(C) 2011 Michael S. Engel. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The family Scelembiidae (Neoembiodea: Embiomorpha: Archembioidea) is recorded from Asia for the first time, based on two individuals preserved in Early Eocene amber from the Cambay Basin, western India. Kumarembia hurleyi Engel & Grimaldi, gen. n. et sp. n., is described, figured, and distinguished from other archembioid genera. The genus shares male genitalic features with scelembiids, otherwise known from South America and Africa.
Polyneoptera, Embioptera, Embiidina, Neoembiodea, Tertiary, taxonomy, India
Embiodea are one of the more infrequently encountered and investigated orders of insects. This is unfortunate given their remarkable morphological specializations, most of which relate to the production of and life within silken galleries. For example, the probasitarsus is greatly swollen and encompasses distinctive silk glands from which the galleries are spun. The wings are unique among the flying insects for their great flexibility, permitting individuals to move in reverse through their silken tunnels, but can be made more rigid by pumping haemolymph into distinctive ‘blood sinuses’, enabling them to gain temporary rigor and permit controlled flight. Females are apterous, while males can be either fully winged or shed their wings, much like termites. Even more fascinating is that where known, all species are gregarious, living in small colonies, much like their putative relatives among the Zoraptera.
The relationship of Embiodea to other orders has been problematic, much like everything pertaining to the phylogeny of webspinners. Among the numerous competing hypotheses, those with the greatest support are a relationship to the Phasmatodea (e.g.,
Herein we provide the description of a new genus and species of fossil webspinner based on two exceptionally well preserved individuals (Fig. 1, 2) recently recovered from Early Eocene amber of the Cambay Basin in western India. These are the first fossil webspinners from Asia (Table 1) and also the first records of their family, Scelembiidae, from the Oriental Region.
Described fossil webspinners (updated from
| Embiodea: Neoembiodea | ||
| Teratembiidae | ||
| Oligembia vetusta Szumik, 1994 | Miocene (Burdigalian) | Dominican Republic |
| Anisembiidae | ||
| Poinarembia rota Ross, 2003b | Miocene (Burdigalian) | Dominican Republic |
| Glyphembia amberica Ross, 2003b | Miocene (Burdigalian) | Dominican Republic |
| Glyphembia vetehae (Szumik, 1998) Ross, 2003b | Miocene (Burdigalian) | Dominican Republic |
| “Embiidae” | ||
| “Embia florissantensis” Cockerell, 1908* | Eocene-Oligocene | Colorado |
| Electroembia antiqua (Pictet, 1854) Ross, 1956 | Eocene (Lutetian) | Baltic |
| Scelembiidae | ||
| Kumarembia hurleyi, gen. et sp. n. | Eocene (Ypresian) | India |
| Pachylembiidae: Sorellembiinae | ||
| Sorellembia estherae Engel & Grimaldi, 2006 | Cretaceous (Albian) | Myanmar |
| Notoligotomidae: Burmitembiinae | ||
| Burmitembia venosa Cockerell, 1919 | Cretaceous (Albian) | Myanmar |
| *Incertae Sedis* | ||
| Sinembiidae | ||
| Sinembia rossi Huang & Nel, 2009 | Jurassic (Bathonian) | Inner Mongolia, China |
| Juraembia ningchengensis Huang & Nel, 2009 | Jurassic (Bathonian) | Inner Mongolia, China |
* This species has been placed in the genus “Lithembia” by
The age, origin, and biotic diversity of the Cambay amber are reviewed by
Mandibles depressed, with incisive teeth well differentiated from molar area. Wings without crossveins between MA1 and MA2; CuA frequently diffuse. Male 10T with a membranous area occupying base and center of sclerite; 10R and 10L connected by thin basal bar; 10RP2 present, short, thumb-like; 10LP1 a curved, apically-forked process; HP rectangular, centered; LC1 with setae on apical area.
Ambonembia Ross (=Ischnosembia Ross), Biguembia Szumik (=Aphanembia Ross), Gibocercus Szumik, Kumarembia Engel and Grimaldi gen. n., Litosembia Ross, Malacosembia Ross, Navasiella Davis, Pararhagadochir Davis, and Rhagadochir Enderlein (=Scelembia Ross) (
urn:lsid:zoobank.org:act:F30F7AFB-D379-4F74-A76F-612169FD3B48
Kumarembia hurleyi Engel and Grimaldi, sp. n.
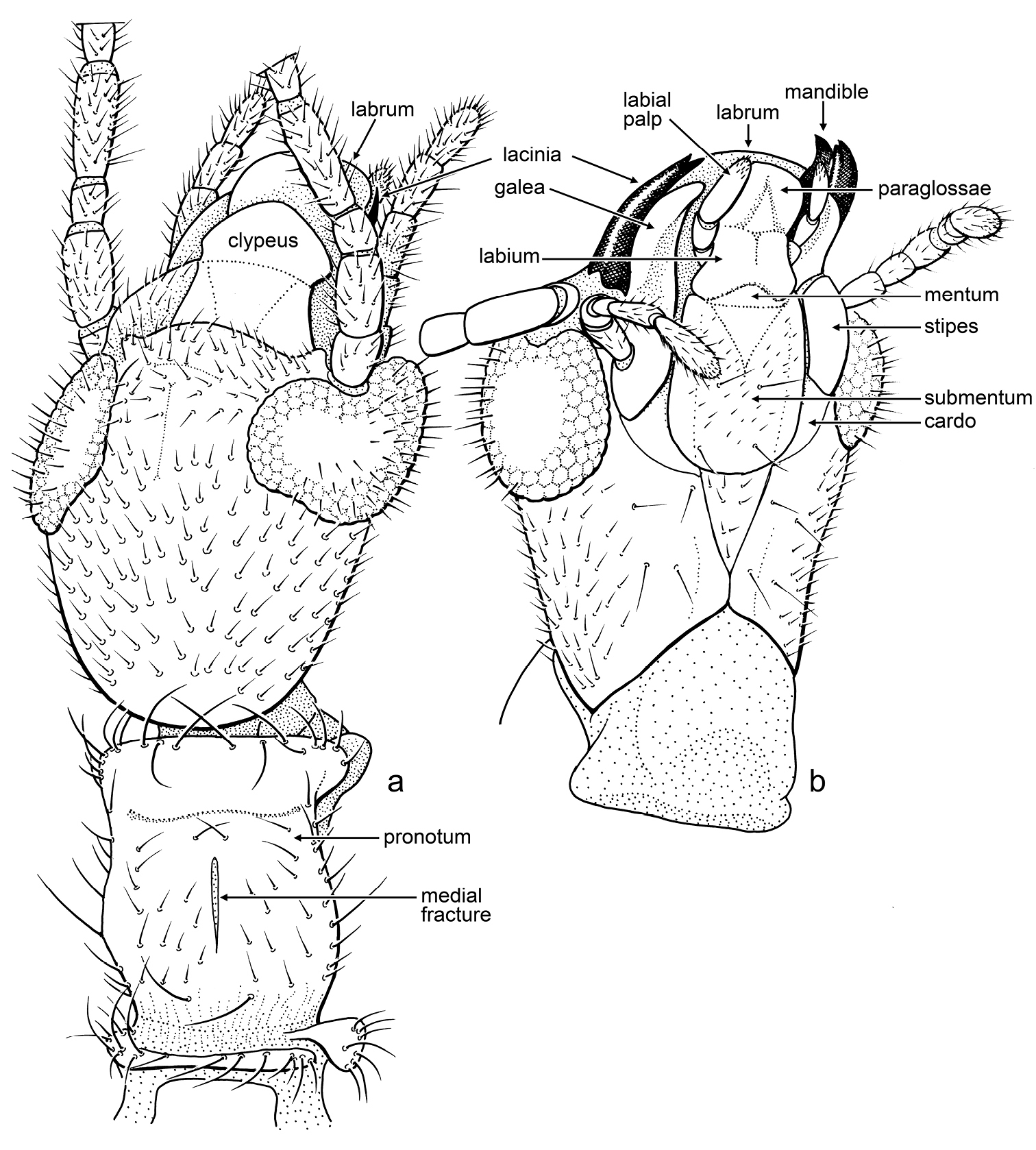
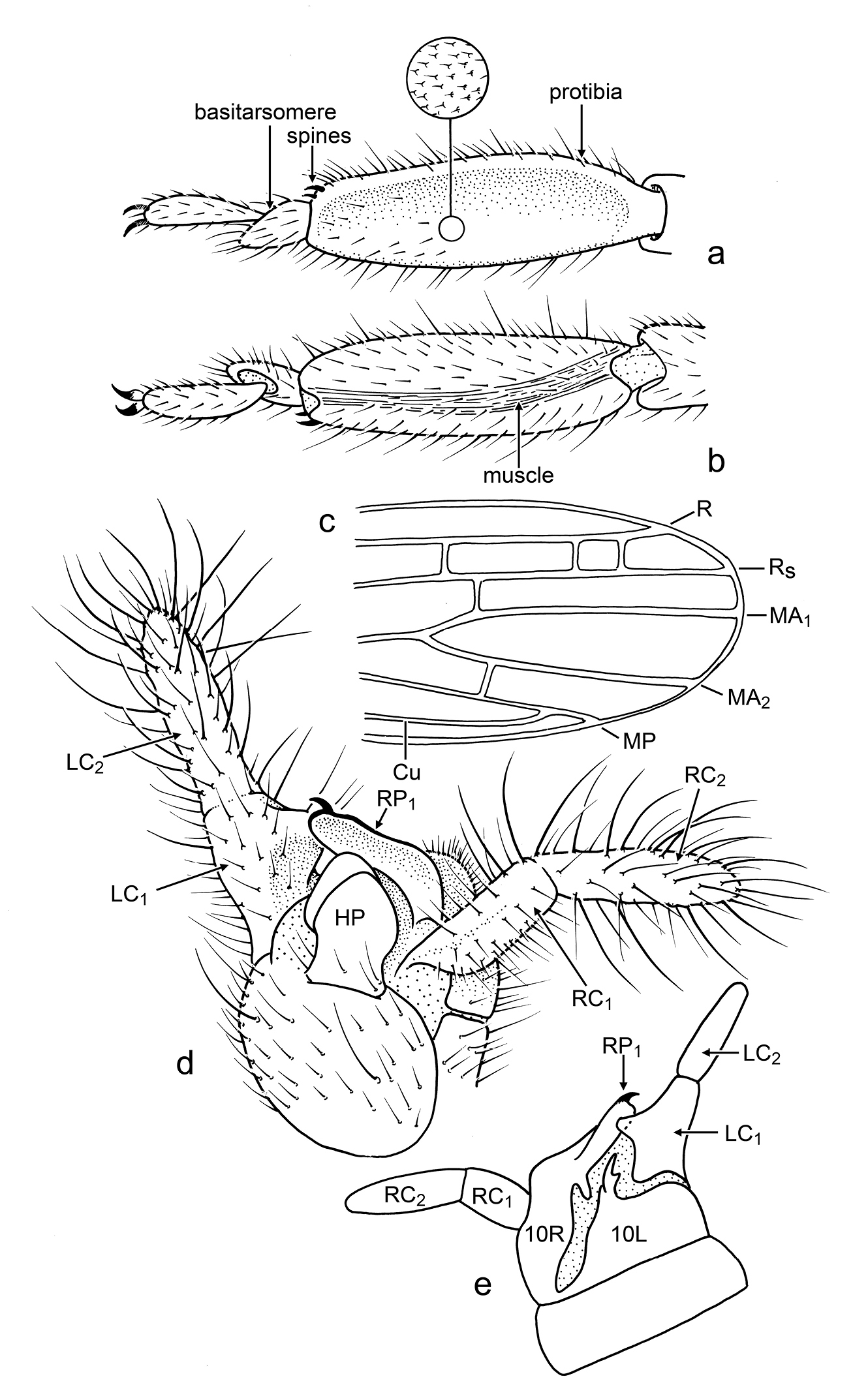
Male: Head relatively slender, longer than wide, elongate oval (Fig. 3a), slightly narrowed posteriad; compound eyes well developed, prominent, emarginate at base of antenna, and less so on posterodorsal margin, setose, with stiff setae, some longer than diameter of facets, and most setae on outermost distal surface (Fig. 3b); ocelli absent; antennae long, with 17 articles (incomplete in holotype, number of articles based on paratype), articles apparently uniformly sclerotized and pigmented (apical articles not differently pigmented or unpigmented); lacinia entirely sclerotized, with two small apical teeth (Fig. 3b), remainder of maxilla generalized; mentum sclerotized, small, approximately one-third length of labium, without setae, tightly joined to submentum; submentum sclerotic, with four stiff, very fine setae, anterior margin straight (appearing to have medial hump owing to mentum), lateral margins relatively straight and converging posteriorly toward base, margins meet before ventral margin of head capsule (Fig. 3b); ventral surface of head capsule, lateral to prementum, with eight (four pairs) fine, stiff, erect setae, head capsule otherwise with numerous decumbent setae, especially dorsally. Cervical area extensively membranous, especially ventrally. Pronotum longer than wide, well sclerotized and apparently pigmented, anterior margin straight, with prominent anterolateral corners, faintly constricted just posterior to anterior margin, posterior margin constricted, with rounded posterolateral corners, dorsally depressed just posterior to anterior margin (resulting in the anterior margin appearing somewhat lip-like), with thin, longitudinal, membranous “fracture” at midline (Fig. 3a). Wings large, mildly infumate; R reaching wing margin, straight apically (not procurved to terminate anteriorly); no c-r crossveins evident; Rs simple, terminating at wing apex (Fig. 4c), several r-rs crossveins present; single rs-ma1 crossvein present shortly after origin of MA1; MA apically forked, MA1 and MA2 both reaching wing margin, without crossveins between them (Fig. 4c); MP simple, reaching to apical wing margin; CuA apparently joining MP apically. Protibia greatest width 0.33× length, silk-producing surface slightly concave (Fig. 4a); distal end with two minute, sclerotized, slightly-curved, spine-like setae on mesal surface (Fig. 4a); metafemur swollen; metabasitarus (= metatarsomere I) elongate, without plantunlae (as in Pararhagadochir); metatarsomere II exceptionally short, without plantula; metadistitarsus (= metatarsomere III) elongate, nearly as long as combined lengths of metabasitarsus and metatarsomere II; pretarsal claws simple; arolium absent (Fig. 4a, 4b). Male terminalia asymmetrical; dorsally with left hemitergite (10L) relatively broad; right hemitergite (10R) relatively narrow, tapering posteriorly; hemitergites separated by membranous area, connected proximally by a thin sclerotic band (Fig. 4e); left tergal process (LP) sclerotized, short, curved, with forked apex, internal (caudad) hook longer than external (proximad) hook, both with tapered and pointed apices (Fig. 4e); right tergal caudal process (RP1) long, extending to LC1, apex with minute hook caudally and gentle lobe proximally; right tergal anterior process (RP2) present, short, thumb-like; ventrally with hypandrium (H) relatively large, broad, with rectangular hypandrial process (HP) positioned medially (Fig. 4d); cercomeres well sclerotized and uniformly covered by stiff, elongate setae in loose whorls (Fig. 4d); apical cercomeres (LC2 and RC2) slightly longer than basal cercomeres (similar to Archembia); left basal cercomere (LC1) medially expanded and lobed (Fig. 4d), left cercal basipodite comprising a sclerotic flange fused to outer rim of LC1 and without evidence of inner lobe or ring; right basal cercomere (RC1) well sclerotized throughout, cylindrical (Fig. 4d, 4e).
Female: Unknown.
The new generic name is a combination of Kumar (honoring Dr. Kumar Krishna, faithful colleague and dear friend, as well as the world’s leading authority on the systematics of Isoptera), and Embia, type genus of and frequent stem for embiodeans. The name is feminine.
urn:lsid:zoobank.org:act:0124F7C1-BB06-4024-8C0A-3CFA053DD4CB
http://species-id.net/wiki/Kumarembia_hurleyi
Fig. 1–4♂; AMNH Tad-261-A (Fig. 1), India: Gujurat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400'N, 73°4.532'E, 17–22 January 2010; to be deposited in the Birbal Sahni Institute of Paleobotany, Lucknow, India.
♂; AMNH Tad-253 (Fig. 2), India: Gujurat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400'N, 73°4.532'E, 17–22 January 2010; in the Division of Invertebrate Zoology, American Museum of Natural History, New York.
As for the genus (vide supra).
Male: Total length (excluding wings and antennae, as preserved) 5.3 mm; forewing length (estimated) 5.1 mm, width 1.1 mm; integument generally light brown except darker on head and antenna, finely imbricate and impunctate where evident (based on paratype, integument of holotype slightly wrinkled owing to apparent desiccation and shrinkage of individual). Head length (to apex of labrum) 1.1 mm, width (just posterior to compound eyes) 0.64 mm, head posterior to compound eyes longer than compound eye diameter, posterior border gently rounded, covered with numerous, short, prominent setae, longer ventrally (Fig. 3). Pronotum length 0.56 mm, width (medial) 0.40 mm, apparently with weak longitudinal strigae in posterior half, with abundant fine setae as follows: anterior margin with row of ~10 setae, medial pair cruciate, lateral to these an upright pair, and lateral to those three pairs medioclinate setae; lateral margins with row ~8 erect setae of variable lengths; dorsal surface with two lateral rows of five short setae each; a short, anteromedial, cruciate pair, and longer posteromedial pair (Fig. 3). Wing membranes micronodulose and with numerous minute setae. LC1 length 0.28 mm, width at level of medial lobe 0.24 mm; LC2 length 0.36 mm; RC1 length 0.26 mm, RC2 length 0.35 mm.
Female: Unknown.
The specific epithet is a patronym honoring Mr. Ailan Hurley-Echevarria for his diligent efforts in processing and screening amber, during which he personally found one of the two specimens.
Photomicrograph of holotype male (Tad-261-A) of Kumarembia hurleyi Engel & Grimaldi, gen. et sp. n., in Early Eocene amber from western India. Total length of individual 5.3 mm.
Photomicrographs of paratype male (Tad-253) of Kumarembia hurleyi Engel & Grimaldi, gen. et sp. n., in Early Eocene amber from western India. A Ventral aspect B Dorsal aspect. Total length of individual 5.2 mm.
Line drawings of Kumarembia hurleyi Engel & Grimaldi, gen. et sp. n. A Head of holotype, dorsal view B Head of paratype, ventral view. Head length (to apex of labrum) 1.1 mm.
Line drawings of Kumarembia hurleyi Engel & Grimaldi, gen. et sp. n. (a, b, and d to same scale). A Protarsus of holotype, dorsal view B Protarsus of holotype, ventral view C Forewing apex of holotype D Male genitalia of holotype, ventral view E Male genitalia of holotype, dorsal view. Refer to description for individual measurements.
The phylogeny of most webspinner lineages remain contentious and in a state of flux. More importantly, numerous undescribed genera and species are known in collections and will likely have a strong influence on any estimations of relationship. It is therefore challenging to make fine determinations of the closest relatives for the Cambay amber fossils. Kumarembia can be placed within the Archembioidea clade by the 10T with a membranous area occupying the base and center of the sclerite, 10R and 10L connected by a thin basal bar, and 10RP2 present, short, and thumb-like. The genus can be placed within the Scelembiidae [= Group C of
Considerable gratitude is extended to Ailan Hurley-Echevarria for his efforts in processing and screening amber, and to A. Sahni for his cooperation and encouragement of paleontological research in India. Photomicrographs were prepared by Ismael A. Hinojosa-Díaz under the direction of MSE. Partial support was provided by University of Kansas Ecology & Evolutionary Biology General Research Fund Allocation #2301465 (to MSE) and by funds provided to DAG by Robert G. Goelet, Chairman Emeritus of the AMNH Board of Trustees. This is a contribution of the Division of Entomology, University of Kansas Natural History Museum.