(C) 2011 Jenna M. Jacobs. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The genus Calosoma (Coleoptera: Carabidae) is a group of large, sometimes ornate beetles, which often voraciously attack caterpillars. Many studies have reported Calosoma beetles being highly conspicuous during defoliator outbreaks. Based on observations of individual beetle behavior, patterns of activity density and phenology we provide a hypothesis on how environmental cues may synchronize Calosoma activity with periods of high defoliation. We have observed that adults of Calosoma frigidum construct underground burrows similar to those reported to be created by larvae for pupation. We propose that small increases in soil surface temperature caused either by defoliation events or decreased albedo of blackened, burned soil causes beetles to leave their underground burrows and begin foraging. Indirect support for this hypothesis comes from high levels of adult Calosoma frigidum collected in relatively small patches of burned forest (<200m2) relative to the surrounding mosaic of unburned forest shortly after a prescribed surface burn.
Calosoma, foraging, behavior, prescribed fire, defoliation
Calosoma frigidum Kirby, also known as “The Cold Country Caterpillar Hunter” (
Prey density has been linked to Calosoma ovarian development, oviposition behaviour, and female activity (
Several studies have noted increased abundance of Calosoma beetles in pitfall traps in response to changes in habitat variables.
Based on comparisons of pitfall trap catch rates Calosoma frigidum in a mosaic of burned and unburned forest, we hypothesize that activity of Calosoma frigidum is triggered by increased soil temperature. We explain that adult Calosoma construct an underground burrow, similar to that made for pupation (
This study took place at the Ecosystem Management by Emulating Natural Disturbance (EMEND) research site in northwestern Alberta, Canada (56°46’13”N, 118° 22’28”W). The 10 ha stand chosen for the study was dominated by trembling aspen (Populus tremuloides Michx.) and balsam poplar (Populus balsamifera L.) with the understory dominated by green alder (Alnus crispa (Aiton))and river alder(Alnus tenufolia Nuttall). Further information on the EMEND site and ground beetles responses can be found in
On April 26th, 2000 a low intensity prescribed burn using drip torches was initiated as an EMEND treatment. The severity of the fire was not sufficient to kill trees and by the end of May evidence of fire was limited to some superficial charcoal deposits on the forest floor and at the base of trees. Surveys of the stand following fire revealed a mosaic of patches where fire had consumed a small portion of the forest floor and scorched tree bases adjacent to patches with no evidence of fire.
Within the stand subjected to prescribed burning we selected four areas (each ca. 100m2) in which burning was clearly evident and four additional areas with no evidence of burning, and installed two pitfall traps per area (16 in total) on June 5th, 2000. Our pitfall traps consisted of an outer permanent (1L) cup, acting as a sleeve to maintain the pit and minimize disturbance at checks, and a removable (500ml) inner cup (
In an effort to make general observations of the beetle, we examined five adult Calosoma frigidum in the lab in 5L glass jars, filled with about 10cm of soil. These jars were left at room temperature for approximately 2 years.
Data AnalysisCommunity level analysis revealed virtually no effect of burning on the larger beetle community or on activity of any individual species except for Calosoma frigidum. We therefore restricted the scope of this study to explaining the response of this species to the EMEND burning treatment. All analyses were conducted in R 2.12.1 (
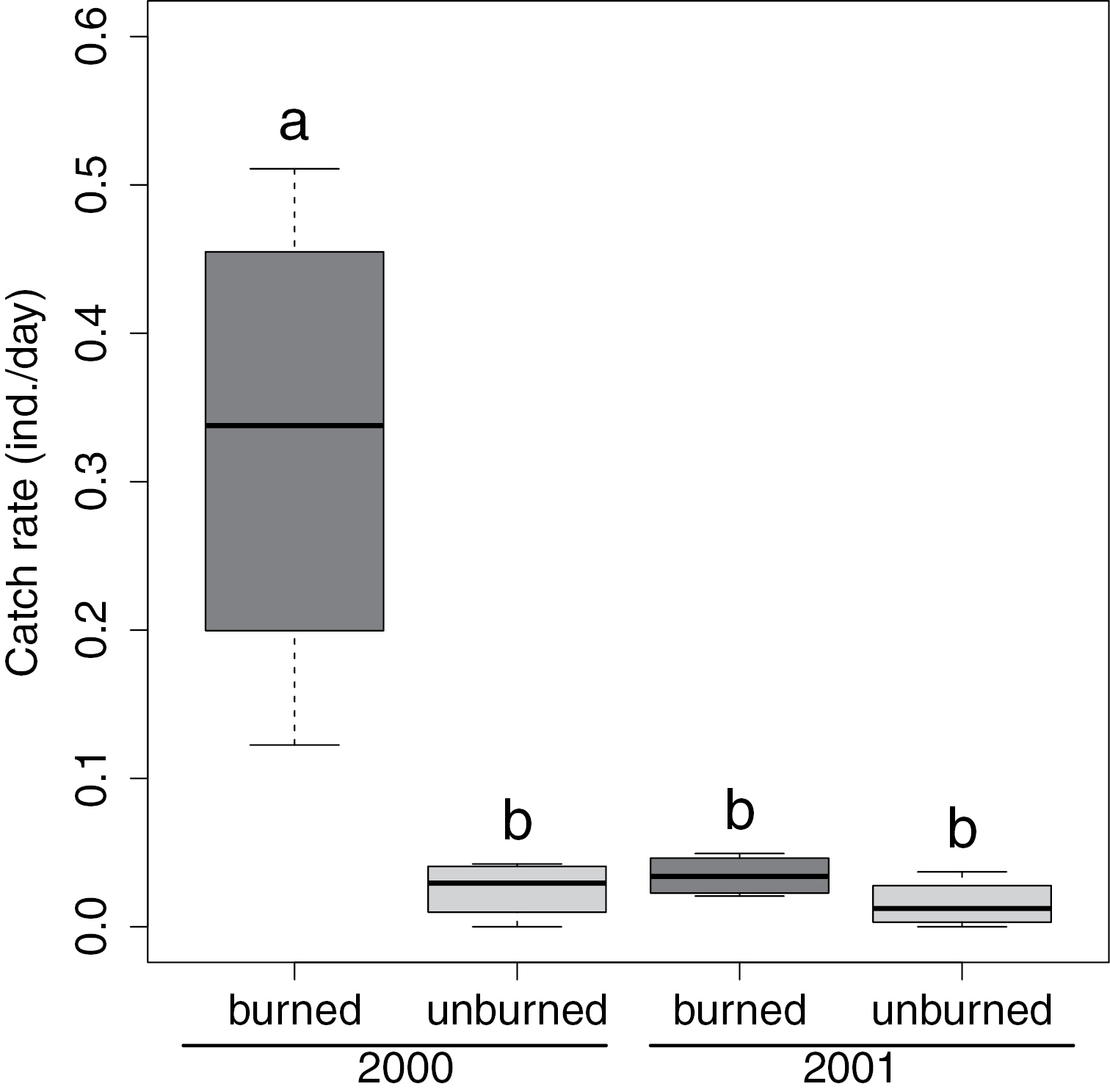
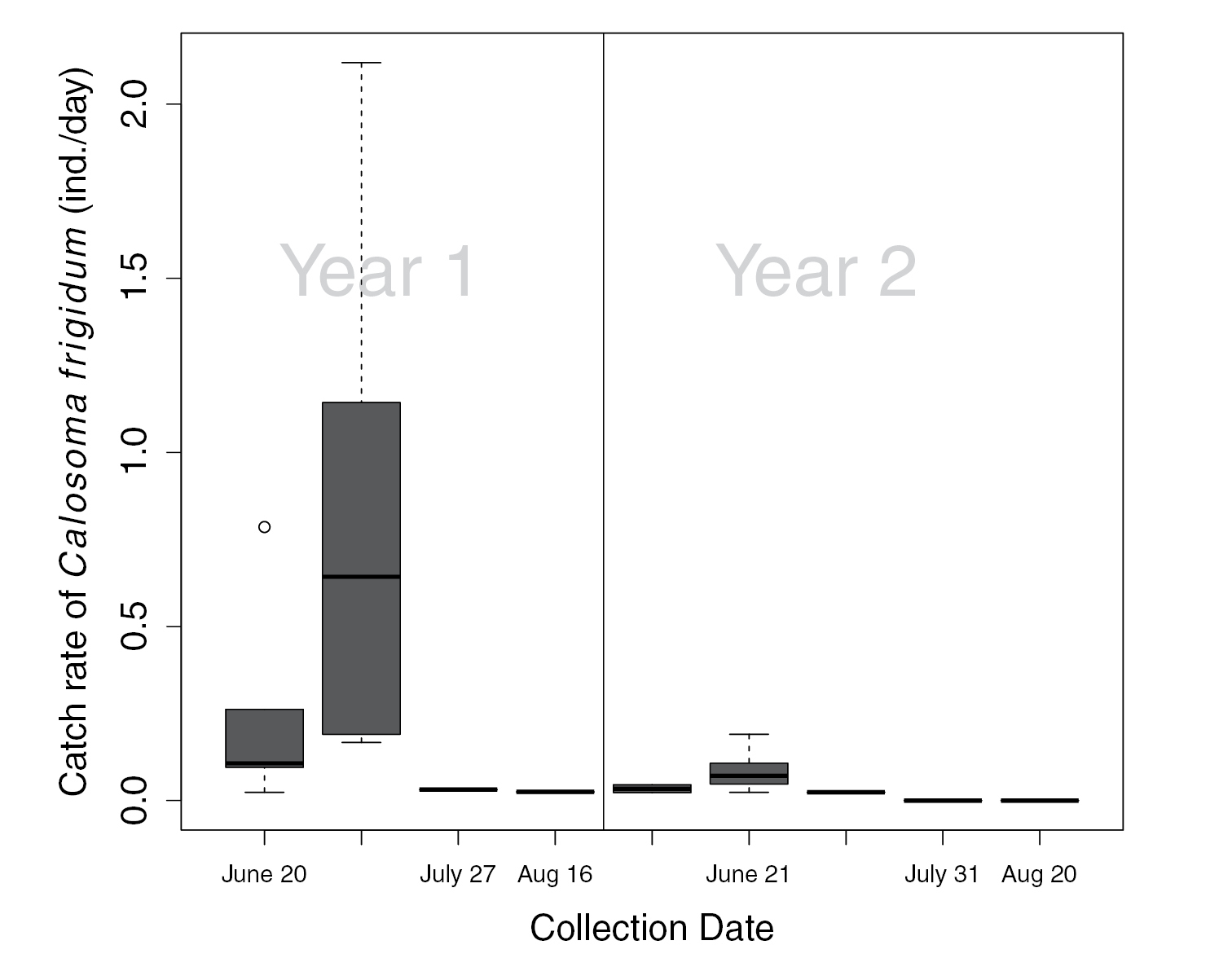
We collected 298 individuals of Calosoma frigidum, representing 14% of the total catch of 74 species. Catch rates of Calosoma frigidum were significantly higher in the burned patches during the year of the fire (2000) than in the unburned patches or anywhere in the stand the following year (Figure 1, RM-ANOVA, Treat x Year, F1, 6=0.08, P=0.017). The highest catch rates were recorded in the July 11th collection in 2000 and for the June 21 in 2001 (Figure 2). However, despite similarity in activity pattern, the overall activity in 2000 was much higher than in the following year.
In the laboratory one of the adult beetles created a burrow ca. 4cm under the soil surface as the soil dried. Whether the other beetles brought into the lab also subsequently created these burrows is unknown, as this behavior was not specifically under study.
Box and whisker plot of catch rates of Calosoma frigidum in burned and unburned patches. Letters denote the results of a Tukey’s HSD post-hoc test following a 2 factor repeated measures ANOVA. Box represents the upper and lower quartiles divided by the median and whiskers are the largest and smallest values.
Box and whisker plot of catch rates in burned patches, by collection date, for the year of the fire (year 1) and the following year (year 2). Box represents the upper and lower quartiles divided by the median and whiskers are the largest and smallest values, excluding outliers represented by circles ○.
Increased catch rates of Calosoma frigidum observed in the same year as the prescribed burn could be explained by either increased activity in burned plots or actual differences in population density between burned and unburned areas. While we are not capable of completely discounting this second explanation, we favor the first for several reasons. First, our study site was very homogeneous and the chances of all four burned patches being located in areas of high Calosoma frigidum density and all four unburned patches in areas of low Calosoma frigidum density seems low. Second, we observed increases in catch rates within the same year as the fire, discounting the possibility of increases due to reproduction. Thirdly, we feel it is unlikely that local densities increased as a result of immigration of Calosoma frigidum into the burned areas for the following reasons. If local density increased, the 3–5 year life span (
We propose that the fire event itself had little direct influence on the beetles because it would have been of very short duration. Instead we posit that charcoal deposited on the soil surface in the wake of the fire resulted in higher soil temperature from decreased surface albedo. Sustained increases in soil temperatures from sun exposure in burned sites may be similar to those during severe defoliation and could serve as a signal to adult beetles to begin foraging when potential prey populations are high. This could explain the results of
Decreasing forest cover caused by forest harvest could also be associated with small increases in temperature of the soil surface, explaining why catch rates of Calosoma frigidum increased in residual strips compared to nearby dense forests in Maine (Jennings et al. 1986). This would also explain why the highest levels of capture were recorded at 50% green tree retention the 2 years following harvest at EMEND (
The capacity of adult beetles to construct underground burrows in conjunction with their long life span may permit Calosoma to effectively exploit cyclic defoliator populations characteristic of the boreal zone (e.g., those of forest tent caterpillar, bruce spanworm and spruce budworm). Calosoma populations could initially build-up during an outbreak through a combination of both localized immigration from neighboring stands and emergence of resident adults from underground burrows within the stand. If defoliator populations remain high, both immigrants and residents could conceivably accumulate in the stand in adult burrows in the soil. When an outbreak collapses and there are no signals to emerge and run, these adults could lay dormant for several years. As the next outbreak unfolds, increased soil temperatures would effectively synchronize adult beetles with an increasing source of prey as well with potential viable congeners to facilitate reproduction. The proportions of these individuals that emigrate following the collapse or remain in adult burrows as a ‘sit and wait’ strategy should vary with outbreak frequency, but such data remain to be collected.
ConclusionsWe have offered a possible mechanism for initiating the foraging response in dormant Calosoma beetles, using specific evidence from Calosoma frigidum in NW Alberta, Canada. Increases in the catch rate and activity patterns of Calosoma beetles have been recorded during defoliation events, herbicide treatments, mechanical canopy thinning and soil charcoal deposits following fire. All these events have the potential to warm the soil surface in a sustained way, and we suggest that such warming could cue beetles to emerge from their underground burrows and begin foraging. Although we acknowledge that this evidence is somewhat circumstantial, we hope that it offers useful insight to inspire further work about what triggers of foraging behavior in this very interesting beetle.
The authors thank Drs. David Langor and Jan Volney for their part in establishing the EMEND project and providing mentorship to JJ, CB and TW during work at EMEND. We also thank Evan Esch, for extremely capable work as a lab/field assistant and for discovering the C. frigidum burrow in the pickle jar. We also thank Canadian Forest Products (CANFOR) and Daishowa-Marubeni International Ltd. (DMI) for financial support of the project through the forest improvement association of Alberta (FRIAA), as EMEND provides a base for many interesting discoveries not in the mainstream of the experiment. Finally, we offer very special thanks to Ross and Joyce Bell whose enthusiasm for carabidology has inspired the three academic generations represented here to pursue carabidology and entomology from an enthusiastic biological perspective.