(C) 2011 Sarah A. Maveety. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Carabid beetles were sampled at five sites, ranging from 1500 m to 3400 m, along a 15 km transect in the cloud forest of Manu National Park, Perú. Seasonal collections during a one year period yielded 77 morphospecies, of which 60% are projected to be undescribed species. There was a significant negative correlation between species richness and altitude, with the number of carabid species declining at the rate of one species for each 100 m increase in altitude. The majority of species (70.1 %) were restricted to only one altitudinal site and no species was found at more than three of the five altitudinal sites. Only one genus, Pelmatellus (Tribe Harpalini), was found at all five sites. Active (hand) collections yielded approximately twice as many species per individuals collected than passive (pitfall trap) collections. This study is the first systematic sampling ofcarabid beetles of a high altitude gradient in the cloud forests of southeastern Perú and supports the need to conserve the zone of extremely high biodiversity present on the eastern slopes of the Peruvian Andes.
Ground beetles, tropical montane forests, Neotropics, pitfall traps, Andes
Tropical forests may support up to 80 percent of the world’s biodiversity (
As with lowland rain forests, montane cloud forests are subject to numerous threats, including logging and land conversion to agriculture and pasturage (
Biological organisms are useful as indicators of local habitat change and as estimators forest fluctuations due to fragmentation or climate change (e.g.,
In many areas of the tropics, where biodiversity is especially high but poorly inventoried, ecological researchers often encounter what has been termed the “taxonomic impediment” (
In the present study, carabid beetles were collected from sites located on an altitudinal cline in the Andes Mountains in order to estimate absolute species diversity and extrapolated species richness. Incidence of rare and altitudinally restricted species is evaluated. Both active and passive collection methods were employed and the efficacy of these methods is compared.
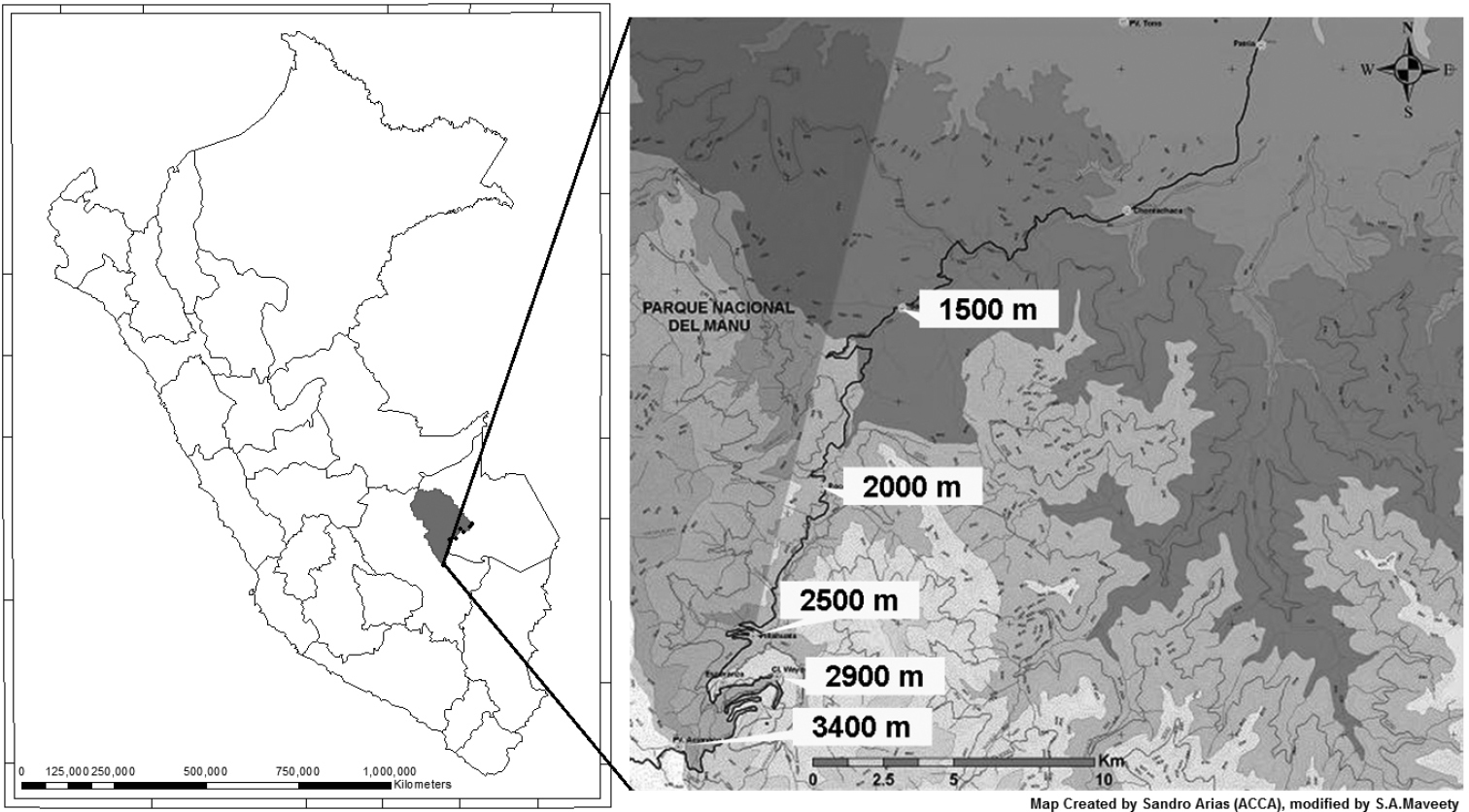
Methods Field studyCarabid beetles were collected at sites adjacent to the Cusco-Pilcopata highway in the Cultural Zone of Manu National Park, Department of Cusco, in southeastern Perú. Five sampling sites were established at approximately 500 m altitudinal intervals: Acjanaco Vigilante Point, (3400 m); Wayqecha Biological Station, (2900 m); Pillahuata Research , (2500 m); Rocotal, (2000 m); and San Pedro Biological Station, (1500 m). The transect decreases 1900 total meters over 15 km in space. Table 1 presents the GPS and altitude locality data for all sites. Geographical locations of the sites are depicted in Fig. 1.
Locality data for the five altitudinal sites.
| Altitude (m) | Altitude Band | Latitude (S)/Longitude (W) | No. Individuals | No. Genera | No. Species | |
|---|---|---|---|---|---|---|
| AJANACO | 3470 | 3400 | 13°11.79'S, 71°37.22'W | 162 | 3 | 7 |
| WAYQECHA | – | – | ||||
| Trap A | 2791 | 2900 | 13°10.99'S, 71°35.07'W | 806 | 6 | 21 |
| Trap B | 2947 | 13°11.55'S, 71°35.28'W | ||||
| PILLAHUATA | – | – | ||||
| Trap A | 2436 | 2500 | 13°09.69'S, 71°35.49'W | 381 | 7 | 21 |
| Trap B | 2425 | 13°09.69'S, 71°35.47'W | ||||
| ROCOTAL | – | – | ||||
| Trap A | 2084 | 2000 | 13°06.80'S, 71°34.25'W | 227 | 13 | 28 |
| Trap B | 2082 | 13°06.68'S, 71°35.00'W | ||||
| SAN PEDRO | – | – | ||||
| Trap A | 1432 | 1500 | 13°03.37'S, 71°32.83'W | 348 | 12 | 28 |
| Trap B | 1427 | 13°03.37'S, 71°32.81'W |
Map of collection sites.
At each site, carabid beetles were collected passively (via pitfall traps) and actively (via hand searches). Pitfall traps consisted of 1 liter plastic cups, the mouth inserted flush with ground level, with a plastic roof anchored by nails over the opening to prevent rain water from entering. Each trap was filled with salt water as a preservative and a small amount of soap to minimize surface tension. In order to account for possible microsite variation, two clusters of traps were placed at least 10 meters apart, with six traps per cluster for a total of 12 traps per site. Collections were conducted monthly from September 2007 to July 2008 by SAM and Peruvian assistants. Active collections consisted of sifting through leaf litter along the forest floor and examining above ground vegetation to approximately 1.5 m in height. Hand collections were done at night due to the generally nocturnal behavior of carabid beetles and included an additional collection during December 2008. All beetles were preserved in 95% ethanol. In order to provide an estimate of search effort, the number of collectors and the time spent searching was recorded for each site (see Table 1 of Online Material).
Since there were no previous collections in this area, a specific key to high altitude Neotropical carabid beetles and a list of species to this area were not available. Specimens where therefore identified to genus using a Neotropical Carabidae key (
Species accumulation curves plot the number of species against the number of individuals in a sample and thus adjust species number for total sampling effort. As more individuals are collected only the rarest species are thought to be excluded from the collection; therefore, when all species are collected, the plotted line reaches a horizontal asymptote. Smoothed species accumulation curves were constructed using EstimateS 7.52 (
The number of rare species was estimated using both a taxonomic and an ecological index. The taxonomic index accounts for rare species as singletons (when a species is represented by one specimen) and doubletons, etc., up to n = 5. Such an index is more often used in studies of diversity from a taxonomic perspective (e.g.,
Nonmetric Multidimensional Scaling (NMDS) in R 2.8.1 <www.r-project.org> was used for ordination analysis. Ordination analysis scores multivariate data in Euclidean space along two principle axes. NMDS uses a community similarity index to score data and places similar data points close in the ordination space. For all NMDS analyses Bray-Curtis similarity indices were used (
A total of 1, 924 carabid beetle specimens were collected, represented by 13 tribes, 22 genera, and 77 morphospecies (Table 2). The most speciose tribes are Harpalini (represented by 27 morphospecies), Platynini (13 morphospecies) and Bembidiini (13 morphospecies). These three tribes also contain the largest number of collected individuals within a tribe: Harpalini (n = 1054), Platynini (n = 528), and Bembidiini (n = 118). Among the five sites the greatest number of individuals collected occurred at 2900 m (n = 802), with the least number of individuals at 3400 m (n = 162).
List of tribes, genera and morphospecies of Carabidae collected.
| Tribe | Morphospecies | No. | Tribe | Morphospecies | No. |
|---|---|---|---|---|---|
| Bembidiini | Bembidion A | 5 | Pelmatellus H | 281 | |
| Bembidion B | 1 | Pelmatellus I | 8 | ||
| Bembidion C | 12 | Pelmatellus J | 113 | ||
| Bembidion D | 3 | Pelmatellus K | 15 | ||
| Bembidion E | 25 | Pelmatellus L | 2 | ||
| Bembidion F | 10 | Trichopselaphus A | 9 | ||
| Bembidion G | 1 | Trichopselaphus B | 1 | ||
| Bembidion H | 8 | Lachnophorini | Anchonoderus A | 12 | |
| Bembidion I | 10 | Psuedophoriticus A | 18 | ||
| Bembidion J | 5 | Psuedophoriticus B | 2 | ||
| Bembidion K | 6 | Lebiini | Calleida A | 3 | |
| Bembidion L | 25 | Calleida B | 1 | ||
| Bembidion M | 7 | Lebia A | 1 | ||
| Cicindelinae | Psuedoxycheila lateguttata peruviana | 5 | Oodini | Lebia B | 1 |
| Dercylus A | 1 | ||||
| Galeritiini | Galerita A | 29 | Ozaeniini | Pachyteles A | 3 |
| Galerita B | 3 | Pachyteles B | 2 | ||
| Galerita C | 1 | Pachyteles C | 1 | ||
| Galerita D | 2 | Perigonini | Diploharpus A | 1 | |
| Harpalini | Goniocellus A | 1 | Platynini | Dyscolus A | 365 |
| Notiobia A | 2 | Dyscolus B | 29 | ||
| Notiobia B | 3 | Dyscolus C | 22 | ||
| Notiobia C | 18 | Dyscolus D | 6 | ||
| Notiobia D | 3 | Dyscolus E | 1 | ||
| Notiobia E | 7 | Dyscolus G | 4 | ||
| Notiobia F | 15 | Dyscolus H | 1 | ||
| Notiobia G | 12 | Dyscolus I | 28 | ||
| Notiobia H | 2 | Dyscolus J | 3 | ||
| Notiobia I | 263 | Dyscolus L | 1 | ||
| Notiobia J | 1 | Dyscolus K | 1 | ||
| Notiobia K | 2 | Dyscolus M | 1 | ||
| Notiobia L | 3 | Glyptolenus A | 66 | ||
| Pelmatellus A | 1 | Pterosticini | Loxandrus A | 1 | |
| Pelmatellus B | 9 | Loxandrus B | 2 | ||
| Pelmatellus C | 276 | Psuedobarys A | 1 | ||
| Pelmatellus D | 3 | Trichonilla A | 1 | ||
| Pelmatellus E | 1 | Scaritini | Ardistomus A | 3 | |
| Pelmatellus F | 2 | Trechini | Trechischibus A | 124 | |
| Pelmatellus G | 1 | Paratrechus sensulatt | 6 |
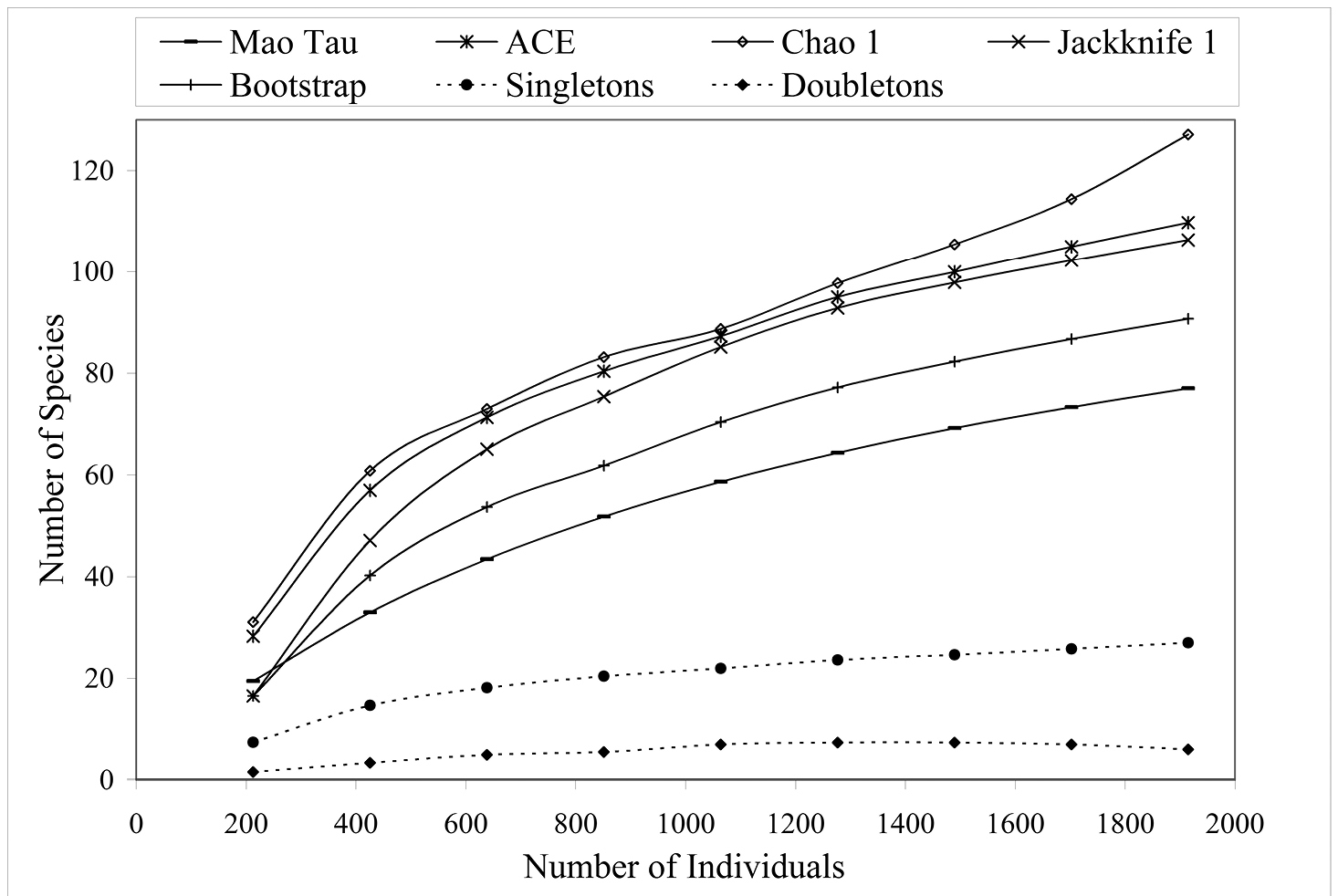
Figure 2 shows the species accumulation curve when all collection sites are combined (n = 1924) using the Mao Tau estimator (
Species accumulation curves for 5 diversity indices when all altitudes and collection types (active or passive) are combined (n = 1924).
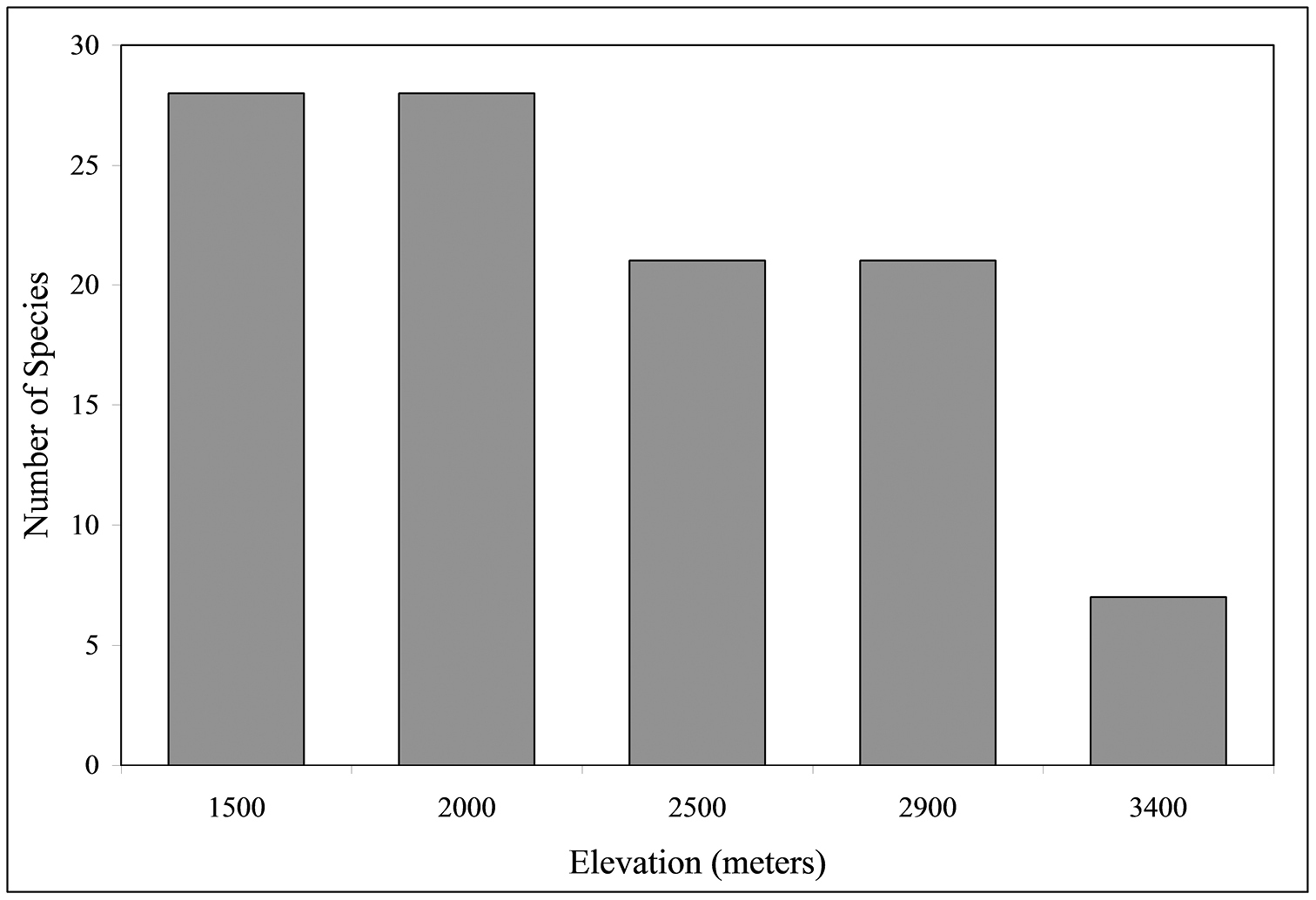
As depicted in Fig. 3, the total number of species collected varied significantly with altitude (χ2 = 14.46, P < 0.01), with the highest species richness (S = 28) found at both 2000 m and 1500 m. There is a significant negative correlation between S and altitude (r = 0.91, P < 0.05). Regression analysis (y = –0.010x + 46.7, Freg = 13.7, p < 0.05) indicates that the number of carabid species declines at the rate of 1.3% of the species diversity for each 100 m increase in altitude. Species numbers differed significantly (via χ2 tests based on magnitude array) among altitudes as follows: 1500 m = 2000 m ≠ 2500 m = 2900 m ≠ 3400 m. Number of genera and tribes are also negatively correlated with altitude (r = 0.94, P <0.05; r = 0.91, P <0.05, respectively).
Number of species at five altitudes when all altitudes and collection types (active or passive) are combined (n = 1924).
There was a high level of site specificity for species, genera and orders (Table 3). The majority of species (70.1 %; 54/77) were collected at only one altitudinal site. Only 6.5% (5/77) of the species were found at three altitudinal sites and no species was found at more than three sites. At higher levels of taxonomic organization, similar trends, though less pronounced, occurred, with 45.5 % (10/22) of genera and 38% (5/13) of tribes limited to one altitude site. One Tribe (Harpalini) and only one genus within (Pelmatellus) were found at all five sites.
Site specificity by taxa (see text for additional descriptions).
| Number of Sites occupied | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Species | 54 | 18 | 5 | 0 | 0 |
| Genera | 10 | 7 | 1 | 2 | 1 |
| Tribes | 5 | 3 | 2 | 2 | 1 |
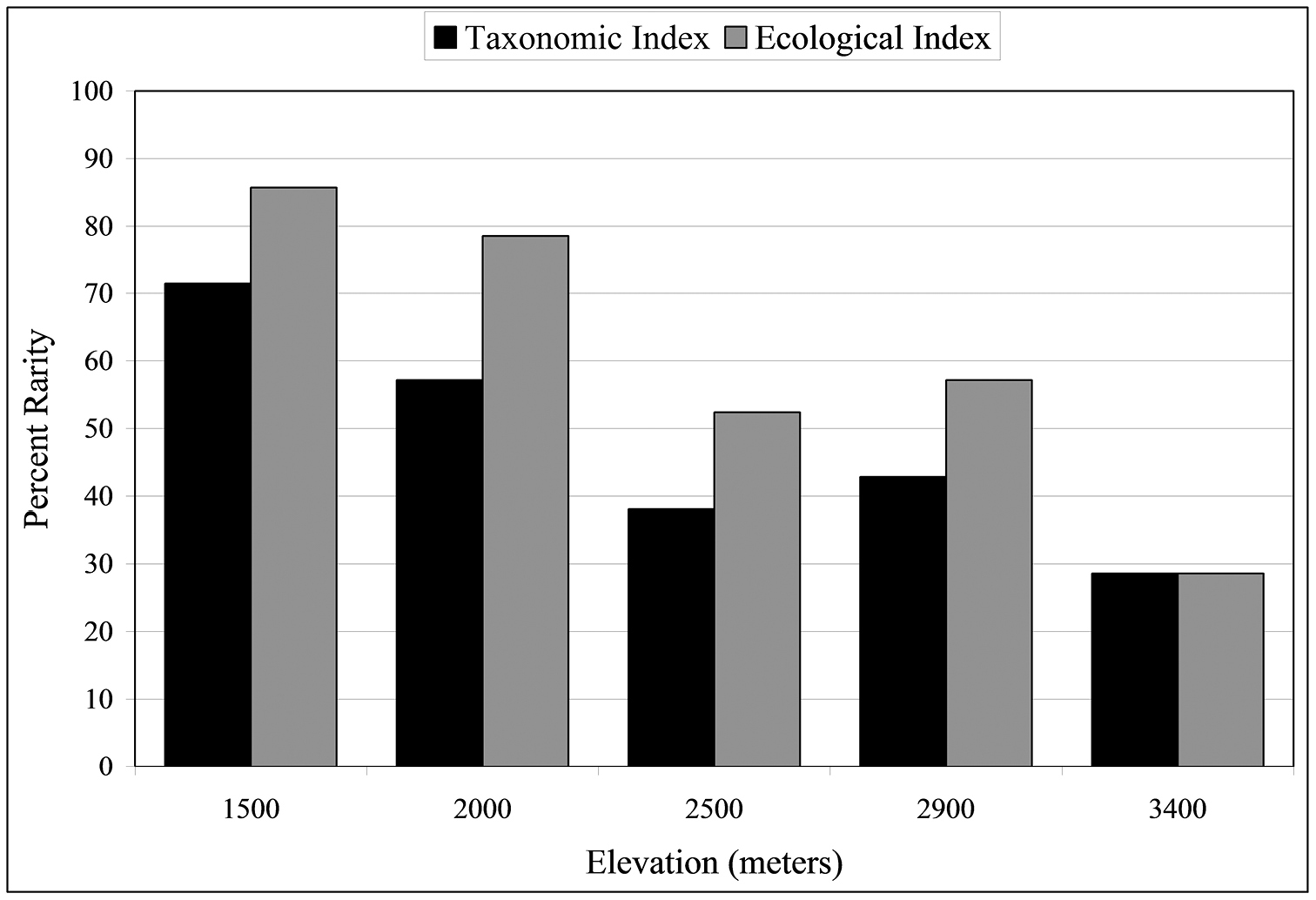
Rarity was estimated from both taxonomic and ecological perspectives (Fig. 4; see Materials and methods for how rarity is estimated). Rare species constitute 29% of the carabid beetle community at the highest altitude, 3400 m, and > 50% at both the two lowest altitudes. At 1500 m, 71% species are rare using the taxonomic index and 85.7% are rare by the ecological index. There are significant negative correlations with altitude for both rarity indices (rtax = 0.951, reco = 0.953, P < 0.05).
Percentage of rare species at five altitudinal sites. Black bars represent the taxonomic rarity index and gray bars represent the ecological rarity index (see text for definitions of rarity).
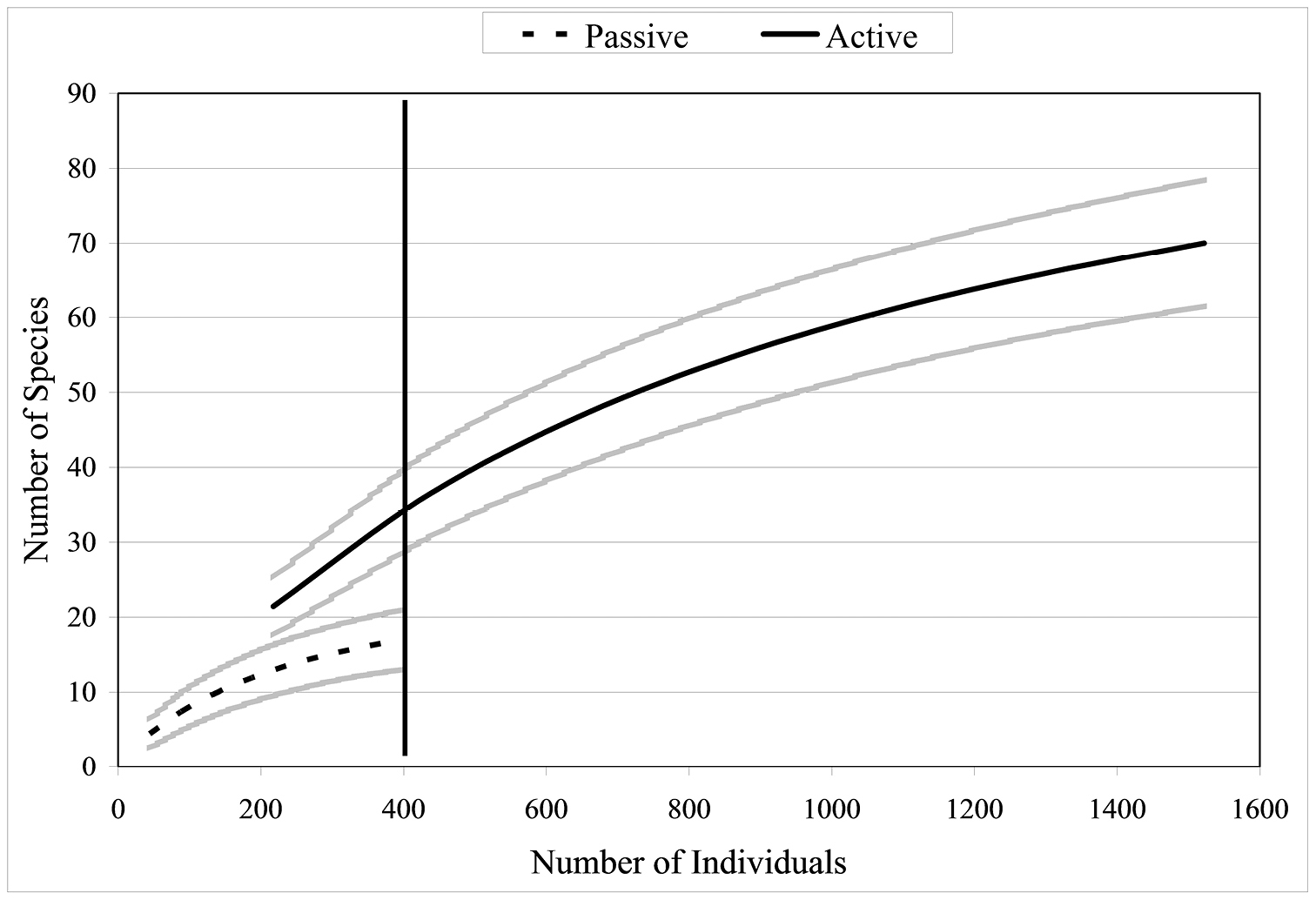
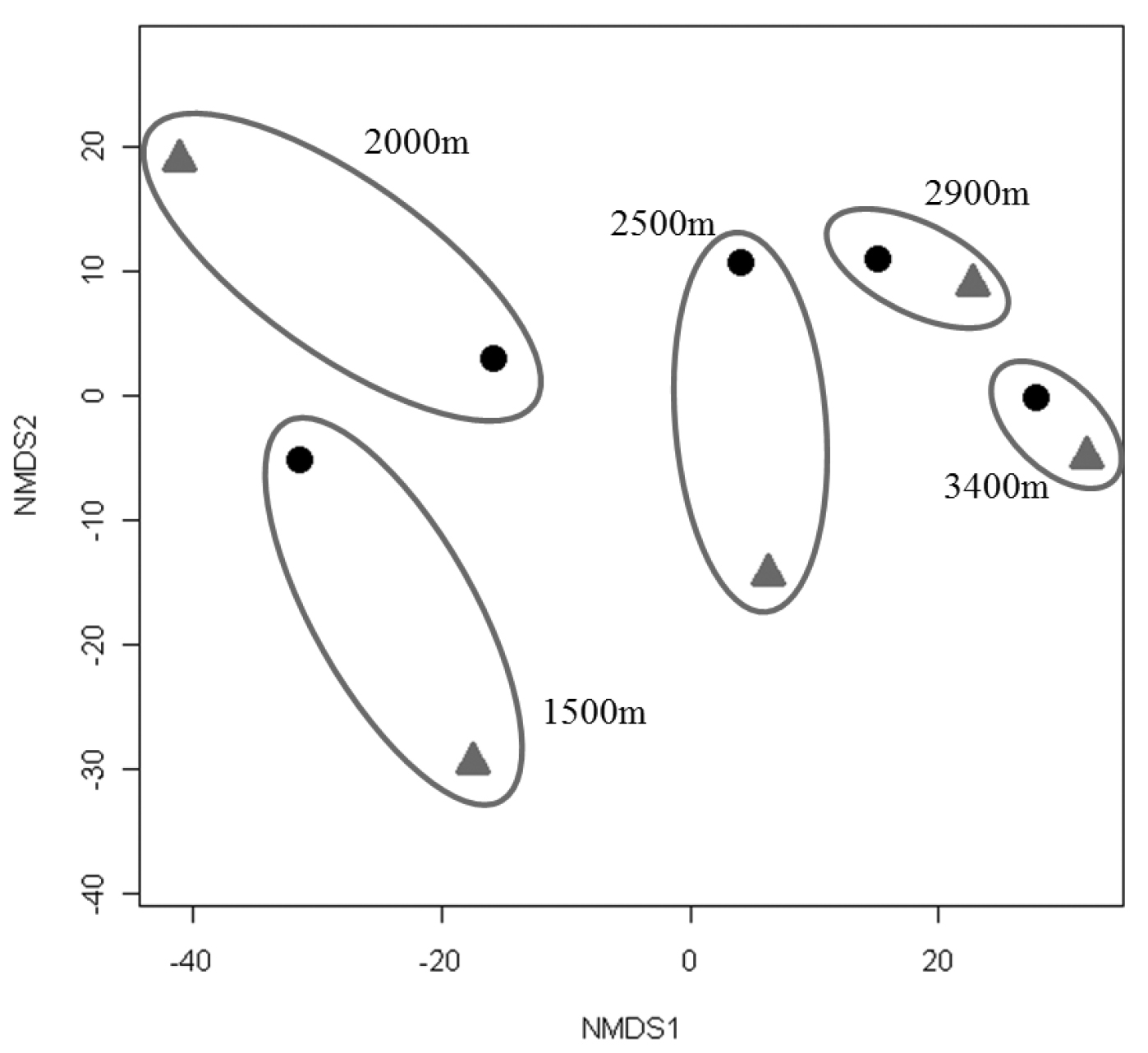
Since collections included both active (hand) and passive (pitfall trap) methods, comparisons can be made between the two techniques. Active collections yielded a greater number of species per individuals collected than passive collections (Fig. 5). Since more than three times the number of individuals was collected actively than obtained via passive collections, the number of species obtained would be expected to be higher for the former. However, the slope of the hand collection curve is also steeper than that generated from passive collections, with the non-overlapping 95% confidence intervals indicating that the number of species actively collected is significantly higher than for comparable size samples collected by passive techniques. For 400 individuals sampled (i.e., n= 400), S = 17 for passive collections while S = 37 for hand collections, suggesting that hand collecting detects more than twice as many species for the same number of individuals. When Nonmetric Multidimensional Scaling (NMDS) analysis is utilized (Fig. 6) the active and passive collection technique data points are not close together in space, suggesting that there is a slight compositional difference between carabid taxa collected actively or passively; however, the clustering of data points by altitudinal zone suggest a stronger altitudinal affect.
Species accumulation curves when all altitudes and collection types (active or passive) are combined (n = 1924). Brackets represent 95% confidence intervals.
Non-Metric Multidimensional Scaling (NMDS) using the Bray Curtis Similarity Index for collections made actively (diamond) and passively (circle). Values for each altitude are grouped by ovals.
This study was the first inventory ofcarabid beetles for a high altitude gradient in the cloud forests of southeastern Perú. For approximately one year of collections, 77 morphospecies were collected. When more formal taxonomic analysis and a description of specimens are complete, approximately 60% of the species are expected to be previously undescribed (TLE). Further sampling will likely result in even more unknown species. Biodiversity inventories of altitudinal gradients are particularly relevant since they can serve as studies of climate change. For example,
Species accumulation curves allow for direct comparison of S at the alpha level, thus avoiding some of the pitfalls with other diversity indicators, such as Shannon and Simpson indices (
As altitude increased, species diversity of carabid beetles decreased significantly. Tree diversity along the same altitudinal transect that was used in this study steadily increases from the Amazonian lowlands (approximately 500 m) to 1500 m, then decreases from 1500 m to 3400 m (
In addition to a significant change in species number with altitude, there is high degree of altitudinal site specificity, suggesting preferences for specific altitudes for the majority of carabid beetle species. Most species are confined to a single altitudinal site. The physical extremes and abrupt changes in abiotic conditions, such as a decrease in temperature and partial pressure of respiratory gases as well as an increase in precipitation (
The dominant tribe collected in the study area was Harpalini; this group is composed of species that are mostly fully winged, and many Neotropical species are seed eaters (
This was one of the first studies in a tropical montane environment to use both active and passive collecting techniques, which several studies suggest is necessary for complete inventories (e.g.,
We thank the Peruvian Ministry of Agriculture, INRENA, and SERNANP for collection and export permits; the Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, especially Dr. Gerardo Lamas for permit coordination; and Amazon Conservation Association (ACCA). Further, we thank the Fulbright Program, Wake Forest University and the National Museum of Natural History at the Smithsonian for support.