(C) 2011 Susan E. Johnson. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Reproductive or neotenic soldiers of the Archotermopsid Zootermopsis nevadensis nevadensis (Hagen) are compared to sterile soldiers and primary male reproductives. Several head capsule morphometrics correlate significantly with gonad size across all forms and both sexes of soldiers. The easily observed field character of ratio of mandible length to labrum length is a consistent and reliable feature of head capsule external morphology for predicting gonad development and reproductive potential of soldier forms regardless of age, sex, or live weight.
evolution of soldier caste, reproductive soldier, neotenic soldier, Zootermopsis, morphometrics
Soldiers are a non-reproductive defensive caste in termites (though they may sometimes have other roles (
Because reproductive soldiers occur only in the most socially and developmentally primitive termites, they are considered probable evolutionary relicts of an early form of soldiers: a stepping-stone toward obligatory sterility and altruistic defense (
Reproductive soldiers, while possessing the generalized soldier form, typically have differences in external morphology that distinguish them from normal soldiers including a slightly rounder head shape and more curved mandibles (
Here we compare external morphology and internal gonad development in normal and neotenic soldiers of Zootermopsis nevadensis nevadensis (Thorne & Haverty, 1989) and in new kings and mature kings. Using measurements of several external features as well as gonad dimensions, differences are quantified between normal soldiers and reproductive soldiers (referred to collectively henceforth as “soldier morphs”). Ratios of these measurements (used to normalize the expected differences due to size and age of individuals) are analyzed for predictive value (
Kings were removed from 64 king and queen right colonies to stimulate production of male replacement reproductives. Colonies were outbred, initiated by alate pairs that emerged from wild colonies collected near Placerville, CA (El Dorado County). At 2 wk intervals beginning 6 wks post king removal colonies were examined for replacement (neotenic) reproductive soldiers. Twelve new male reproductive soldiers were weighed live then individually preserved in Pampel’s fixative (composed of 2 - 4 parts glacial acetic acid , 15 parts 95% ethyl alcohol, 30 parts distilled water, 6 parts formalin (40% formaldehyde in water): BioQuip Products). Ten mature (molted to soldier morph at least 3 months previously) male reproductive soldiers and ten mature normal/sterile (molted to soldier morph at least 3 months previously) male soldiers from similar sized colonies were also weighed live and individually preserved. After 24 h in fixative external characters (width of head capsule, length of head capsule, length of left mandible from condyle to apex, length of labrum, width of labrum) of specimens were measured using an eyepiece-mounted micrometer on a Leica MZ MPO dissecting microscope. Each termite was then pinned to a paraffin-filled Petri dish and a longitudinal incision was made on the dorsal side. The open body cavity was flooded with a solution of Nile Blue dye and water then rinsed with 70% ethanol after several seconds leaving enough ethanol to partially cover the specimen. Widest and narrowest diameter of the left testis was measured. Testis width subsequently refers to the widest measurement of the left testis for specimens of known age and weight.
Characterization of dealate and mature male primary reproductivesNewly sclerotized male alates (new kings) were individually isolated with a 2 cm square of moistened paper towel until they shed their wings. After wing abscission they were weighed, preserved in Pampel’s fixative and analyzed as above. A subsample of the kings removed from colonies to generate secondary reproductives were also weighed, preserved and analyzed as above. All these kings were at least two years old.
Characterization of archived specimens of reproductive and normal soldiersIn addition to the production of known age reproductive soldiers, previously collected individuals were classified as “normal soldiers” (n = 144; 84 male, 60 female) or “reproductive soldiers” (n = 47; 38 male, 9 female) based upon external morphology and observed colony role. The majority (192) were from outcrossed laboratory colonies, which were bred from alates maturing in colonies initiated by alate pairs that emerged from wild colonies near Placerville, CA (El Dorado County). Ten individuals developed in and were collected directly from the field-collected stock colonies. Sixteen were collected directly from the field in October 2007 from Eldorado National Forest (El Dorado County, CA). The field collected individuals were preserved in ethanol without fixative, 79 were fixed in Bouin’s solution (composed of 37% formaldehyde (24% by weight), picric acid (71%), and glacial acetic acid (5%): BBC Biochemical Corporation) for at least an hour before transfer to 80% ethanol, and 115 were fixed and stored in Pampel’s. Eight had previously been preserved in an unidentified fixative (ethanol and/or Pampel’s).
The external measurements of each soldier morph individual included: dorsal width of head capsule at widest point, dorsal length of head capsule without mandibles from the posterior margin to the base of the labrum, length of left mandible from condyle to apex, length of labrum, width of labrum, wingbud length (if present), width of postmentum at narrowest point, width of postmentum at widest point, length of postmentum, length of eye, and width of eye. Sex was recorded as well. After external measurements were completed the following measurements were taken: females—width of ovary at midpoint, width of ovary at widest point, length of ovary from tip to base of posterior ovariole, number of eggs; males—widest diameter of testis, narrowest diameter of testis. Ratios of head, labrum, and testis lengths and widths were made for each individual as a measurement of roundness. The ratio of the mandible length to the labrum length was also calculated. These morphometrics were suggested as useful differentiating characteristics for soldier morphs by laboratory experience and the published anatomical work on the reproductive system of Zootermopsis nevadensis nevadensis and other termites by
All data were analyzed using SAS 9.1 for Windows (Correlation Analysis, MANOVA, ANOVA). Results were considered significant at the 0.05 level. Correlations were univariate, and thus may result in an overall type I error rate greater than the pair-wise rate of 0.05.
Results Specimens of known age and live weightContrasting live weight of normal soldiers to reproductive soldiers and kings
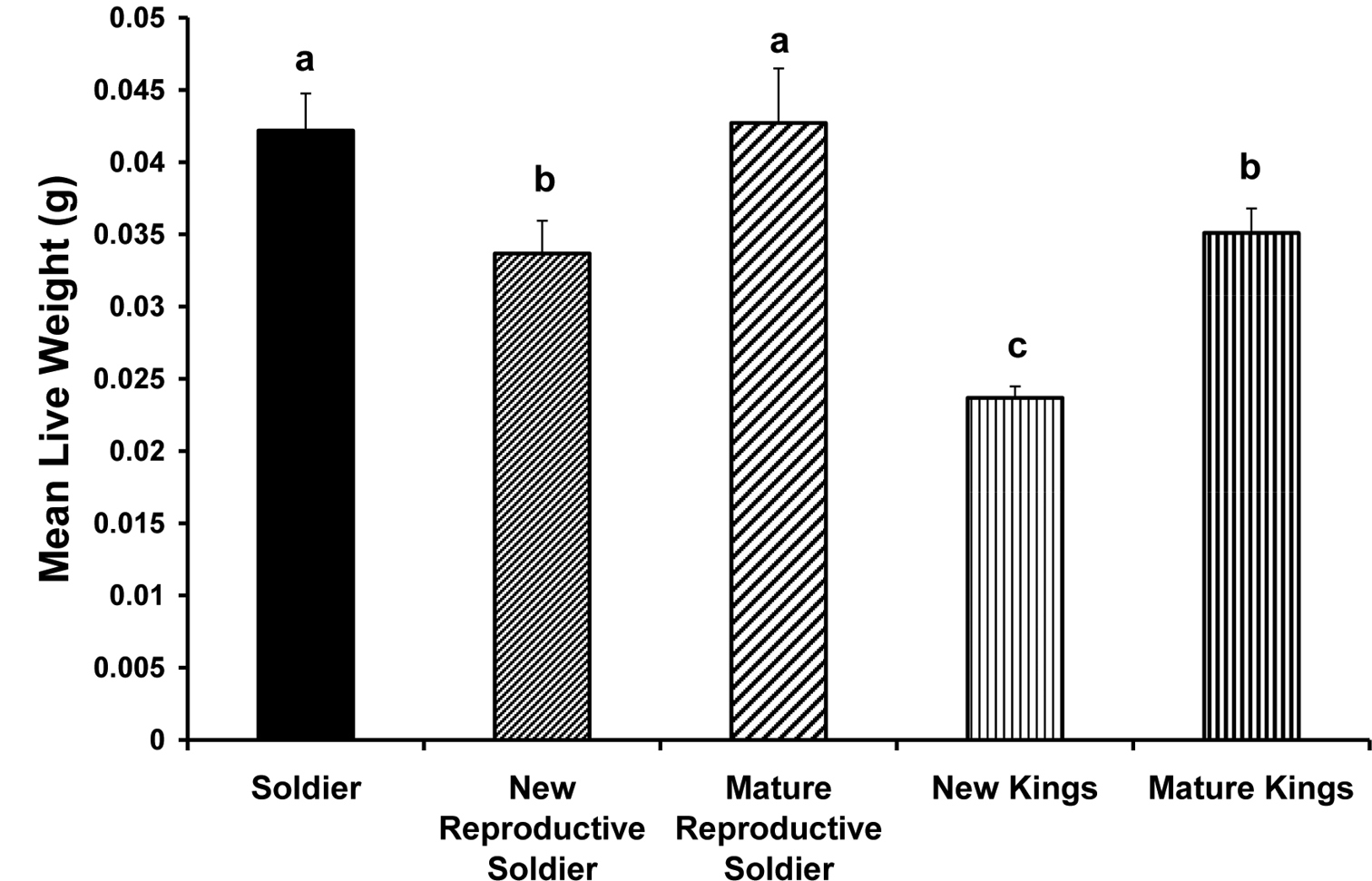
Newly differentiated male reproductive soldiers were smaller (mg live weight) than either normal sterile soldiers or mature reproductive soldiers (p < 0.0001) but not different from mature kings. Normal sterile soldiers and mature reproductive soldiers did not differ in weight. Dealate kings were smaller than the other four morphs/castes (p < 0.0001) (Fig. 1).
Comparison of means for live weight (g) of male soldiers, reproductive soldiers and primary reproductives (new and mature kings). Means with the same letter are not significantly different. Error bars indicate standard errors.
Contrasting external morphology and gonad size of normal and reproductive soldiers
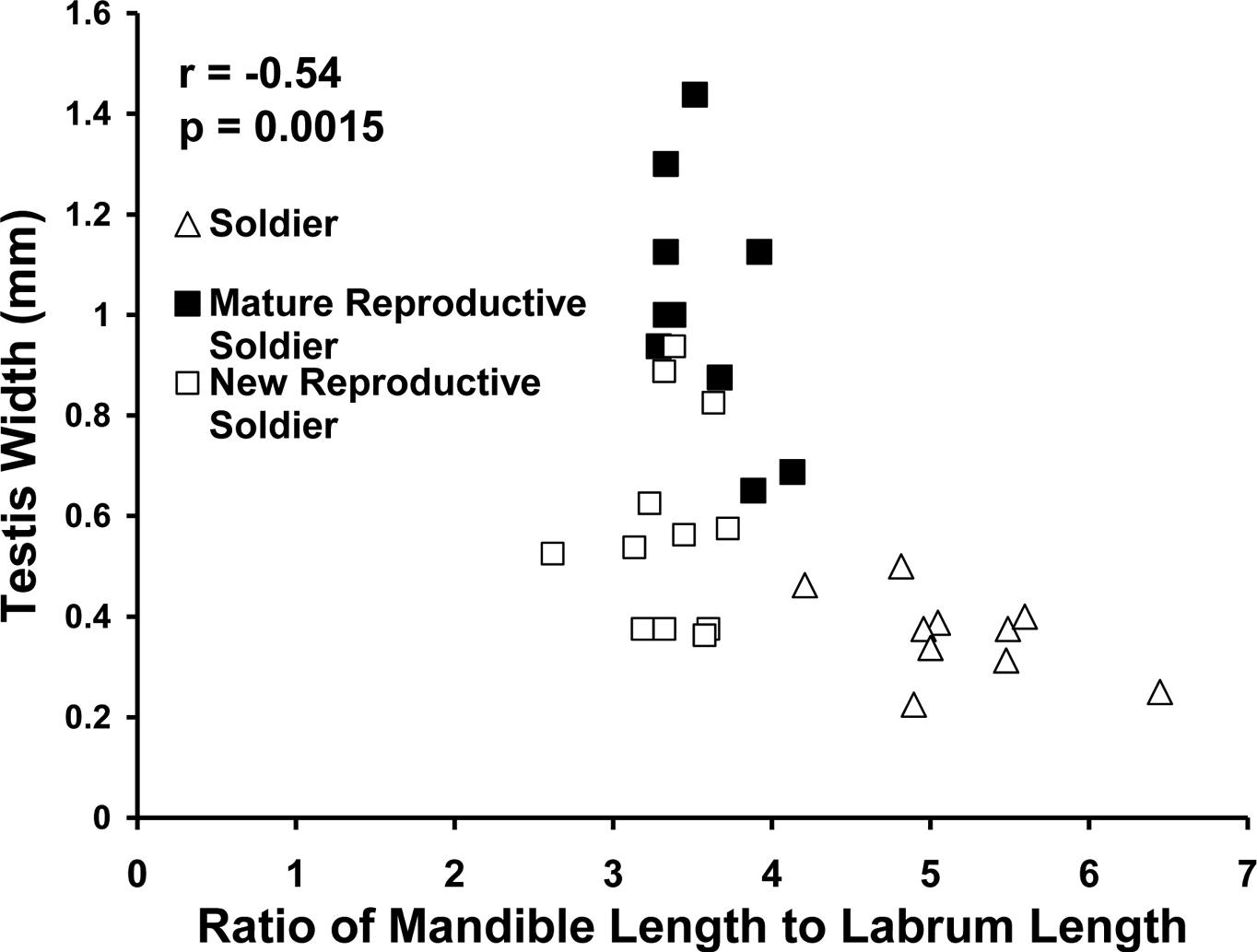
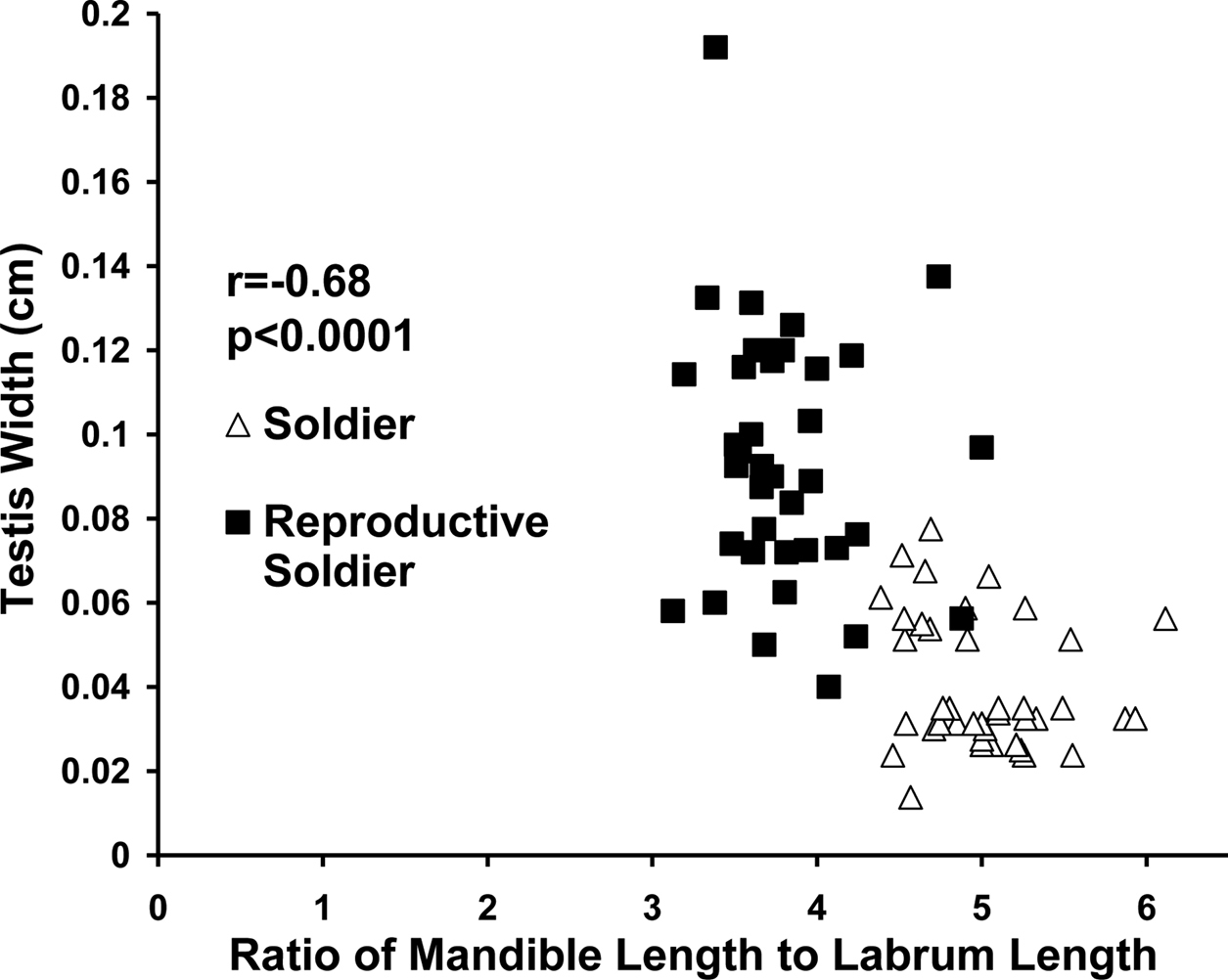
The ratio of labrum length to left mandible length distinguished sterile from reproductive soldier morphs (p < 0.0001). Reproductive soldiers had larger testes than sterile soldiers regardless of live weight or age of reproductive soldier (p < 0.0001). Testis width correlated with the ratio of left mandible length to labrum length (p = 0.0015) (Fig. 2).
Correlation between testis width and ratio of mandible length to labrum length in male soldiers and new and mature reproductive soldiers.
Age effects on gonad development
Testes (width) in recently eclosed reproductive soldiers were larger than mature sterile soldiers but smaller than mature reproductive soldiers (p < 0.0001). Dealate kings testes’ were equivalent in width to sterile soldiers’, while mature kings’ testes were equivalent to newly differentiated reproductive soldiers (Fig. 3).
Comparison of means for testis width (mm) of male soldiers, reproductive soldiers and new and mature kings. Means with the same letter are not significantly different. Error bars indicate standard errors.
Correlation of live weight and testis width
There was no correlation between live weight and testis width in sterile soldiers (p = 0.2952), new reproductive soldiers (p = 0.8225), mature reproductive soldiers (p = 0.0639) or new kings (p = 0.3071). Mature kings (n = 21) testis width and live weight correlated positively (p = 0.0448).
Specimens of unknown age and live weightMorphological differences between soldiers and reproductive soldiers
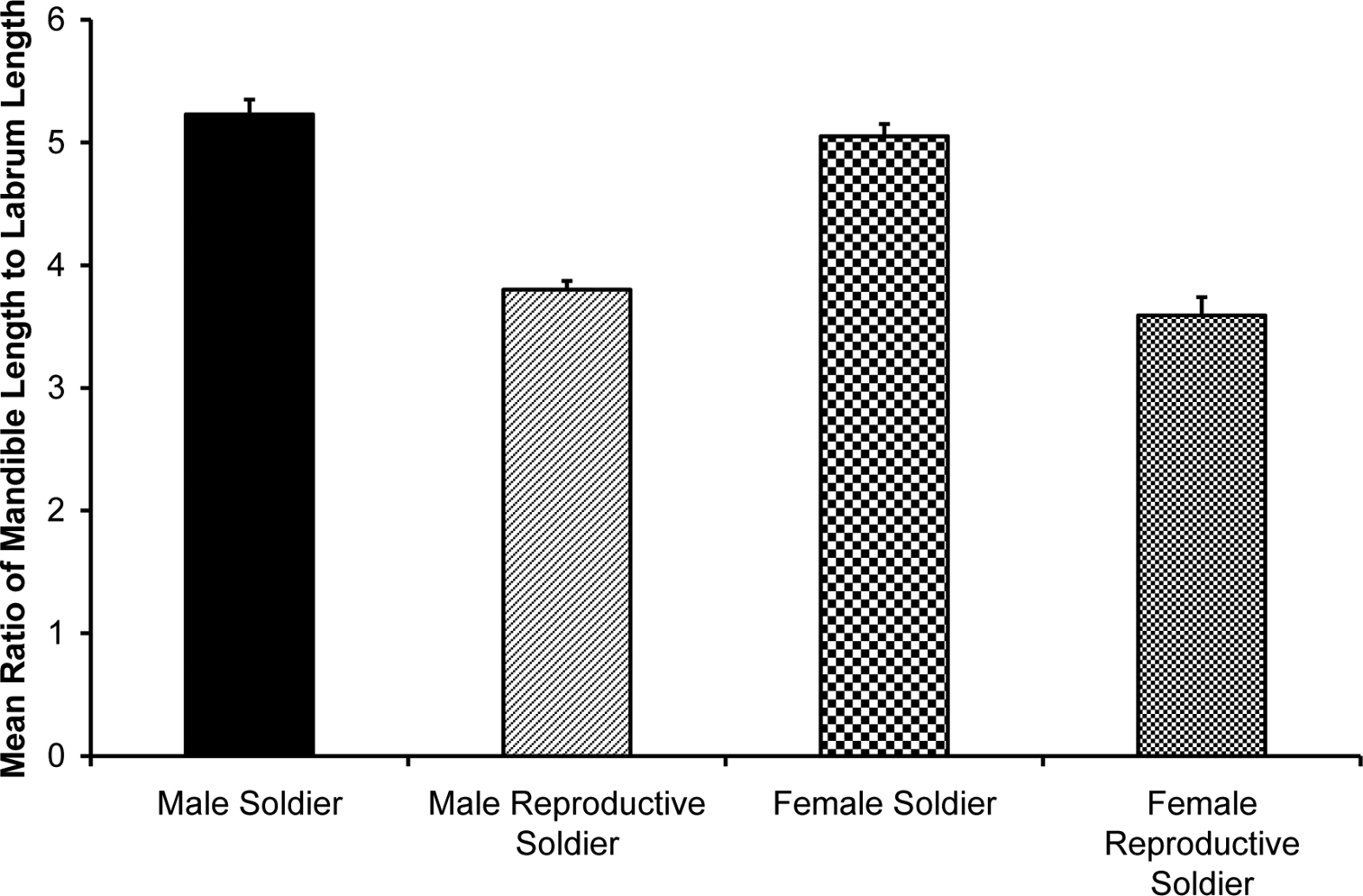
Figure 4 shows pooled means by caste, after grouping male and female data because there was no significant sex effect. Morphological differences between soldiers and reproductive soldiers by sex are listed in Table 1. Multivariate ANOVA (MANOVA) for both male and female morphology indicated no overall caste by sex interaction (p > 0.4) and a significant overall caste effect (Wilks’ Lambda statistic, p < 0.01). (See supplementary material, Table 1, for comparison of means for significant quantitative measurements of castes using pooled male and female data with no significant sex or sex-by-caste interaction).
Comparison of means for ratio of mandible length to labrum length of male and female soldiers and reproductive soldiers. Within sex, soldiers had statistically significantly different mean ratios than reproductive soldiers (p < 0.01). Error bars indicate standard errors. Source data for the figure are listed in a table in the Supplementary Material.
Morphological differences between soldiers and reproductive soldiers by sex in Zootermopsis n.nevadensis
| Variable | P-value of caste effect (males) | P-value of caste effect (females) | ||
|---|---|---|---|---|
| Mean (cm) ± SE, (n) [male soldiers] | Mean (cm) ± SE, (n) [male reproductive soldiers] | Mean (cm) ± SE, (n) [female soldiers] | Mean (cm) ± SE, (n) [female reproductive soldiers] | |
| Eye Length | 0.0002 | 0.2373 | ||
| 0.02 ± 0.0006 (84) | 0.03 ± 0.0016 (38) | 0.02 ± 0.0009 (60) | 0.02 ± 0.002 (9) | |
| Head Length | < 0.0001 | < 0.0001 | ||
| 0.40 ± 0.0066 (84) | 0.31 ± 0.0057 (38) | 0.40 ± 0.0061 (58) | 0.31 ± 0.010 (9) | |
| Head Width | 0.0004 | 0.0088 | ||
| 0.30 ± 0.0040 (84) | 0.27 ± 0.0047 (38) | 0.30 ± 0.0036 (60) | 0.26 ± 0.0090 (9) | |
| Labrum Length | 0.7438 | 0.1484 | ||
| 0.06 ± 0.0009 (82) | 0.06 ± 0.001 (38) | 0.06 ± 0.0008 (60) | 0.07 ± 0.002 (9) | |
| Labrum Width | < 0.0001 | < 0.0001 | ||
| 0.07 ± 0.0008 (82) | 0.08 ± 0.001 (38) | 0.07 ± 0.0008 (59) | 0.08 ± 0.001 (9) | |
| Mandible Length | <0.0001 | < 0.0001 | ||
| 0.32 ± 0.0047 (82) | 0.24 ± 0.0044 (38) | 0.32 ± 0.0040 (60) | 0.24 ± 0.010 (9) | |
| Postmentum Length | < 0.0001 | 0.0009 | ||
| 0.28 ± 0.0055 (83) | 0.20 ± 0.0053 (38) | 0.28 ± 0.0062 (59) | 0.21 ± 0.012 (9) | |
| Postmentum Width (at widest point) | < 0.0001 | 0.0019 | ||
| 0.11 ± 0.0014 (83) | 0.099 ± 0.0015 (38) | 0.11 ± 0.0012 (60) | 0.099 ± 0.0039 (9) | |
| Postmentum Width (at narrowest point) | 0.0010 | 0.1168 | ||
| 0.064 ± 0.00066 (83) | 0.069 ± 0.0014 (38) | 0.065 ± 0.00094 (60) | 0.067 ± 0.0024 (9) | |
| Ratio Mandible length: labrum length | < 0.0001 | < 0.0001 | ||
| 5.23 ± 0.12 (81) | 3.80 ± 0.072 (38) | 5.05 ± 0.10 (60) | 3.59 ± 0.15 (9) | |
| Testes Diameter (smallest) | < 0.0001 | |||
| 0.033 ± 0.0012 (60) | 0.074 ± 0.0036 (38) | |||
| Testes Diameter (largest) | < 0.0001 | |||
| 0.042 ± 0.0015 (72) | 0.094 ± 0.0050 (38) | |||
| Ovary Length | < 0.0001 | |||
| 0.15 ± 0.0068 (53) | 0.36 ± 0.051 (7) | |||
| Ovary Width (midpoint) | < 0.0001 | |||
| 0.019 ± 0.0013 (50) | 0.070 ± 0.01 (7) | |||
| Ovary Width (widest point) | < 0.0001 | |||
| 0.025 ± 0.0013 (51) | 0.087 ± 0.012 (7) | |||
ANOVA results and descriptive statistics for each variable measured, by sex. Variables with p-values less than 0.05 were considered significant and are highlighted in bold. MANOVA indicated a significant overall caste effect for both males (p < 0.01) and females (p < 0.01) based on Wilks’ Lambda statistic.
Four of eight female reproductive soldiers had at least one egg; none of the 50 female normal soldiers examined had eggs. There was no significant difference between possession of wingbuds by caste.
Correlations between external and internal morphology
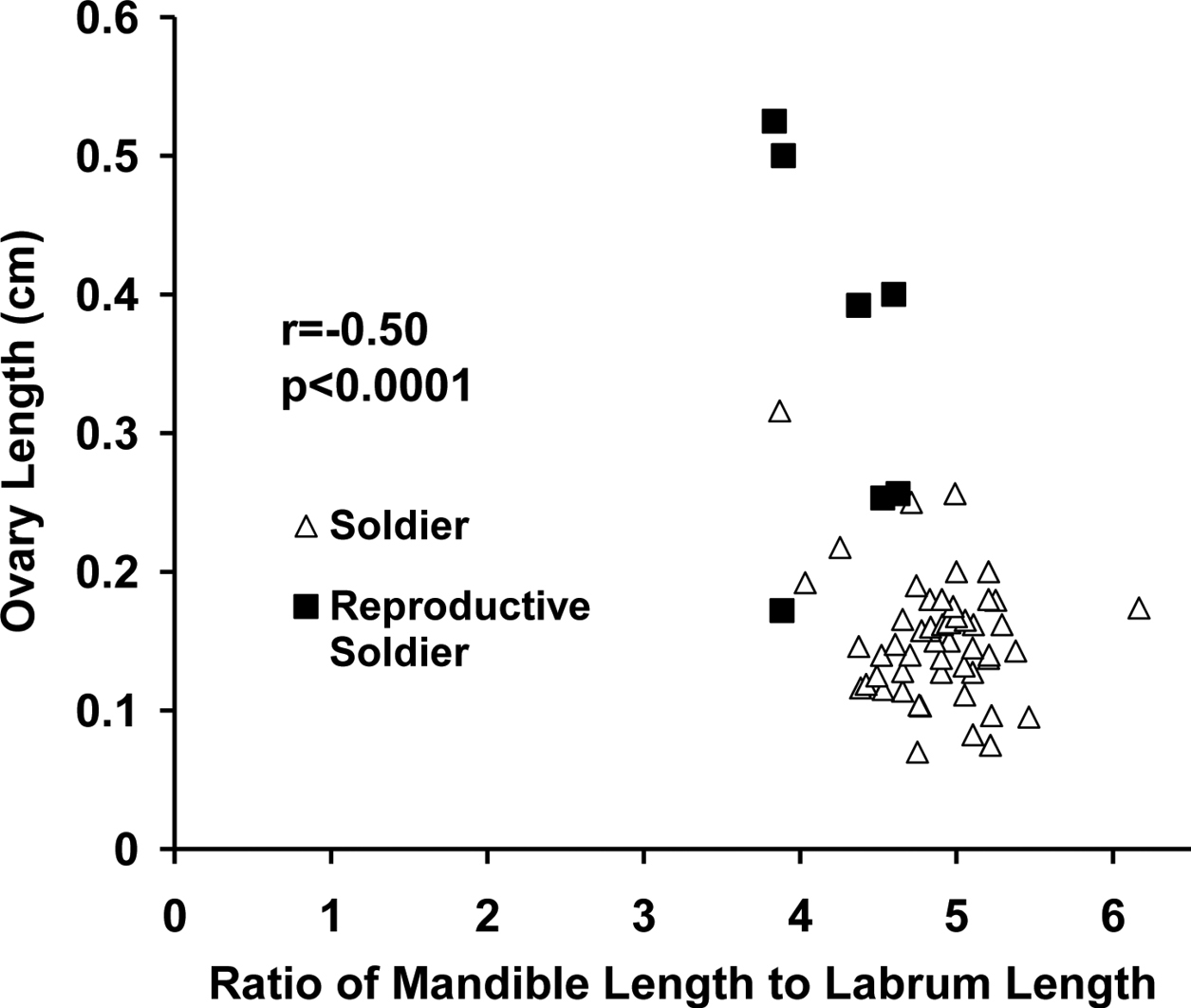
In female soldier morphs the ratio of mandible length to labrum length (Fig. 5) was correlated with ovary length. For male soldier morphs, the ratio of mandible length to labrum length (Fig. 6) was correlated with testis width. The lack of clear, discrete groups in Fig. 5, 6 was because newly differentiated RS were intermixed with developed RS in the archived material. Ovary length and testes width would have been much greater and the groups discrete following a few weeks of development. Figure 2 shows the progression and distinct separation of known age male soldiers and reproductive soldiers. (See Appendix I for the correlation table.)
Correlation between ovary length and the ratio of mandible length to labrum length in female soldier morphs (soldiers and reproductive soldiers).
Correlation between testis diameter at the widest point and the ratio of mandible length to labrum length in male soldier morphs (soldiers and reproductive soldiers).
Homogeneity of gonad size variance between soldiers and reproductive soldiers
Variance of ovary length was 0.0182 (mm2) in female reproductive soldiers which was much greater (p < 0.0001) than that for soldiers (0.000246) . Variance in ovary width at the widest point was also greater (p < 0.0001) in female reproductive soldiers (0.000106) than in normal soldiers (0.0000109). Variance in the widest diameter of the testis was greater (p < 0.0001) for male reproductive soldiers (0.0000955 mm2) than that for soldiers (0.0000231).
DiscussionNeotenic soldiers of both sexes had smaller, rounder heads than soldiers (also observed although not formally analyzed by
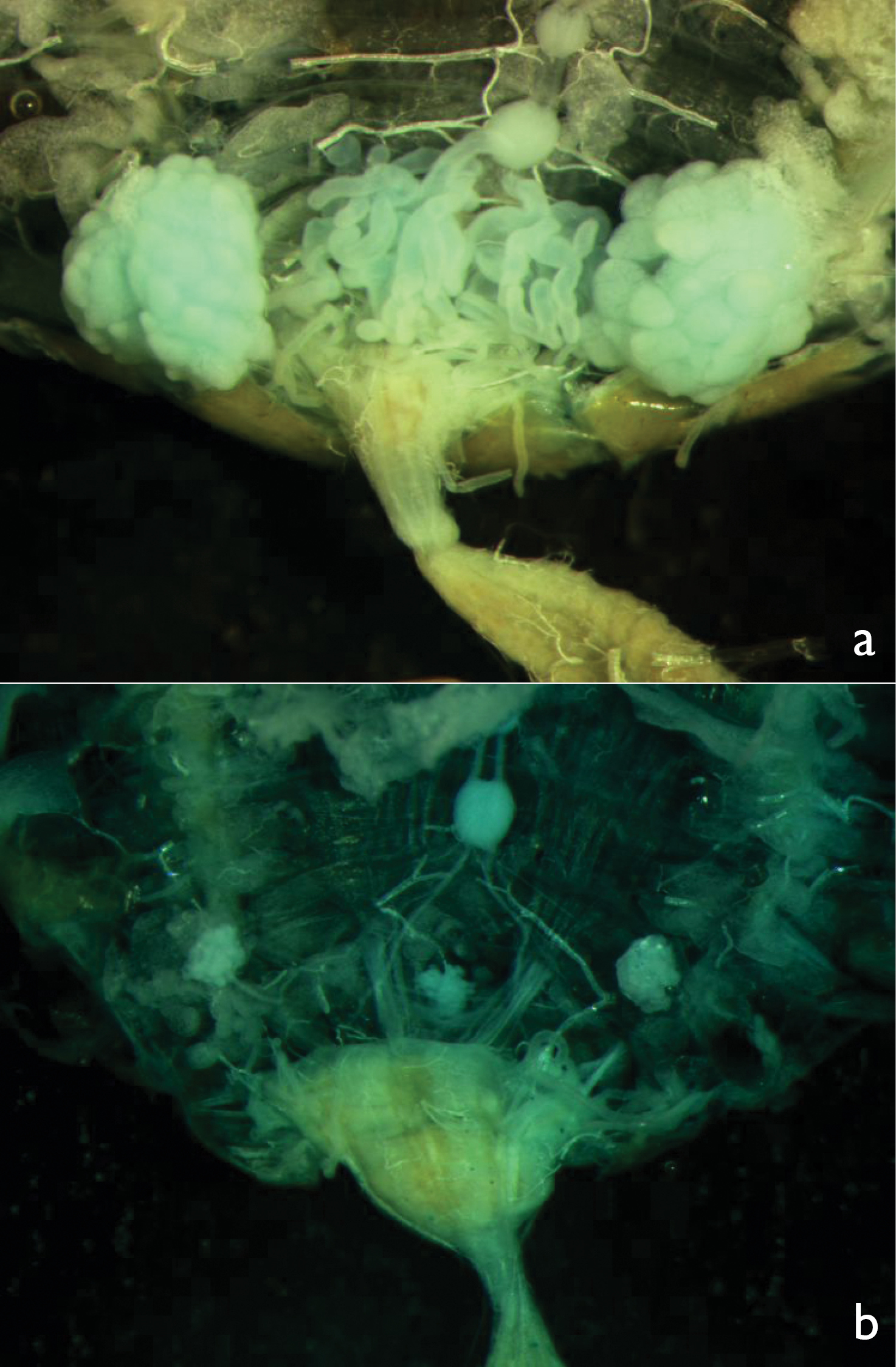
Testes and accessory glands of a male reproductive soldier and b male soldier. Both preparations were photographed under the same magnification.
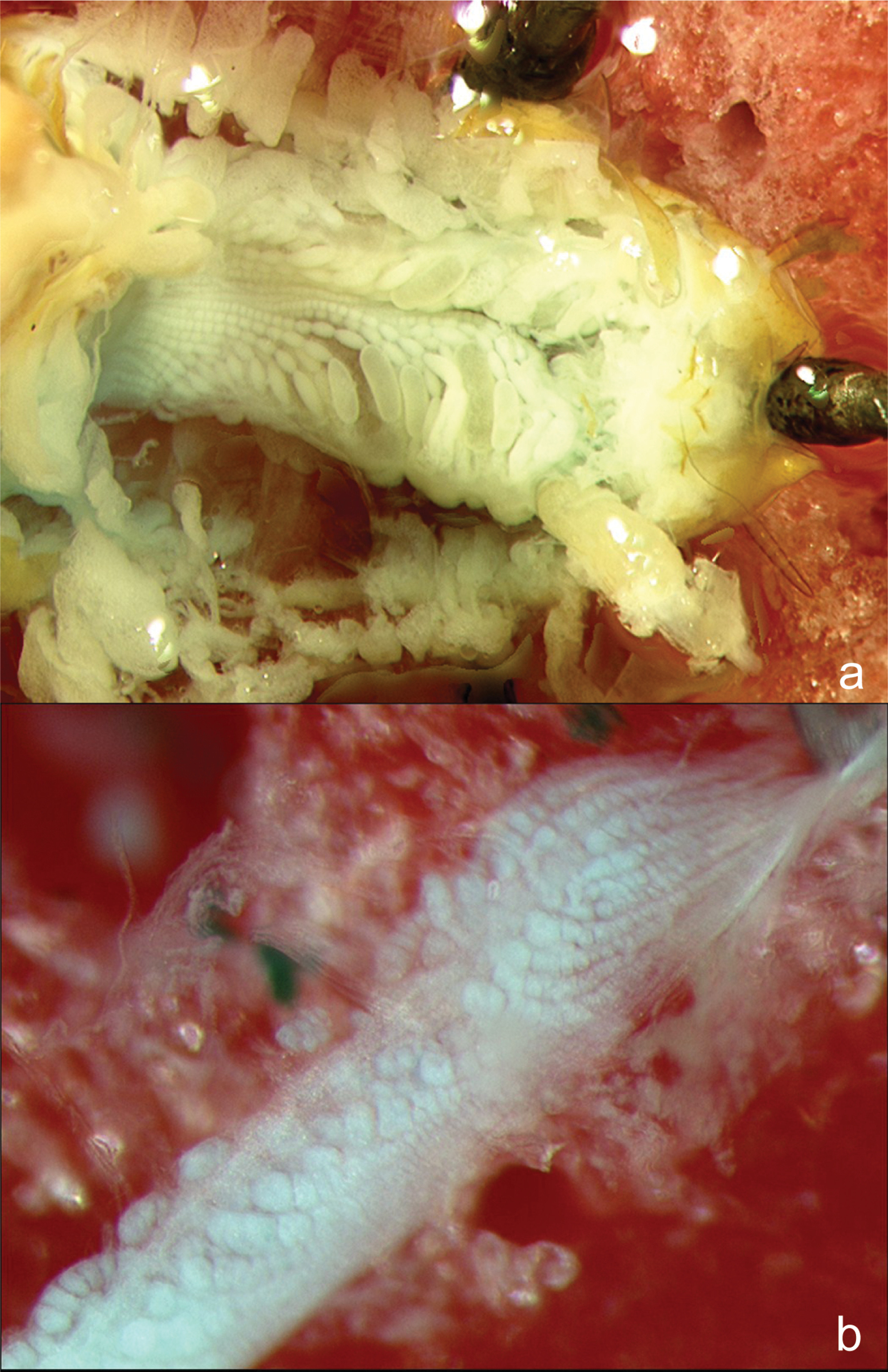
Ovaries of a a female reproductive soldier and b a female soldier. The female reproductive soldier ovarioles are much more developed and contain several eggs, while the female soldier ovarioles are reduced, with no evidence of egg development.
The external, ratio-based morphological measurement of mandible length to labrum length is a strong indicator of gonad size in soldier morphs of both sexes. This ratio accounts for body size and age differences and is visible before other characteristics (e.g. color and shape of abdomen) are apparent, serving as a useful, reliable correlate of gonad size in soldier morphs of a variety of ages. This ratio can easily be estimated—a labrum that extends less than a quarter of the length of the mandibles indicates a normal, sterile soldier. If the labrum is close to a third of the length of the mandible the individual is a reproductive soldier (Fig. 9).
Male reproductive soldier (top) and male soldier (bottom). Note coloration of abdomen, shape of head capsule, and ratio of mandible to labrum length.
Variation in gonad size may be due to age, as newly developed male reproductive soldiers had significantly smaller gonads than those that had been fertile for a longer period. Female reproductive soldiers had much greater variance in ovary length and ovary width at the widest point than did normal soldiers. This may be because of the inclusion of these younger, less sexually developed reproductive soldiers in the sample.
It is likely that modern reproductive soldiers represent an early step in soldier evolution, and that the loss of fertility in soldiers was secondary to the development of large mandibles and heavily sclerotized heads, advantageous for primitive termites in intercolony interactions (
Further study is needed to elucidate the developmental pathway of reproductive soldiers and to determine whether they result from a combination of developmental or social signals, or whether they develop in response to a single stimulus. Because reproductive soldiers are considered relictual transitional forms reflecting the evolutionary history of soldiers (
We thank John Aidan Manubay and Matthew Uchino for laboratory assistance and Al Greene for insightful comments on earlier drafts of this paper. The authors respectfully dedicate this paper to Dr. Kumar Krishna whose innovative research and inspired mentoring have substantially advanced knowledge of termite biology.
External morphology and gonad size: Complete means comparisons and correlations. (doi: 10.3897/zookeys.148.1672.app) File format: PDF
Explanation note: Table 1: Morphological differences between soldiers and soldier neotenics of Zootermopsis nevadensis and Table 2: Correlations between external morphology and gonad size in soldier morphs of Zootermopsis nevadensis
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.