(C) 2012 André Nemésio. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three new species of orchid bees are described and figured from the Amazon and Atlantic forests of Brazil. Euglossa clausi sp. n., Euglossa moratoi sp. n., and Euglossa pepei sp. n. are distinguished from their close congeners Euglossa crassipunctata Moure, Euglossa parvula Dressler, and Euglossa sapphirina Moure, previously placed in the subgenus Euglossa (Glossurella) Dressler, 1982, a demonstrably paraphyletic assemblage requiring serious reconsideration. Their affinities with related species are discussed and pertinent characters are figured.
Amazon Basin, Atlantic Forest, Apoidea, Anthophila, Euglossini, orchid bees, new species, taxonomy
The taxonomy of the Neotropical orchid bees (Apinae: Euglossini sensu
Herein we continue this tradition with the recognition and description of three new species of Euglossa Latreille. All three species are closely related to species until recently placed in the paraphyletic subgenus Glossurella Dressler (
Material considered herein is deposited in the collections of the Universidade Federal de Minas Gerais, Belo Horizonte, Brazil (UFMG); Florida Museum of Natural History, Gainesville, Florida, USA (FMNH); and the Division of Entomology, University of Kansas Natural History Museum, Lawrence, Kansas, USA (SEMC). General morphological terminology for bees follows
All three species described herein are left as incertae sedis in regard to subgenus (following the suggestion of
urn:lsid:zoobank.org:act:D52A8C97-75AD-4F25-8450-B0CC4DA5D103
http://species-id.net/wiki/Euglossa_clausi
Figures 1–11♂, with the following data: “Euglossini do PERD, Pq. E. Rio Doce, 3859-11105” and “Marliéria, MG, Brasil, 04/07/1999, A. Nemésio” (UFMG). Details of the type locality are: Parque Estadual do Rio Doce (19°43'S, 42°34'W; 200 m a.s.l.), in the municipality of Marliéria, state of Minas Gerais, southeastern Brazil.
3♂♂, with the following label data: “Euglossini do PERD, Pq. E. Rio Doce, 3859-11106” and “Marliéria, MG, Brasil, 04/07/1999, A. Nemésio”; “idem, 3872-11131” and “idem” (UFMG); “idem, 3876-11137” and “idem” (UFMG). 1♂, “Brazil, E. Santo, No. Linhares, 12.xi.1968, R.L. Dressler” (FMNH). 1♂, “Brazil, Bahia, Res. Mte. Pascoal, 8.xi.1968, R.L. Dressler” (FMNH). 1♂, “Brazil, E. Santo, Conceicao da Barra, 10.xi.1968, R.L. Dressler” (FMNH). 1♂, “Brazil, Bahia, Itabuna, 19.vi.1971, H. Kennedy, cineole” (SEMC). 1♂, “Brazil, Bahia, Itabuna, 6.xi.1968, R.L. Dressler” (SEMC).
Euglossa clausi can be distinguished readily from both Euglossa crassipunctata and Euglossa sapphirina owing to its larger size (ca. 15% larger than both species), and a combination of integumental coloration that exactly matches neither of the aforementioned species (and for this reason has been confused with both: vide
♂: Body length ca. 10.0 mm; forewing length ca. 7.7 mm; head width 4.4 mm; interorbital distance at level of antennal sockets 2.5 mm; maximum interorbital distance 2.7 mm; labiomaxillary complex in repose reaching tip of body; scape length 0.8 mm; compound eye length 2.7 mm; mesoscutellum width 2.5 mm, length 1.2 mm; abdominal width 4.2 mm.
Coloration and vestiture: Clypeus and upper frons dark blue, remainder of head greenish-blue (Fig. 3); ivory paraocular markings well developed, reaching malar area, wider below; anterior surface of antennal scape black with very minute ivory marking in some specimens (including holotype); mesoscutum, mesoscutellum, and metasoma bluish-green (Figs 1, 2). Wing membranes lightly infumate. Pubescence very sparse, predominantly fulvous setae on metasoma and around antennal sockets, black and fulvous setae on mesosoma, black setae especially on mesoscutum (compared to predominantly fulvous setae in Euglossa moratoi). Protibia and probasitarsus fringed with dense fulvous setae; velvet area occupying all ventral surface of mesotibia, posterior mesotibial tuft approximately one-third size of anterior tuft, almost an isosceles triangle in shape, merging with anterior tuft; anterior mesotibial tuft oval, about three times larger than posterior tuft (Figs 4, 5); metatibia oblong-rhomboid, inflated (Fig. 6).
Punctation: Mesoscutum with punctation separated by a puncture width or less, with large circular punctures; punctures on mesoscutellum sparser than on mesoscutum medioposteriorly, separated there by a puncture width or greater, with larger circular punctures. Punctation on discal base of T1 with large circular punctures of roughly same size more clearly defined medially than in other species and separated by less than a puncture width; punctures of T1–T6 dense, comprised of minute circular punctures; punctures on T7 sparser than on preceding terga, with large circular punctures; S2 with small, widely-separated tufts.
Terminalia: Male terminalia as in figures 7–11. S7 slightly invaginated mesally, forming a shallow incision with converging sides forming angle of ~110°, lateral sections faintly curved; apical setae throughout invaginated section, comprising seven alveoli (with one seta each) on each side; notospiculum weak, slightly divided apically, posterolateral projections of anterior section weak, not prominent; posterior section triangular, sharply pointed, with basolateral points not as sharply developed as in Euglossa moratoi, slightly more rounded; anterior-most section of gonobase projected ventrally, forming angle of ~100° with remainder of ventral edge; gonostylus simple (‘type V’ of
♀: Unknown.
The specific epithet is a patronym honoring Dr. Claus Rasmussen, noted corbiculate bee biologist and systematist, in recognition of his years of kind collegiality.
Specimens of this species have been collected mostly from baits of cineole and vanillin, while a few specimens were collected from skatole.
Euglossa clausi sp. n. is a widespread bee in the Atlantic forest. Males have been collected from the state of Pernambuco in the north, to the northern portion of the state of São Paulo in the south (vide
Specimens of this species had been labeled in collections under the nomen nudum “cyanifrons”. It may be that additional material is located in other institutions under this name. In addition, individuals of this species were treated in the literature as Euglossa sapphirina (
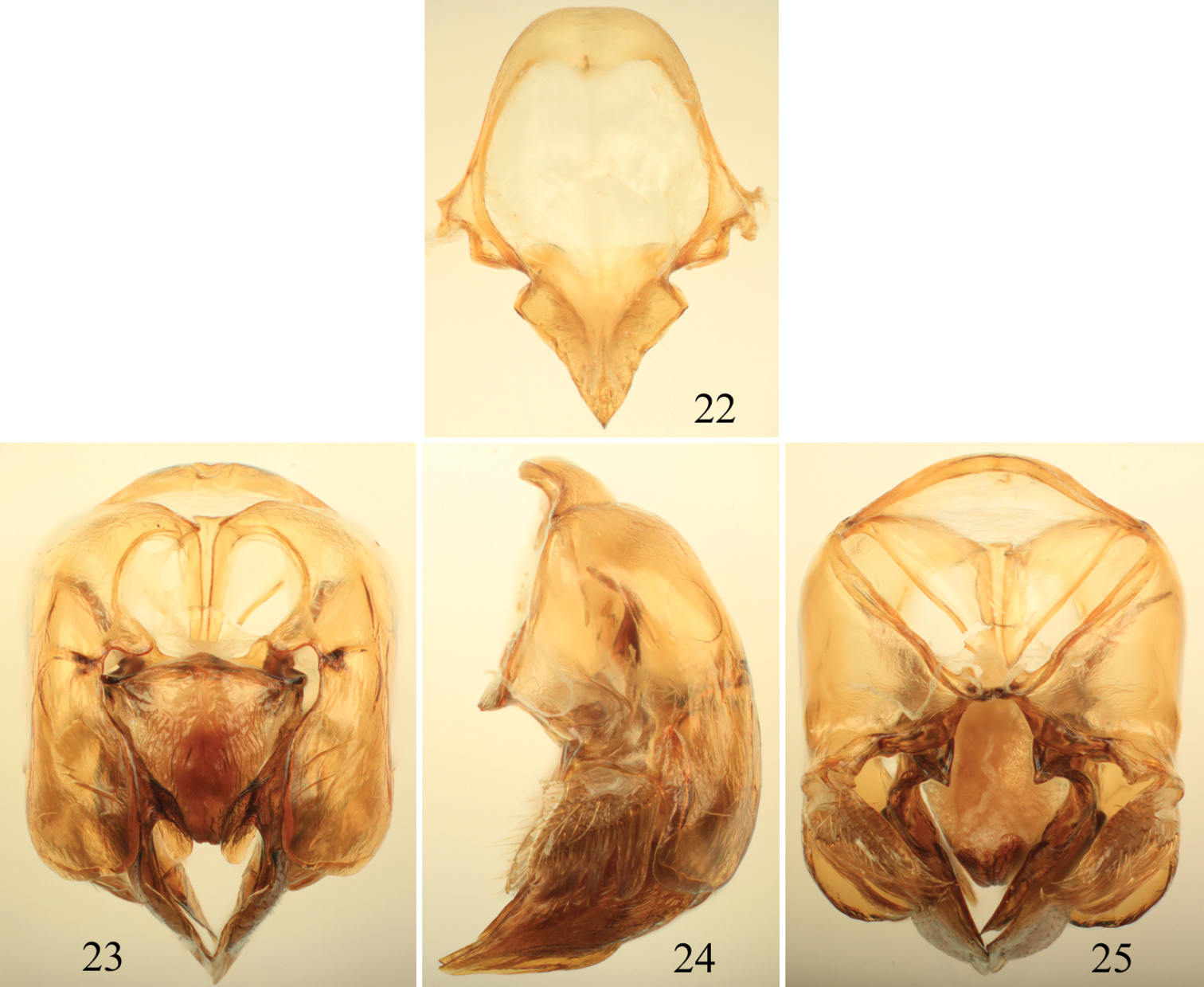
Photomicrographs of paratype male of Euglossa clausi Nemésio and Engel, sp. n. 1 Lateral habitus 2 Dorsal habitus (arrow points to rounded pronotal angle) 3 Facial aspect.
Tibial characters of Euglossa clausi Nemésio and Engel, sp. n. 4 Outer surface of mesotibia 5 Detail of mesotibial tufts 6 Outer surface of metatibia.
Male terminalia of Euglossa clausi Nemésio and Engel, sp. n. 7 Seventh metasomal sternum 8 Eighth sternum (note that relative proportions of the anterior section to the posterior section may be distorted owing to position of sclerite when photographed) 9 Genital capsule, dorsal view 10 Genital capsule, lateral view 11 Genital capsule, ventral view.
urn:lsid:zoobank.org:act:78CE101D-7A27-47AD-8C6D-A980E636A432
http://species-id.net/wiki/Euglossa_moratoi
Figures 16–25♂, with the following data: “EIA Porto Trombetas, Cipó I, Zona Leste, 12200-36025” and “Oriximiná, PA, Brasil 25/02/2007, R. B. Martines” (UFMG). The type locality is: Porto Trombetas, in the municipality of Oriximiná, state of Pará, northern Brazil.
10 ♂♂, with the following label data: “EIA Porto Trombetas, Monte Branco 2, Zona Leste, 11567-34328” and “Oriximiná, PA, Brasil 11/12/2006, R. B. Martines” (UFMG); “idem, 11575-34366” and “idem” (UFMG); “idem, 11578-34374” and “idem” (UFMG); “idem, Cipó 2, Zona Leste, 11634-34512” (SEMC) and “idem, 13/12/2006” and “idem” (UFMG); “idem, Teófilo 2, Zona Leste, 11545-34254” and “idem, 10/12/2006” (UFMG); “ParNa S. do Divisor, 12512-36708” and “Mâncio Lima, AC, Brasil, 21/11/1996, E. F. Morato” (UFMG); “idem, 12541-36759” and “idem” (UFMG); “14507-42692” and “Santarém, PA, Brasil, 11/12/1978, A. Raw”, (UFMG); “14917-43369” and “Manaus, AM, Brasil, 08/10/1988, E. F. Morato” (UFMG); “Santa Maria, 04°13'S, 55°58'W, 14396-42535” and “Itaituba, PA, Brasil, 18/01/1979, J. M. F. Camargo” (UFMG).
Euglossa moratoi sp. n. can be distinguished most easily from Euglossa crassipunctata, Euglossa sapphirina, and Euglossa clausi due to its small size (ca.20% smaller than the other species), the projecting pronotal dorsolateral angle which is more acute (slightly pointing) at its apex (differing from the rather bluntly rounded and non-projecting angle in all other species in the crassipunctata group) (Fig. 17; cf. figure 2), and the longer posterior mesotibial tuft relative to those in Euglossa crassipunctata, Euglossa sapphirina, and Euglossa clausi (Figs 19, 20); photographs of the holotypes of Euglossa crassipunctata and Euglossa sapphirina are in
♂: Body length ca. 8.0 mm; forewing length ca. 6.7 mm; head width 3.7 mm; interorbital distance at level of antennal sockets 2.1 mm; maximum interorbital distance 2.2 mm; labiomaxillary complex in repose reaching tip of body; scape length 0.56 mm; compound eye length 2.4 mm; mesoscutellum width 2.0 mm, length 0.93 mm; abdominal width 3.4 mm.
Coloration and vestiture: Clypeus and upper frons dark blue, remainder of face greenish (Fig. 18); ivory paraocular markings well developed, reaching malar area, not very wide below; anterior surface of antennal scape black; mesoscutum bluish-green, mesoscutellum and metasoma green (Figs 16, 17). Wing membranes lightly infumate. Pubescence very sparse, predominantly fulvous on metasoma and around antennal sockets, black and fulvous setae on mesosoma (compared to predominantly black setae in Euglossa clausi). Protibia and probasitarsus fringed with dense, fulvous setae; velvet area occupying all ventral surface of mesotibia, posterior mesotibial tuft approximately nearly one-third size of anterior tuft, triangular, slightly long and merging with anterior tuft; anterior mesotibial tuft oval, 2.5 times larger than posterior tuft (Figs 19, 20); metatibia oblong-rhomboid, inflated (Fig. 21).
Punctation: Mesoscutum with large circular punctures separated by a puncture width or less except anteromedially separated by a puncture width or greater particularly medially; punctures on mesoscutellum sparser than on disc of mesoscutum, with larger circular punctures separated by a puncture width or greater except along borders punctures separated by less than a puncture width. Punctation on discal base of T1 with large circular punctures of roughly same size more clearly defined medially and separated by less than a puncture width; punctation on T1–T6 dense, comprised of small hexagonal punctures; on T7 sparse relative to preceding terga, with large circular punctures; S2 with very small, widely-separated, semicircular tufts.
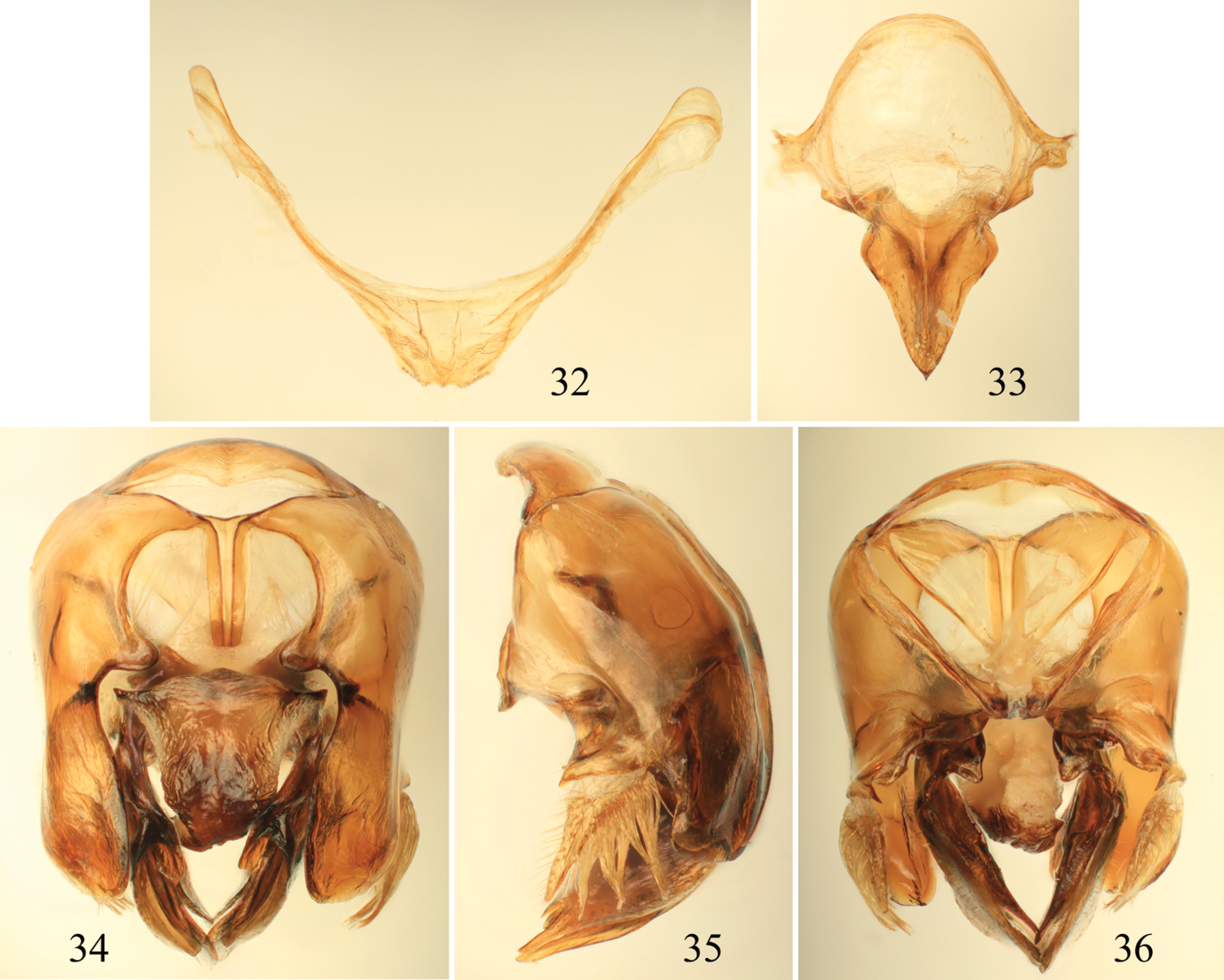
Terminalia: Male terminalia as in figures 22–25. S7 largely as in Euglossa clausi, with posterior margin of S7 slightly invaginated mesally, forming a shallow incision with converging sides forming an angle of ~110°, lateral sections slightly curved; apical setae only on outer sides of invaginated section, comprising four alveoli (with one seta each) on each side; notospiculum weak, slightly divided apically, posterolateral projections of anterior section large and pronounced; posterior section triangular, sharply pointed apically, with prominent basolateral points; anteriormost section of gonobase curved ventrally forming an angle of ~100° with remainder of ventral edge; gonostylus simple (‘type V’ of
♀: Unknown.
The specific epithet is a patronym honoring Dr. Élder Ferreira Morato, noted entomologist and close colleague of the senior author.
Specimens of this species have been collected mostly at baits of vanillin, although a few specimens were also attracted to cineole, eugenol, and skatole.
Euglossa moratoi seems to be widespread in the Amazon Basin. Males have been collected from the westernmost part of the Brazilian Amazon (
Specimens of this species have been treated as Euglossa crassipunctata in the literature (
Male terminalia of Euglossa crassipunctata Moure. 12 Eighth metasomal sternum (note that relative proportions of the anterior section to the posterior section may be distorted owing to position of sclerite when photographed) 13 Genital capsule, dorsal view 14 Genital capsule, lateral view 15 Genital capsule, ventral view.
Photomicrographs of paratype male of Euglossa moratoi Nemésio and Engel, sp. n. 16 Lateral habitus 17 Dorsal habitus (arrow points to projected pronotal angle) 18 Facial aspect.
Tibial characters of Euglossa moratoi Nemésio and Engel, sp. n. 19 Outer surface of mesotibia 20 Detail of mesotibial tufts 21 Outer surface of metatibia.
urn:lsid:zoobank.org:act:FFEAFD21-E49B-4313-9A5D-27313107095B
http://species-id.net/wiki/Euglossa_pepei
Figures 26–36♂, with the following data: “Euglossina da Hileia Baiana, PN Pau Brasil, 19679-56729” and “Porto Seguro, BA, Brasil, 19/04/2009, A. Nemésio” (UFMG). Details of the type locality are: Parque Nacional do Pau Brasil (16°31'S, 39°17'W; 90 m a.s.l.), in the municipality of Porto Seguro, state of Bahia, northeastern Brazil.
4♂♂, with the following label data: “Euglossina da Hileia Baiana, PN Pau Brasil, 19641-56644” and “Porto Seguro, BA, Brasil, 17/04/2009, A. Nemésio” (UFMG); “idem, 19659-56671” and “idem, 18/04/2009” (UFMG); “idem, 19706-56790” and “idem, 20/04/2009” (UFMG), and “Euglossina da Hileia Baiana, PN Descobrimento, 20601-58992” and “Prado, BA, Brasil, 18/12/2008, A. Nemésio” (SEMC).
Euglossa pepei is the most distinctive of the species of crassipunctata group. The shape and size of the anterior mesotibial tuft and the presence of a minute posterior tuft (Figs 29, 30) is most similar to that observed in Euglossa parvula Dressler. However, both species can be separated by the larger size of the oval anterior tuft and the smaller glandular scar of the metatibia in Euglossa pepei. In regards to the terminalia, S8 in Euglossa pepei is distinctly more slender (cf. figures 33 and 37), and the gonostylus is more pronounced (cf. figures 34–36 versus 38–40). In addition, the bluish coloration is practically restricted to the head and discal base of the mesoscutum, and the sterna are golden green, the latter feature contrasting with other species in the group for which there are at least present some bluish hues.
♂: Body length ca. 9.5 mm; forewing length ca. 7.7 mm; head width 3.7 mm; interorbital distance at level of antennal socket 2.1 mm; maximum interorbital distance 2.6 mm; labiomaxillary complex in repose reaching apex of body; scape length 0.7 mm; compound eye length 2.7 mm; mesoscutellum width 2.3 mm, length 1.1 mm; abdominal width 3.8 mm.
Coloration and vestiture: Clypeus and upper frons dark blue, remainder of head greenish (Fig. 28); ivory paraocular markings well developed, reaching malar area but not particularly wide below; anterior surface of antennal scape black; discal base of mesoscutum blue, remainder of mesoscutum, mesoscutellum, and metasoma green (Figs 26, 27). Wing membranes lightly infumate. Pubescence very sparse, predominantly fulvous setae on metasoma and around antennal sockets, black and fulvous setae on mesosoma, black setae especially prominent on mesoscutum (compared to predominantly fulvous setae in Euglossa parvula). Protibia and probasitarsus fringed with dense, fulvous setae; velvet area occupying all ventral surface of mesotibia, posterior mesotibial tuft very small, less than 1/30 of area of anterior tuft; anterior mesotibial tuft oval, very large, occupying approximately one quarter of velvet area length (Figs 29, 30); metatibia oblong-rhomboid, inflated (Fig. 31).
Punctation: Mesoscutum with circular punctures of two different sizes separated by less than a puncture width, those anterolaterally nearly contiguous; punctures on mesoscutellum more widely spaced than those of mesoscutal disc, with larger circular punctures separated by a puncture width or slightly less in medial third otherwise separated by less than a puncture width. Punctation on discal base of T1 with large circular punctures, punctures weak and separated by less than a puncture width; on distal part of T1 and T2–T6 dense, consisting of minute circular punctures; on T7 dense, with large circular punctures; S2 with very small, almost inconspicuous, widely-separated tufts.
Terminalia: Male terminalia as in figures 32–36. Posterior margin of S7 deeply invaginated mesally, lateral sections almost straight; apical setae only on two apexes of invaginated section; notospiculum weak, slightly divided apically, posterolateral projects distinct (in this regard more similar to Euglossa clausi, Euglossa moratoi, and Euglossa parvula); posterior section triangular, elongate, pointed apically, with basolateral projections not as prominent as in Euglossa clausi and Euglossa moratoi; anteriormost section of gonobase curved ventrally, forming angle of ~110° with remainder of ventral edge; gonostylus simple (‘type V’ of
♀: Unknown.
The specific epithet is a patronym honoring Leandro Mattos Santos, nicknamed “Pepê”, in recognition of his accomplishments in melittology.
All four of the known males were collected at baits of vanillin.
Euglossa pepei is known only from the small type series, all collected at Parque Nacional do Pau Brasil, municipality of Porto Seguro, Bahia, Brazil.
Male terminalia of Euglossa moratoi Nemésio and Engel, sp. n. 22 Eighth metasomal sternum (note that relative proportions of the anterior section to the posterior section may be distorted owing to position of sclerite when photographed) 23 Genital capsule, dorsal view 24 Genital capsule, lateral view 25 Genital capsule, ventral view.
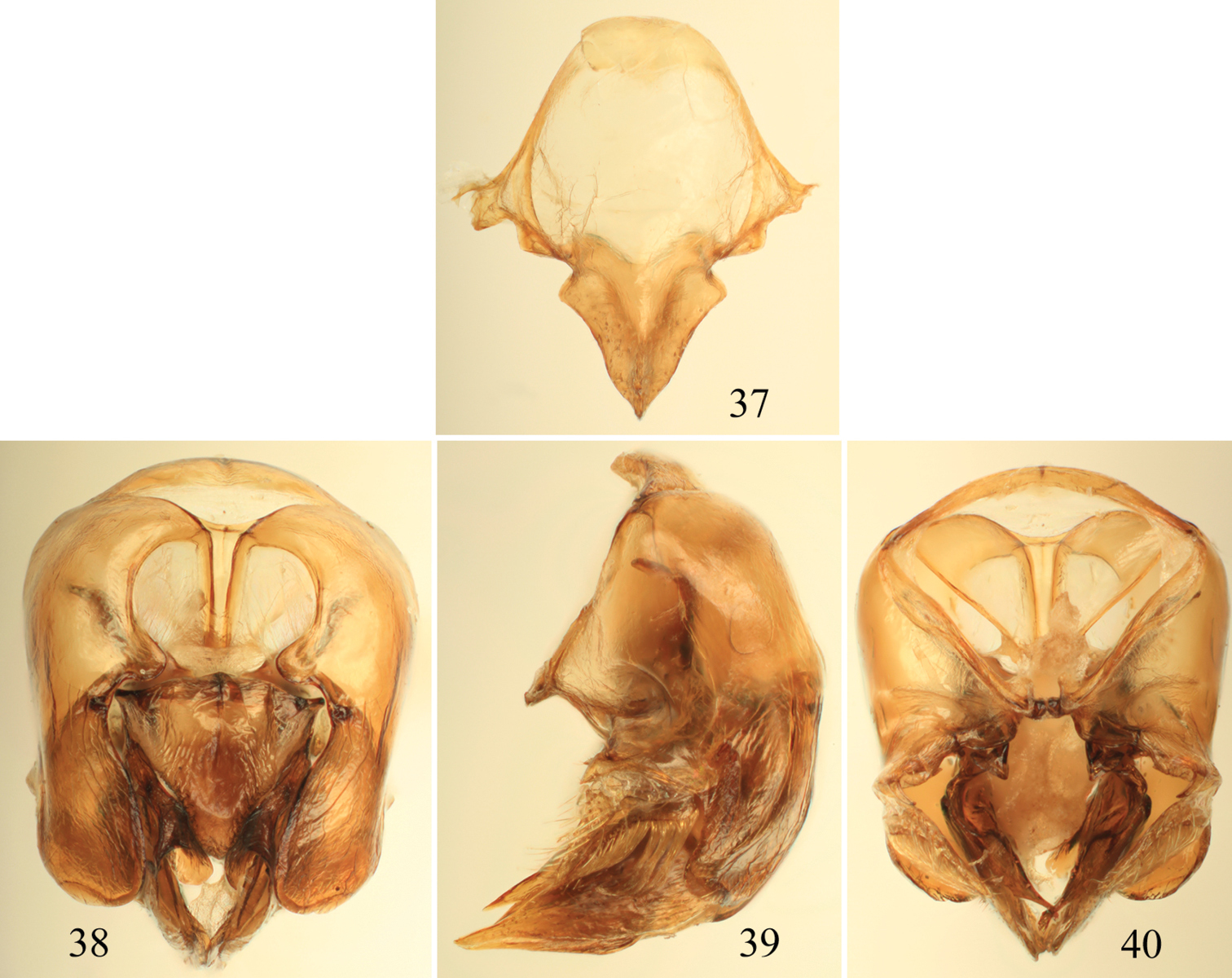
Photomicrographs of paratype male of Euglossa pepei Nemésio and Engel, sp. n. 26 Lateral habitus 27 Dorsal habitus 28 Facial aspect.
Tibial characters of Euglossa pepei Nemésio and Engel, sp. n. 29 Outer surface of mesotibia 30 Detail of mesotibial tufts 31 Outer surface of metatibia.
Male terminalia of Euglossa pepei Nemésio and Engel, sp. n. 32 Seventh metasomal sternum 33 Eighth sternum (note that relative proportions of the anterior section to the posterior section may be distorted owing to position of sclerite when photographed) 34 Genital capsule, dorsal view 35 Genital capsule, lateral view 36 Genital capsule, ventral view.
Male terminalia of Euglossa parvula Dressler. 37 Eighth metasomal sternum (note that relative proportions of the anterior section to the posterior section may be distorted owing to position of sclerite when photographed) 38 Genital capsule, dorsal view 39 Genital capsule, lateral view 40 Genital capsule, ventral view.
The following key is based on males given that females are not yet known for all of the included species.
| 1 | Posterior mesotibial tuft at most small and inconspicuous, at most as wide as bordering posterior area of depressed integument (e.g., Figs 29, 30) | 2 |
| – | Posterior mesotibial tuft well developed and triangular, much larger and encompassing nearly entire bordering area of depressed integument (e.g., Figs 4, 5, 19, 20) | 3 |
| 2 | Mesoscutellum with punctures medially separated by a puncture width or less (rarely more so); male terminalia as in figures 32–36 | Euglossa pepei sp. n. |
| – | Mesoscutellum with punctures medially a puncture width or frequently more; male terminalia as in figures 37–40 | Euglossa parvula Dressler, 1982 |
| 3 | S8 with posterolateral projections of anterior section prominently angled (e.g., Fig. 12) | 4 |
| – | S8 posterolateral projections of anterior section not developed, rounded (e.g., Figs 8, 22) | 5 |
| 4 | Pronotal dorsolateral angle rounded, not projecting (Fig. 2); mesoscutellum with punctures of most of disc separated by a puncture width or less except medioposteriorly some wider than a puncture width; posterolateral projection of anterior section of S8 angled but not prominent (Fig. 8) | Euglossa clausi sp. n. |
| – | Pronotal dorsolateral angle projecting, acute (Fig. 17); mesoscutellum with punctures of most of disc separated by a puncture width or slightly more and distinctly more medioposteriorly; posterolateral projection of anterior section of S8 angled and strongly prominent (Fig. 22) | Euglossa moratoi sp. n. |
| 5 | Mesoscutum, mesoscutellum, and majority of mesoscutum brilliant metallic green; S8 apically coming to a sharp, narrow point; gonostylus with broader base | Euglossa crassipunctata Moure, 1968 |
| – | Integument entirely dark metallic blue to bluish violet; S8 apically coming to a broad point; gonostylus with narrow base | Euglossa sapphirina Moure, 1968 |
Recent phylogenetic studies on the interrelationships among species of Euglossa and based on both morphological and DNA sequence data (
When establishing Glossurella,
After describing Euglossa crassipunctata and Euglossa sapphirina, Moure (1968: 43) mentioned that he was unable to find morphological features distinguishing both species outside of their coloration, preferring to erect the two taxa given that he could not find intermediates. The possibility of polymorphic species occurred to
“... in favor of this hypothesis is the fact that both species are morphologically indistinguishable, except for coloration... and that they occur sympatrically – at least Eg. crassipunctata is sympatric with Eg. sapphirina in the entire distributional range of the latter. Against this hypothesis is the fact that, strangely, the possible polymorphism is restricted to a relatively small area of the wide geographic range of Eg. crassipunctata.” (
At the time the above statements were made the populations considered herein as two distinct species, Euglossa moratoi and Euglossa clausi, were treated as Euglossa crassipunctata. Our revised interpretation of this material restricts the geographic distribution of Euglossa crassipunctata to Central America, where it is sympatric with Euglossa sapphirina. More importantly, there are slight differences in the structure of the male terminalia of both species, particularly in the form of S8 between Central American populations (MSE pers. obs.). As noted above, variation within a species for some genitalic structures is known (e.g.,
While Euglossa clausi and Euglossa moratoi are remarkably similar superficially to Euglossa crassipunctata, the form of the male terminalia serves to most strongly distinguish these species. For instance, the posterolateral projection of the anterior section of S8 in Euglossa crassipunctata is scarcely developed and gently rounded, while this process if more developed in the new species, each forming a noticeable angle, although it is most extremely developed in Euglossa moratoi. Lastly, the basolateral projections of the posterior section are much more prominent in the two new species relative to that of Euglossa crassipunctata (it should be noted that these same differences hold for comparisons between the new species and Euglossa sapphirina). In addition, the lateral section of the gonostylus is significantly shorter and narrower in Euglossa crassipunctata, with a noticeably slender and elongate lateral lobe, while all of these structures are much broader and more prominent in Euglossa moratoi and Euglossa clausi. Undoubtedly, all of these species are closely related, but each is clearly distinct as evidenced by the male terminalia.
With the addition of the species described here, the crassipunctata species group comprises six species, which appear to fall into two subgroups. The first, the crassipunctata subgroup (sensu strictissimo) includes Euglossa crassipunctata, Euglossa sapphirina, Euglossa moratoi, and Euglossa clausi all with a triangular and well developed posterior mesotibial tuft. The second, or the parvula subgroup, consists of Euglossa parvula and Euglossa pepei, both with a posterior mesotibial tuft lacking or at most very small and inconspicuous (nearly vestigial). Both subgroups are represented in the Amazon and the Atlantic forests, but only the first subgroup is present in Central America.
In closing, it is significant to note the distinctiveness and apparent endemicity of Euglossa pepei, while Euglossa moratoi and Euglossa clausi are likely more common in collections, although undoubtedly misidentified as Euglossa crassipunctata. Euglossa pepei is presently known only from five specimens in a restricted area in Bahia, and the same region where species such as Euglossa cyanochlora Moure and Exaerete salsai Nemésio are also endemic. Among all species of the crassipunctata group, Euglossa pepei is the most distinctive in terms of both its external morphology and genitalia. It is greatly hoped that future collecting will bring more material of this species, particularly the unknown female, and permit a more thorough understanding of its biology and distribution.
We are grateful to Prof. Dr. Marcelo O. Gonzaga (Universidade Federal de Uberlândia) and Dr. Ismael A. Hinojosa-Díaz (Emory University) for assistance with photomicrography, to Prof. Dr. Charles D. Michener (University of Kansas) for his continued mentorship, to two anonymous reviewers for their helpful comments which improved the final version, and to Dr. Michael Ohl (Museum für Naturkunde) for his valuable assistance and advice as editor. Partial support was provided by US National Science Foundation grant DBI-1057366 (to MSE).