(C) 2012 Didier VandenSpiegel. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Ammodesmidae are represented in western Africa by two species of a single genus, Ammodesmus Cook, 1896 (= Cenchrodesmus Cook, 1896, syn. n.). The type-species Ammodesmus granum Cook, 1896 (= Cenchrodesmus volutus Cook, 1896, syn. n.) is redescribed, based on neotype selection, as well as on additional samples, often containing numerous specimens, from Liberia, Guinea and the Ivory Coast. A new species is described from Mount Nimba, Guinea: Ammodesmus nimba sp. n.
Diplopoda, Polydesmida, Ammodesmidae, Ammodesmus, taxonomy, new species, new synonymy, western Africa
The small Afrotropical family Ammodesmidae has hitherto been known to comprise only three genera. One of these is Elassystremma Hoffman & Howell, 1981, a recently reviewed oligotypic genus currently comprising four species from Kenya, Tanzania and Malawi (
The taxonomic history of the family Ammodesmidae is rather confusing (
The diagnoses of the two genera and species were rather anecdotal and provided little useful information (
As no genital structures had been mentioned,
Between 2008 and 2011, a rich material of diplopods was taken or amassed by the first author from Liberia, Guinea and the Ivory Coast. This large collection appears to contain a proportion of Ammodesmidae, fortunately also with males from each locality becoming available for study. Three different morphotypes could be distinguished at once, including the two forms described by
The present paper provides a review of Ammodesmus, the sole ammodesmid genus that appears to populate western Africa. Its gonopod and numerous other characters are documented here for the first time, and compared to those of Elassystremma, the sole eastern Afrotropical counterpart ammodesmid. The type species Ammodesmus granum is redescribed, based on neotype designation, and a new species is added to this genus. A distribution map of and a key to the Ammodesmus species are also given.
Material and methodsThe bulk of material belongs to the collection of the Royal Museum for Central Africa (MRAC), Tervuren, Belgium, with only a few duplicates shared with the collections of the Zoological Museum, State University of Moscow (ZMUM), Russia and the Muséum national d’Histoire naturelle, Paris (MNHN), France, as indicated hereafter. All samples are stored in 70% ethanol. Photographs were made with a Leica digital camera Leica DFC 500 mounted on a Leica MZ16A stereomicroscope. Images were processed with a Leica Application Suite program. Specimens for scanning electron microscopy (SEM) were air-dried, mounted on aluminium stubs, coated with gold and studied in a JEOL JSM-6480LV scanning electron microscope.
TaxonomyAn oligotypic family of minute polydesmidans (1.4–5.0 mm long) with 18 or 19 body segments in both sexes, capable of rolling into a tight sphere. Conglobation pattern becoming typical from paratergum 4 onwards (
Sterna very narrow, coxae usually subcontiguous medially. Last male legs either modified (Ammodesmus) or not (Elassystremma). Gonopod aperture rather modest in size, transversely oval, not reaching sides of metazona ventrally.
Gonopods mostly complex; coxae globose, usually but not always strongly enlarged and deeply excavate in the middle (= gonocoel), cannulae very evident. Telopodites basically unipartite, slender or stout, sometimes with a small lateroparabasal outgrowth, only seldom strongly exposed (Ammodesmus granum), more usually deeply sunken into gonocoel, leaving only tips exposed. Seminal groove mostly running on mesal face, turning laterad due to telopodite twisting only distally to subapically, with a very evident (Ammodesmus granum) or small solenomere either devoid of or supplied with a hairy pulvillus; accessory seminal chamber absent.
Liberia, Guinea and Ivory Coast (western Africa), as well as Tanzania, Kenya and Malawi (the African eastern Arc Mountains).
| 1 | Last pair of male legs not modified. Eastern tropical Africa | Elassystremma |
| – | Last pair of male legs strongly modified (Fig. 23). Western tropical Africa | Ammodesmus |
http://species-id.net/wiki/Ammodesmus
Ammodesmus granum Cook, 1896
(after
Western Africa (Liberia, Guinea and Ivory Coast).
Ammodesmus granum and Ammodesmus nimba sp. n.
| 1 | Coloration pinkish to brownish with darker metaterga (Fig. 1). Male with metatergal tubercles; gonopod with a small coxa, leaving most of telopodite exposed | Ammodesmus granum |
| 2 | Colour pattern of metaterga spotty (Fig. 28). Male devoid of metatergal tubercles; gonopods with strongly enlarged coxae supplied with a prominent gonocoel | Ammodesmus nimba sp. n. |
http://species-id.net/wiki/Ammodesmus_granum
Figs 1–27Neotype ♂ (MRAC 21667), Liberia, Bong Range Forest, 06°49'N, 010°17'W, rainforest, pitfall trapping, 13.III.2005, leg. D. Flomo.
This male specimen has been chosen as neotype, because it is in perfect condition and represents a near-topotype. A neotype of Cenchrodesmus volutus has also been selected to substantiate this taxon as well.
1 ♂, 2 ♀ (ZMUM), 1 ♂ (MNHN), same locality, date and collector; 6 ex. (MRAC 21966) GUINEA, Mt Nimba, Gouela forest, 07°37'N, 008°21'W, Winkler extraction, 12.X.2008; 39 ex. (MRAC 21981), including a ♀ neotype of Cenchrodesmus volutus, same locality, Winkler extraction, 15–17.X.2008; 6 ex. (MRAC 21991), same locality, Winkler extraction, 13–15.X.2008; 4 ex. (MRAC 22004), same locality, Winkler extraction, 17–19.X.2008; 12 ex. (MRAC 22045), same locality, Winkler extraction, 13–16.X.2008; 4 ex. (MRAC 22007), Mt Nimba, Zié forest, 07°40'N, 008°22'W, Winkler extraction, 16–18.X.2008; 2 ex. (MRAC 22040), same locality, Winkler extraction, 14–16.X.2008; 2 ex. (MRAC 22050), Mt Nimba, Ziela forest, 07°43'N, 008°21'W, litter, Winkler extraction, 19.X.2008; all leg. D. VandenSpiegel; 1 ♂, 1 ♀ (MRAC 22284), Taï forest, 05°50'N, 007°21'W, Winkler extraction, 01–03.IX.2010; 1 ex. (MRAC 22285), same locality, Winkler extraction, 13–15.X.2010; 4 ex. (MRAC 22286), same locality, Winkler extraction, 01–03.IX.2010; 2 ex. (MRAC 22288), same locality, Winkler extraction, 01–03.IX.2010; 1 ex. (MRAC 22289), same locality, Winkler extraction, 01–03.IX.2010; 2 ex. (MRAC 22290), same locality, Winkler extraction, 13–15.X.2010, all leg. A. Kablan; 3 ex. (MRAC 22287), same locality, forest on clayey soil, Winkler extraction, 22–24.II.2010; 4 ex. (MRAC 22291), Taï forest, Ecological Research Centre, 05°50'N, 007°21'W, secondary forest, Winkler extraction, 21–22.II.2010, all leg. M. Diarassouba & R. Jocqué.
Minute polydesmidans (1.4–2.0 mm in length) showing evident sexual dimorphism in tergal structure: ♂ with a transverse row of up to ten ovoid tubercles arising from posterior part of each metatergum, ♀ with nearly smooth metaterga. Gonopod with a small globular coxa reaching in length only about one-third of telopodite; the latter slender, flattened and twisted mesad in distal part, with a small sac-shaped outgrowth laterally at base.
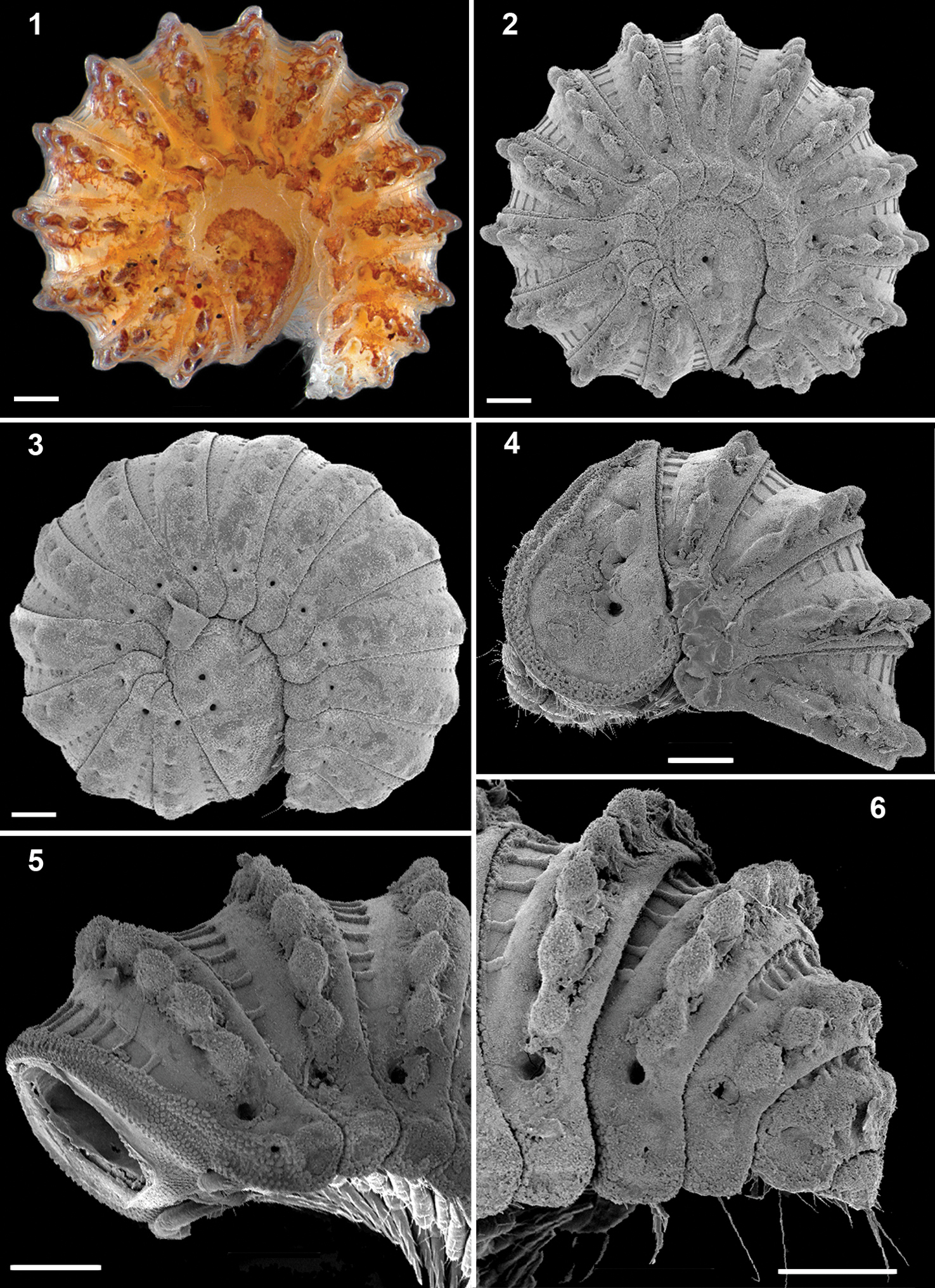
♂ ca 1.9 mm long; maximum width, 0.9 mm. Entire dorsal surface covered with a thin layer of secretions (= cerategument), under which the body integument is light brown to pinkish with metaterga of each segment brownish (Fig. 1). Body with 18 or 19body rings (16 or 17+1+T), shape as in Figs 1, 2 & 3, with caudal body end tapering towards a relatively small telson not concealed by paraterga (Fig. 6).
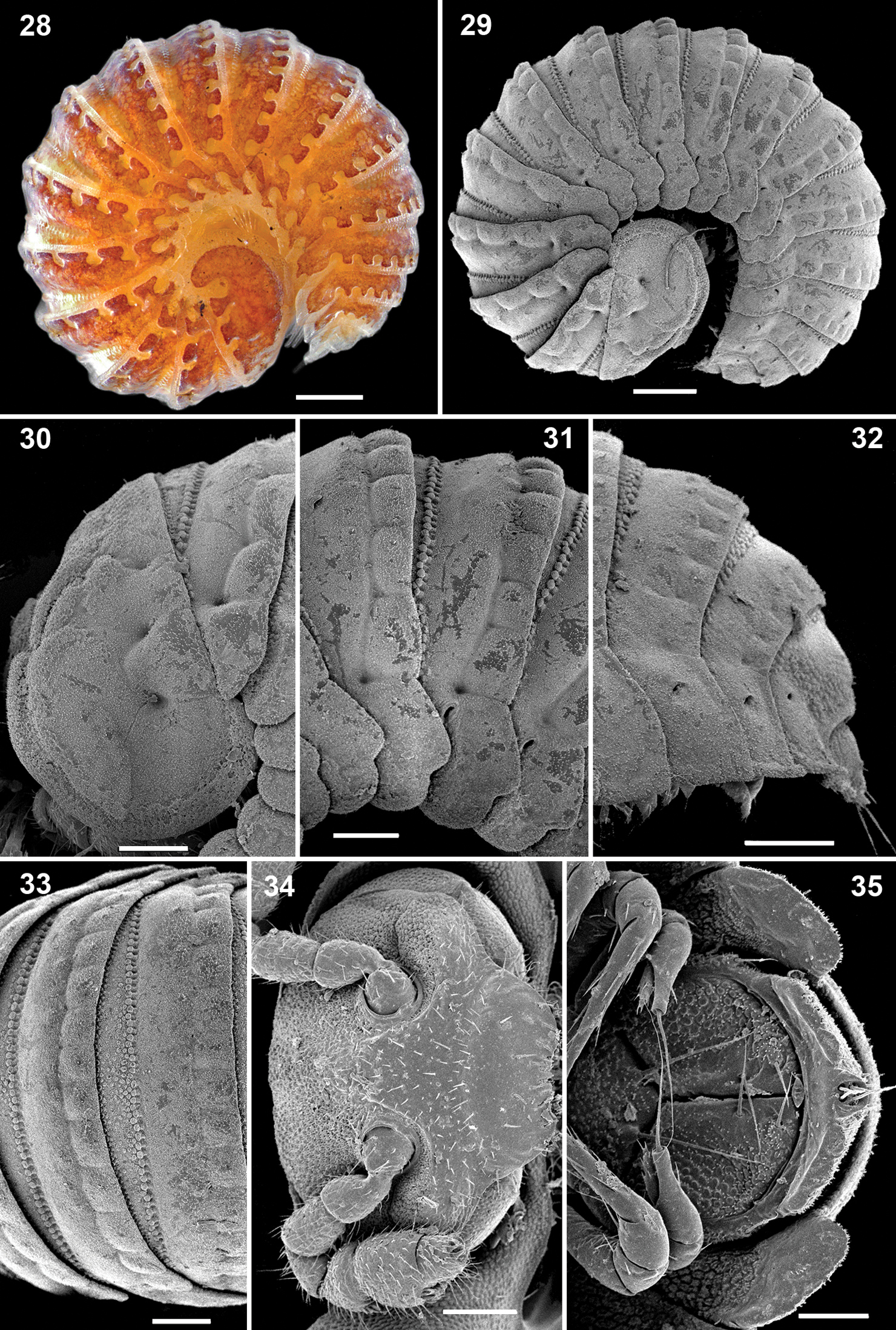
Ammodesmus granum1, 2 habitus of male, lateral view 3 habitus of female, lateral view 4–6 male, anterior, middle and caudal parts of body, respectively, lateral view. Scale bars: 100 µm.
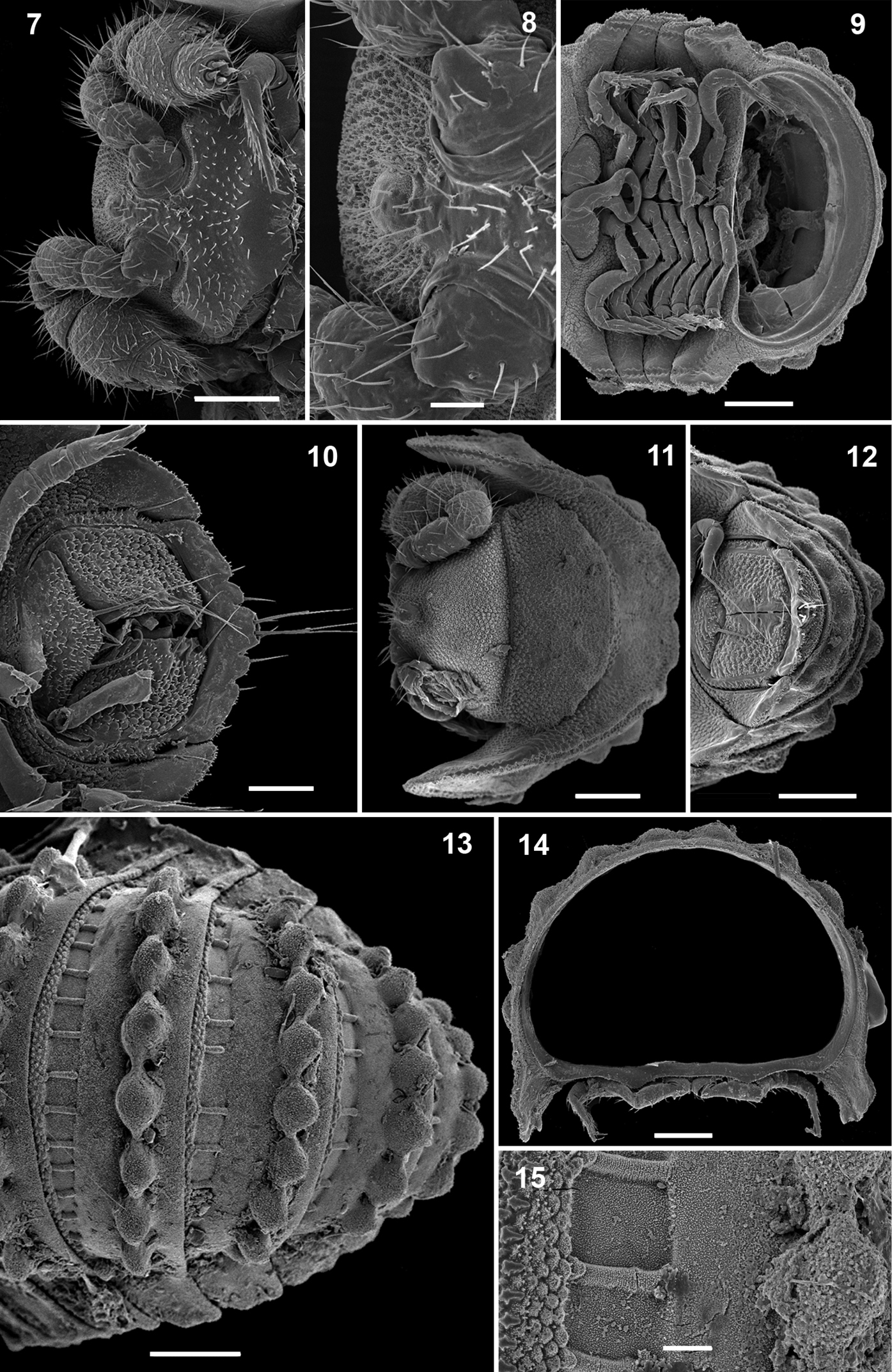
Head small, only partly concealed under front edge of collum (Figs 7, 11); preceding half of head densely granular, lower part smooth and densely setose (Figs 7, 8). Interantennal isthmus about as wide as antennomere 1, surmounted by a small tubercle (Fig. 8). Antennae as in Fig. 7. Collum covered with low rounded tubercles (Fig. 11), tergum 2 as usual, hypertrophied, with strongly enlarged, spatuliform paraterga concealing the head in lateral view, ventral edge with a line of granules (Fig. 3). Limbus smooth; 2nd and following metaterga with 7–10 large oblong tubercles along caudal margin (Figs 1, 2, 4, 5, 6, 13). Each tubercle surmounted by a short seta (Fig. 17). Prozona rugose anteriorly, with a row of square areas along anterior edge of metatergum (Figs 13, 15), these square areas being reduced in ♀ or absent in juveniles (Fig. 3). Paraterga set at segments’ midheight just below a deep pit (Fig. 16, pt), continuing the highly convex outline of dorsum, their ends rounded, projecting far below venter/coxae (Fig. 14), increasingly angular towards telson (Figs 5, 6). Anteroventral edges of paraterga 3 to 15 with a notch forming a groove for paratergum 2 to hinge into during volvation (Figs 18, 19, g). Ozopore formula: 5, 7, 9, 10, 12, 13, 15, 17; ozopores opening flush on tergal surface about midheight of paraterga, most of openings concealed by preceding paraterga (Fig. 16). Telson small (Fig. 6).
Ammodesmus granum 7 male head, ventral view 8 interantennal isthmus, ventral view 9 midbody segments, ventral view 10 caudal part of body, ventral view 11 head and collum, dorsal view 12, 13 posterior part of body, caudal and dorsal views, respectively 14 cross-section of a midbody segment, caudal view 15 tegument texture, dorsal view. Scale bars: 7, 9–14, 100 µm; 8, 15, 20 µm.
Ammodesmus granum, male. 16 midbody paraterga, lateral view 17 metatergal tubercle, lateral view 18, 19 paratergal groove, laterocaudal and ventral views, respectively 20 first left leg, ventral view 21 modified setae of first leg 22 midbody leg, lateral view 23 last right leg, lateral view. Scale bars: 16, 100 µm; 17, 23, 10 µm; 18, 19, 20, 22, 20 µm; 21, 5 µm (g: groove, o: ozopore, pt: pit, p2: paraterga 2).
Legs rather slender, but short, barely reaching tips of paraterga (Fig. 14); femoral and tarsal segments longest, subequal in length; claw normal, simple, very slightly curved ventrad (Fig. 22); first pair of legs in ♂ with modified setae (Figs 20, 21); last pair of ♂ legs modified, typical of Ammodesmus (Fig. 23).
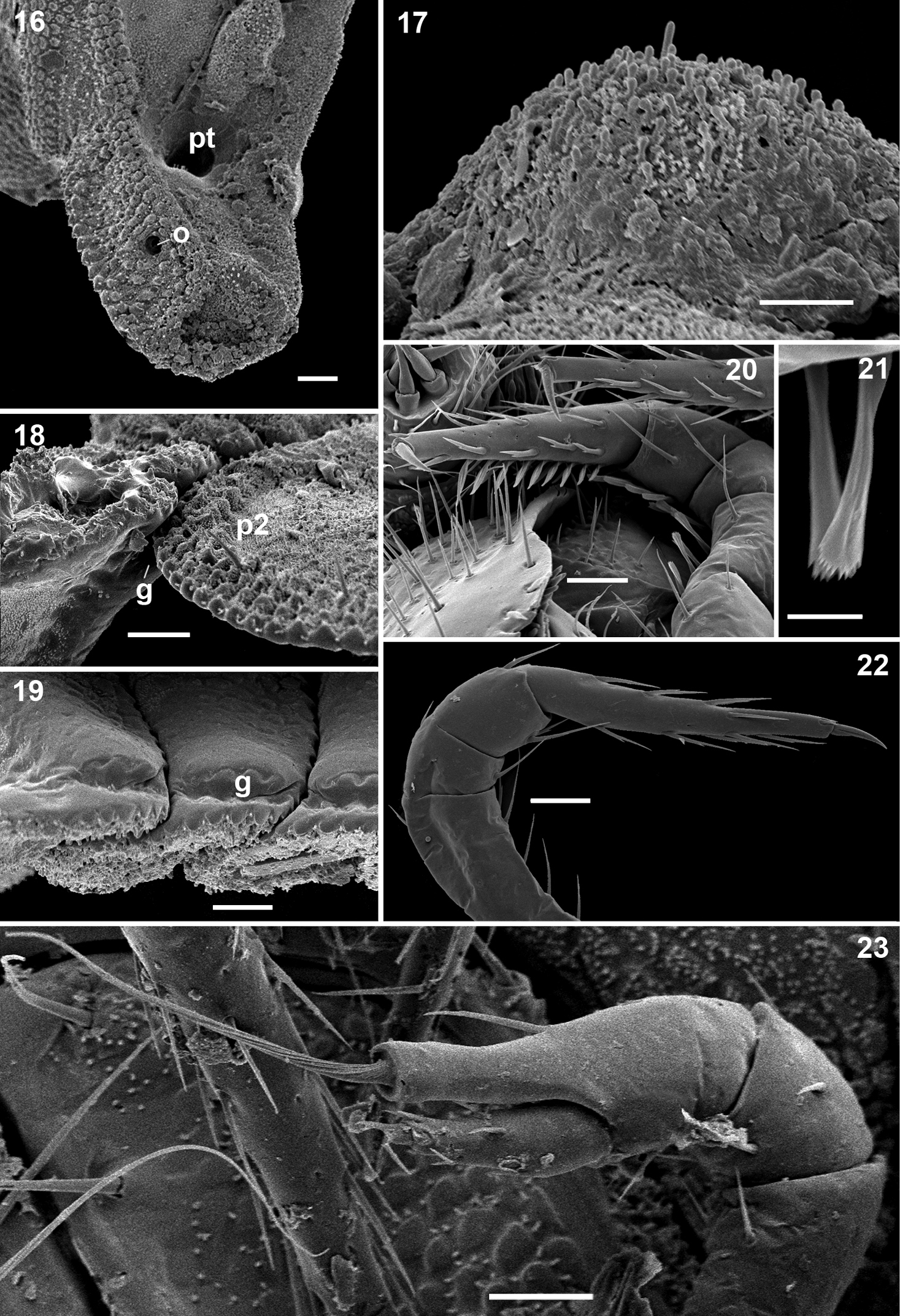
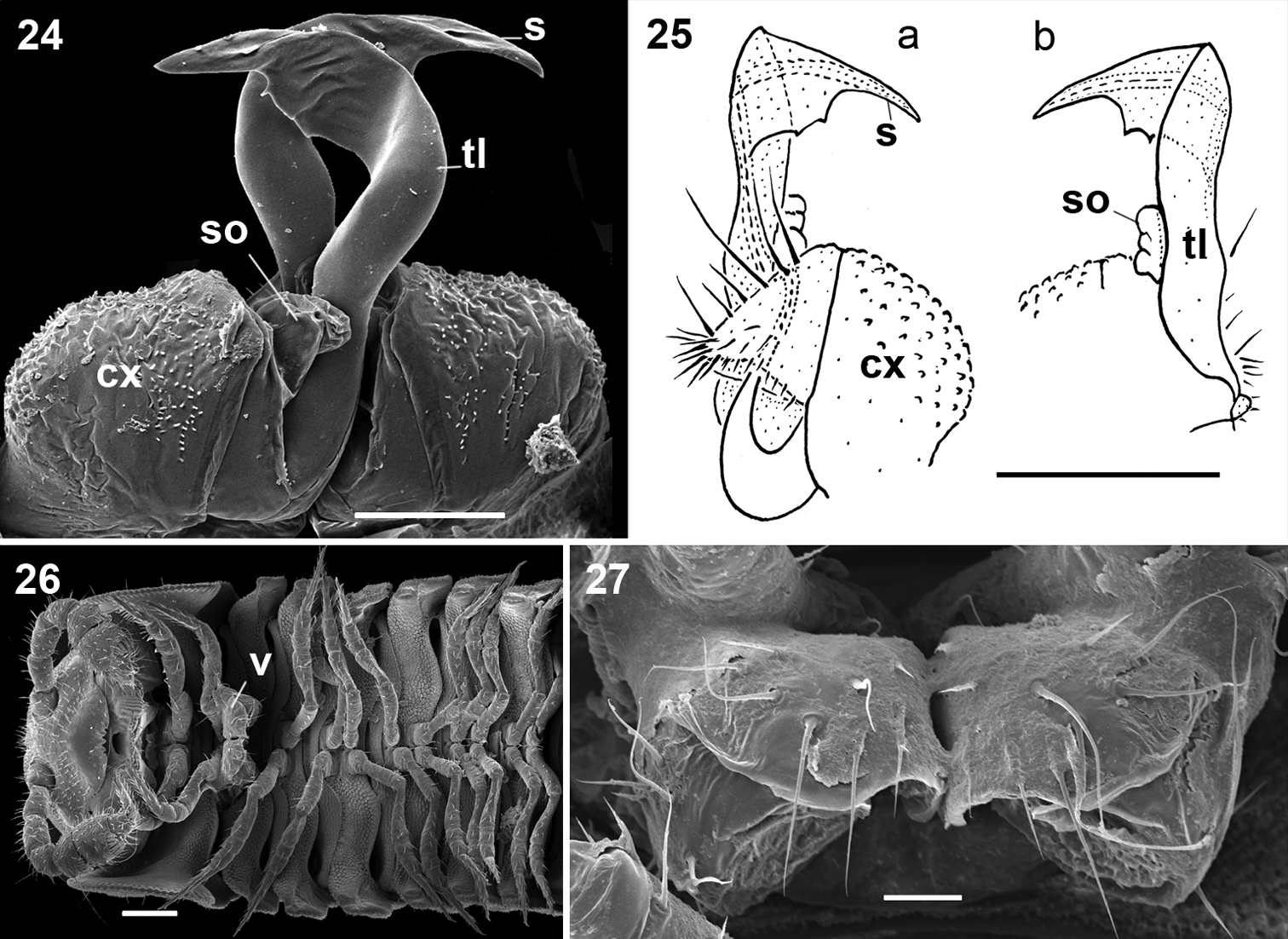
Gonopods (Figs 24, 25) relatively simple. Coxae rather small, globular, scaly, without setae. Telopodite long and well exposed beyond small gonocoxae; apical part of the main body of telopodite (= solenomere) smooth, flattened, pointed and twisted medially, devoid of a hairy pulvillus; a small, sac-shaped, lateral outgrowth at base of telopodite.
♀ usually slightly larger than ♂, segments rather smooth, without metatergal tubercles. Vulva small, poorly sclerotized, edge of bursa supplied with long setae (Figs 26, 27).
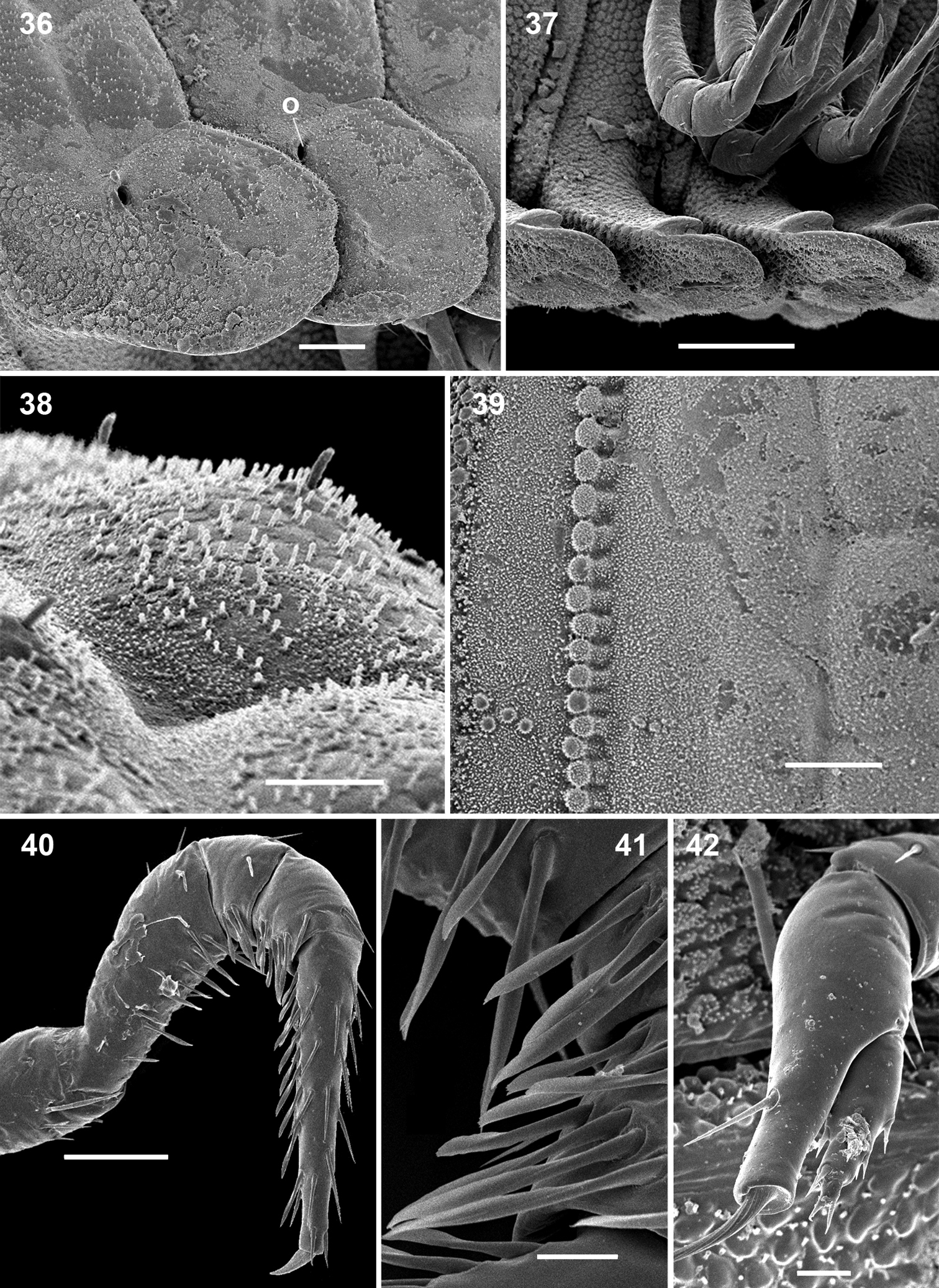
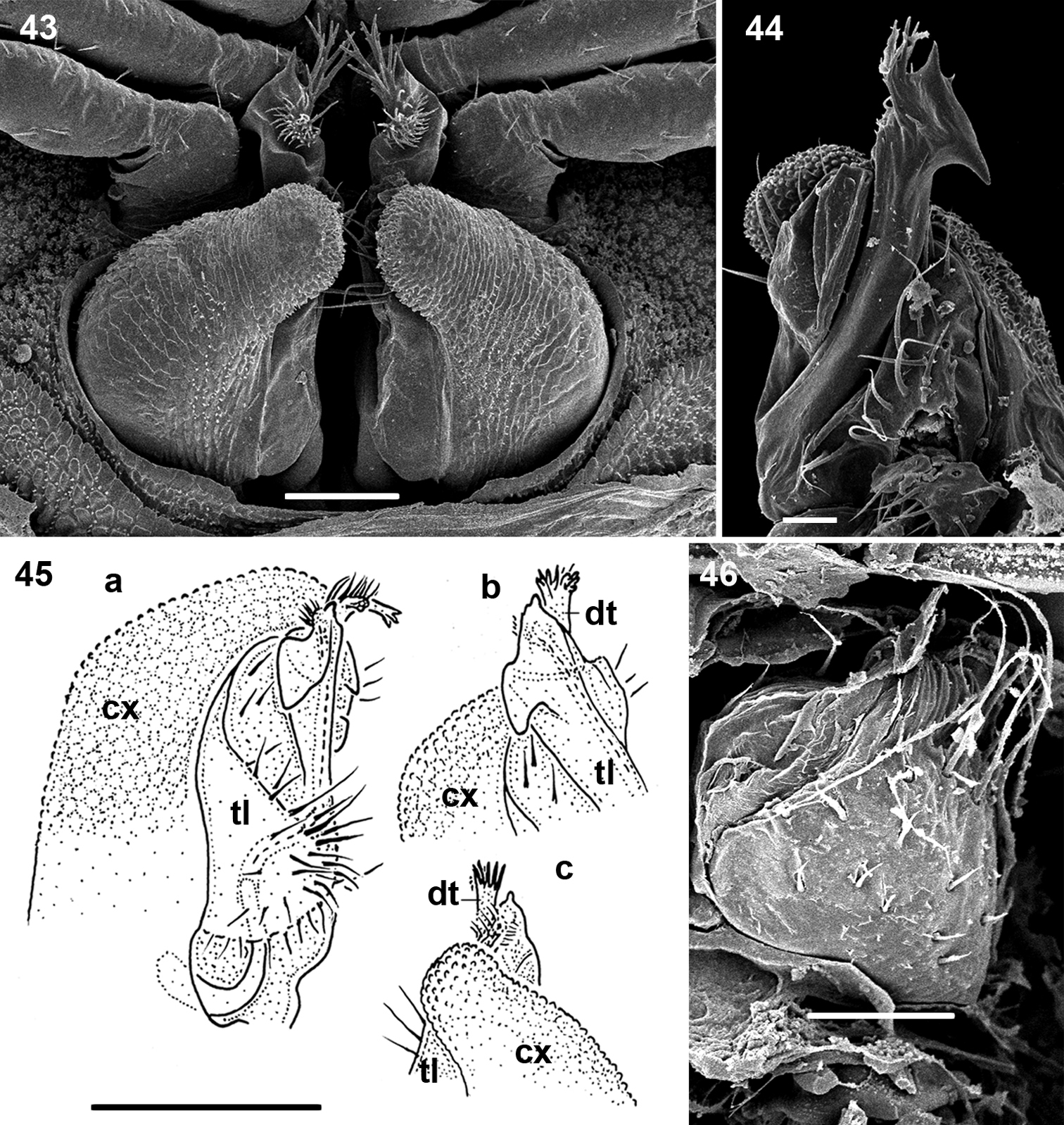
Ammodesmus granum. 24 gonopods, caudal view 25 drawing of right gonopod, frontal (a) and caudal (b) views, respectively 26 anterior part of female body, ventral view 27 vulvae, ventral view. Scale bars: 24, 50 µm; 25, 26, 100 µm; 27, 20 µm. (cx: coxae, s: solenomere, so: sac-shaped outgrowth, tl: telopodite).
Known from Liberia, Guinea and the Ivory Coast. It is noteworthy that at Mt Nimba this species occurs parapatrically with the new congener described below.
Ammodesmus granum is striking in being perhaps the only species in Polydesmida in which both sexes vary in the number (18 or 19) of body rings. Infraspecific variations in the number of body segments in this order are usually quite rare, always being stable per sex. Thus, in such cases males always have fewer body rings (18 or 19) than females (19 or 20), a situation not too uncommon, e.g., in Haplodesmidae (
Neotype designations for both Ammodesmus granum and Cenchrodesmus volutus are necessary, because the original types can be presumed as being lost. A special search undertaken among Cook’s diplopod collections, currently housed in the Smithsonian Institution, National Museum of Natural History, Washington, D.C., had failed already before the description of Elassystremma pongwe by
urn:lsid:zoobank.org:act:B0262CE7-D6AC-434D-BC93-A502EAC2D999
http://species-id.net/wiki/Ammodesmus_nimba
Figs 28–46Holotype ♂ (MRAC 22510), GUINEA, Mt Nimba, Freton forest, 07°37'N, 008°29'W, soil and litter, Winkler extraction, 10.III.2012, leg. A. Henrard, C. Allard, P. Bimou & M. Sidibé. Paratypes: 12 ex. (MRAC 22511), 1 ♂, 1 ♀ (ZMUM), 1 ♂ (MNHN), same locality, together with holotype.
Minute polydesmidans with a characteristic, spotty colour pattern of the caudal edge of each segment. Gonopods with extremely large coxae concealing the telopodites inside a deep gonocoel.
♂ca 2.8 mm long; maximum width, 0.9 mm. Body integument light brown to pinkish. Colour pattern of metaterga characteristic, spotty (Fig. 28). Body with 19 body rings (17+1+T), shape as in Figs 28, 29.
Ammodesmus nimba sp. n., male paratype. 28, 29 habitus, lateral view 30–32 anterior, middle and caudal parts of body, respectively, lateral view 33 midbody segments, dorsal view 34 head, frontal view 35 posterior part of body, ventral view. Scale bars: 100 µm.
Head small, partly concealed under front edge of collum; upper half of head densely granular, lower half smooth and densely setose. Interantennal isthmus without knob, about as wide as antennomere 1 (Fig. 34). Antennae as in Fig. 34. Collum relatively large, rather convex, surface slightly granular. Tergum 2 as usual, hypertrophied, with strongly enlarged, spatuliform paraterga concealing the head in lateral view (Figs 28, 29, 30), ventral edge with up to 4 rows of granules (Figs 29, 30). Limbus smooth, 2nd and following metaterga with a row of up to 13 low bosses lining the caudal margin; each boss obviously supporting a small apical seta (Figs 30–33, 38). Prozona rugose anteriorly, with a row of small granules along anterior edge of metatergum (Fig. 39). Paraterga set below segments’ midheight, continuing the convex outline of dorsum, with a notch basally at posterolateral edge; ends rather regularly rounded, increasingly angular towards telson (Figs 31, 32, 36). Anteroventral parts of paraterga 3 to 15 with a notch forming a groove for paraterga 2 to hinge into during volvation (Fig. 37). Ozopore formula: 5, 7, 9, 10, 12, 13, 15, 17; ozopores opening flush on tergal surface at about anterior third of paraterga, openings oblong and not concealed by preceding paraterga (Fig. 36). Telson small (Figs 32, 36).
Ammodesmus nimba sp. n., male paratype. 36, 37 midbody paraterga, lateral and ventral views, respectively 38 metatergal bosses, sublateral view 39 tegument texture, dorsal view 40 first leg 41 modified setae of first leg 42 last right leg, lateral view. Scale bars: 36, 39, 40, 50 µm; 37, 100 µm; 38, 20 µm; 41, 42, 10 µm (o: ozopore).
Sterna and legs as in Ammodesmus granum (Figs 40, 41, 42). Gonopod aperture relatively modest in size, transversely oval (Fig. 43).
Gonopods highly complex (Figs 43–45); coxae oblong, strongly enlarged to protect telopodites (Figs 43, 45). Telopodite only a little longer than coxa, showing a hook-shaped apical part (Figs 44, 45b) carrying a digitiform tubercle (Figs 43, 44, 45c). Solenomere very small and short, supplied with a distinct hairy pulvillus (Fig. 45a).
♀ agrees precisely in colour and structural details with ♂, also being (nearly) of the same size and counting 19 body rings. Vulva small, setose, poorly sclerotized, edge of bursa with some particularly long setae (Fig. 46).
Ammodesmus nimba sp. n. 43 gonopods, caudal view 44 right gonopod, frontal view 45 drawing of left gonopod, frontal view (a), apicofrontal (b) and caudal (c) views, respectively 46 right vulva, ventral view. Scale bars: 43, 46, 50 µm; 44, 20 µm; 45, 100 µm. (cx: coxae, dt: digitiform tubercle, tl: telopodite)
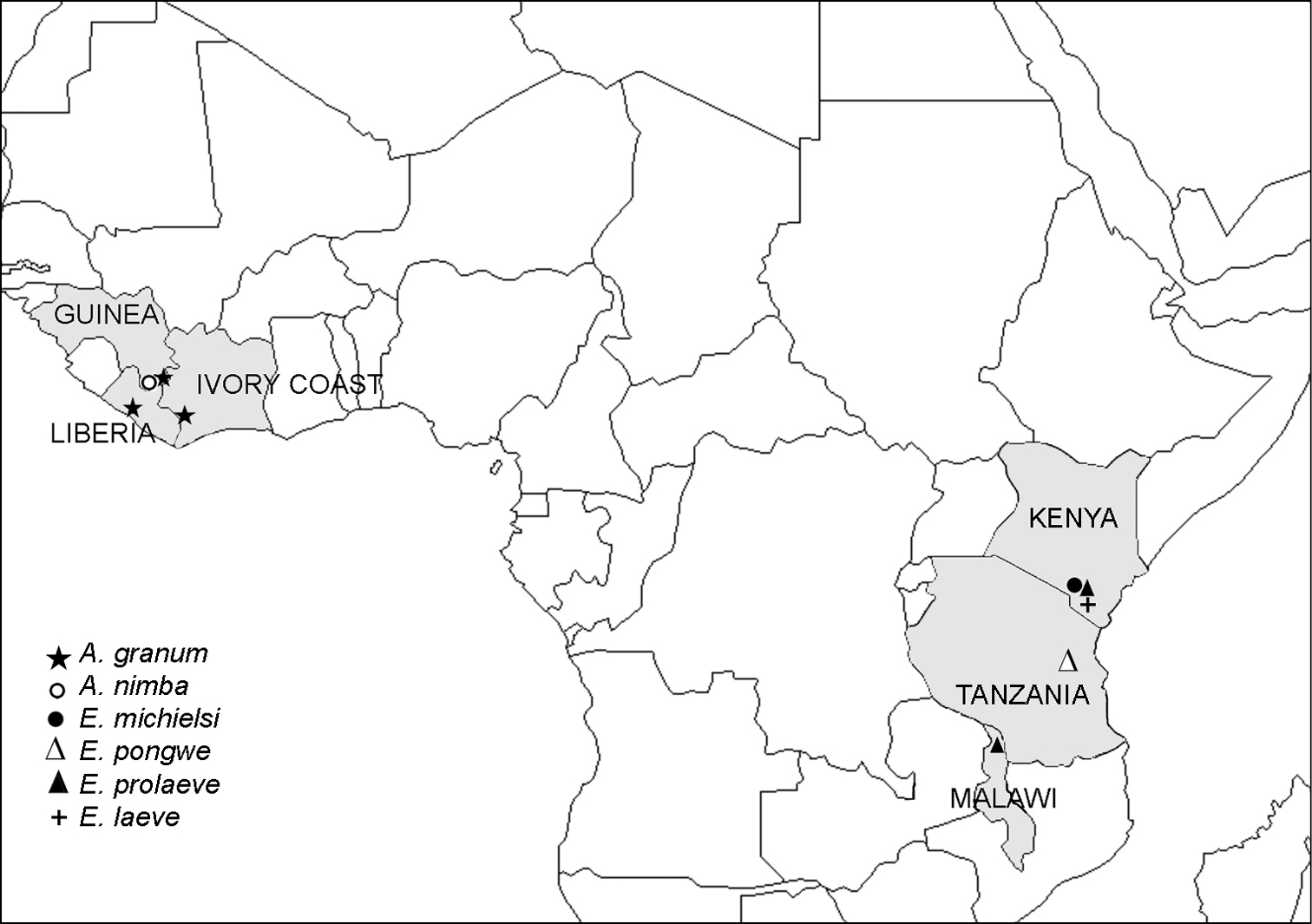
Superficially, both species of Ammodesmus might look sufficiently different to consider them as representing different genera, especially as regards the absence of sexual dimorphism in metatergal structure and the presence of a deep gonocoel in Ammodesmus nimba as opposed to Ammodesmus granum. The main distinctions can also be summarized in a tabular form (Table). However, based on all evidence, we are rather inclined to recognize only two valid genera in Ammodesmidae, both quite disjunct also geographically (Fig. 47).
Principal differences between genera of Ammodesmidae
| Key characters in genera of Ammodesmidae | Ammodesmus granum | Ammodesmus nimba sp. n. | Elassystremma spp. |
|---|---|---|---|
| Sexual dimorphism in metatergal structure | yes | no | no |
| Size | up to 2 mm long | idem | up to 5 mm long |
| Ozopore formula | 5, 7, 9, 10, 12, 13, 15–17(18) | idem | 5, 7, 9, 12, 15, 17(18) |
| First male leg with modified setae | yes | yes | no |
| Last male leg modified | yes | yes | no |
| Gonopod telopodite deeply sunken into a very evident gonocoel | no | yes | yes |
Referring to the type locality, a noun in apposition.
Known only from the type locality and probably endemic to Mt Nimba.
Despite extensive efforts applying the same collecting techniques in similar habitats in many places, Ammodesmus nimba appears to occur, and to be apparently common, only at the single locality whence it has been taken, whereas Ammodesmus granum has a surprisingly wide distribution. The vast range of Ammodesmus granum, currently reported from the western part of Liberia, at Mt Nimba in Guinea and in the Taï forest in the western part of Ivory Coast, is rather unusual for such a tiny and hygrophilous animal. Certainly being likewise rather poorly vagile, this species can be assumed to represent a relict which must have been widely distributed in the past when woodlands werecontinuous in western tropical Africa (
Geographically, the family Ammodesmidae seems to be purely Afrotropical, Ammodesmus being obviously confined to western Africa while Elassystremma to eastern Africa (Fig. 47). All four Elassystremma species (Elassystremma pongwe Hoffman & Howell, 1981, Elassystremma michielsi VandenSpiegel & Golovatch, 2004, Elassystremma leave VandenSpiegel & Golovatch, 2004 and Elassystremma prolaeve VandenSpiegel & Golovatch, 2004) are slightly larger than Ammodesmus (up to 5 mm long), and their gonopods are invariably complex, sunken inside a deep gonocoel (
Distribution of the family Ammodesmidae.
The use of Winkler-Mocsarski apparatuses, or Winkler apparatuses for short, appears to be the most appropriate technique in sampling particularly cryptic soil/litter fauna. This technique has allowed for material to be collected in almost any East and West African tropical forest prospected by the first author and it is most likely that new species will be revealed in the central parts of the continent, if the collecting efforts use appropriate techniques. At least the wide geographical gap between both genera certainly invites further studies (
We are most grateful to all of the collectors whose material has been used for the present study, particularly to A. Henrard, Ch. Allard, P. Bimou & M. Sidibé. Special thanks are expressed to G. Rondeau and O. Diallo, from the OKAPI Environnent Conseil, for the logistic help rendered in Guinea.
This research was undertaken by Golder Associates and sub-consultants as a component of the Nimba Project ESIA for Société des Mines de Fer de Guinée.