| Taxon names | Citations | Map localities | Turn highlighting On/Off |







(C) 2011 James R. LaBonte. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
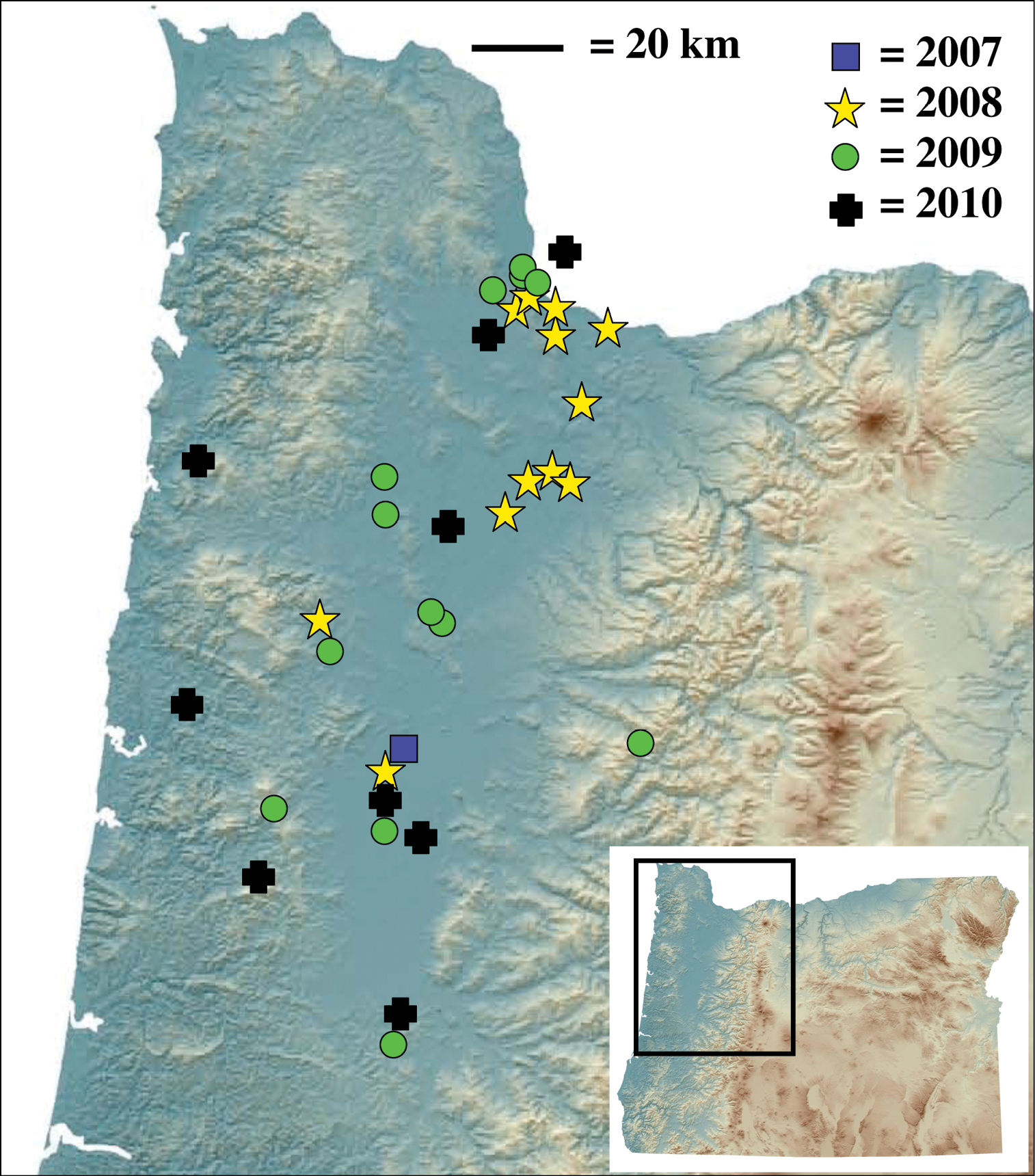
Nebria brevicollis (Fabricius) is one of the most frequently encountered and widely distributed carabid beetles in Europe. Until recently, the only North American records were based on two single specimens, both from the 1930’s in southeastern Canada. In 2008, this species was found at thirteen different sites in five counties in northwestern Oregon. As of the end of 2010, it has been found in thirty-four different sites in ten Oregon counties, with a north-south range of ~150 km and an east-west range of ~90 km. It was also detected in 2010 in southwestern Washington (Vancouver), just north of Portland and the Columbia River.
The ecological amplitude of Nebria brevicollis in Oregon rivals that of the most eurytopic native carabid species, e.g., Pterostichus algidus LeConte and Scaphinotus marginatus (Fischer von Waldheim). It has been found in highly degraded heavy industrial sites, agricultural fields, city parks, gardens, second growth woodlands, mature conifer forests, montane rock gardens, and otherwise pristine stands of old growth noble fir, with elevations ranging from essentially sea level to 1, 249 meters. Climates at these locales vary from that of the Mediterranean Willamette Valley floor, where snow rarely occurs and summers are hot and dry, to the summit of the Oregon Coast Range, where deep snow may be present from November through April and summers are cool. The carabid communities in which Nebria brevicollis has been found range from those predominantly of fellow exotic species, e.g., at heavily perturbed sites, to those where it is the only exotic species, such as at the Coast Range summit.
Nebria brevicollis is clearly an invasive species in that it is not restricted to anthropogenic habitats, is rapidly expanding its North American range, and can be abundant in essentially pristine settings. What is not yet clear is whether it is or will become a damaging species. Although it is already the most abundant carabid species in some settings, based upon pitfall catches, it is unknown whether this represents competitive superiority, trap vulnerability, or utilization of previously untapped or non-limiting resources. Deleterious ecological effects could include not only competition with other predators (including other carabid species) in agricultural and natural settings but also predation upon non-adult stages of threatened and endangered species of butterflies.
Nebria brevicollis, invasive species, Carabidae, North America, Oregon, Washington
There are approximately sixty species of exotic Carabidae established in North America (Y. Bousquet in prep,
Research on exotic species of carabids in North America to this point indicates that most appear to be ecologically neutral or benign, having little, if any, deleterious or beneficial effects on native carabids, on other biotic elements, or on native biota in native habitats. This is largely a consequence of the strong synanthropic associations of most of the exotic carabids, which are perceived as filling and being more-or-less resticted to a largely empty set of anthropogenic niches otherwise occupied by indigenous species of carabids typical of open habitat or by a few indigenous habitat generalists (
Given their presence in non-anthropogenic habitats, can either Carabus nemoralis or Pterostichus melanarius be considered invasive species rather than relatively benign, more-or-less ecologically neutral additions to our fauna? There are a great many definitions of invasive species. However, a practical definition I favor is that an exotic species is invasive if it establishes and reproduces in natural, relatively undisturbed, non-anthropogenic habitats as well as those of human origins AND also has detrimental effects on the native biota OR has deleterious economic (including detrimental effects on desirable exotic biota) or human health effects. Based on this definition, it seems that neither species meets the preceding definition of invasive exotic species. Although both species have been found in and are presumably reproducing in relatively undisturbed forest habitats, to this point neither appear to have detrimental effects on the native carabid species or other native organisms in those habitats. Both species have been associated with reduced native carabid species diversity in anthropogenic habitats but this was not felt to be a pronounced overall effect even in those situations (
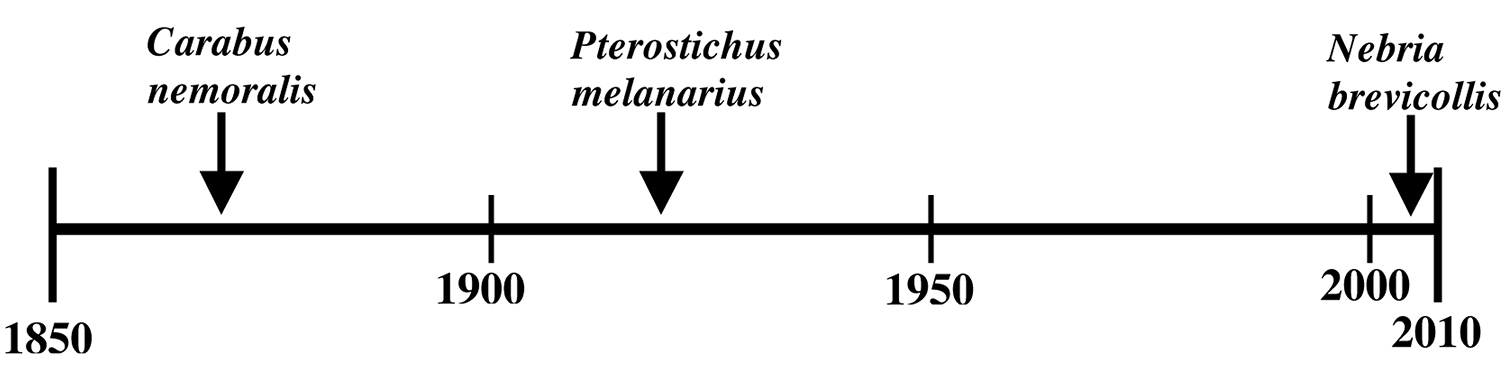
Approximate timeline of detections of Carabus nemoralis, Pterostichus melanarius, and Nebria brevicollis in North America.
Nebria brevicollis (Fabricius) (Figs 2, 3) was found to be established in western Oregon in 2008, with the earliest specimens extending only back to 2007 (
Lateral habitus of Nebria brevicollis.
Dorsal habitus of Nebria brevicollis.
Given the habitat breadth demonstrated by Nebria brevicollis in Oregon, the question was raised in
Although not strictly part of my definition of an invasive species, rapid range expansion is certainly characteristic of invasive species (e.g.,
Known localities of Nebria brevicollis in North America through 2010 and first year of detection per site.
While rate of detection is not necessarily equivalent to rate of range expansion, there is strong evidence for rapid range expansion of this exotic species following recent introduction into Oregon (
This rapid range expansion has been fueled by both the pronounced eurytopy of Nebria brevicollis and effective mechanisms of dispersal. Members of this species in our region not only have flight capability but indeed disperse in this manner. All specimens I’ve seen of Nebria brevicollis in Oregon have fully developed wings and I have found numerous individuals in Lindgren flight intercept funnel traps. Furthermore, it is likely that these insects have been, and continue to be, dispersed over long distances through anthropogenic pathways such as firewood, sod, miscellaneous yard debris, potted plants, and hitchhiking in or on vehicles. Dispersal into remote areas may have been via the latter means. Such stratified dispersal, combining short range diffusive dispersal with satellite populations established far ahead of the main expansion front via anthropogenic means and subsequently coalescing with core populations unless separated by unsuitable habitat, is typical of exotic invasive insect species (
Kavanaugh and LaBonte (2008) found Nebria brevicollis flourishing in at least lightly disturbed mixed forest at low elevation (Fig. 5). Nonetheless, it could be legitimately argued that this was not a natural and non-anthropogenic setting as the area had undergone rural residential development with homes (Fig. 6) and agricultural endeavors imbedded therein and the forest had been cut some decades previously. In this manner, Nebria brevicollis was behaving in a manner similar to that documented for Carabus nemoralis and Pterostichus melanarius. However, since
Second-growth mixed forest near Dallas, Oregon.
Rural homesite amid second-growth mixed forest near Dallas, Oregon.
Sites outside of the eastern and western margins of the Willamette Valley (Fig. 4) are amid non-anthropogenic and largely natural habitats. The easternmost site is at a remote locale in the western foothills of the Cascade Mountains at an elevation of 1, 220 m and consists of old growth coniferous closed canopy forest of noble fir (Abies procera), western hemlock (Tsuga heterophylla), and Douglas-fir (Pseudotsuga menziesii). The three westernmost locales in which Nebria brevicollis was found in 2010 are all in forested areas in the Coast Range. One of these, Prairie Peak, is quite remote and accessible only by a dirt road and is amid mature second-growth Douglas-fir and western hemlock forest. Nonetheless, Nebria brevicollis was found to be very abundant in the essentially undisturbed native grass bald habitat (Figs 7–10) at 1, 040 m elevation, even via casual collecting.
Oregon Coast Range, Prairie Peak 7 (top left), view of Oregon Coast Range to north from summit 8 (top right), grass bald at summit 9 (bottom left), glacier lily (Erythronium oregonum) patch at summit 10 (lower right), rock garden at summit..
Perhaps the most disturbing locale, in an ecological context, where Nebria brevicollis has been found in abundance is at and below the summit of Marys Peak. This mountain is at the crest of the Oregon Coast Range, at an elevation of 1, 250 m (Fig. 11). Higher elevations on the peak often remain snow covered from mid-November through March or April. An extensive grass bald and meadow system extends throughout the subsummit and the vicinity of the summit (Fig. 12). A large rock garden is present among the exposed bedrock at the summit. A forest dominated by mature and old growth noble fir with some western hemlock and Douglas-fir is present from near the summit down to about 900 m (Figs 13 and 14). Most of the stands are closed canopy, although numerous gaps are present. This forest has not been logged, although most of the trees date from a stand-replacing fire in the late 1840’s. Although a few exotic species of carabids can be found in disturbed areas along the access road, manual collecting and a 1984-1985 pitfall study have demonstrated that the forest carabid fauna is pristine, consisting only of indigenous species. In the pitfall study, twenty-one species were found, with eight species comprising ~96.5% of all individuals captured (J.R. LaBonte, unpublished data).
Oregon Coast Range, Marys Peak 11 (upper left), Marys Peak in May, seen from the Willamette Valley south of Corvallis 12 (upper right), meadow at summit (courtesy D.R. Maddison) 13 (lower left), old growth noble fir stand on north flank 14 (lower right), old growth noble fir stand on northwest flank.
In September 2009, I was appalled to find large numbers of Nebria brevicollis under cover in the closed canopy stands along the north flank of Marys Peak (Figs 13 and 14). This was at one of the sites I had pitfalled in 1984–1985 and I had frequently collected there since that time. Subsequent collecting found Nebria brevicollis to be extremely abundant throughout forest, meadow, and summit areas. In many situations, it was the most common carabid found. During one collecting trip in late June 2010, it was the only abundant carabid to be found at the summit in the rock garden and adjacent meadows. Turning rocks often revealed a dozen or more individual Nebria brevicollis.
In summary, Nebria brevicollis exhibits tremendous ecological amplitude in Oregon, being found from virtually at sea level to over 1, 200 m in elevation and is abundant in habitats ranging from desolate industrial sites to pristine montane old growth forest. Such ecological range is unrivaled by any other exotic carabid species in Oregon and is only matched by two extremely eurytopic indigenous species, Pterostichus algidus LeConte and Scaphinotus marginatus (Fischer).
Is Nebria brevicollis a competitor of other carabid species?Exotic species newly present in a habitat can cause populations of indigenes or previously established exotics to decline through competition for limiting resources such as food or shelter, via predation, or by introduction of novel parasites and pathogens (e.g.,
There is no question that Nebria brevicollis is extremely abundant in some Oregon habitats. Such hyper-abundance is typical of invasive insect generalist predators, which often reach densities several orders of magnitude greater than similar indigenous species (
If Nebria brevicollis is indeed causing other carabid species to decline in its presence, there are several mechanisms through which deleterious impacts could be effected, including competitive displacement (for resources or via interference) or intraguild predation (Snyder and Crowder 2010). Given the modest size of final instar larvae and adults of this insect, it seems unlikely that intraguild predation upon other adult carabids by Nebria brevicolllis would be significant, except perhaps upon substantially smaller species, such as members of Bembidion Latreille or Trechus Clairville. Predation by immature and adult Nebria brevicollis upon non-imagines of other carabid species seems much more likely to have substantive effects thereon, but the frequency of this behavior is unknown. Interference competition by Nebria brevicollis upon other carabid taxa is certainly possible, but has not been documented.
The most probable means by which Nebria brevicollis would negatively affect populations of other carabid species would be via competition for food or shelter. Displacement of ecologically similar indigenous species by introduced invasive insect generalist predators occurs with striking regularity (
Competition would seem most likely to be greatest for food (presuming that it is not in unlimited supply) and most intense among those species with diets and phenologies most similar to that of Nebria brevicollis. Although there appears to be surprisingly little data available on the diet of Nebria brevicollis, it appears to fit the archetypal carabid profile as an opportunistic predator taking a wide array of prey within the limitations imposed by morphology, phenology, habitat, and behavior. Nebria brevicollis has been documented as feeding on molluscs (e.g., slugs), earthworms, mites, Opiliones, spiders, Collembola, and the eggs, larvae, pupae, and adults of various small insects, including Coleoptera (e.g., Elateridae), Diptera (e.g., Anthomyiidae), and Lepidoptera (small caterpillars) (
Although there is rather little published feeding data on the indigenous species found in Oregon with Nebria brevicollis, it appears that most are opportunistic predators or omnivores and the same is true for the exotic species (e.g.,
The capabilities for opportunistic predation of Nebria brevicollis are apparently greater than those of other Nebria, for this species has been observed foraging over grasses (Fig. 15) and forbs (e.g., lupine, Fig. 16) in mid-day on Marys Peak (J.R. LaBonte and D.R. Maddision, personal observations). This behavior has not been formerly documented for Nebria brevicollis, let alone for any other species of Nebria (D.H. Kavanaugh, pers. comm.). Numerous individuals at several locales were observed behaving in this manner. The possibility that vegetation was used as “launch pads” for dispersal flights was considered, but no flight or pre-flight behavior was observed. The beetles were traveling up and down grass stalks and wandering over lupine leaves and were clearly engaged in investigative and foraging behavior. Access to non-prostrate vegetation expands the potential prey resources for Nebria brevicollis beyond the epigean habitats to which many other predatory carabids are restricted. Combined with the marked eurytopy of this beetle, such foraging behavior may enable Nebria brevicollis to achieve higher population densities and subsequent competitive advantage over sympatric carabid species (e.g.,
Oregon Coast Range, Marys Peak, rock garden meadow at summit. Images courtesy of D.R. Maddison, 15 Nebria brevicollis on grass stems, 16 Nebria brevicollis on lupine vegetation.
A final question in this regard is whether the phenology of Nebria brevicollis in North America differs sufficiently from sympatric carabids to provide temporal refugia from competition for those species. For instance, several studies have concluded that spring-active native carabid species can coexist with the predominantly summer active Pterostichus melanarius (
It is important to remember that the phenological patterns discussed above refer to adult phenologies. Larvae of carabid species which have imaginal temporal refugia from interaction with adult Nebria brevicollis are still vulnerable to potential competition, not only from adults but from the larvae. For instance, since the larvae of Nebria brevicollis are active from fall through spring (
There is little question that Nebria brevicollis has great potential for deleterious effects on sympatric species of other carabids. This exotic species is extraordinarily eurytopic, can attain extremely high populations, is polyphagous, and is active during periods when other carabid species would be vulnerable to competitive interactions and, possibly predation. However, at this time, there is no unequivocal evidence that negative impacts have occurred.
Does Nebria brevicollis have deleterious effects on other, non-carabid, taxa?The apparently opportunistic predation of adult Nebria brevicollis upon small and modest-sized invertebrates combined with the great abundance achieved by this exotic predator in many habitats could pose threats to some non-carabid taxa, especially some threatened and endangered Lepidoptera. It has been found at Mount Hebo (Tillamook County) (D.R. Maddison, personal observation), one of the few remaining locales of the federally listed threatened Oregon silverspot butterfly, Speyeria zerene hippolyta (Edwards). Eggs of this butterfly are laid on low vegetation near host plants, blue violet (Viola adunca) and larvae and pupae are on violet foliage (
Icaricia icarioides fenderi (Macy), the Fender’s blue butterfly, is a federally listed endangered subspecies that is also conceivably threatened by Nebria brevicollis. During spring and summer, non-adult stages are on the host plant, Kincaid’s lupine (Lupinus sulphureus kincaidii) (
Whether the occurrence of Nebria brevicollis at sites where threatened and endangered butterflies exist constitutes a risk to those insects is unknown. However, these opportunistic predators are known to feed on the eggs, larvae (including caterpillars), and pupae of a wide variety of insects. There is no a priori reason to presume that they would refrain from feeding on any available prey of suitable size. Of course, this also raises the question of what other threatened or endangered insects (or those that are potentially so) within the current or future North American range of Nebria brevicollis would be threatened by this exotic predator. For instance, the Oregon Biodiversity Information Center (2010) currently lists 102 species and subspecies of insects as being rare, threatened, or endangered. Although many of these taxa are outside of the known distribution of Nebria brevicollis or are found in situations that would render them at low risk from this species (for instance, twenty-four species are caddisflies, Trichoptera), some could conceivably be preyed upon by this beetle. Of course, the effects of a hyper-abundant, eurytopic, opportunistic exotic predator on non-threatened species of other insects or invertebrates are even more speculative.
ConclusionsNebria brevicollis is rapidly expanding its range in western Oregon and Washington. It is also an extraordinarily eurytopic species, demonstrating a habitat breadth surpassing any previously established exotic species of Carabidae in Oregon. Only a few indigenous carabid species rival its eurytopy. Very high populations can be attained in all these habitats. It is also a very polyphagous opportunistic predator within the prey size constraints imposed by morphology. Unlike most carabid species sharing its habitats, Nebria brevicollis is also capable of foraging on erect vegetation. These are all earmarks of an exotic species with great potential for invasiveness. While Nebria brevicollis clearly meets the criteria of an invasive species with regard to range expansion and incursions into non-anthropogenic habitats, it is not known whether it is having deleterious effects on sympatric carabid species or non-carabid taxa.
This is a golden opportunity to assess the effects of a recently introduced and established exotic species. It is rare that the early stages of invasion are well documented, as in this case. I encourage those colleagues with the interest and resources to investigate this intriguing situation.
I wish to thank Jana C. Lee, USDA-ARS, Horticultural Crops Research Laboratory, Corvallis, OR, who generously provided information with regard to Nebria brevicollis from her research on the predators and pests of blueberry fields. The U.S. Department of Agriculture, Animal Pest and Health Investigative Service, Plant Pest and Quarantine, provided funding through the Cooperative Agricultural Pest Surveys for the Exotic Terrestrial Plant Pest and Grape Pest surveys, which yielded many of the records of Nebria brevicollis in Oregon. I also wish to recognize the Oregon Department of Agriculture (ODA) and my colleagues therein for conducting the aforementioned surveys. Steven A. Valley, ODA’s peerless imaging technician, acquired the habitus images of Nebria brevicollis included in this paper. David R. Maddison, Zoology Department and curator of the Oregon State Arthropod Collection (OSAC), Oregon State University, Corvallis, OR, provided his observations on Nebria brevicollis, accompanied me on many of the sojourns into the field yielding data thereon, and provided several key images used in this paper. Christopher Marshall, collection manager of OSAC, enabled me to readily access the collection in search of Nebria brevicollis records. Terry L. Erwin and David H. Kavanaugh generously reviewed earlier versions of the manuscript and their comments markedly improved it. Dave also provided much needed encouragement at a critical juncture.