.3–3.7 (3.5±0.16) mm, October 5, 1982, collected from the type locality.
The grassland of Zhalaiteqi, Inner Mongolia, China (46°43'59"N, 123°19'20"E).
Dextral, small sized, thin but solid, semi-translucent, lustrous, globular. Whorls 6 on average, with conical spire. Shell light yellow or white in color, with some dense and well-developed growth lines. Spiral lines on body whorl weak. Apex sharp. Suture deep. Last whorl constricted, expanded towards the base, convex, comprising about 3/4 of shell high. Aperture elliptical. Peristome reflected, with white, thickened callus inside. Inner lip and columellar lip reflected, partly covering umbilicus. Umbilicus narrow, deep.
Penis long. Flagellum absent. Penis retractor muscle slender, moderately long. Vas deferens moderately long. Oviduct short and thick. Vagina short. Dart sac inflated, thick. Accessory sac small. Two mucus glands. Pedunculus of bursa copulatrix slender, but not long. Bursa copulatrix oval.
The snail usually lives on damp pastures, especially in tall and dense grass, i.e., Achnatherum splendens. However, it is difficult to collect this animal because of serious grassland degradation in Inner Mongolia.
The new subspecies resembles Bradybaena virgo virgo in morphology, but the two subspecies can be differentiated by the following characteristics: (1) The subspecies mongolia has a smaller shell (shell height 5.5–7.0 mm, width 6.4–7.5 mm) than Bradybaena virgo virgo (shell height 12.0 mm, width 13.5 mm), (2) it has two mucus glands instead of one in the nominate form, and (3) its umbilicus is wider (about 1/9 of the shell width) than in the nominate form (about 1/12 of the shell width).
Twenty-three partial sequences of COI were analyzed. The aligned sequences contained no indels and were deposited in GenBank (Table 1). The molecular phylogeny was based on the analysis of 615 unambiguously aligned nucleotide sites, of which 253 were variable and 233 were parsimony informative. According to the Akaike information criterion, the general time reversible model with a proportion of invariable sites and a gamma shaped distribution of rates across sites (GTR + I + G) was the best-fitting model of sequence evolution. All other settings for ML analysis were kept as default.
The ML tree (Fig. 5) presented two major clades corresponding to the families Camaenidae and Bradybaenidae, respectively. Bradybaena virgo virgo originally classified in Ganesella belonged to a clade of taxa in Bradybaena, and this agreed with the anatomical result. Thus the placement of this species in Bradybaena is suggested.
The clade of taxa in the family Camaenidae contained three subclades, Satsuma species from Taiwan in group A, Satsuma largillierti from Japan and species from southeast China and north Taiwan in group B. In addition, species in group B were divided into two subgroups, including subgroup B1 with sinistral shell and subgroup B2 with dextral shell, and this is consistent with the study on the reproductive system above. Therefore, the two species Bradybaena meridionalis from Luofushan, Guangdong and Bradybaena uncopila from Hangzhou, Zhejiang in subgroup B1, which were originally classified in Bradybaenidae, should be assigned to the family Camaenidae. On the other hand, Satsuma stenozona from Fuzhou and Ganesella citrina from Wuyi Mountain in subgroup B2 appeared monophyletic. There were low amounts of morphological difference between species from Fujian and Satsuma mellea from Taiwan with geographic isolation. In view of the above, the two taxa from Fujian are revised as a subspecies of Satsuma mellea (Fig. 5).
In the present study, three camaenids and one bradybaenid from China were revised on the base of morphological and molecular characters, but the systematics of the remaining Chinese species in the superfamily Camaenoidea are still problematic. Camaenids and bradybaenids may be more complex than we have previously suspected. In the future, more samplings will be required to resolve this problem.
We sincerely thank associate professor Wei-Hong Zhang of the University of Xinjiang, and professor De-Niu Chen of Institute of Zoology, Chinese Academy of Science for helpful comments on the manuscript. This research is supported by National Natural Science Foundation of China (31372162; 31040072), Natural Science Foundation in Fujian Province (2012J01063) and National Science Council, Taiwan (NSC 98-2621-B-390-001).