(C) 2013 Stuart H. McKamey. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The nymphs of Antillotolania Ramos and Deiroderes Ramos are described for the first time, along with the first host record for the genus Antillotolania, represented by Antillotolania myricae, sp. n. Nymphal features of both genera, such as a ventrally fused, cylindrical tergum IX (anal tube), the presence of abdominal lamellae, and heads with foliaceous ventrolateral lobes confirm their placement in Membracidae and are consistent with phylogenetic analyses placing them in Stegaspidinae but in conflict with a cladistic analysis showing a closer relationship to Nicomiinae. Head processes and emarginate forewing pads in the last instars of both genera support an earlier estimate, based on nuclear genes, that the two genera form a monophyletic group in Stegaspidinae. Distinguishing features of the four species of Antillotolania are tabulated.
Caribbean, Antilles, new species, immature stages, host plant
Treehoppers of the family Membracidae are best known for an enlarged, often extravagant pronotum expanded posteriorly over the scutellum (completely or partially concealing the scutellum) or more usually over the entire body. Indeed, until recently this was a diagnostic feature of the family Membracidae.
Other treehoppers lacking a posteriorly projecting pronotum, but with an acuminate or truncate scutellum, were placed in the treehopper families Aetalionidae, Biturritiidae, and Nicomiidae (
Antillotolania and Deiroderes (
Several attempts have been made to determine the phylogenetic placement of Deiroderes and Antillotolania within Membracidae. In a molecular phylogenetic investigation of Membracidae,
In a separate phylogenetic analysis based on morphological evidence,
Both cladistic estimates incorporating morphology (
In the present paper we describe a new species of Antillotolania, with host and habitat based on multiple series of adults and immatures collected along the central mountain range of Puerto Rico and describe its immature stages. We also describe the fifth instar of Deiroderes, based on one specimen collected from the reported host and adjacent to the type locality of Deiroderes inermis Ramos in the xeric region of Guánica, Puerto Rico. We also discuss the subfamiy placement of the two genera in the light of the new evidence.
Prior to this work, this genus contained three species: Antillotolania doramariae Ramos and Antillotolania extrema Cryan & Bartlett from Puerto Rico, and Antillotolania microcentroides Cryan & Bartlett from Guadeloupe and Tortola (British West Indies). These are represented by a total of seven specimens and nothing is known of their biology. No male of Antillotolania extrema has been collected. The new species is represented by 11 adult specimens and nymphs.
The originally monotypic genus was described based on one female, lost, and one male, both from Maricao, Puerto Rico. The forewing venation, which contains phylogenetically important characters, differed in the two illustrations. In recent years, a few additional Antillotolania have been captured by sweeping vegetation in the U.S. Virgin Islands (J. Cryan, C. Bartlett, pers. comm.), which has enabled their incorporation into phylogenetic estimates using molecular data (
urn:lsid:zoobank.org:act:9588EEE0-5578-4335-8EAD-282468C3434E
http://species-id.net/wiki/Antillotolania_myricae
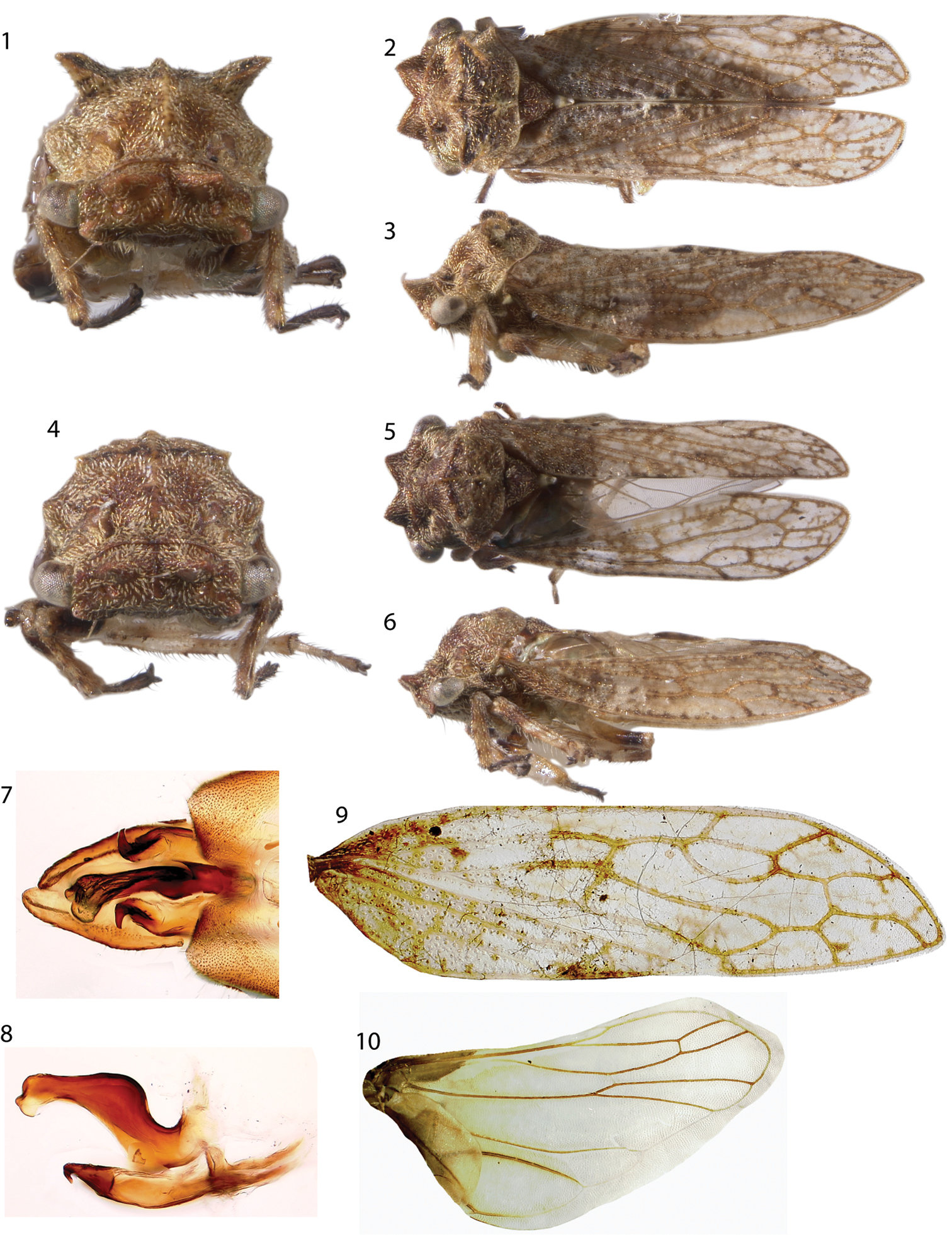
Figs 1–17, 28–31Dimensions (mm): Length with forewings in repose female 6.2, male 5.8, width between humeral angles female 1.8, male 1.6. Head and thorax densely pilose. Head quadrate in anterior view, in dorsal view with two subtriangular projections, longitudinally carinate behind eyes. Forewing (Fig. 9) M and Cu fused at base, 3 m-cu crossveins, 2 r-m veins, R branched into R1-3 and R4+5 basad of fork of vein M. Hindwing (Fig. 10) with 1 r-m crossvein and 1 m-cu crossvein, cubital vein un-branched, anal vein branched. Pro- and mesothoracic legs lacking cucullate setae. Metathoracic tibia with cucullate setae in rows I, II, and III as follows: ca. 20 in row I along entire length; ca. 10 in row III throughout distal half; and fewer than 10, larger cucullate setae in row II irregularly spaced in conjunction with darkly pigmented sections of tibiae (pale row II edge densely pilose but setae without cucullate bases). Abdomen lacking abdominal lamellae, vestiture (see
Male (Figs 4–8): Pronotum with small shelf like suprahumeral developments, little more than carinae that do not extend from the pronotal surface (Fig. 4–6). Pygofer and subgenital plates bare, not setose, lacking projections. Styles with base long and subparallel, acute apices recurved laterally (Fig. 7). Aedeagus asymmetrical in dorsal view, lobe of apex curving to the right (Fig. 7); shaft sinuate, directed dorsally then posteriorly, apex expanded (Fig. 8).
Female (Figs 1–3): Resembling male but pronotum with prominent suprahumeral horns, subtriangular, projecting dorsolaterally (Figs 1, 28).
Nymph (Figs 11–16): Fifth instar length 6.2 mm. Densely pilose and dorsoventrally compressed throughout. Head with subtriangular projections directed anteriorly as in adult, in anterior view ventral margin carinate, excavated, with ventrolateral lobes, in lateral view posterolaterally emarginate. Thoracic nota lacking scoli. Forewing ventrally emarginate. Abdominal terga IV-VII with large lateral lamellae directed posterolaterally, smaller on IV and subequal on V-VIII; tergum IX fused ventrally, forming ‘anal tube’, length subequal to remaining terga combined in last instar, as long as remainder of abdomen and thorax combined in earlier instars. Terga III-VIII with 2 pairs of enlarged chalazae, the first near mid line and the second between the first and the abdominal lamellae. Tergum IX slightly wider at base, otherwise parallel-sided, completely fused ventrally. Nascent genitalia barely exceeding posterior limit of tergum VIII lamellae (Fig. 15).
Antillotolania myricae, sp. n. 1–3 female habitus in anterior, dorsal, and lateral views, respectively 4–6 male, same views 7 Dorsal views, distal half of pygofer with aedeagus and styles in resting position over subgenital plates. 8 Lateral view, aedeagus and styles 9–10 left forewing and hind wing, respectively.
Antillotolania myricae sp. n. 11–15 fifth instar in anterior, dorsal, lateral and detail ventral views, respectively 16 third instar, with proportionately longer ‘anal tube’ (ventrally fused tergum IX) 17 surface vestiture of adult abdominal tergum IV.
Holotype male (USNM), Puerto Rico, municipio Maricao, km 63.1 rt. 120, ca. 4 air km S Maricao. 18°08.429N; 66°58.322W, 777m, 2 May 2005. S. McKamey & B. V. Brodbeck. Paratypes (USNM, NCSU): 3 females, 11 nymphs, same locality as holotype. 3 early instar, mun. Patillas-San Lorenzo, km 5 rt.7740 nr. jxn. rt. 181, 18.10002°N; 66.01812°W, 664 m, 27 Feb 2007, S. McKamey & L.L. Deitz. 2 males, 2 females, 3 nymphs, mun. Guayama, km. 0.7 rt. 742 off rt. 7741 nr. El Chino, ca. 6 air km N Guayama, 18.05422°N; 66.10001°W, 632 m, 27 Feb 2007, S. McKamey & L.L. Deitz. 2 females, 1 nymph, mun. Cayey, rt. 7737, 1.5 air km SE Cayey, 18.08516°N; 66.17194°W, 730 m, 27 Feb 2007, S. McKamey & L.L. Deitz. 1 male, 2 nymph, mun. Cayey, rt. 184 just S jxn. 173, nr. Carite Recreational Area, 18.13181°N; 66.07427°W, 497 m, 2 March 2007, S. McKamey.
All specimens collected from Myrica splendens (Sw.) DC., Myrtaceae, a weedy species of the West Indies, Mexico, Central and South America.
Moist highlands of Puerto Rico.
Based on our series of 11 adults and over 20 immatures, the venation and nymphal characters coded ambiguously in phylogenetic estimates of the family have been determined, as discussed below. No adults of the new species were obtained from rearing, but both adults and nymphs were repeatedly obtained from the same host at the same time, at a variety of localities, without finding any other membracids. Note that the male of Antillotolania extrema, if discovered, may have smaller suprahumeral horns, as evidenced by the strong sexual dimorphism exhibited by the new species. The first couplet in the key provided by
Characters and states:
1. Suprahumeral horns present only as carinae (0) or projecting from adjacent pronotal surface (1).
2. Forewing vein R4+5 fused with R1 basad (0) or distad (1) of fork of vein M.
3. Forewing crossvein m-cu3 originating basad (0) or distad (1) of fork of vein M.
4. Forewing vein A1 smoothly merging with claval vein (0) or bent at a right angle and perpendicularly connecting to clavela vein (1).
5. Metathoracic tibia with cucullate setae in rows I, II, and III (0) or row II only (1).
Deiroderes contains three species: Deiroderes inermis Ramos from Puerto Rico and nearby islands of the British West Indies, Deiroderes inornatus Cryan & Deitz from Jamaica, and Deiroderes punctatus Metcalf & Bruner from Cuba. These were represented by a total of 13 specimens, with nothing known of their biology except one host record for Deiroderes inermis: Capparis indica (L.) Fawc. & Rendle (Capparaceae) (but see Cryan & Deitz [2002] regarding a conflict with this host record).
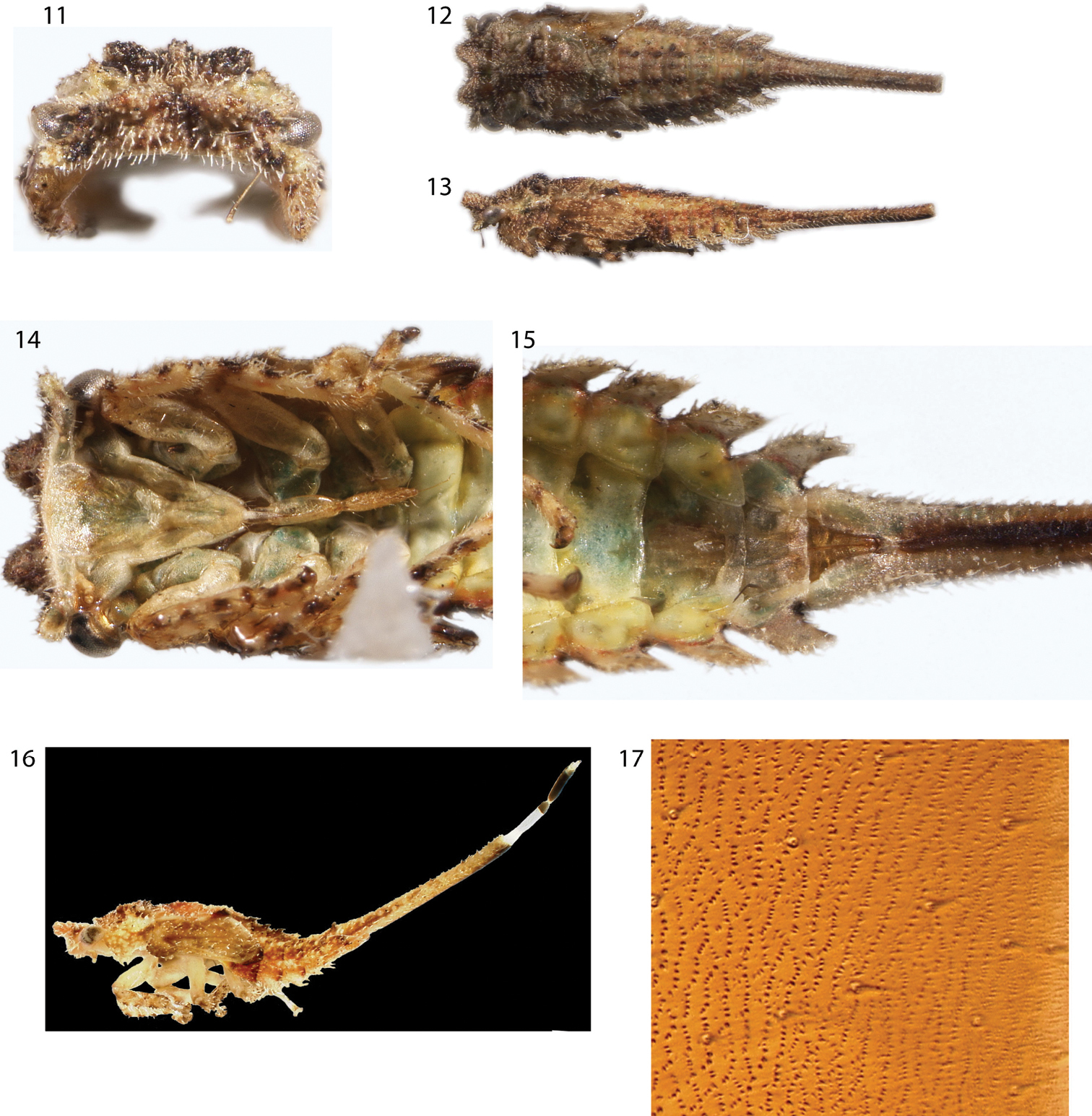
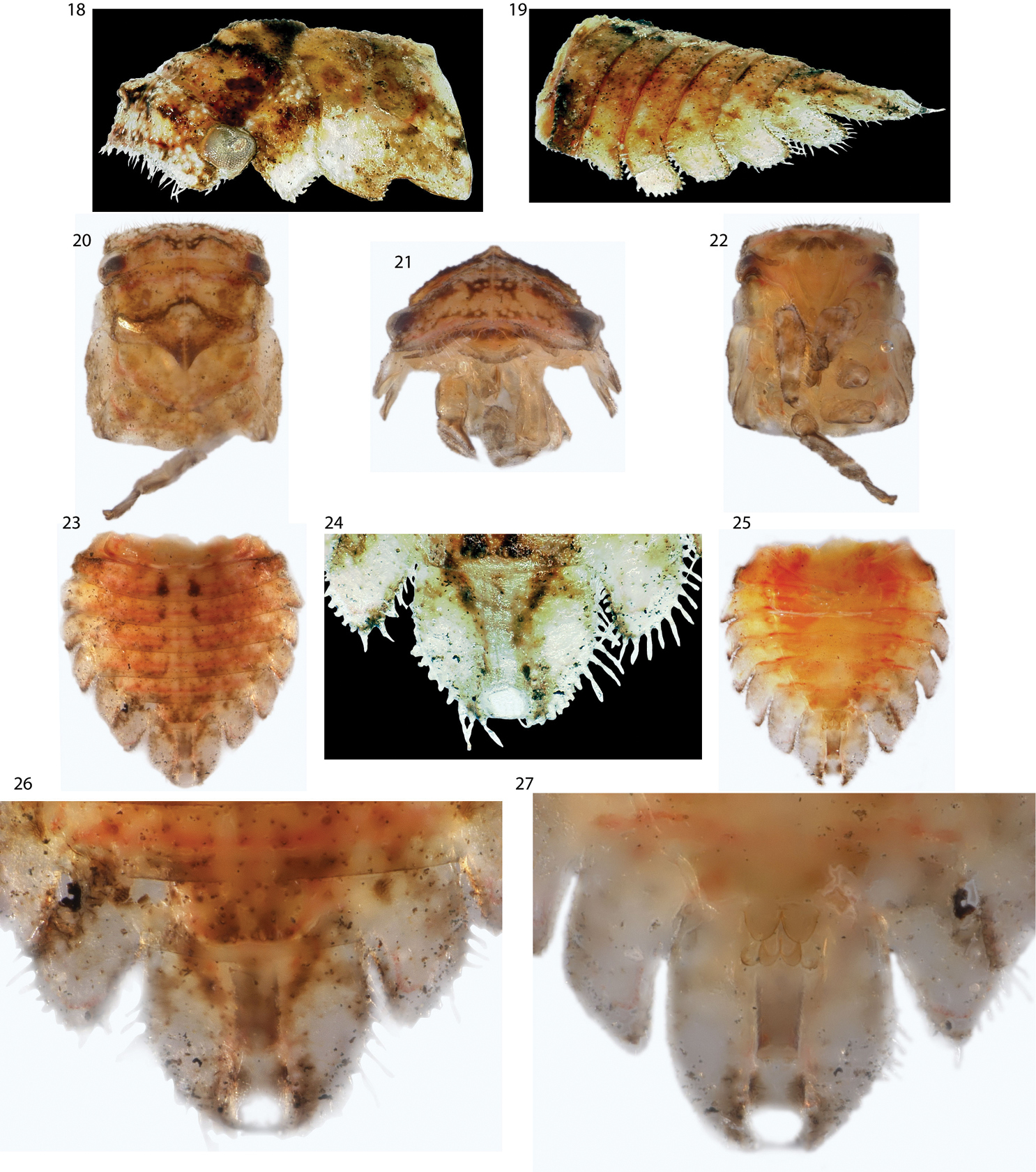
Nymph (fifth instar): Length 3.5 mm. Glabrous throughout. Head with small protrusions (Fig. 18) in same placement as the large subtrianglar projections of Antillotolania, in anterior view ventral margin carinate, head ventrally excavated, with foliaceous ventrolateral lobes (Figs 20, 21), in dorsal view quadrate, in lateral view not emarginate. Thoracic nota lacking scoli. Forewing emarginate. Abdominal terga IV–VII with large lateral lamellae, directed posterolaterally, smallest on tergum IV and increasing in size posteriorly; tergum IX fused ventrally, forming short ‘anal tube’, length about 2 × longer than tergum VIII. Terga III–VIII each with 1 pair of enlarged chalazae near mid line. Tergum IX dorsoventrally compressed. Nascent genitalia barely exceeding posterior limit of tergum VII lamella.
Deiroderes inermis immature, bisected during capture. 18, 20–22 head and thorax in oblique, dorsal, anterior, and ventral views, respectively 19, 23–27 abdomen in oblique (19), dorsal (23, 24, 26) and ventral (25, 27) views. Head processes visible in 18, anal tube opening visible in 24, and nascent genitalia visible in 27.
Photographs of live Puerto Rican Membracidae. 28–31 Antillotolania myricae, sp. n. adult female (28), adult male (29), third instar (defensive position) (30), and fifth instar (31), all on Myrica splendens (Myrtaceae) 32 Nessorhinus abbreviatus Ramos in same xeric habitat of Deiroderes inermis type locality (Guánica) on an unidentified host.
Caught sweeping a uniform stand of Capparis indica, a recorded host of Deiroderes inermis, adjacent to Guánica State Forest, which is the type locality of Deiroderes inermis. The Guánica area of Puerto Rico is arid and other membracids were previously unknown from there until L.L. Deitz (North Carolina State University) and SHM discovered Nessorhinus abbreviatus Ramos (Fig. 32) in February, 2007, on a different, unidentified host in February, 2007. Nymphs of Nessorhinus gibberulus Stål are known (McKamey unpubl.) and the fifth instars are several millimeters longer than that of Deiroderes inermis.
The anal tube (a ventrally fused abdominal segment IX, from which the anal segments protrude when defecating) present in nymphs of both Antillotolania and Deiroderes is a diagnostic feature of Membracidae. The nymphs of both genera display many features characteristic of other cryptic membracid nymphs: a flattened body and large abdominal lamellae that break up their body outline and an emarginate forewing pad providing a space for the mesothoracic tibia to rest, increasing their crypsis, suggesting that the two genera are correctly placed in that family. In some other membracid immatures with emarginate forewing pads, the tibiae are also flattened, but this is not the case in Antillotolania or Deiroderes. Instead, these have a pronotum that is posterolaterally emarginate, providing a resting place for the prothoracic tibia as well, as also occurs in some other membracids, such as some Darninae.
Placing Antillotolania and Deiroderes to subfamily and tribe is more problematic. The two possible subfamilies (with short pronotum) are Nicomiinae and Stegaspidinae. There are few nicomiine immatures known and all have been associated indirectly with adults due to the solitary nature of the species and difficulty of rearing adults. An illustration of a Tolania Stål nymph, which lacks any trace of abdominal lamellae, was provided by
In a phylogenetic study,
It may be that the head processes and emarginate forewing pads (Figs 13, 16) found in Antillotolania, Deiroderes, and some other cryptic membracids (but not in stegaspidine immatures) are homologous, giving morphological support to the hypothesis of
We thank M. Metz for some of the digital images of preserved specimens; D. Kolterman and G. Brekon of the Herbarium, University of Puerto Rico, Mayagüez for host determination; and Jason Cryan and Natalia Vandenberg for comments on the manuscript. We thank USDA-CREES TSTAR grant # 34135-15669 for the grant enabling the trip to Puerto Rico during which the discoveries of Antillotolania and Deiroderes were simply collateral benefits.