(C) 2012 Arnaud Faille. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A molecular phylogeny of the species from the Trechus brucki clade (previously Trechus uhagoni group)based on fragments of four mitochondrial genes and one nuclear gene is given. We describe Trechus (Trechus) bouilloni sp. n. from the western pre–Pyrenees: Sierras de Urbasa–Andía, Navarra, Spain. The species was collected in mesovoid shallow substratum (mss), a subterranean environment. Molecular as well as morphological evidences demonstrate that the new species belongs to the Trechus brucki clade. A narrow endemic species of high altitude in western French Pyrenees merged with Trechus brucki Fairmaire, 1862a, Trechus bruckoides sp. n., is described. A lectotype is designated for Trechus brucki and Trechus planiusculus Fairmaire, 1862b (junior synonym of Trechus brucki). The species group is redefined based on molecular and morphological characters, and renamed as the brucki group, as Trechus brucki was the first described species of the clade. A unique synapomorphy of the male genitalia, a characteristic secondary sclerotization of the sperm duct, which is shared by all the species of the brucki group sensu novo, is described and illustrated. The Trechus brucki group sensu novo is composed of Trechus beusti (Schaufuss, 1863), Trechus bouilloni sp. n., Trechus brucki, Trechus bruckoides sp. n., Trechus grenieri Pandellé, 1867, T. uhagoni uhagoni Crotch, 1869, T. uhagoni ruteri Colas, 1935 and Trechus pieltaini Jeannel, 1920. We discuss the taxonomy of the group and provide illustrations of structures showing the differences between the species, along with distribution data and biogeographical comments.
Carabidae, Trechini, Trechus brucki group , new species, molecular phylogeny, subterranean environment, Pyrenees, France, Spain
The genus Trechus (Coleoptera, Carabidae, Trechinae) includes more than 800 species, most of them in the Palearctic area (Moravec et al.2003;
In this paper we describe a species collected by traps in a MSS (mesovoid shallow substratum, “Milieu Souterrain Superficiel” sensu
Trechus uhagonii was described by
In the Monographie des Trechinae,
The uhagoni group sensu
Trechus brucki is an alpine species located at high altitude in the central and western Pyrenees, and it is until now not recorded from the Spanish slope of the chain (
Specimens were collected by hand or by means of pitfall traps containing water saturated in salt or propylene glycol, known to preserve DNA (
Extractions of single specimens were non–destructive, using the DNeasy Tissue Kit (Qiagen GmbH, Hilden, Germany). After extraction, specimens were mounted on cards and genitalia stored in water–soluble dimethyl hydantoin formaldehyde resin (DMHF) on transparent cards, pinned beneath the specimen. Vouchers and DNA samples are kept in the collections of ZSM, IBE and MNHN.
We included examples of most species of the Trechus uhagoni group, with the exception of Trechus bruckoides sp. n., Trechus carrilloi and Trechus sharpi and some examples of Trechus of the angusticollis group sensu
We amplified fragments of four mitochondrial genes: 3’ end of cytochrome c oxidase subunit (cox1); a single fragment including the 3’ end of the large ribosomal unit (rrnL), the whole tRNA–Leu gene (trnL) and the 5’ end of the NADH dehydrogenase 1 (nad1);and one nuclear gene (internal fragment of the large ribosomal unit 28S rRNA, LSU) (see Table 2 for primers used). Sequences were assembled and edited using Sequencher TM 4.8 (Gene Codes, Inc., Ann Arbor, MI). Parts of the sequences for 14 of the species were taken from
New sequences have been deposited in the EMBL database with Accession Numbers HE817887–HE817940 (Table 1).
Sequenced specimens, with localities, collectors, codes and sequence accession numbers (unpublished sequences in bold).
| sp | locality | collector | code | LSU | cox1 | rrnL | trnL | NAD1 |
|---|---|---|---|---|---|---|---|---|
| Aphaenops Bonvouloir, 1862 | ||||||||
| Aphaenops leschenaulti Bonvouloir, 1861 | Grotte de Castelmouly – Bagnères–de–Bigorre (France–65) | C. Bourdeau, P. Déliot, A. Faille | MNHN–AF1 | GQ293593 | HE817919 | GQ293739 | GQ293757 | GQ293822 |
| Trechus Clairville, 1806 | ||||||||
| Trechus grenieri Pandellé, 1867 | Résurgence de la Hèche, Fréchet–Aure (France–65) | J.P. Besson, C. Bourdeau, A. Faille | ZSM–L13 | HE817904 | HE817920 | HE817887 | HE817887 | HE817887 |
| Trechus brucki Fairmaire, 1862 | Pic du Gabizos, Arrens (France–65) | C. Bourdeau | ZSM–L329 | HE817906 | HE817921 | HE817888 | HE817888 | HE817888 |
| Trechus brucki Fairmaire, 1862 | Pic du Gabizos, Arrens (France–65) | C. Bourdeau | ZSM–L329b | HE817907 | HE817889 | HE817889 | HE817889 | |
| Trechus brucki Fairmaire, 1862 | Pic de Sesques, Laruns (France–64) | C. Bourdeau | ZSM–L446 | HE817905 | HE817922 | HE817890 | HE817890 | HE817890 |
| Trechus brucki Fairmaire, 1862 | Pic de Gaziès, Laruns (France–64) | C. Bourdeau | ZSM–L190 | HE817908 | HE817923 | HE817891 | HE817891 | HE817891 |
| Trechus brucki Fairmaire, 1862 | Caperan d´Anéou, Laruns (France–64) | C. Bourdeau | ZSM–L449 | HE817909 | HE817924 | HE817892 | HE817892 | HE817892 |
| Trechus uhagoni Crotch, 1869 | Cueva de Orobe – Alsasúa (Spain–Navarra) | C. Bourdeau, J. Fresneda | ZSM–L161 | HE817910 | HE817925 | HE817893 | HE817893 | HE817893 |
| Trechus bouilloni Faille, Bourdeau & Fresneda, sp. n. | Puerto de Lizarraga, Lizarraga (Spain–Navarra) | C. Bourdeau, J. Fresneda | ZSM_L201b | HE817911 | HE817926 | HE817894 | HE817894 | HE817894 |
| Trechus bouilloni Faille, Bourdeau & Fresneda, sp. n. | Puerto de Lizarraga, Lizarraga (Spain–Navarra) | C. Bourdeau, J. Fresneda | ZSM_L201t | HE817927 | HE817895 | HE817895 | HE817895 | |
| Trechus beusti (Schaufuss 1863) | Cueva de San Adrián, Zegama (Spain–Guipúzcoa) | C. Bourdeau, J. Fresneda | ZSM–L199 | HE817912 | HE817928 | HE817896 | HE817896 | HE817896 |
| Trechus pieltaini Jeannel, 1920 | Cueva de Mairuelegorreta, Gorbea (Spain–Álava) | C. Bourdeau | ZSM–L395 | HE817913 | HE817929 | HE817897 | HE817897 | HE817897 |
| Trechus navaricus (Vuillefroy, 1869) | Grotte de Sare – Sare (France–64) | C. Bourdeau | MNHN–AF103 | GQ293603 | GQ293687 | FR729578 | FR729578 | FR729578 |
| Trechus bordei Peyerimhoff, 1909 | Grotte d´Ayssaguer – Larrau (France–64) | C. Bourdeau, P. Déliot, A. Faille | MNHN–TBA | HE817914 | HE817930 | HE817898 | HE817898 | HE817898 |
| Trechus bonvouloiri Pandellé, 1867 | Pic de Montaigu – Baudéan (France – 65) | C. Bourdeau | ZSM–L218 | HE817915 | HE817931 | HE817899 | HE817899 | HE817899 |
| Trechus abeillei Pandellé, 1872 | Cirque d´Anglade Couflens (France–09) | C. Vanderbergh | ZSM–L15 | HE817916 | HE817932 | HE817900 | HE817900 | HE817900 |
| Trechus distinctus Fairmaire & Laboulbène, 1854 | Col Sobe Ariel – Laruns (France–64) | C. Bourdeau | ZSM–L216 | HE817917 | HE817933 | HE817901 | HE817901 | HE817901 |
| Trechus aubryi Coiffait, 1953 | Cirque d´Anglade Couflens (France–09) | B. Junger | ZSM–L370 | HE817934 | HE817902 | HE817902 | HE817902 | |
| Trechus jeannei Sciaky, 1998 | Bosque de Saja, Saja (Spain–Cantabria) | C. Bourdeau | ZSM–L516 | HE817918 | HE817903 | HE817903 | HE817903 | |
| Trechus saxicola Putzeys, 1870 | Braña Caballo – Piedrafita (Spain–León) | C. Bourdeau, P. Déliot, A. Faille | MNHN–AF100 | GQ293614 | HE817935 | FR729577 | FR729577 | FR729577 |
| Trechus escalerae Abeille de Perrin, 1903 | Cueva de Porro Covañona – Covadonga (Spain–Asturias) | J.M. Salgado | MNHN–AF104 | GQ293612 | FR733912 | GQ293731 | GQ293793 | GQ293839 |
| Trechus ceballosi Mateu, 1953 | Aven de Licie Etsaut – Lanne–en–Barétous (France–64) | C. Bourdeau, A. Faille | MNHN–AF128 | GQ293610 | FR733914 | GQ293728 | GQ293791 | GQ293850 |
| Trechus distigma Kiesenwetter, 1851 | Aven de Nabails – Arthez d’Asson (France–64) | C. Bourdeau, P. Déliot, A. Faille | MNHN–AF94 | GQ293611 | HE817936 | FR729575 | FR729575 | FR729575 |
| Trechus barnevillei Pandellé, 1867 | Cueva del Pis – Penilla, Santiurde de Toranzo (Spain–Cantabria) | C. Bourdeau, P. Déliot, A. Faille | MNHN–AF97 | GQ293607 | GQ293680 | GQ293727 | GQ293783 | GQ293848 |
| Trechus obtusus Erichson, 1837 | Estrada de Nicho (Portugal–Madeira) | A. Arraiol | IBE–AF2 | FR733997 | HE817937 | FR729579 | FR729579 | FR729579 |
| Trechus quadristriatus (Schrank, 1781) | Collau de la Plana del Turbón – Egea (Spain– Huesca) | P. Déliot, A. Faille, J. Fresneda | MNHN–AF96 | GQ293619 | FR733908 | GQ293743 | GQ293745 | GQ293841 |
| Trechus fulvus Dejean, 1831 | Cueva del Pis – Penilla, Santiurde de Toranzo (Spain–Cantabria) | C. Bourdeau, P. Déliot, A. Faille | MNHN–AF98 | GQ293613 | HE817938 | GQ293729 | ||
| Trechus martinezi Jeannel, 1927 | Cova de les Meravelles – Cocentaina (Spain– Alicante) | C. Andújar, P. Arribas, A. Faille | IBE–AF1 | FR733996 | HE817939 | FR729576 | FR729576 | FR729576 |
| Trechus schaufussi ssp. comasi Hernando, 2002 | Cueva Basaula – Barindano (Spain–Navarra) | J. Fresneda | MNHN–AF127 | GQ293617 | HE817940 | FR729580 | FR729580 | FR729580 |
| Apoduvalius Jeannel, 1953 | ||||||||
| Apoduvalius alberichae Español, 1971 | Cova de Agudir – Cardano de abajo – Palencia (Spain–Asturias) | J.M. Salgado | MNHN–AF105 | GQ293618 | GQ293632 | GQ293732 | GQ293794 | GQ293840 |
| Apoduvalius anseriformis Salgado et Peláez, 2004 | Cueva de Entrecuevas – Caravia Alta (Spain– Palencia) | A. Cieslak, A. Faille, J. Fresneda, I. Ribera, J.M. Salgado | MNCN–AF2 | FR733999 | FR733916 | FR729582 | FR729582 | FR729582 |
Primers used in the study. F, forward; R, reverse.
| Gene | Name | Sense | Sequence | Reference |
| cox1 | Jerry (M202) | F | CAACATTTATTTTGATTTTTTGG |
|
| Pat (M70) | R | TCCA(A)TGCACTAATCTGCCATATTA |
|
|
| Chy | F | T(A/T)GTAGCCCA(T/C)TTTCATTA(T/C)GT |
|
|
| Tom | R | AC(A/G)TAATGAAA(A/G)TGGGCTAC(T/A)A |
|
|
| rrnL–nad1 | 16saR (M14) | F | CGCCTGTTTA(A/T)CAAAAACAT |
|
| 16Sa | R | ATGTTTTTGTTAAACAGGCG |
|
|
| 16Sb | R | CCGGTCTGAACTCAGATCATGT |
|
|
| ND1A (M223) | R | GGTCCCTTACGAATTTGAATATATCCT |
|
|
| LSU | D1 | F | GGGAGGAAAAGAAACTAAC |
|
| D3 | R | GCATAGTTCACCATCTTTC |
|
We aligned the sequences using the MAFFT online v.6 and the Q–INS–i algorithm (
The aedeagus and genital duct were extracted and included in a drop of Canada balsam or dimethyl hydantoin formaldehyde resin (DMHF) on a transparent slide. Preparations were mounted below the specimen, on the same pin. Pictures were taken with microscopes Olympus ch and Olympus szx16, coupled with a camera Olympus c5060wz. Serial pictures were combined using the CombineZP software, and finally processed using Adobe Photoshop CS.
Institutional codes and abbreviations used in the taxonomic treatment and private collectorsIBE Institute of Evolutionary Biology (CSIC-UPF), Barcelona (Spain).
MNCN Museo Nacional de Ciencias Naturales (CSIC), Madrid (Spain)
MNHN Muséum National d´Histoire Naturelle, Paris (France).
MZB Museu de Ciències Naturals (Zoologia), Barcelona (Spain).
ZSM Zoologische Staatssammlung, München (Germany).
MFN Museum für Naturkunde, Berlin (Germany).
CAF coll. A. Faille (Paris, France).
CCB coll. C. Bourdeau (Rebigue, France).
CJF coll. J. Fresneda (Llesp, Spain).
CMT coll. M. Toribio (Madrid, Spain).
LE Length of elytra.
LP Length of pronotum.
WE Width of elytra.
WH Width of head.
WP Width of pronotum.
WPB Width of pronotal base.
Resultsurn:lsid:zoobank.org:act:C967CB33-C16A-468F-B786-E6F376B2D978
http://species-id.net/wiki/Trechus_bouilloni
Figs 1, 8, 15, 16,29Spain, Navarra, Sierra de Urbasa–Andía, Lizarraga, Puerto de Lizarraga, UTM (WGS 84): 30 T, X: 580, Y: 4746, Z: 900 m.
Holotype (MNHN): 1 ♂, Spain, Navarra, Sierra de Urbasa–Andía, Lizarraga, Puerto de Lizarraga, MSS, trap: 1–5–1980/15–8–1980, Bourdeau and Fresneda leg., voucher number ZSM–L201, MNHN]. DNA aliquotes preserved in the DNA and tissue collections of the ZSM, MNHN and IBE; Genitalia dissected and mounted in a separate label pinned with the specimen. Paratypes: 52 ♂♂, 62 ♀♀, same label data as holotype (MNCN, MNHN, MZB, ZSM, CCB, CJF, CAF, CMT).
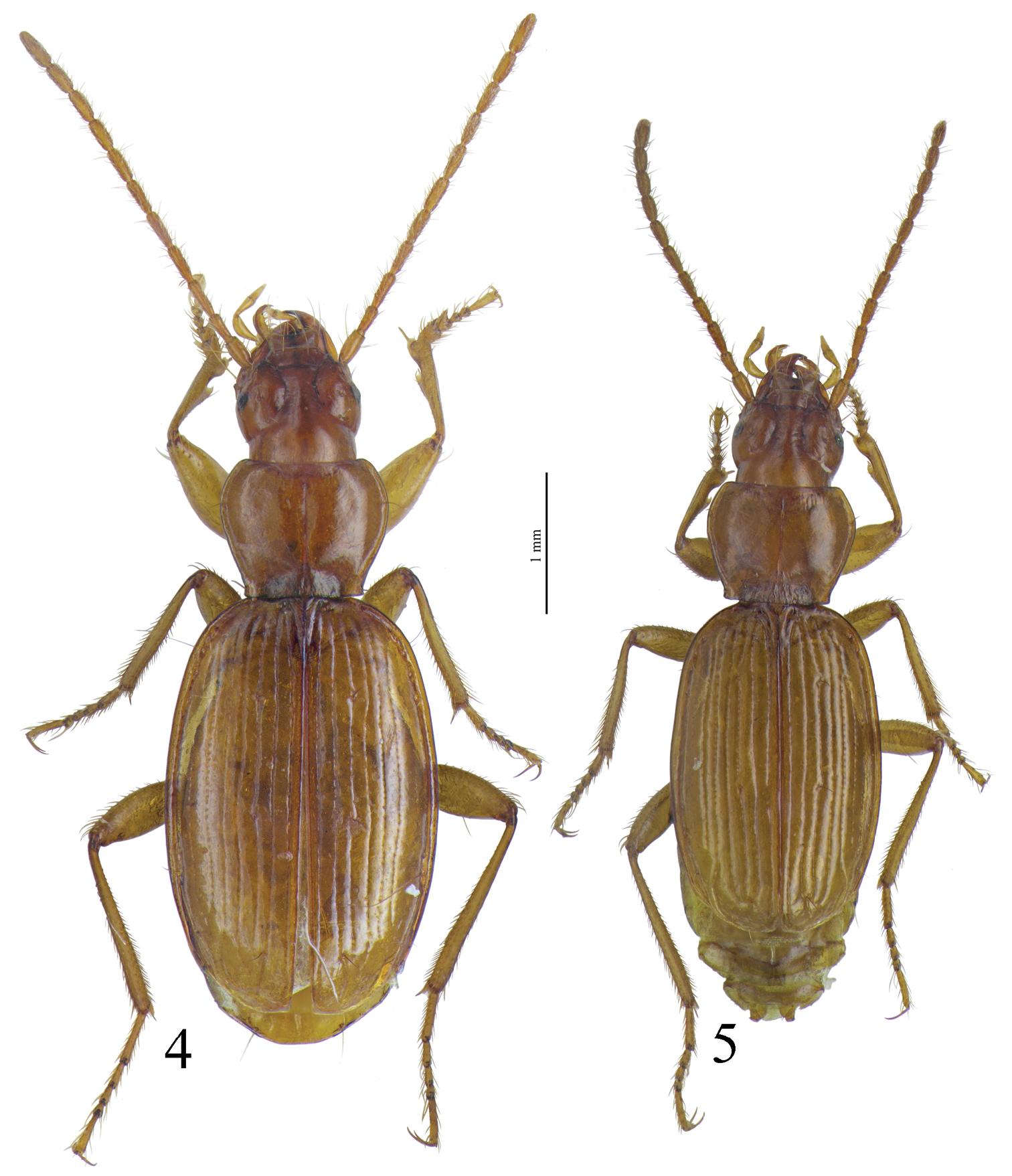
Large size (ca 5 mm) and round shape (Fig. 1). Median lobe of aedeagus slender, in lateral view (Fig. 15) the basal third curved, the central part straight and the apex with a curved hook assymetrical in dorsal view (Fig. 8). Inner sac of aedeagus (=endophallus) with an elongate and well-sclerotized piece, forming a gut and armed with internal scales. Characteristic secondary sclerotization of the sperm duct (Fig. 15: CP2) forming a kind of second copulatory piece outside base of the median lobe.
Habitus as in Fig. 1.Elongated, round–sided. Body surface with a very thin, hardly visible, dense microreticulation, with more distinguishable meshes on the head.
Colour. Dorsal surface dark brown, moderately shiny. Antennae, palpi and legs light brown.
Chetotaxy. Surface of elytra glabrous with the exception of a periscutellar seta, two discal setae on the third stria, four humeral setae, four setae along lateral margin and two preapical setae. Marginal setae of pronotum present, the anterior ones located before the first third of the length. Ventral pubescence limited to one seta on each half sternite.
Head. Eyes reduced, flat; ommatidia well defined; maximum diameter of about eight ommatidia, temples approximately twice the length of eyes, strongly wrinkled to the neck. Frontal furrows deeply impressed. Antennae moderately long, five antennomeres extend beyond the pronotal base. Antennomere III distinctly longer than antennomeres II and IV, which are similar in length.
Pronotum. Proportions (M–F): WP/LP = 1.3–1.28, WP/WPB = 1.3–1.3, WP/WH = 1.38–1.3, WE/WP = 1.57–1.53. Transverse, with lateral margins finely bordered; wider in anterior part, narrower than elytra; posterior part much narrower than base of elytra. One seta in the marginal gutter at about a third of pronotum length, another one close to hind angle. Sides evenly rounded and straight just between hind angles and insertions of posterior setae. Hind angles well developed, salient.
Elytra. Proportions (M–F): WE/LE = 0.65–0.69. Oval, broadest almost at mid–length; surface moderately convex, flattened on disc. Shoulders distinct but rounded. Striae very finely punctuated, sixth inner striae deeply impressed on disc, but reduced at apex and base; seventh striae shallower, but distinct, the eighth reduced to the posterior quarter of elytra. Apical striola strongly impressed continuing the fifth stria.
Hind wings. Very reduced, not functional.
Male genitalia. Median lobe of aedeagus slender, in lateral view (Fig. 15) the basal third curved, the central part straight and the apex showing a curved hook; assymetrical in dorsal view (Fig. 8). Parameres slender, each with 4 to 6 setae at apex. Internal sac of aedeagus with an elongate well-sclerotized piece, forming a symmetrical gut and armed with internal scales (Fig. 16). Characteristic secondary sclerotization of the sperm duct forming a kind of second copulatory piece out of the base of the median lobe (Fig. 15: CP2).
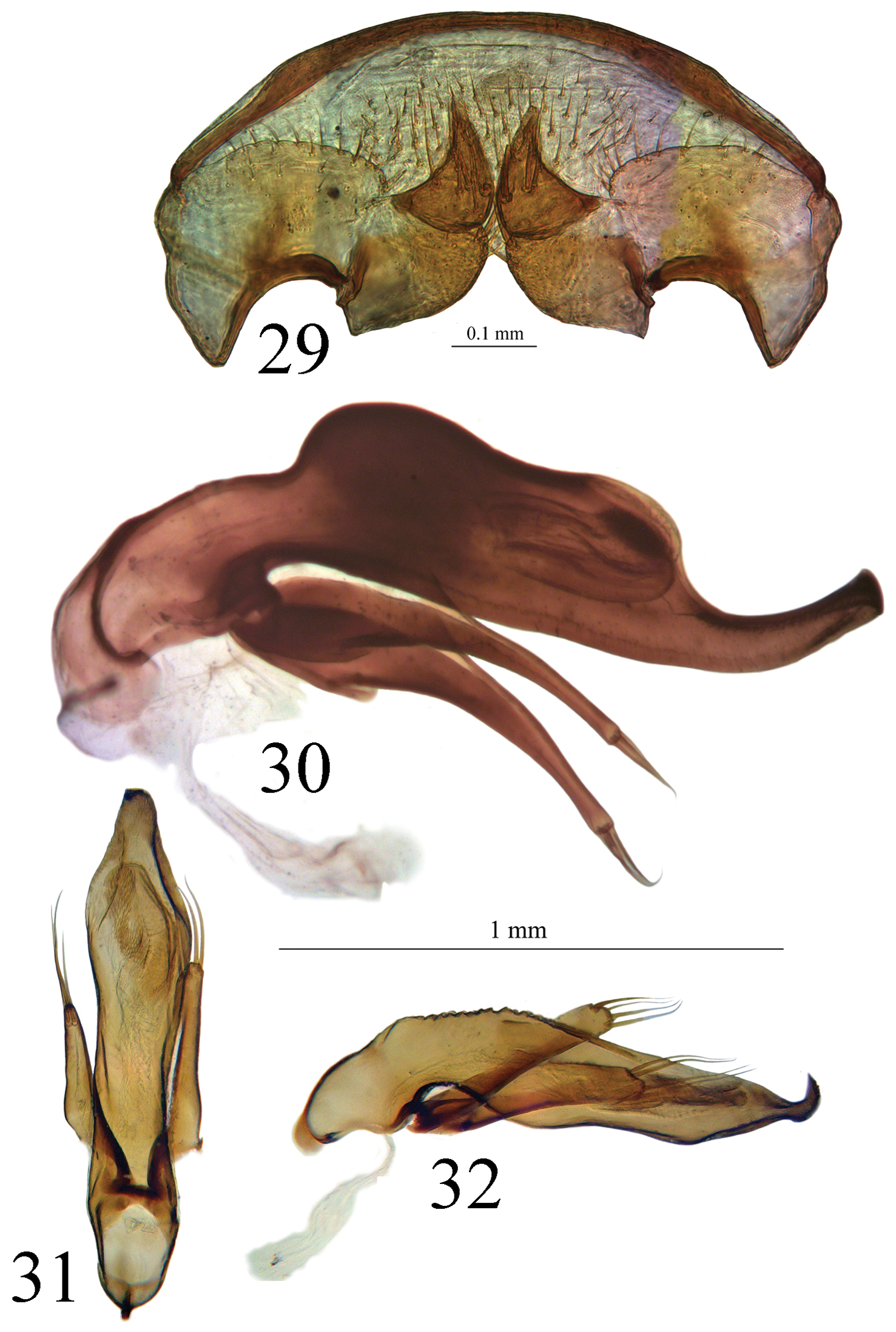
Internal genitalia membranous. Gonocoxites unguiform, with 4 to 5 large setae, and 2 small near apex. Gonosubcoxites with 2 to 3 large setae near the internal edge. Laterotergite IX with 12 setae at the basal margin, and 4 to 6 scattered (Fig. 29).
Mean length (5 exemplars): 5.25 mm (male), 4.56 mm (female).
The new species is dedicated to Michel Bouillon, Pyrenean speleologist, who was the first to discover the existence of cave beetles in MSS.
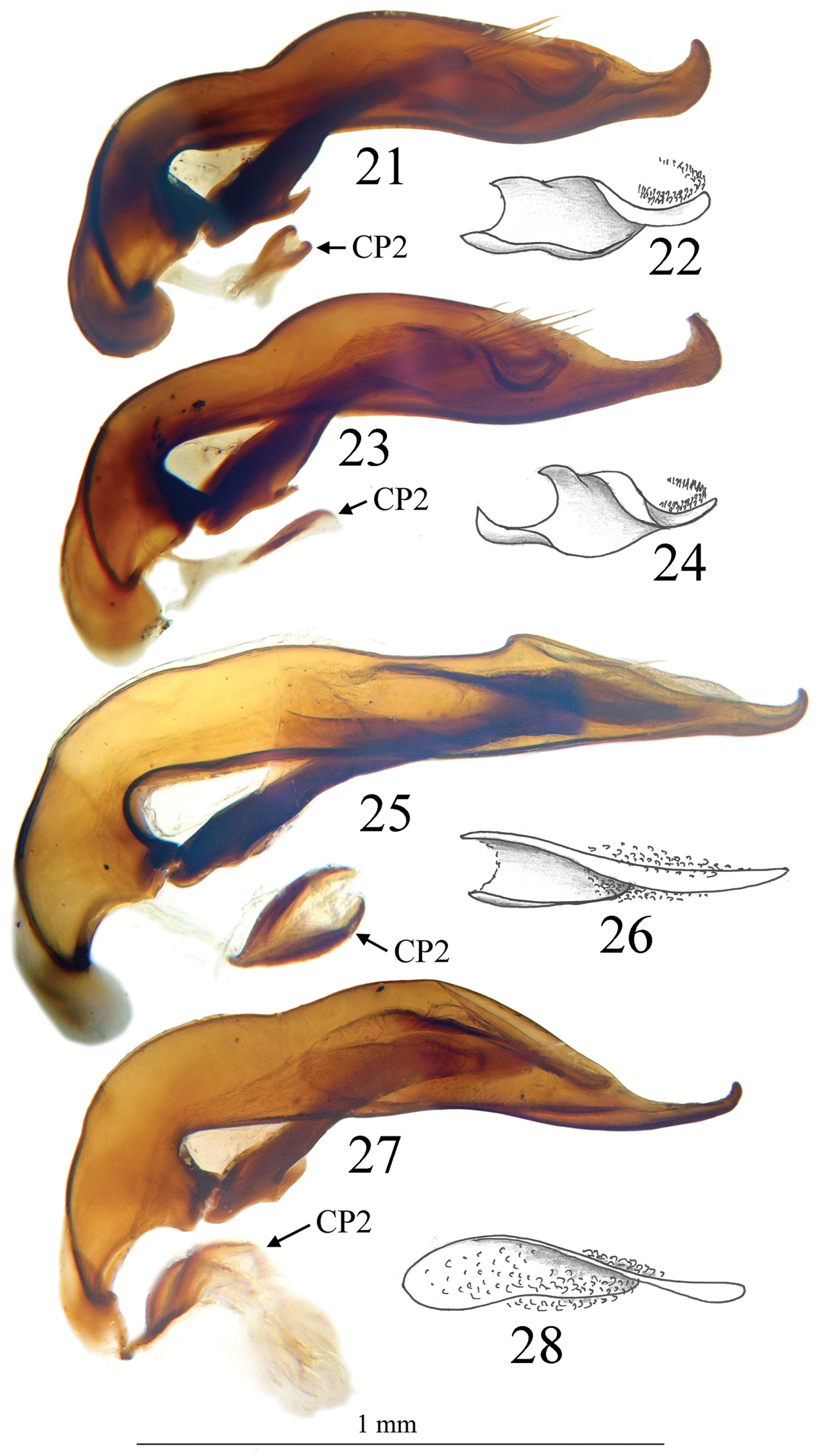
Trechus bouilloni sp. n. is a representative of the Trechus brucki group sensu novo as defined in the present paper. It shares with Trechus grenieri, Trechus uhagoni, Trechus beusti, and Trechus pieltaini the same kind of aedeagus morphology, especially the apex with a curved hook in lateral view, and an internal sac showing two sclerotized parts, the internal copulatory piece and another triangular piece forming a kind of second copulatory piece (CP2, Figs 17–24), also existing in Trechus brucki and Trechus bruckoides sp. n. (Figs 25–28). Similar secondary sclerotized structures of endophallus are known in some groups of insects including Coleoptera, and described as a “sperm pump” (
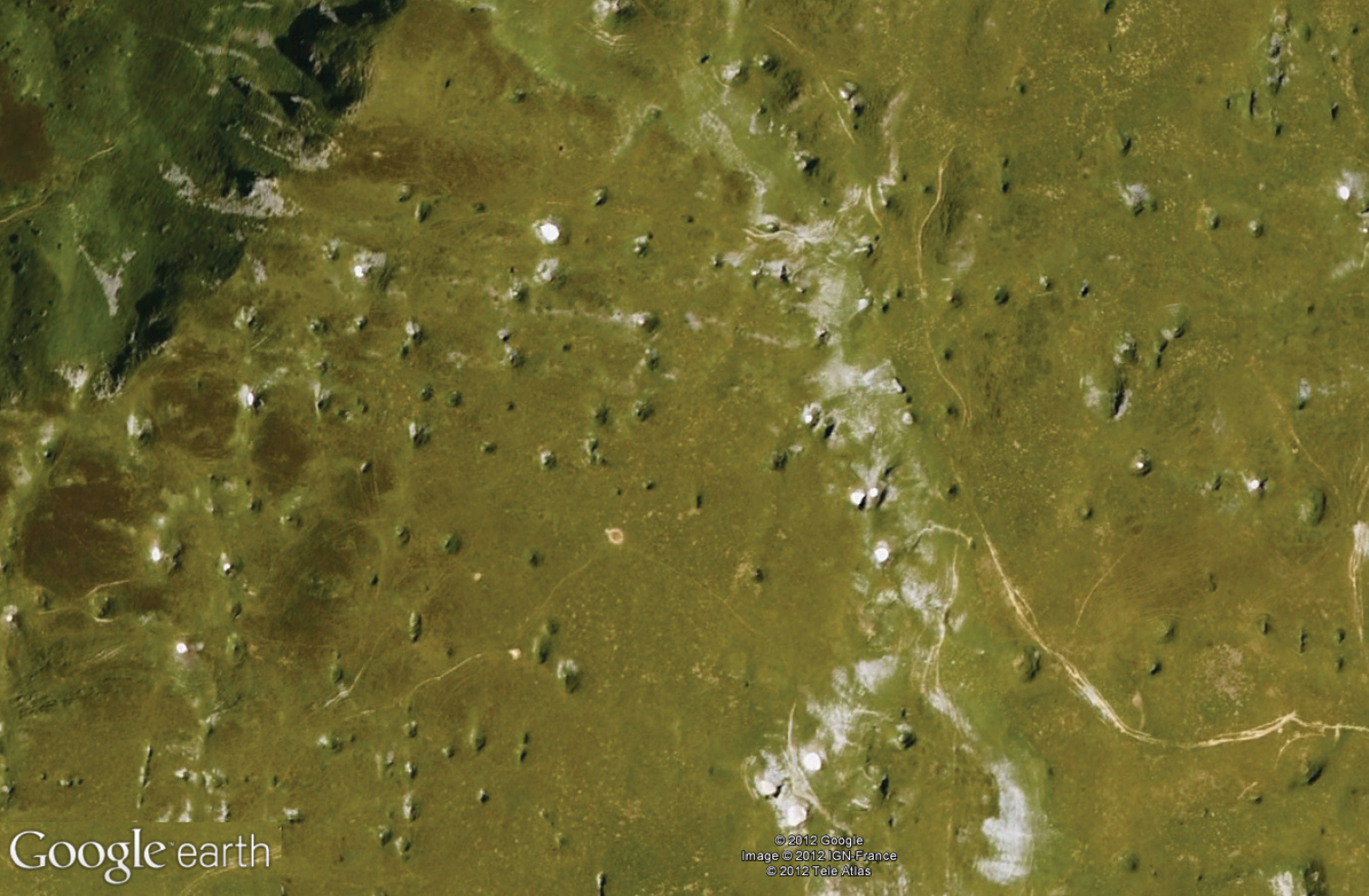
Trechus bouilloni sp. n. is only known from the type locality, the MSS of Lizarraga pass (Navarra, Spain) (Fig. 36). The type locality is a MSS located on a northern slope at the eastern extremity of the Sierra de Andía–Urbasa, close to the Lizarraga pass.
Trechus were collected by means of traps in a zone of scree (altitude: 900 m) extending from east to west at the feet of cliffs of Albian limestone lining the northern slope of the plateau of the Sierra de Andía–Urbasa. This scree slope consists of a mass of fallen rocks resulting from the erosion of calcareous cliffs and constitutes a steeply sloped (45°) MSS, filling one of the numerous gullies of a beech forest covering the entire northern side of the plateau lining the southward depression of the Río Arakil (Sakana valley).
On this unstable ground, beeches are replaced by grassy and mossy vegetation dotted with shrubs. The layer of humus is irregular and very thin and only partly covers the blocks of white, angular, medium–sized limestone, rarely exceeding the size of 1 dm³.
The traps were placed 50 centimeters deep in a “C–type” horizon (sensu
The other Coleoptera collected with Trechus bouilloni sp. n. were Leiodidae, Cholevinae: Catops subfuscus Kellner, 1846, Sciodrepoides watsoni (Spence, 1813) (Catopini) and Bathysciola sp. (Leptodirini).
Some specimens of Trechus bouilloni sp. n. were parasitized by an undetermined Ascomycete.
Trechus bouilloni sp. n. was not found in caves of the area north of Larraona (cueva de los Cristinos, cuevas de Erbeltz, Txintxoleze, Noriturri, Akuandi, del Queso, Iniriturri, Arleze, Laminatitur), suggesting that it is strictly located in MSS (CB personal observation).
«Eaux–Bonnes, M. vom Bruck» (Fairmaire, 1862b). France, Pyrénées–Atlantiques.
Lectotype (MNHN), present designation: 1 ♂, labelled: «oblongulus Bonnes» [white rectangular label (ms, Fairmaire)], «Bruckii» [white rectangular label (ms, Fairmaire)], «MUSEUM PARIS Collection Léon Fairmaire 1906» [white rectangular label (printed)], «TYPE» [red rectangular label (printed)], «Lectotypus / Trechus bruckii Fairmaire / Faille, Bourdeau & / Fresneda des. 2012” [red rectangular label (printed)], genitalia dissected and mounted in a separate label pinned with the specimen. Paralectotype (MNHN): 1 ♀, same label data and pin as lectotype except “Paralectotypus / Trechus bruckii Fairmaire / Faille, Bourdeau & / Fresneda des. 2012” [red rectangular label (printed)].
Lectotype (MNHN), present designation: 1 ♀ (red dot), labelled: “oblongus” [white rectangular label (ms, Fairmaire)], “planiusculus” [white rectangular label (ms, Fairmaire)], “Bruckii” [white rectangular label (ms, Fairmaire)], “2203” [white rectangular label (ms, Fairmaire)], “MUSEUM PARIS Collection Léon Fairmaire 1906” [white rectangular label (printed)], “TYPE” [red rectangular label (printed)], “Lectotypus / Trechus planiusculus Fairmaire / Faille, Bourdeau & / Fresneda des. 2012” [red rectangular label (printed)]. Paralectotypes (MNHN): 1 ♀, same label data and pin as lectotype except «Paralectotypus / Trechus planiusculus Frm / Faille, Bourdeau & / Fresneda des. 2012” [red rectangular label (printed)]; 1 ♂, “H Pyrenees 1856 M. Pandellé” [white rectangular label (printed)], “Bruckii” [white rectangular label (ms, Fairmaire)], “COTYPE” [white and red rectangular label (printed)], “R. Jeannel Brucki Fr” [white rectangular label (ms, Jeannel)], “MUSEUM PARIS coll. R. JEANNEL 1931” [white rectangular label (printed)], “Paralectotypus / Trechus planiusculus Frm / Faille, Bourdeau & / Fresneda des. 2012” [red rectangular label (printed)], genitalia dissected and mounted in a separate label pinned with the specimen.
1 ♀ (MNHN) labelled: “planiusculus” [white rectangular label (ms, Fairmaire ?)], “Bruckii” [white rectangular label (ms, Fairmaire)], “MUSEUM PARIS Collection Léon Fairmaire 1906” [white rectangular label (printed)], “R. Jeannel Brucki Fr” [white rectangular label (ms, Jeannel)]. We do not consider this specimen as a syntype of Trechus planiusculus as it is not labeled « oblongus » as the specimen of the type series, suggesting that the specimen arrived in the Fairmaire collection after the description of planiusculus. A second female specimen (MNHN) labelled: “oblongus Arrens” [white rectangular label (ms, Fairmaire)], “TYPE” [white and red rectangular label (printed)], “MUSEUM PARIS Collection Léon Fairmaire 1906” [white rectangular label (printed)]. This specimen could be the reference specimen of Trechus oblongus Schaum, 1862. Reference of the name comes from
The study of specimens of Trechus brucki pecoudi from Orhy and of numerous exemplars of Trechus brucki, including types of the previously described subspecies of Trechus brucki, demonstrated that none of the characters quoted either by
Trechus politus and Trechus planiusculus were described by Fairmaire in the volume of the Annales de la Société Entomologique de France of 1861 published in 1862 (Fairmaire, 1862b). As the name Trechus politus was already used for an American species (today Trechisibus politus Brullé, 1842),
urn:lsid:zoobank.org:act:030DC2D3-4509-4877-8C21-9D83AA8563B5
http://species-id.net/wiki/Trechus_bruckoides
Figs 6, 13, 25, 26France, Pyrénées Atlantiques, Ossau, Sède de Pan UTM (WGS 84): 30 T, X:704, Y:4768.
Holotype (MNHN): 1 ♂, France, Pyrénées Atlantiques, Ossau, Sède de Pan, labelled: «Ossau, Sède–Pan» [white rectangular label (printed)], «MUSEUM PARIS coll. R. JEANNEL 1931» [white rectangular label (printed)], «R. Jeannel Brucki Fr.» [white rectangular label (ms, Jeannel)], «Holotypus / Trechus bruckoides sp. n. / Faille, Bourdeau & / Fresneda det. 2012” [red rectangular label (printed)], genitalia dissected and mounted in a separate label pinned with the specimen. Paratypes: 1 ♂, “Pic Montagnoü (v. d´Ossau) Mascaraux” [white rectangular label (ms)], “MUSEUM PARIS 1932 coll. Sainte–Claire Deville” [white rectangular label (printed)], “angusticollis Kiesw.” [white rectangular label (ms)] (MNHN); 1 ♂, “Pic Massibe B. PYR. 1938” [white rectangular label (ms)], “Trechus Brucki” [white rectangular label (ms)], “Collection H. Coiffait” [white rectangular label (printed)] (MNHN); 1 ♂, “Bielle/ B. Pyr.” “Trechus brucki/det. Tedeschi” “coll. Tedeschi/ZSM 2009” (ZSM); Pic Montagnon, 15–VII–1979, Bourdeau leg., 6 ♂♂ and 1 ♀ (CAF, CCB, CJF); Sède de Pan, Bielle, VII–1995, Bourdeau leg., 1 ♂ (CCB); Sède de Pan, Bielle, 2–VIII–1980, Bourdeau leg., 3 ♂♂ (CCB); Sède de Pan, Bielle, 10–VII–1981, Bourdeau leg., 1 ♂ and 2 ♀♀ (CCB). All the paratypes with the label “Paratypus / Trechus bruckoides sp. n. / Faille, Bourdeau & / Fresneda det. 2012” [red rectangular label (printed)].
1 ♀, «Pied du pic Lauriolle près Bielle Bas. Pyr. 29.6.37», coll. Bonnaire (MNHN). Sède de Pan, Mascaraux, 2 exx. (coll. Nègre, MNHN). Pic Montagnon: 4 exx. Sède de Pan: 3 exx (MNHN). Sède de Pan, 23–6–1943, 1 ♂, 2 ♀♀ (MNHN, coll. Coiffait). Pic Massibe: VII–1941, 1 ♀ (MNHN, coll.Coiffait).
Large size (ca 4 mm) and round shape (Fig. 6). Median lobe of aedeagus slender, subparallel and decreasing in width from the apical tenth to the apex, which is softly curved in lateral view (Fig. 25), nearly symmetrical and with apex regularly rounded in dorsal view (Fig. 13). Endophallus with an elongate and well-sclerotized piece, forming a twisted gut. Characteristic secondary sclerotization of the sperm duct (Fig 25: CP2) present. External appearance very close to Trechus brucki.
Habitus as in Fig. 6.Elongated, round–sided. Body surface with a very thin, hardly visible, dense microreticulation, no more distinguishable meshes on the head.
Colour. Dorsal surface dark brown, moderately shiny. Antennae, palpi and legs light brown.
Chetotaxy. Surface of elytra glabrous with the exception of a periscutellar seta, two discal setae on the third stria, four humeral setae, four setae along lateral margin and two preapical setae. Marginal setae of pronotum present, the anterior ones located at the first anterior third of the length.
Head. Eyes flat, well–developed, temples smaller than the length of eyes, strongly wrinkled to the neck. Frontal furrows moderately deep. Antennae short (2–2.3mm) and thick.
Pronotum. Proportions (M): WP/LP = 1.3, WP/WPB = 1.35, WP/WH = 1.34, WE/WP = 1.63. Transverse, with lateral margins bordered, wider in anterior part, much less wide than elytra. Posterior part much narrower than base of elytra. One seta in the marginal gutter at about a third of pronotum length, another one just before hind angle. Sides evenly rounded and straight just between hind angles and insertions of posterior setae. Hind angles well developed, right.
Elytra. Proportions (M): WE/LE = 0.64. Subrectangular, broadest after the mid–length; surface moderately convex, flattened on disc. Shoulders distinct but rounded. Striae almost impunctuate, sixth inner discal striae distinct, but reduced at apex and base, especially in callus area; seventh striae shallower, nearly indistinct, the eighth only distinct close to apex of elytra. Apical striola well impressed continuing the fifth stria.
Hind wings. Very reduced, not functional.
Male genitalia. Median lobe of aedeagus slender, in lateral view (Fig 25) basal third curved, central part straight, parallel and elongated towards apex. Nearly symmetrical in dorsal view (Fig 13). Parameres slender, each with 4 setae at tip. Inner sac of aedeagus armed with scales with an elongate well sclerotized piece, forming a twisted gut (Fig 26). Characteristic secondary sclerotization of the sperm duct forming a kind of second copulatory piece out of the base of the median lobe (Fig 25: CP2).
Not examined.
Mean length (4 exemplars): 4.78 mm (male).
The specific epithet refers to Trechus brucki, species with which the new species was merged.
Trechus brucki and Trechus bruckoides sp. n. are externally very similar but strong differences isolate the two taxa especially in shape of male genitalia (Figs 25, 27). The aedeagus shape of Trechus bruckoides sp. n. is exactly as indicated in
Trechus bruckoides sp. n. is only known from the calcareous plateau of Esturou located at 1860 m, north of Montagnon peak (1973 m) and Mailh Massibé (1973 m), at the northern extremity of the massifs separating Aspe and Ossau valleys (Fig. 37). South of this area (Sesques and Gaziès peaks (2600 m)), it is replaced by Trechus brucki which occurs together with Trechus distinctus. During Pleistocene glacial cycles, this plateau was covered by a névé which shaped an area of sinks of nivo–karstic origin (
This mid altitude nivicolous environment could have led to isolation of populations of the species from southern glaciated areas and glacial tongues of the northern slope of Ossau glacier and led to the differentiation of this population of cryophilic and highly hygrophilic Trechus. Such a hypothesis could also explain the presence of the hypogean Trechini Aphaenops bessoni Cabidoche, 1962, endemic to this karstic plateau (pits of Col d’Aran), and closely related to Aphaenops loubensi Jeannel, 1953, an endemic species of the Pierre Saint Martin massif, western to the Aspe Valley. Some other endemic nivicolous Carabidae with morphologically distinct populations occur in the area, like Carabus (Iniopachus) pyrenaeus Audinet–Serville, 1821 (the population of Sède de Pan was first described as a distinct subspecies, Carabus pyrenaeus cephalicus Csiki, 1927), Nebria lafresnayei Audinet–Serville, 1821, Pterostichus (Cryobius) amoenus mascarauxianus Pupier, 2008, Pterostichus (Lianoe) nadari mascarauxi Jeannel, 1928 and Pterostichus (Lianoe) dufourii (Dejean 1828). The peculiarities of this fauna suggest that this restricted area is an important center of diversification.
The molecular phylogeny (Fig. 34) suggests a well–supported clade gathering the following species:
Trechus beusti (Fig. 4), Trechus bouilloni sp. n.(Fig. 1), Trechus grenieri (Fig. 2), Trechus brucki (Fig. 7), Trechus pieltaini (Fig. 5) and Trechus uhagoni (Fig. 3). This result is in accordance with morphology: all the species of the clade share the aedeagal median lobe long and strongly curved just behind basal bulb, with terminal lamella well–developed. Moreover, the clade is supported by a strong synapomorphy: all the species share a strongly sclerotized part of the sperm duct, forming a second copulatory piece (Figs 15, 17, 19, 21, 23, 27: CP2). This synapomorphy is also present in Trechus bruckoides sp. n.(Fig. 25). Consequently, molecular and morphological results allow us to define the Trechus brucki group sensu novo: Trechus beusti, Trechus bouilloni sp. n., Trechus brucki, Trechus bruckoides sp. n., Trechus grenieri, Trechus uhagoni and Trechus pieltaini.
Two species, Trechus pieltaini and Trechus beusti, were included by
Our molecular results as well as genital morphology, in dorsal and lateral view and shape of copulatory piece (Figs 10, 19, 20), suggest that Trechus uhagoni could be considered a distinct species from Trechus grenieri. Morphological differences between the two species are the following:
– Trechus grenieri: aedeagus in dorsal view (Fig. 9) with subparallel sides, round apex with a short triangular tip; in lateral view (Fig. 17) basal third strongly rounded, median lobe slightly angular in the middle; apical hook with a thin tip. The copulatory piece is an asymmetrical gut slightly tapering and filled with a densely scaly area (Fig. 18).
– Trechus uhagoni: aedeagus in dorsal view (Fig. 10) with the left side narrowed or sinuate from the middle to apex, the left side of apical quarter deeply narrowed, forming a long triangular tip; in lateral view (Fig. 19) only the basal quarter rounded, median lobe without dorsal angle in the middle, short with apical hook with massive tip. The copulatory piece is similar to the one of grenieri but the gut is parallel and shortened in its apical part (Fig. 20).
With 7 subspecies recognized in the last catalogues (Moravec et al 2003,
The study of specimens from the whole range of Trechus grenieri including all the subspecies, most of the types and material from intermediate localities (see distribution) leads us to conclude that the characters used to discriminate the subspecies (size, eyes size, shape of elytra and pronotum) are inconstant and overlapping between populations. The shape of the male genitalia is similar for all the populations between Gave de Pau and Ariège valley, including the one (ssp aulaensis) which was said to be different (
Trechus beusti was described by
Trechus brucki and Trechus bruckoides sp. n.do not have the peculiar hooked apex of the median lobe observed in the other species of the clade, but the apex is nevertheless strongly curved (Figs 25, 27).
The case of two further species remains doubtful: Trechus carrilloi was included by its descriptor in the uhagoni group especially because of the structure of the aedeagus, with an apex with an apical hook (Fig. 31). However, the secondary sclerotization of the ejaculatory duct is lacking in this species and it is characterized by a homogenous elytral pubescence which is present in other species of the area (
Finally, Trechus sharpi was included in the Trechus uhagoni group by
The species of the Trechus brucki clade are humicolous (grenieri, uhagoni), orophilous (grenieri, brucki, bruckoidessp. n.), or troglobitic/subterranean (bouilloni sp. n., pieltaini, beusti). We can notice a coincidence in the ecology of Trechini and Leptodirini with Basque–Pyrenean distribution: whereas the species are humicolous (or nivicolous for some Trechini) in the Pyrenees, the species occurring in the Basque country are mainly hypogean (
If we use the standard mitochondrial mutation rate for insects of 2.3% divergence per Myr (0.0115 substitutions ⁄ site ⁄ Myr) (
Strong erosion leading to a deep excavation of Pyrenean valleys associated with climate variations led to the dispersal and diversification of the brucki clade. The main events are (
1. Persistence of the Ebro depression between the Basque–Pyrenean area and the Iberian central plateau. The persistence of the Ebro salty basin from the late Oligocene (25 Ma) until the late Miocene (6 Ma) isolated groups with an Iberian distribution from those with a Pyrenean or Basque–Pyrenean distribution. This flat and shallow lagoon area received the tributaries of the Ebro river, from Reinosa to the Mediterranean Sea.
2. Impact of Quaternary erosion on karst fragmentation. On the northern slope, the folds which have an east–west orientation are narrow and divided by north–south valleys. On the southern slope, orogenesis caused the formation of two folds with an east–west orientation (internal and external “sierras”) parallel to the axial chain. Similarly, Quaternary erosion separated these sierras by narrow north–south valleys. Near the Atlantic, these “sierras” meet with Basque folds which have a complex north–west/south–east orientation, divided by narrow north–south valleys, from Bilbao to Alsasua. Between Vitoria and Pamplona, these Basque “sierras” are separated by the Pre–pyrenean middle depression, a broad valley excavated by the Zadorra (westward) and Arakil (eastward) rivers (Fig 35). These rivers flow into the Ebro Basin, separating the northern massifs of Aralar, Urquilla and Gorbea from the southern Sierra of Urbasa–Andía. The hydrographic system was set mainly by significant erosion due to numerous glaciation cycles during the Pleistocene (2.5 Ma).
Our molecular study suggests that the brucki lineage could have originated in the area delimited by the northern sierras of Gorbea and Urquilla and the edge of the sierra de Andía. The sierras de Andía, Urbasa and Entzia form the exact border between the hypogean fauna of the Pyrenees and Iberia. North of this limit occur Trechus bouilloni sp. n., Troglorites breuili Jeannel, 1919 (Carabidae, Pterostichini) –Urbasa–Andía–Entzia, Aralar, Ernio and Pagoeta massifs, between the Deba and Urola rivers (
Trechus bouilloni sp. n. has a subterranean lifestyle among the scree–covered northern slope (900 m) of Sierra de Andía, whereas the type locality of Trechus uhagoni is the Orobe doline (700 m), located at the eastern limit of Sierra de Urquilla. Early Pleistocene climate variations could have led to drastic changes in biome composition, limiting dispersal possibilities and leading to the isolation of the population of Trechus bouilloni sp. n. (potentially forestal), south of the Arakil River. One hypothesis could be that the hygrophilous species were colonizing high altitude or hypogean habitats during interglaciar warming as observed in other species of Coleoptera (
Trechus brucki lives in the alpine zone (above 1700 m) of the axial ridge from Pic d´Orhy to Col du Pourtalet, in the high Ossau Valley. On the north ridge, Trechus brucki can be encountered in the same biotopes, near snow tongues melting on scree-covered slopes, from Aspe to Gave de Pau Valleys. As for Trechus grenieri and Trechus uhagoni, both are mainly forestal and occur at lower altitude except for Trechus grenieri in the eastern part of the range (Mount Valier area). The Ariège Valley is the eastern limit of the group.
The distribution area of the Trechus brucki group coincides with the one of the Basque–Pyrenean Leptodirini clade (Fig 35). In the Pyrenees, both groups are made up of forestal, endogean, humicolous, lapidicolous or orophilous, but not hypogean, species. It is only in Basque relief, the western part of their distribution, that both groups include subterranean species. Regarding Leptodirini, the basal group of the Basque–Pyrenean clade is the Bathysciola schiodtei group (endogean/humicolous elements); its distribution area is extended from Ariège, Trechus mystica Fresneda & Fery, 2009 (France: Haute–Garonne and Ariège; Spain: Val d’Aran) to the Basque relief, Bathysciola breuili Bolívar, 1921 in Peña Gorbea or Bathysciola rugosa (Sharp, 1873) in Sierra de Urbasa and Urquilla. A high degree of troglobiomorphy is only found in some hypogean species of the Basque area: Aranzadiella Español, 1972 (basin of Deba River), Euryspeonomus Jeannel, 1919 (Aralar, Urbasa/Andía and Baztan Valley), Josettekia Bellés & Déliot, 1983 (Ernio and Aralar massifs), Nafarroa Fresneda & Dupré, 2010 (Kintoa Massif) and Speocharidius Jeannel, 1919 (between the Urola and Orio Rivers). In the Pyrenees, the species of the Trechus brucki clade are epigean, forestal (Trechus grenieri, Trechus uhagoni ruteri) or orophilous(Trechus brucki, Trechus bruckoides sp. n.). Pyrenean speciation events in the group are more recent and are probably closely related to late Pleistocene climatic changes, as already observed in alpine Trechus (
Habitus of Trechus bouilloni sp. n. (Lizarraga pass).
Habitus of 2 Trechus grenieri (grotte de l´Eglise) and 3 Trechus uhagoni (Orobe doline).
Habitus of 4 Trechus beusti (Cueva de San Adrián) and 5 Trechus pieltaini (Cueva de Mairuelegorreta).
Habitus of 6 Trechus bruckoides sp. n. (Montagnon) and 7 Trechus brucki (Jaout).
Aedeagus in dorsal view of 8 Trechus bouilloni sp. n. (Lizarraga pass) 9 Trechus grenieri (Lapiaz de Lazur) and 10 Trechus uhagoni (Orobe doline). CP2, secondary copulatory piece.
Aedeagus in dorsal view of 11 Trechus beusti (Cueva de San Adrián) 12 Trechus pieltaini (Cueva de Mairuelegorreta) 13 Trechus bruckoides sp. n. (Montagnon) and 14 Trechus brucki (Lac d’Anglas). CP2, secondary copulatory piece.
Aedeagus in lateral view and detail of internal sac of 15, 16 Trechus bouilloni sp. n. (Lizarraga pass) 17, 18 Trechus grenieri (Eglise cave) and 19, 20 Trechus uhagoni (Orobe doline). CP2, secondary copulatory piece.
Aedeagus in lateral view and detail of internal sac of 21, 22 Trechus beusti (Cueva de San Adrián), 23, 24. Trechus pieltaini (Cueva de Mairuelegorreta), 25, 26 Trechus bruckoides sp. n. (Montagnon) and 27, 28 Trechus brucki (Lac d’Anglas). CP2, secondary copulatory piece.
29 Genital armature of the female of Trechus bouilloni sp. n. (Lizarraga pass) 30 Aedeagus in lateral view of Trechus sharpi (Cueva la Cuevona) 31, 32 Aedeagus in dorsal and lateral view of Trechus carrilloi (Bosque de Saja).
Lectotype and paralectotype of Trechus brucki.
Phylogram of Trechus of the brucki group obtained in RAxML, using the combined data matrix. Number in nodes: ML bootstrap (>50%) (see Material and Methods for details). In blue, the Trechus brucki group sensu novo. In purple, Trechus bordei group. In red: Trechus bouilloni sp. n.
Distribution map of Trechus brucki group and related species. Material studied: symbols with cross.
The MSS of Lizarraga pass (Navarra, Spain).
Sinkhole area of the Plateau of Esturou (Hautes–Pyrénées, France).
We thank the collectors mentioned in Table 1 for providing material, M. Toribio and J.M. Salgado for the loan of material, I. Ribera and two referees for helping with their comments, T. Deuve and A. Taghavian (MNHN) for the loan of types and specimens of the T. brucki group and M. Balke (ZSM) who let us study the material of Trechini from the ZSM.
We are grateful to B. Jaeger (Museum für Naturkunde, Berlin) for providing information on the material from the Schaufuss collection and to J. Judge (University of California, Berkeley) for the linguistic revision of the manuscript. AF was supported by a postdoctoral Research Fellowship from the Alexander von Humboldt Foundation.
Distribution of the Trechus brucki group sensu novo: Material studied and bibliographic records (Fig. 35, map). Material not studied indicated by (!)
Trechus brucki group sensu novoTrechus beusti Schaufuss, 1863
Anophthalmus beusti Schaufuss 1863: 149
Spain: Guipúzcoa: 1. Zegama, Cueva de San Adrián (Schaufuss 1863; Bolívar y Pieltain and Jeannel 1921; Jeannel 1921, 1927; Jeanne and Zaballos 1986; Zaballos and Jeanne 1994; Serrano 2003; Ortuño and Marcos 2003); 4–VIII–2009, Fresneda leg., 1 ♀, voucher number label “ZSM_L199” (ZSM); 14–VIII–2009, Bourdeau and Fresneda leg., 1 ♂, voucher number label “ZSM_L200” (CAF); 31–XII–2009, Bourdeau leg., 4 exx. (CCB); 18–IV–2010, Bourdeau leg., 1 ex. (CCB); 2–V–2011, Bourdeau leg., 1 ♂ and 1 ♀ (CJF), 5 exx. (CCB). 2. Oñate, cave Iritegui (= Integui) (!) (Serrano 2003; Ortuño and Marcos 2003). 3. Oñate, cave Tortuga (!) (Serrano 2003; Ortuño and Marcos 2003).
Trechus bouilloni Faille, Bourdeau & Fresneda, sp. n.
See results
Trechus bruckoides Faille, Bourdeau & Fresneda, sp. n.
See results
Trechus brucki Fairmaire, 1862
Trechus brucki Fairmaire, 1862a: 548
Trechus politus Fairmaire, 1862b (nec politus Brullé, 1842): 578
Trechus planiusculus Fairmaire, 1862b (nec planiusculus Costa, 1858): 578
Trechus brucki microthorax Coiffait, 1952: 190
Trechus brucki pecoudi Colas & Gaudin, 1935: 248 n. syn.
Trechus brucki truilheti Coiffait, 1952: 189
Trechus brucki vandeli Coiffait, 1952: 189
France: Pyrénées–Atlantiques: 1. Val d’Ossau, les Eaux Bonnes (Fairmaire 1862a; Jeannel 1927, 1941). 2. Sommet du Pic d’Orhy, 1900 m (ssp. pecoudi, Colas and Gaudin 1935; Zaballos and Jeanne 1994; in Spain, Navarra, Macizo de Orhí after Serrano 2003); Pic d’Orhy, 5–1925, 1 ♂ and 1 ♀, coll. Nègre (MNHN); Basses Pyrénées, pentes du Pic d’Orry, 29–V–1936, G. Tempère / pierres, 1900–2000 m, 1 ex., coll. Nègre (MNHN), 1 ♂, coll. Coiffait (MNHN). 3. Pic de Jaout, dans la zone subalpine, vers 1500 m (!) (Jeannel 1941, Bonadona 1971). 4. Col de Mahourat, au fond de la vallée d´Ossau, près du col du Pourtalet, 1 ♀ (Coiffait 1952); Col du Pourtalet, Basses Pyr, pic de Maourat, alt 2000 m N°776, 28–9–1959, M. LAVIT (Holotype of Trechus brucki truilheti, coll. Coiffait, MNHN), 1 ♂ (Paratype of Trechus brucki truilheti, coll. Coiffait, MNHN). 5. Sesques, Lac Isabe, 10–8–1979, 8 exx., Bourdeau leg (CCB). Laruns, Pic de Sesques, bord de névé, 2300 m, 30–VI–2011, 1 ♀, voucher number label “ZSM–L446”, Bourdeau leg. (CAF). 6. Lescun, 1 ♂ (Coiffait 1952, Bonadona 1971). 7. Pic d´Anie, 1 ♀ (Coiffait 1952); Pic d´Anie, 1 ♀ bois de Braca, 15–VI–1951, Coll Coiffait (Holotype of Trechus brucki vandeli, coll. Coiffait, MNHN). 8. Pic d’Arlas, 1800 à 2000 m (!) (Jeanne 1984). 9. Col de la Pierre Saint Martin (!) (Jeanne 1984, in Spain after Zaballos and Jeanne 1994 and Serrano 2003). 10. Cirque d´Azuns, 1800 m (!) (Jeanne 1984). 11. Gourette, Lac d’Anglas, 20–8–2000, 6 exx., Bourdeau leg. (CCB). 12. Jaout, 15–8–1989, 1 ♂, Bourdeau leg. (CCB); 20–VI–1996, 1 ex., Bourdeau leg. (CCB); VIII–1981, 8 exx., Bourdeau leg. (CCB). 13. Col du Pourtalet, VI–2007, 3 exx., Bourdeau leg. (CCB). Pic d´Anéou, 2000 m, du col du Pourtalet au col d´Anéou, 2000 à 2100 m (Jeanne 1984). Pourtalet, Caperan d´Anéou, 5–VII–2011, 1 ♂, voucher number label “ZSM–L449”, 1 ♀, Bourdeau leg. (CAF). 7–1959, 2 exx, J. Aubry leg (coll. Coiffait, MNHN). 14. Pène Blanque, 2500 m (!) (Jeanne 1984). 15. Ossau, Pic de Gaziès, VII–2009, 1 ♀, voucher number label “ZSM–L190”, Bourdeau leg. (CAF). Hautes–Pyrénées: 16. Arrens, Pic du Gabizos, 2000 m, 10–VII–2010, 1 ♂, voucher number label “ZSM–L329”, 1 ♀, voucher number label “ZSM–L329bis”, Bourdeau leg. (CAF, ZSM), 1 ♀ (CAF). 17. Pic Granquet, ♂♂ et ♀♀ (Coiffait 1952); Pic Granquet, 1600 m, 5–9–1943, 8 exx. (Holotype and Paratypes of Trechus brucki microthorax, coll. Coiffait, MNHN); 1 ex, cotype, 1600 m, 5–9–1943, coll. Nègre (MNHN). Soum de Granquet, Lac d´Ourrec, en haute vallée de l´Esponne (!) (Queinnec and Ollivier 2011).
Spain: Huesca: 18. Candanchú (!) (Zaballos and Jeanne 1994). After Serrano (2003) in Spain, «Lérida» (mistake), Macizo de Aneu (!).
Trechus grenieri Pandellé, 1867
Trechus grenieri Pandellé, 1867: 147
Trechus bepmalei Jeannel, 1921: 176
Trechus despaxi Jeannel, 1922: 341
Trechus uhagoni prepyrenaeus Coiffait, 1974: 24
Trechus uhagoni aulaensis Aubry, 1981: 251
France: Hautes–Pyrénées: 1. Gazost, 1200 m, au bord des ruisseaux en forêt (Pandellé 1867; Jeannel 1927, 1941, Bonadona 1971), 1 ♀, coll. Fairmaire 1906 (MNHN). 2. Val de Lesponne, sous les amas de feuilles mortes en bordure immédiate des ruisseaux (!) (Bonadona 1971). 3. Barèges, 1 ♂, ex coll. Jeannel ex coll. Castelnau (MNHN). 4. Fréchet–Aure, résurgence de la Hèche, 14–V–2008, 1 ♂, voucher number label “ZSM–L13”, 1 ♀, Besson, Bourdeau & Faille leg. (CAF). 5. Nistos, Bas–Nistos, Grotte de l’Eglise (Bonadona 1971, Corbaz & Jauzion 1988); 2–III–1980, 2 exx., Bourdeau leg (CCB); 27–V–1945: 1 ♂, 17–VI–1945: 1 ♂, coll. Fourès (MNHN); 1 ♂, M. Bouillon rec. (CAF); VI–46, 4 exx., Bourgoin (MNHN); 15–XII–45, 3 exx., Bourgoin Colas (MNHN); 28 exx. (MNHN, coll. Coiffait). 6. Doline de la Bayelle de Gazave, 6 exx. (MNHN). Haute–Garonne: 7. Saint–Béat, Cap de Tus, 1200 m, près de la fontaine ferrugineuse au–dessus du col de Couret (Jeannel 1922, 1927, 1941, Bonadona 1971); été 1922, R. Despax, 3 exx., coll. R. Jeannel (MNHN). Saint Béat, Août 1922, 1 ex., coll. Despax in coll. Nègre (MNHN). 8. Boutx, forêt de Mourtis (Jeannel 1927, 1941, Bonadona 1971). Ft de Mourtis, St Béat, 1450 m, VIII–1926, 9 exx., coll. R. Jeannel, 1931 (MNHN). Ft de Mourtis, 5 exx., coll. Nègre (MNHN). 9. Arbas, 4–8–1980, 9 exx., Bourdeau leg. (CCB). 10. Val d’Espingo, 1800 m, au–dessus du lac d’Ôo (!) (Jeannel 1921, 1927, 1941). 11. Haute vallée du Lys, Superbagnères (Jeannel 1941, Bonadona 1971). Station de Superbagnères, 1650–1700 m, 3–X–1929, 1 ♂, Jeannel (MNHN). « Station de Superbagnères/ pierres 1700 m, 3 oct 1929 », 1 ♂, Gadeau de Kerville, coll. Nègre (MNHN). Ariège: 12. Rimont, Maison forestière, VII–1962 (Trechus uhagoni prepyrenaeus, Holotype ♂, coll. Coiffait MNHN). 13. Forêt d´Andronne, Le Bosc, vers 1000 m, 1 ex., 2 ♂♂ and 4 ♀♀ (Coiffait 1974). IV–1961, 2 ♂♂, Trechus uhagoni prepyrenaeus, paratypes; XI–1961, 3 ♀♀, paratypes (MNHN, coll. Coiffait). 14. Riverenert, vers 1100 m sur le versant Nord du col de la Crouzette, 2 ♀♀ (Coiffait 1974). La Crouzette, Sentenac de Serou, IX–1960, 2 ♀♀, coll. Coiffait (MNHN). 15. Lapiaz de Lazur, flanc NE du Mont Valier, VII–1978, 2 exx., Bourdeau leg. (CCB). 16. Port d’Aula, 2200 m (!) (Aubry 1981). Spain: Lérida: Port d’Aula (!) (Zaballos and Jeanne 1994; Serrano 2003).
Trechus pieltaini Jeannel, 1920
Trechus pieltaini Jeannel, 1920: 155
Spain: Álava: 1. massif of Gorbea (Serrano 2003), Cueva de Mairuelegorreta (Jeannel 1920, 1927, Bolívar y Pieltain and Jeannel 1921, Ortuño and Marcos 2003); 22–V–2011, 1 ♀, voucher number label “ZSM–L395”, Bourdeau leg. (CAF). 22–VII–2011, 4 ♂♂ and 4 ♀♀, Bourdeau leg. (CJF, CAF). 9.IV.1977, 2 exx., Garde leg. (coll. Lagar). 2. Cueva del Manantial (!) (Bolívar y Pieltain and Jeannel 1921). 3. Cueva de Arcegui (Monte Gorbea 1000 m, 30TWNI1963), T.M. Zuya–Zuia (!) (Ortuño and Marcos 2003). 4. Cueva de Sogusti–2 (Monte Gorbea 1000 m, 30TWNI1963), T.M. Zigoitia (!) (Ortuño and Marcos 2003). Vizcaya: 5. Cueva del Polvorino (= Polvorón) de Elorrea, Ceánuri en el macizo de Gorbea, a 1050 m sobre el nivel del mar (Español 1965, Zaballos and Jeanne 1994); 21–IX–1962, 1 ♀, Nolte leg., coll. Daffner (ZSM). 6. Ceánuri, Sima A–S–109 (!) (Zaballos and Jeanne 1994).
“Hisp”, “Alte Sammlung”, 3 ♂♂, 1 ♀ (ZSM). We found in the ZSM collection a specimen labelled “Asturien, collection Strasser” which is most probably an erroneous locality.
Trechus uhagoni Crotch, 1869
Trechus uhagoni Crotch, 1869: 14
Spain: Navarra: 1. Zegama, Alsasua (Crotch 1869) Cueva de Orobe (Jeannel 1927; Español 1965, Zaballos and Jeanne 1994; Serrano 2003); 1–VI–2004, 7 exx., Bourdeau leg. (CCB); 13–VII–2004, 1 ♂, voucher number label “MNHN–AF102”, Bourdeau leg. (MNHN), 1 ♀ (CAF); 2–V–2009, 3 exx. (CJF, CAF); 1 ♂, voucher number label “ZSM–L161” Bourdeau and Fresneda leg. (CAF).
We found 4 specimens labelled «Andara Escalera» (MNHN). A locality called Endara exists in SE of Oiartzun (Guipúzcoa). We cannot exclude that the species also occurs in this area. It is also quoted in Guipúzcoa, Macizo de Izarraitz, Ekain (Galán 2003) but the specific attribution of these Trechus should be verified.
Trechus uhagoni ruteri Colas, 1935
Trechus uhagoni ruteri Colas, 1935: 253
France: Pyrénées–Atlantiques: 1. Larrau, Cañon d´Holçarté, pont d´Amuby, 7–1934, G. Colas (Holotype, aedeagus not with the specimen (MNHN) (Colas and Gaudin 1935, Jeannel 1941, Bonadona 1971). 2. Larrau, Bois de Saint Joseph (!) (Jeanne 1984). 3. Forêt d´Iraty (Jeanne 1984); Iraty, 21–IX–1949, 6 exx., H. Coiffait (MNHN, coll. Coiffait). 4. Col de Bentarté, près du Mont Urculo, 5–1925, 4 ♂♂ and 1 ♀♀, coll. Jeannel (MNHN) (Jeannel 1941, Bonadona 1971, Jeanne 1984).
Spain: Navarra: 5. Aoiz, Acueducto de Orbaiceta (Español 1977). 6. Orbaiceta, Bosque de Irati (Jeanne and Zaballos 1986, Zaballos and Jeanne 1994). 3. Espinal, puerto de Ibañeta (Zaballos and Jeanne 1994). 7. Col de Roncevaux, juillet 1934, 2 ♀♀, L. & A. Gaudin (MNHN). 5–1925, 2 exx. (Coll Nègre in MNHN). 8. Entrada C. de Espinal, C. Bolívar, 2 ♂♂ (MNHN). 6. Peña Escaori, 1600 m, 5–1925, 1 ♂, Gaudin (MNHN).
Species of uncertain phylogenetic affinities
Trechus carrilloi Toribio & Rodríguez, 1997
Trechus (Trechus) carrilloi Toribio & Rodríguez, 1997: 283
Spain: Cantabria: 1. Campoo de Cabuérniga, Bosque de Saja, UTM 30TUN967728 (Toribio and Rodríguez 1997; Serrano 2003); type series (!) (Toribio and Rodríguez 1997): Holotype ♂, 23–VIII–1997, M. Toribio leg. (CMT); paratypes (CMT, MNCN, col. Carabajal, col. García and col. Rodríguez): 4–X–1997, 11 ♂♂ and 9 ♀♀, J. García leg.; 4–X–1997, 3 ♂♂ and 4 ♀♀, F. Rodríguez leg.; 31–X–1997, 2 ♂♂, M. Toribio leg.; 31–X–1997, 2 ♂♂ and 2 ♀♀, F. Rodríguez leg.; 21–XI–1998, 2 ♂♂, F. Rodríguez leg. Same locality, 27–VIII–2001, 1 ♂, Toribio leg. (CJF).
Trechus sharpi Jeannel, 1921
Trechus sharpi Jeannel, 1921: 179
Spain: Cantabria: 1. Santander, Población, sur le mont Hijedo, au sud–est de Reinosa (Jeannel 1921, 1927; Zaballos and Jeanne 1994). 2. Santander, Collection Strasser, 1 ♂ (ZSM). 3. Puerto de San Glorio (Zaballos and Jeanne 1994). 4. Pico Tres Mares (Zaballos and Jeanne 1994). 5. Montes cantábricos orientales (Serrano 2003). 6. Sejo (T.M.Valdaliga), Cva. La Mina, 20–XII–2003, 2 ♂♂, C.G. Luque leg. (Col. Salgado). 7. Quijas, Cueva la Cuevona, 13–IX–1995, 1 ♂, C.G. Luque leg. (Col. Salgado). 8. “Trechus cantaber”, Monts Cantabriques, Sierra de la Sagra, 3 exx., coll. de la Cruz in coll. Coiffait (MNHN).