(C) 2011 Christine A. Nalepa. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The literature on pairing and mating behavior in termites indicates that a number of distal antennal segments in dealates of both sexes are often removed during colony foundation, with terms such as amputation, mutilation and cannibalism typically employed to report the phenomenon. Here we propose the use of the phrase ‘antennal cropping’ to describe the behavior, and assess naturally occurring levels of its occurrence by comparing the number of antennal segments in museum specimens of alates and dealates in 16 species of Australian termites (four families), supplemented by analyzing published data on Coptotermes gestroi. Dealates had significantly fewer antennal segments than alates in 14 of the 16 termite species, with both exceptions belonging to the family Termitidae. Levels of antennal cropping were not significantly different between the sexes but did vary by family. Dealates in the Kalotermitidae removed the most segments (41.3%) and those in the Termitidae removed the fewest (8.9%). We discuss the biological significance of this phylogenetically widespread termite behavior, and suggest that controlled antennal cropping is not only a normal part of their behavioral repertoire but also a key influence that changes the conduct and physiology of the royal pair during the initial stages of colony foundation.
mutilation, cannibalism, density effects, incipient colony
Several studies of colony foundation in termites note that the antennae of newly flown alates are typically undamaged, but the terminal antennal segments in both sexes are removed during colony establishment (
The Australian National Insect Collection (ANIC) at CSIRO Ecosystem Sciences, formerly CSIRO Entomology (Canberra, Australia), was systematically searched for termite species in which samples of both the alate and dealate stage were represented. Antennal segments of these stages were counted at 25× on a Wild M5A stereomicroscope (Meerbrugg, Switzerland), and included the scape, pedicel, and individual segments of the flagellum (= antennomeres or flagellomeres). Cropped antennae are easily distinguished from unaltered antennae as they typically have a melanized, healed wound at the distal tip. Because these are adult insects, wound healing occurs but there is no regeneration of lost segments. Data from the longer of the two antennae of each individual was used in the analysis. A dealate primary reproductive was included in the analysis only if it was collected with its mate or with colony members, or if it was physogastric, indicating that it was collected from an established colony. An individual was excluded from analysis if it exhibited any bodily damage resulting from the collection process. Individuals were sexed based on the shape of the terminal abdominal sternites (
The mean (± S.E.) number of of antennal segments in reproductives from 17 termite species. The t-tests are unpaired between alates and dealate.<br/>
| Family | Length of antennae (# of segments) | Change in length of antennae | t | df | p | ||
|---|---|---|---|---|---|---|---|
| Species | Alates (n) | Dealates (n) | # of segments | % | |||
| Stolotermitidae | |||||||
| Porotermes adamsoni | 16.3 ± 0.9 (9) | 11.6 ± 1.1 (5) | -4.7 | -32.1 | 8.784 | 12 | <0.001 |
| Stolotermes victoriensis | 14.9 ± 1.8 (8) | 10.2 ± 1.6 (12) | -4.7 | -31.5 | 6.038 | 18 | <0.001 |
| Kalotermitidae | |||||||
| Neotermes papua | 18.5 ± 0.7 (2) | – | – | ||||
| Neotermes insularis | 18.7 ± 1.3 (14) | 11.8 ± 1.3 (12) | -6.9 | -36.9 | 13.806 | 24 | <0.001 |
| Ceratokalotermes spoliator | 13.2 ± 0.8 (9) | 8.3 ± 1.2 (6) | -4.9 | -33.3 | 9.316 | 13 | <0.001 |
| Kalotermes convexus | 13.6 ± 1.0 (10) | 7.8 ± 1.3 (15) | -5.8 | -42.6 | 12.277 | 23 | <0.001 |
| Glyptotermes brevicornis | 13.5 ± 0.8 (6) | 8.4 ± 1.6 (14) | -5.1 | -37.8 | 7.380 | 18 | <0.001 |
| Cryptotermes secundus | 16.5 ± 1.4 (14) | 8.5 ± 1.0 (12) | -8.0 | -48.5 | 16.490 | 24 | <0.001 |
| Bifiditermes condonensis | 17.7 ± 2.0 (9) | 9.1 ± 1.7 (11) | -8.6 | -48.6 | 10.372 | 18 | <0.001 |
| Rhinotermitidae | |||||||
| Heterotermes ferox | 16.9 ± 1.0 (10) | 13.5 ± 2.1 (2) | -3.4 | -20.1 | 3.792 | 10 | 0.004 |
| Schedorhinotermes actuosus | 18.8 ± 2.4 (12) | 13.0 ± 2.3 (5) | -5.8 | -30.9 | 4.533 | 15 | <0.001 |
| Coptotermes gestroi | 20.2 ± 0.4 (80) | 12.9 ± 0.2 (80) | -7.3 | -36.1 | 15.541 | 158 | <0.001 |
| Coptotermes lacteus | 18.4 ± 1.8 (16) | 13.2 ± 0.5 (4) | -5.1 | -27.9 | 5.585 | 18 | <0.001 |
| Termitidae | |||||||
| Microcerotermes turneri | 13.8 ± 0.4 (9) | 12.7 ± 2.2 (18) | -1.1 | -8.0 | 1.454 | 25 | 0.158 |
| Drepanotermes perniger | 15.6 ± 2.2 (11) | 16.5 ± 1.7 (13) | 0.8 | +6.7 | 1.058 | 22 | 0.302 |
| Xylochomitermes occidualis | 14.9 ± 0.3 (14) | 13.2 ± 1.2 (19) | -1.8 | -11.4 | 5.548 | 31 | <0.001 |
| Tumulitermes nastilis | 16.1 ± 1.0 (8) | 12.2 ± 0.4 (5) | -3.9 | -23.0 | 8.242 | 11 | <0.001 |
We supplemented our data with that obtained from Coptotermes gestroi by
The antennae lengths of the 17 species were analysed in a four factor Generalised Linear Model (GLM). The four factors used in analysis were species nested in families, families, sex, and wing status (alate or dealate). Planned posthoc pairwise comparisons were used to find differences between species and families; all comparisons were Tukey’s-adjusted to account for potential errors. The posthoc comparisons were unnecessary for sex and status as there were only two levels in these factors. Interactions between families, sex and wing status were also considered in the GLM. Finally, unpaired t-tests were performed on wing-status for each family.
Statistical analyses were performed using
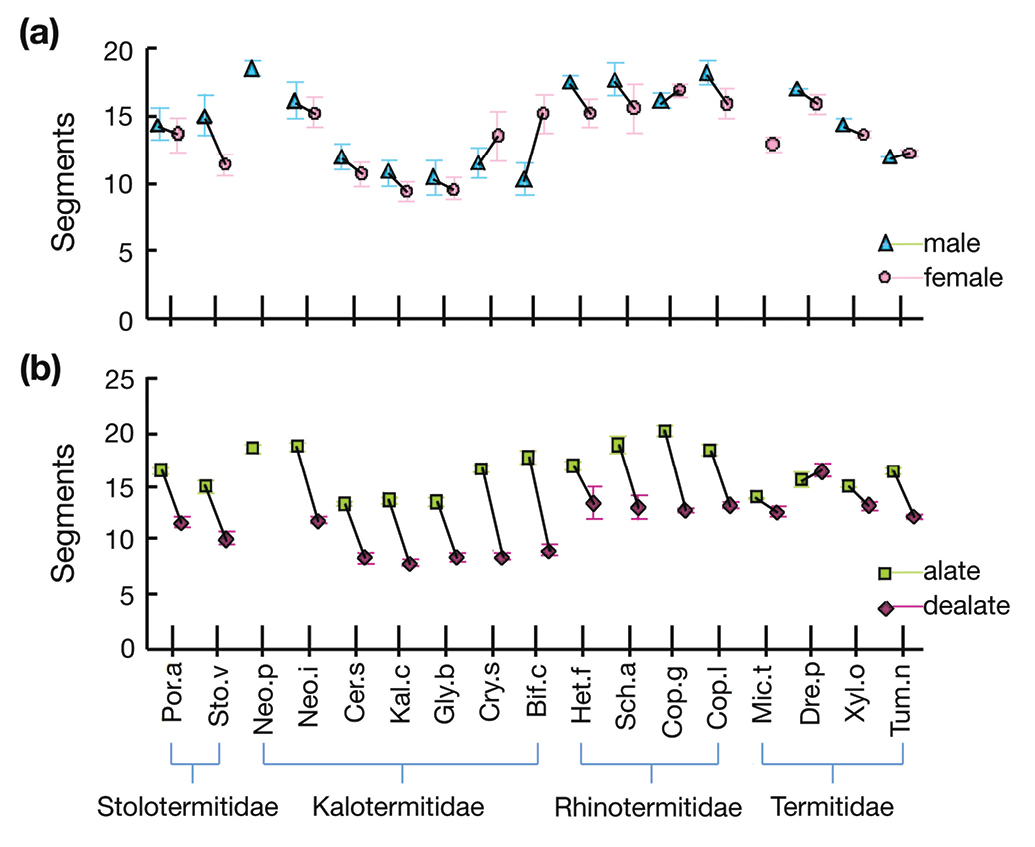
We documented a wide range of antennal lengths in the imaginal stage of termites (Table 1). Among alates, Schedorhinotermes actuosus had the highest number of antennal segments, around 19, and Ceratokalotermes spoliator had the fewest, with about 13. Among dealates segments were most numerous (around 16) in Drepanotermes perniger, and Kalotermes convexus had the fewest, with around eight.
Overall, the difference between the sexes was small, about one antennal segment, with overlapping standard errors; males had 14.5 ± 0.7 antennal segments whereas females had 13.5 ± 0.6. However the difference between winged and wingless adults was substantial, about five antennal segments, with non-overlapping standard errors. Alates averaged 16.3 ± 0.5 antennal segments, whereas dealates averaged 11.4 ± 0.6 (all averaged across species; Fig. 1).
Average (± standard error) antenna length measured in number of antennal segments of 17 termite species for a male and females; and b alates and delates. Species names abbreviated as in Table 3.
In the GLM analysis, significant differences were found between species (nested within families) (F12, 379 = 8.151; p < 0.001), termite families (F3, 379 = 25.586; p < 0.001), and wing status (F1, 379 = 164.940; p < 0.001), but no significant differences between the sexes (F1, 379 = 0.133; p = 0.715) (Table 2). The GLM analysis explained three quarters of the variation (r2 = 0.757). The mean differences in antennal length and Tukey-corrected posthoc pairwise comparisons between species are listed in Table 3. The general pattern is Ceratokalotermes spoliator, Kalotermes convexus, Glyptotermes brevicornis and Cryptotermes secundus, allin the Kalotermitidae, are different from Schedorhinotermes actuosus, Coptotermes gestroi and Coptotermes lacteus in the Rhinotermitidae, and Microcerotermes turneri, Drepanotermes perniger and Tumulitermes nastilis in the Termitidae. Differences between species therefore can be clustered into differences between families.
The results of the generalised linear model run on antennal length.
| Factor | Sum-of-Squares | df | Mean-Square | F-ratio | p |
|---|---|---|---|---|---|
| Species(Family) | 499.010 | 12 | 41.584 | 8.151 | 0.000 |
| Family | 391.608 | 3 | 130.536 | 25.586 | 0.000 |
| Sex | 0.679 | 1 | 0.679 | 0.133 | 0.715 |
| Wing status | 841.514 | 1 | 841.514 | 164.940 | 0.000 |
| Family × Sex | 16.775 | 3 | 5.592 | 1.096 | 0.351 |
| Family × Wing status | 183.450 | 3 | 61.150 | 11.986 | 0.000 |
| Sex × Wing status | 0.140 | 1 | 0.140 | 0.027 | 0.868 |
| Family × Sex × Wing status | 4.612 | 3 | 1.537 | 0.301 | 0.824 |
| Error | 1933.631 | 379 | 5.102 |
The matrix of pairwise mean differences in antennal length between species. Pairs that were significantly different in Tukey adjusted pairwise posthoc comparisons from the GLM posthoc are indicated as * p < 0.05, † p < 0.01, ‡ p < 0.001. Nb. Neotermes papue was excluded due to a lack of data. Abbreviations: Por.a = Porotermes adamsoni; Sto.v = Stolotermes victoriensis; Neo.i = Neotermes insularis; Cero.s = Ceratokalotermes spoliator; Kalo.c = Kalotermes convexus; Glypt.b = Glyptotermes brevicornis; Cry.s = Cryptotermes secundus; Bif.c = Bifiditermes condonensis; Het.f = Heterotermes ferox; Sch.a = Schedorhinotermes actuosus; Cop.g = Coptotermes gestroi; Cop.l = Coptotermes lacteus; Mic.t = Microcerotermes turneri; Dre.p = Drepanotermes perniger; Xyl.o = Xylochomitermes occidualis; Tum.n = Tumulitermes nastilis.
| Por.a | Sto.v | Neo.i | Cer.s | Kal.c | Gly.b | Cry.s | Bif.c | Het.f | Sch.a | Cop.g | Cop.l | Mic.t | Dre.p | Xyl.o | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sto.v | 0.7 | ||||||||||||||
| Neo.i | 1.1 | 1.8 | |||||||||||||
| Cer.s | 3.1 | 2.5 | 4.2‡ | ||||||||||||
| Kal.c | 3.3* | 2.7 | 4.5‡ | 0.2 | |||||||||||
| Gly.b | 3.1 | 2.4 | 4.2‡ | 0.0 | 0.3 | ||||||||||
| Cry.s | 1.5 | 0.9 | 2.6† | 1.6 | 1.8 | 1.6 | |||||||||
| Bif.c | 0.8 | 0.2 | 2.0 | 2.3 | 2.5 | 2.3 | 0.7 | ||||||||
| Het.f | 0.5 | 1.2 | 0.6 | 3.6 | 3.8 | 3.6 | 2.0 | 1.3 | |||||||
| Sch.a | 2.2 | 2.9 | 1.1 | 5.3‡ | 5.5‡ | 5.3‡ | 3.7† | 3.0 | 1.7 | ||||||
| Cop.g | 2.5 | 3.2* | 1.4 | 5.6‡ | 5.9‡ | 5.6‡ | 4.0‡ | 3.4‡ | 2.0 | 0.3 | |||||
| Cop.l | 1.9 | 2.6 | 0.8 | 5.0‡ | 5.2‡ | 5.0‡ | 3.4* | 2.7 | 1.4 | 0.3 | 0.6 | ||||
| Mic.t | 2.0 | 2.7 | 0.9 | 5.1‡ | 5.3‡ | 5.1 | 3.5* | 2.8 | 1.5 | 0.2 | 0.5 | 0.1 | |||
| Dre.p | 1.8 | 2.5 | 0.7 | 5.0‡ | 5.2‡ | 4.9† | 3.4* | 2.7 | 1.3 | 0.4 | 0.7 | 0.1 | 0. | ||
| Xyl.o | 0.2 | 0.9 | 0.9 | 3.4 | 3.6 | 3.3 | 1.8 | 1.1 | 0.2 | 1.9 | 2.3* | 1.6 | 1.8 | 1.6 | |
| Tum.n | 0.3 | 1.0 | 0.8 | 3.4† | 3.7‡ | 3.4 | 1.8 | 1.1 | 0.2 | 1.9 | 2.2 | 1.6 | 1.7 | 1.5 | 0.0 |
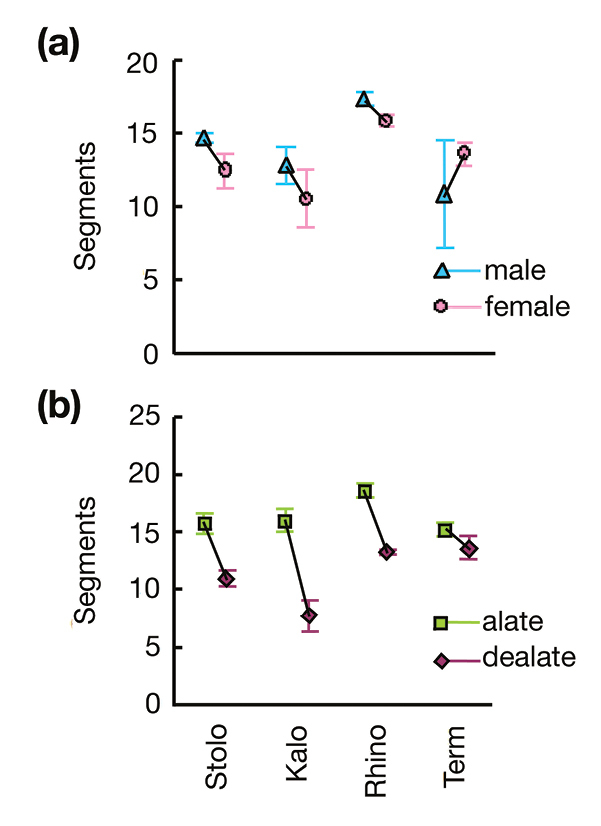
This pattern is also seen in the results of the GLM, as the F ratios suggest that the effect of family was about three times more important than the effect of species. In particular the Rhinotermitidae had longer antennae than the other families. Species in the Termopsidae had 13.3 ± 1.3 antennal segments, those in Kalotermitidae 13.0 ± 1.2, the Rhinotermitidae 16.7 ± 0.3, and the Termitidae 14.4 ± 0.6. The mean pairwise differences in antennal length between families, and the Tukey-corrected posthoc pairwise comparisons, were significantly different for Kalotermitidae × Rhinotermitidae (mean difference 3.3, p < 0.001), Kalotermitidae × Termitidae (md 2.1, p = 0.002) and Rhinotermitidae × Termitidae (md 2.0, p = 0.004); the remaining comparisons were not significant (Kalotermitidae × Termopsidae md 1.2, p = 0.098; Rhinotermitidae x Termitidae md 1.2, p = 0.239; Termitidae × Termopsidae md 0.8, p = 0.688).
The largest F ratio from the GLM was for wing status, which was about six times more important than family, and 20 times more important than species differences in determining antennae length. This is clear from the paired t-tests: 14 of the 16 possible alate vs. delate comparisons were significant (Table 1, Fig. 1). The two species without a difference in alate and delate antennal length were Microcerotermes turneri and Drepanotermes perniger, which both belong to the same branch of the Termitinae in the Termitidae, whereas Xylochomitermes occidualis lies in another branch of the Termitinae and Tumulitermes nastilis is in the Nasutitermitinae (Inwood et al. 2007, Legendre et al. 2008).
Only one interaction was significant: family × wing status (F3, 389 = 11.986, p < 0.001), showing that antennal cropping varies among families. This variation is clear in Fig. 2, with alates in Stolotermitidae, Kalotermitidae and Rhinotermitidae all losing five to seven antennal segments after dealation, whereas in Termitidae dealates lose perhaps two. Expressed as a percentage, kalotermids cropped on average the most antennal segments: Stolotermitidae 32.0%, Kalotermitidae 41.3%, Rhinotermitidae 28.8% and Termitidae 8.9%. The lack of an effect due to sex either as a main effect, or in the interaction terms (Table 2) is clear from Figs. 1 and 2, with mostly small and inconsistent differences between males and females.
Average (± standard error) antenna length measured in number of antennal segments of four termite families for a male and females; and b alates and delates. Abbreviations: Stolo = Stolotermitidae; Kalo = Kalotermitidae, Rhino = Rhinotermitidae; Term = Termitidae.
The mean antennal length for Coptotermes gestroi alates from the tree stump (i.e., prior to swarming) was 20.7 ± 0.7 for males and 19.8 ± 1.1 for females, and from the swarm it was 19.2 ± 0.9 for males and 21.0 ± 0.6 for females. There were no significant differences found either for alate source (F1, 76 = 0.032; p = 0.858) or sex (F1, 76 = 0.228; p = 0.635), and the interaction was not significant (F1, 76 = 2.594; p = 0.111).
The mean antennal length for Coptotermes gestroi dealates at nine months after colony initiation was 12.2 ± 0.6 for males and 13.6 ± 0.3 for females, and from 2 years after colony initiation it was 12.6 ± 0.3 for males and 13.3 ± 0.3 for females. There were no significant differences found for age (F1, 76 = 0.035; p = 0.853) but there was a significant difference for sex (F1, 76 = 7.122; p = 0.009), as females had longer antennae than males, albeit only one segment longer; the interaction was not significant (F1, 76 = 0.651; p = 0.422), indicating that the difference between the sexes did not change over time.
DiscussionOur data suggest that antennal cropping is a phylogenetically widespread, fairly precise behavior. There was a significant decrease in the number of antennal segments in dealates when compared to alates in termites from all families except two species of Termitidae. No more than half of the antenna was trimmed in any case, although our data may slightly underestimate differences since we used the longer of the two antennae in our analysis. There is some variation in both the number of segments in the right and left antennae of individuals (
The sole description of the behavioral process leading to the loss of antennal segments is by
Antennal cropping was proposed to play a key role in the transition to pair behavior by decreasing the amount of physical contact perceived by the male and female (
The dual nature of the antenna as both transmitter and receiver dictates that regardless of whether a paired individual crops its own or its partner’s antenna, both members of the pair are likely to be affected (Table 4). In its role as receiver, antennal cropping would decrease an individual’s ability to detect environmental stimuli, including pheromones. In its role as transmitter, shorter antennae result in decreased tactile stimulation of the partner.
The dual nature of antennal cropping: both partners are affected regardless of whether an individual crops its own or its partner’s antennae.
| Crop self | Crop partner | |
|---|---|---|
| Effect on self | Decreases self ability to detect environmental stimuli | Decreases tactile stimulation of self |
| Effect on partner | Decreases tactile stimulation of partner | Decreases partner’s ability to detect environmental stimuli |
Antennal cropping has been recorded in several cockroach taxa, but its functional significance is unknown. Nymphs of Blattella germanica self-prune their antennae – the ends are nipped off just prior to molting (
Antennal cropping should be considered a key factor when studying changes in behavior and physiology during termite colony foundation, as density dependent effects result at least in part from sensory input mediated by the antennae in both crickets and locusts (