(C) 2011 Ovidiu Alin Popovici. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Triteleia peyerimhoffi comb. n. (Kieffer, 1906) is redescribed taking into account its great variability and is considered the senior synonym of Triteleia dubia (Kieffer, 1908), Calliscelio lugens (Kieffer, 1910) and Triteleia striolata Kononova & Petrov, 2000, syn. n. Neotypes are designated for Triteleia dubia and Triteleia peyerimhoffi. Triteleia peyerimhoffi is a new record for Greece, France and Croatia and was reared for the first time from eggs of Orthoptera laid in the dead wood of Quercus sp. and Tilia sp. in Romania.
Hymenoptera, Platygastroidea, microhymenoptera, egg parasitoids, Caloteleia peyerimhoffi, Triteleia dubia, variability
Jean-Jacques Kieffer (b. 1857 – d. 1925) was an Abbé, a clergyman, and taught natural history and religion at the Collége Saint-Austin at Bitche in Lorraine (
Triteleia peyerimhoffi was described by
In 1908, Kieffer, described Ceratoteleia, including species with a characteristic long metasoma with a horn on the first metasomal tergite, and with the length of the marginal vein varying from punctiform to the same length as the stigmal vein. Two years later (
Kieffer obviously did not study Ashmead’s type of Caloteleia grenadensis (in BMNH) and therefore his concept of Ceratoteleia is very heterogeneous, containing species of several genera (e.g. Triteleia, Calliscelio, Holoteleia, Probaryconus, etc.).
Triteleia dubia was described by
Two years later
Our goal in this paper is to provide a modern description of this species, document its unusual variability and provide new data about its biology. The contributions of the authors are as follows: O.A. Popovici (character definition, species concept development, imaging, collection of new material, manuscript preparation); F. Bin (had the idea for the paper, provided most of the Italian material, and contributed to the section on biology); L. Masner (character definition, species concept development, provided new material from Italy, Hungary and France and elaborated the plan for this paper); M. Popovici (geometric morphometric analysis); David Notton (provided specimens from the BMNH and corrected the English of the paper). Any nomenclatural acts in this paper are to be attributed to O. A. Popovici and L. Masner.
Material and methodsMost of specimens seen were caught with a Malaise trap in various parts of Europe, especially the south and west including: France, Greece, Hungary, Italy and Romania. One specimen each from Croatia and Romania were swept. The remaining specimens were reared from the dead wood of Tilia species and Quercus species. Specimens were glued to triangular card points. For better examination, the maxillo-labial complex, one antenna, legs and wings of some specimens were removed and mounted on microscope slides. Specimens were examined using a Kruss MSZ54 stereomicroscope. Microscope slides were analyzed with a Euromex GE 3045 microscope, and drawings were made using a Reichart drawing tube.
Abbreviations and morphological terms used in text:A1, A2, ... A12: antennomeres 1-12; DPO: diameter of posterior ocellus; fmc: foramen magnum capitis; gen: gena; ha: hypostomal area; HE: height of compound eye; hf: hypostomal folds; Hfd: height of frontal depression; ihc: inner hypostomal carina; Lck: length of central keel; LE: length of compound eye; Lfw: length of fore wing; LH: length of head (measured at level of anterior ocellus); Lhw: length of hind wing; LOL: lateral ocellar line, the shortest distance between inner margins of anterior and posterior ocellus; Lscut: length of scutellum; Lt: length of temple; MLC: maxillo-labial complex; ocp: occiput; ocpc: occipital carina; ohc: outer hypostomal carina; OOL: ocellar ocular line, the shortest distance from inner orbit of compound eye to the outer margin of lateral ocellus; pg: postgena; pgb: postgenal bridge; POL: posterior ocellar line, the shortest distance between inner margins of posterior ocelli; sgp: sub-genal process; T1, T2, ... T6: metasomal terga 1-6; tb: tentorial bridge; Wfw: maximum width of fore wing (measured perpendicular to fore wing margin); WH: maximum width of head; Whw: width of hind wing; Wscut: width of scutellum.
Morphometric analysis. In total 82 specimens of Triteleia were measured. 60 females: Croatia (1); France (18); Greece (7); Hungary (3); Italy (27); and Romania (4), and 22 males: Greece (3); and Italy (19).All measurements were made using a Kruss MSZ54 stereomicroscope at 90× magnification.
The following characters were measured: body length; LH; WH; POL; LOL; DPO; HE; LE; distance between compound eyes (measured at level of anterior ocellus); Lt; distance between toruli; Lck; Hfd; surface of frontal depression covered with transversal striae; distance between compound eye and frontal depression; length of cheek; length and width for all antennal segments (A1….A12); length of mesosoma; width of mesosoma; length of mesoscutum; Lscut; Wscut; length of metascutellum; width of metascutellum; distance between lateral propodeal carina; width of lateral propodeal area; Lfw; Wfw; length of marginal vein; length of stigmal vein; length of postmarginal vein; Lhw; Whw; length of marginal fringe of hind wing (at level of hamuli); length of metasoma; length of T1; minimum width of T1; maximum width of T1; length of T2; maximum width of T2; length of T3; maximum width of T3; length of T4; minimum width of T4; length of T5; minimum width of T5; length of T6; minimum width of T6.
For each ratio in the description of species we used minimum – maximum (mean ± standard deviation).
The relationships between specimens were analyzed using Principal Component Analysis (PCA). This was performed using log-transformed data on a variance–covariance matrix (
The metasoma was analyzed using geometric morphometric methods based on the Kendall theory of shape (
Relationships between metasoma shapes were investigated using Principal Component Analysis (PCA). PCA was performed on all shape variables in order to define the greatest axes of metasoma shape variation in the dataset. The visualization of the shape differences was made with thin-plate spline deformation grids. Overall differences between metasoma shapes have been tested by a Multivariate Analysis of Variance (MANOVA) and the permutation tests (1000 permutations) for Mahalanobis distances among populations were performed to confirm the significant differences. The effect of size on shape was investigated by multiple regression.
Statistical analysis was performed using Morpho J (
Morphological terminology follows (
CNCI Canadian National Collection of Insects, Ottawa, Canada
OPPC O. Popovici personal collection, University ‘Al. I. Cuza’ Iasi, Romania
FBIN Collection of F. Bin, Università di Perugia, Perugia, Italy
BMNH Natural History Museum, London, United Kingdom
MNHN Muséum national d’Histoire naturelle, Paris, France
HNHM Hungarian Natural History Museum, Budapest, Hungary
Resultshttp://species-id.net/wiki/Triteleia_peyerimhoffi
Body size: female 3.0–4.6 mm (3.9 ± 0.4, n = 60); male 3.4–4.1 mm (3.6 ± 0.2, n = 22).
Colour: body black; antenna brown with reddish tint on some parts: radicle yellow with reddish tint; A1–5 with reddish tint on the ventral side; wing veins brown; legs light brown, sometime yellowish; middle of femora with dark tint.
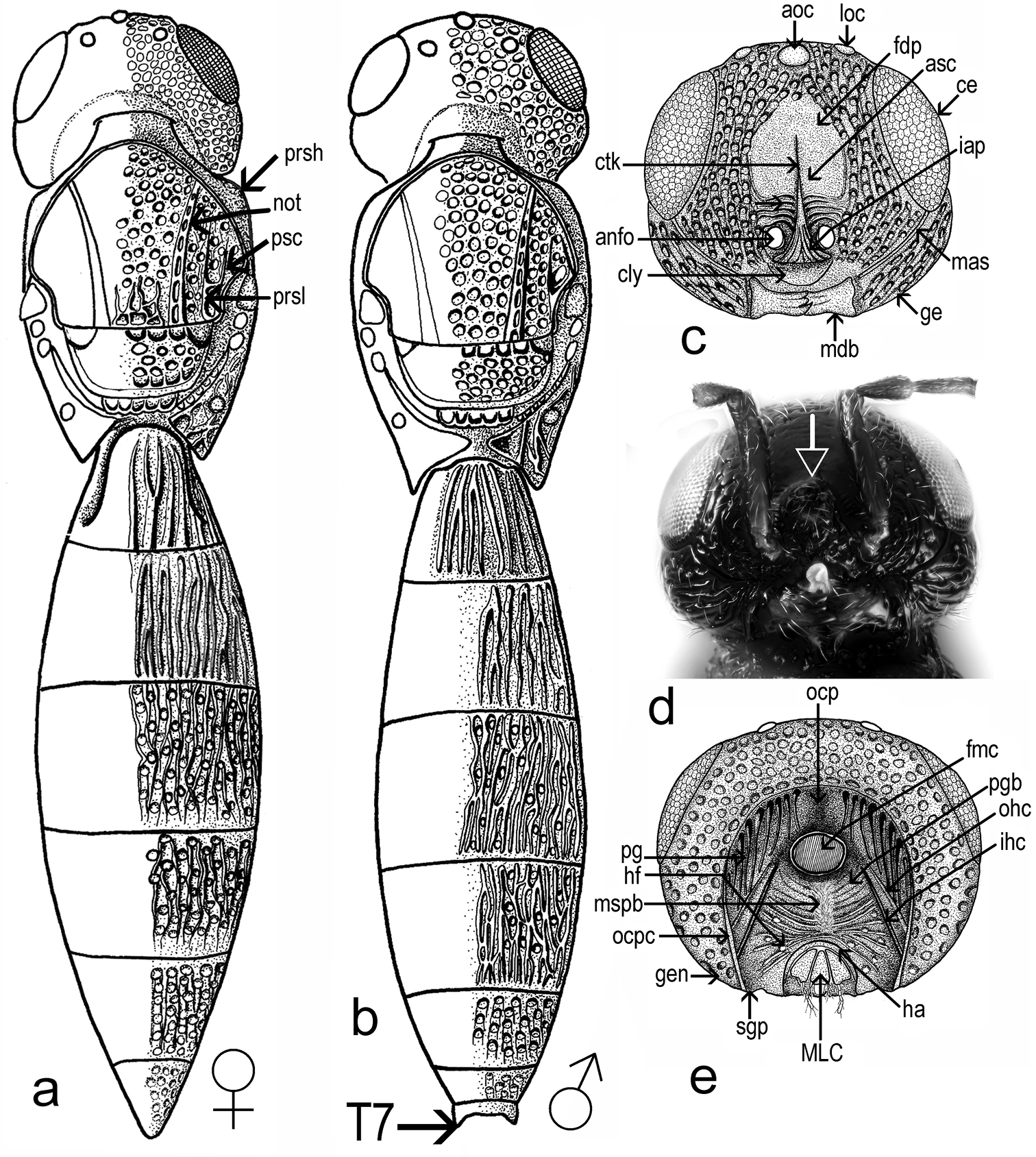
Head shape: dorsal view transverse, width 1.6–2.0 times length in female (1.8 ± 0.1, n = 60), 1.6–1.8 times length in male (1.7 ± 0.05, n = 22), 1.0–1.1 times width of mesosoma in female (1.02 ± 0.03, n = 60). Hyperoccipital carina absent. Occipital carina present, smooth, almost absent in median part. Compound eye large, glabrous. Eye width 1.6–2.8 times temple width in female (2.2± 0.3, n = 60), 1.5–2.3 times temple width in male (1.9 ± 0.2, n = 22) and 1.7–4.1 times distance between eye and frontal depression in female (3.1 ± 0.5, n = 60), 2.0–3.3 times distance between eye and frontal depression in male (2.5 ± 0.3, n = 22). Eye height 1.2–1.4 times width of eye in female (1.2 ± 0.05, n = 60), 0.8–1.0 times width of eye in male (0.9 ± 0.06, n = 22) and 1.6–3.2 times length of cheek in female (2.3 ± 0.25, n = 60), 1.9–2.4 times length of cheek in male (2.1 ± 0.1, n = 22). Inner orbits nearly parallel, diverging only in ventral half. Length of diameter of posterior ocellus 1.3–2.7 times OOL in female (2.0 ± 0.3, n= 60), 1.3–2.5 times OOL in male (2.2 ± 0.3, n = 22). POL 1.3–2.3 times LOL in female (1.7 ± 0.19, n= 60), 1.3–2.0 times LOL in male (1.7 ± 0.2, n = 22). Distance between compound eyes (measured at level of anterior ocellus) 1.5–2.1 times POL in female (1.8 ± 0.12, n = 60), 1.6–2.1 times POL in male (1.9 ± 0.09, n = 22). Orbital carina absent; frontal depression shallow, unmargined, submedian carina absent; antennal scrobe present, shining; central keel on frons (ctk Fig. 1c), present, not bifurcate, only a weak trace in some specimens. Length of central keel 0.2–0.9 (0.5 ± 0.2, n = 60) times height of frontal depression in female. Base of frontal depression transversely striate (Fig. 1c). The transverse striation is very variable 0.1–0.5 (0.3 ± 0.09, n = 60) times height of frontal depression in female. Interantennal prominence (iap Fig. 1c) moderately produced, torulus opening on antero-frontal surface of prominence (in one specimen, the interantennal prominence was hypertrophied, so that the distance between the toruli was twice than of normal specimens (Fig. 1d). Malar sulcus (mas, Figs 1c; 1d; 2a; 2b) present, fine, deeply incised, almost straight, running from lower margin of eye to mandibular articulation. Genal carina absent. Cheek without costae arising from anterior mandibular articulation. Clypeus (cly Fig. 1c) very small, narrow, semicircle, without corners produced laterally. Mandible strong, relatively short and broad, apex tridentate, teeth subequal in length, acute, ventral toothslightly longer. Number of maxillary palpomeres 4; labial palpomeres 2.
Sculpture of head (Figs 1a; 1b; 1c; 1d; 1e; 2a; 2b): vertex, interocellar space, cheek and space between compound eye and frontal depression foveolate. Frontal depression shining in apical half, transversely striate basally (Fig. 1c). In some specimens the lateral sides of frontal depression are longitudinally striate.
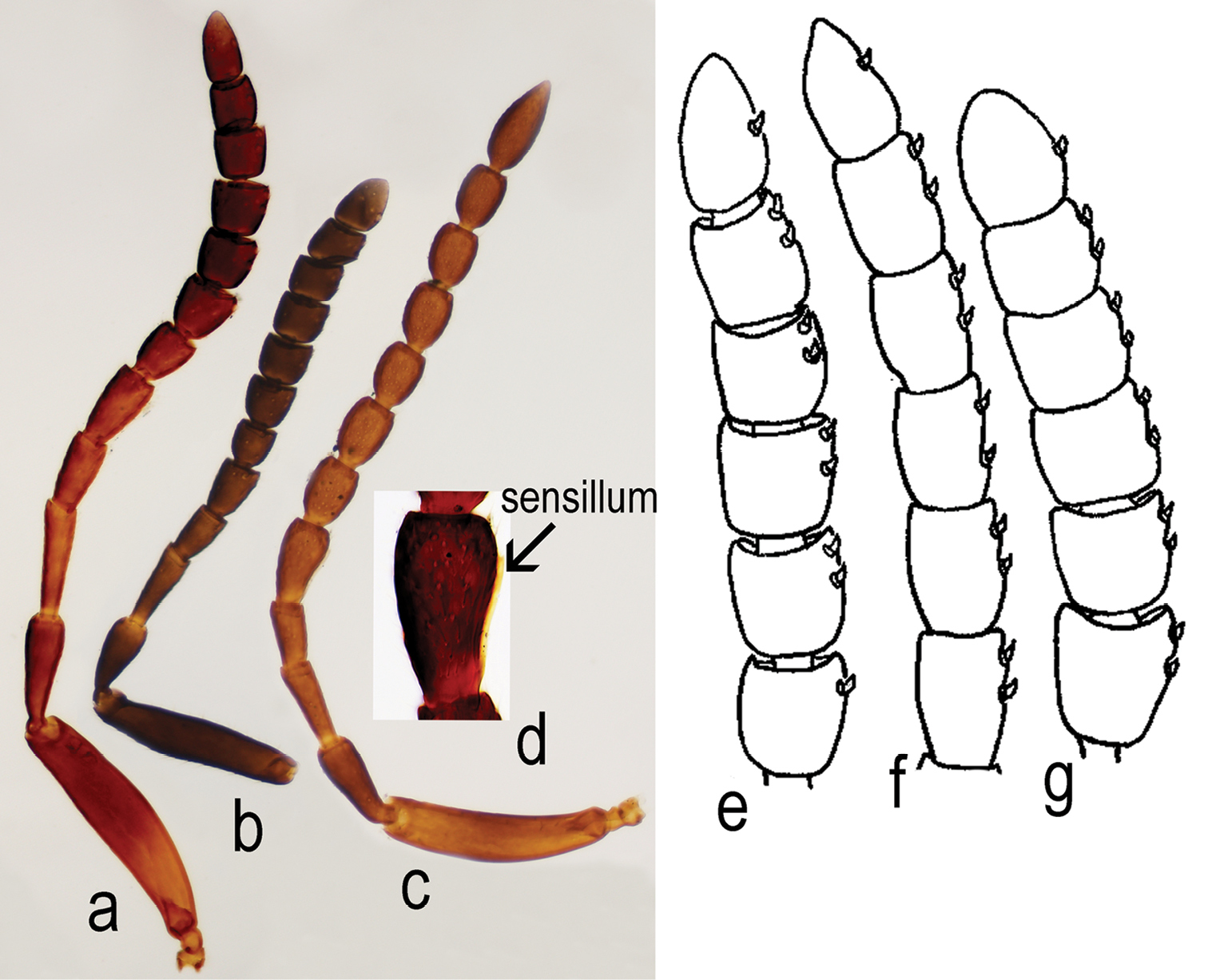
Antenna 12-segmented in both sexes (Figs 2a; 3a; 3b; 3c). Length of A1 4.0–6.25 times width in female (4.8 ± 0.38, n= 60), 4.0–4.6 times width in male (4.3 ± 0.2, n = 22), 2.0–2.8 times length of A2 in female (2.4 ± 0.18, n= 60), 2.1–2.6 times length of A2 in male (2.4 ± 0.13, n = 22). Length of A2 2.0–3.6 times width in female (2.5 ± 0.29, n= 60), 1.8–3.3 times width in male (2.2 ± 0.3, n = 22) and 0.7–1.57 times length of A3 in female (0.9 ± 0.14, n= 60), 0.9–1.1 times length of A3 in male (1.0 ± 0.07, n = 22). A3, in female, the longest funicular segment, 2.3–4.6 times width (3.4 ± 0.4, n= 60), 2.0–3.3 times width in male (2.5 ± 0.3, n = 22), 1.0–2.25 times length of A4 in female (1.4 ± 0.18, n= 60), 1.3–1.7 times length of A4 in male (1.5 ± 0.13, n = 22). Length of A4 1.3–3.6 times width in female (2.1 ± 0.4, n= 60), 1.25–2.0 times width in male (1.5 ± 0.2, n = 22) and 0.8–1.4 times length of A5 in female (1.0 ± 0.12, n= 60), 0.6–0.9 times length of A5 in male (0.8 ± 0.06, n = 22). Width of A4 0.6–1.0 times width of A5 in female (0.88 ± 0.1, n= 60), 0.7–1.0 times width of A5 in male (0.8 ± 0.06, n = 22). Length of A5 1.3–2.5 times width in female (1.8 ± 0.3, n= 60), 1.4–2.0 times width in male (1.7 ± 0.1, n = 22) and 1.0–1.75 times length of A6 in female (1.3 ± 0.15, n= 60), 1.1-1.5 times length of A6 in male (1.3 ± 0.08, n = 22). Length of A6 1.0–2.0 times width in female (1.2 ± 0.2, n= 60), 1.2–1.8 times width in male (1.4 ± 0.1, n = 22) and 0.8–1.4 times length of A7 in female (1 ± 0.14, n= 60), 0.9–1.0 times length of A7 in male (1.0 ± 0.01, n = 22). Clava in female non-abrupt; claval formula A7–12: 1:2:2:2:2:1, differing from claval formula of Apegus and Macroteleia (in both cases 2:2:2:2:2:1; (Figs 3e; 3f; 3g)). Male antenna non-clavate; A5 sexually modified (Figs 3c; 3d). Length of A12 1.0–1.75 times width in female (1.4 ± 0.02, n= 60), 1.8–2.5 times width in male (2.3 ± 0.3, n = 22) and 1.0–1.75 times length of A11 in female (1.2 ± 0.15, n= 60), 1.5–2.0 times length of A11 in male (1.7 ± 0.1, n = 22).
Back of head (Fig. 1e): occipital carina present, with vertical part well developed and with horizontal part shallow. Temples well developed behind eyes; occiput smooth, deeply concave. Foramen magnum capitis well developed, surrounded by a deep fossa, distance between foramen and occipital carina c. 1.5 times its diameter. Postgena covered with vertical folds. Postgenal bridge smooth. Hypostomal folds present. Median sulcus of the postgenal bridge present. Inner hypostomal carina well developed, more distinct that outer hypostomal carina. Maxillo-labial complex with stipes, prementum, maxillary and labial palpi visible. Subgenal process weakly developed. Hypostomal area narrow. Hypostomal tooth not visible.
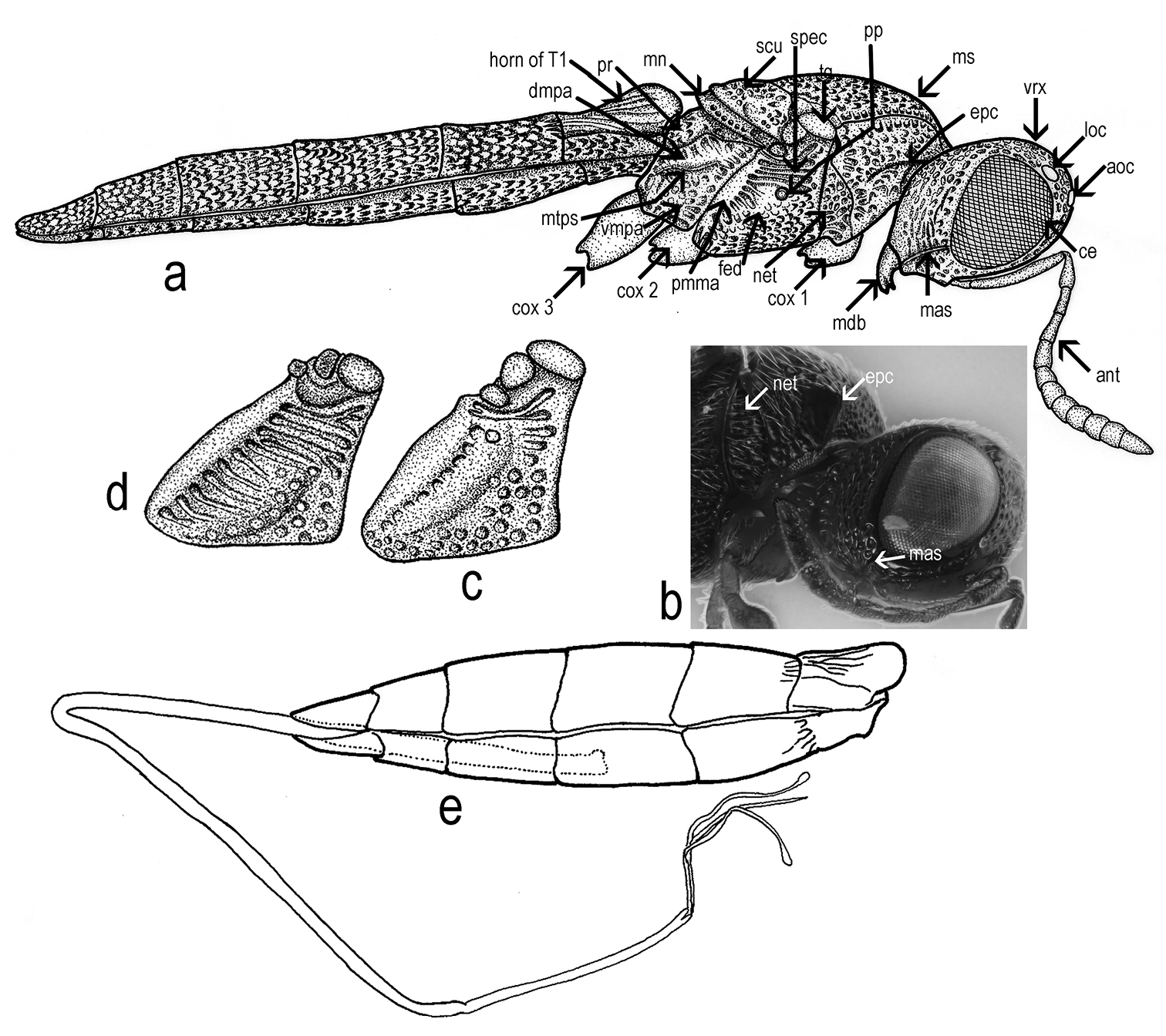
Mesosoma (Figs 1a; 1b) length 1.2–1.4 times width in female (1.3 ± 0.05, n = 60), 1.3–1.5 times width in male (1.4 ± 0.04, n = 22). Dorsal margin of mesosoma weakly convex in lateral view.
Transverse pronotal carina absent, pronotal shoulders strongly developed, rounded anteriorly. Vertical epomial carina present; horizontal epomial carina present (Figs 2a; 2b). Cervical pronotal area oblique, largely hidden in dorsal view. Lateral pronotal area broad, weakly concave. Netrion present (net Figs 2a; 2b), broad, approximately triangular, open ventrally, with foveolate sculpture.
Mesoscutum (Figs 1a; 1b), weakly convex, 2.1–2.8 times as long as scutellum, (2.4 ± 0.2, n = 60). Skaphion absent. Admedian lines absent. Notauli present, percurrent, usually deeply incised, crenulate. Notauli converging, closely approximated posteriorly, slightly dilated posteriorly. Humeral and suprahumeral sulci crenulate, but indistinct. Parapsidal lines present. Parascutal carina distinct. Mesoscutum foveolate. Transscutal articulation deep, crenulate. Mesoscutellum transverse, width 1.9–2.4 times length, (2.1 ± 0.13, n = 60); weakly convex, unarmed, posterior rim crenulate, sculpture like mesoscutum; length 3.3–8.0 times length of metascutellum in female (5.0 ± 0.75, n = 60), 3.8–5.7 times length of metascutellum in male (5.0 ± 0.6, n = 22). Metascutellum produced into a distinct rectangular plate, 4.0–8.0 times wider than long in female (5.0 ± 0.8, n = 60), 3.2–5.3 times wider than long in male (4.7 ± 0.6, n = 22).
Mesopleuron (Figs 2a; 2c; 2d) almost glabrous, with some scattered hairs. Speculum visible above the femoral depression, with a variable number of transverse ridges. Femoral depression large, deep, shining or with very smooth sculpture. Pleural pit distinct. Mesopleural carina indistinct. Posterodorsal corner of mesopleuron obtuse. Posterior mesepimeral area broad and shining. Sternaulus indistinct.
Propodeum (Figs 1a; 1b) in dorsal view, reduced and deeply excavate medially, lateral propodeal carinae separate the lateral propodeal areas from the deep and large metasomal depression which accommodates the horn of T1. The antero-dorsal ends of the carinae extend over the dorsal margin of the propodeum to form a projection.
Metapleuron entirely sculptured, divided by metapleural sulcus into a small dorsal area and in a large ventral area (Fig. 2a).
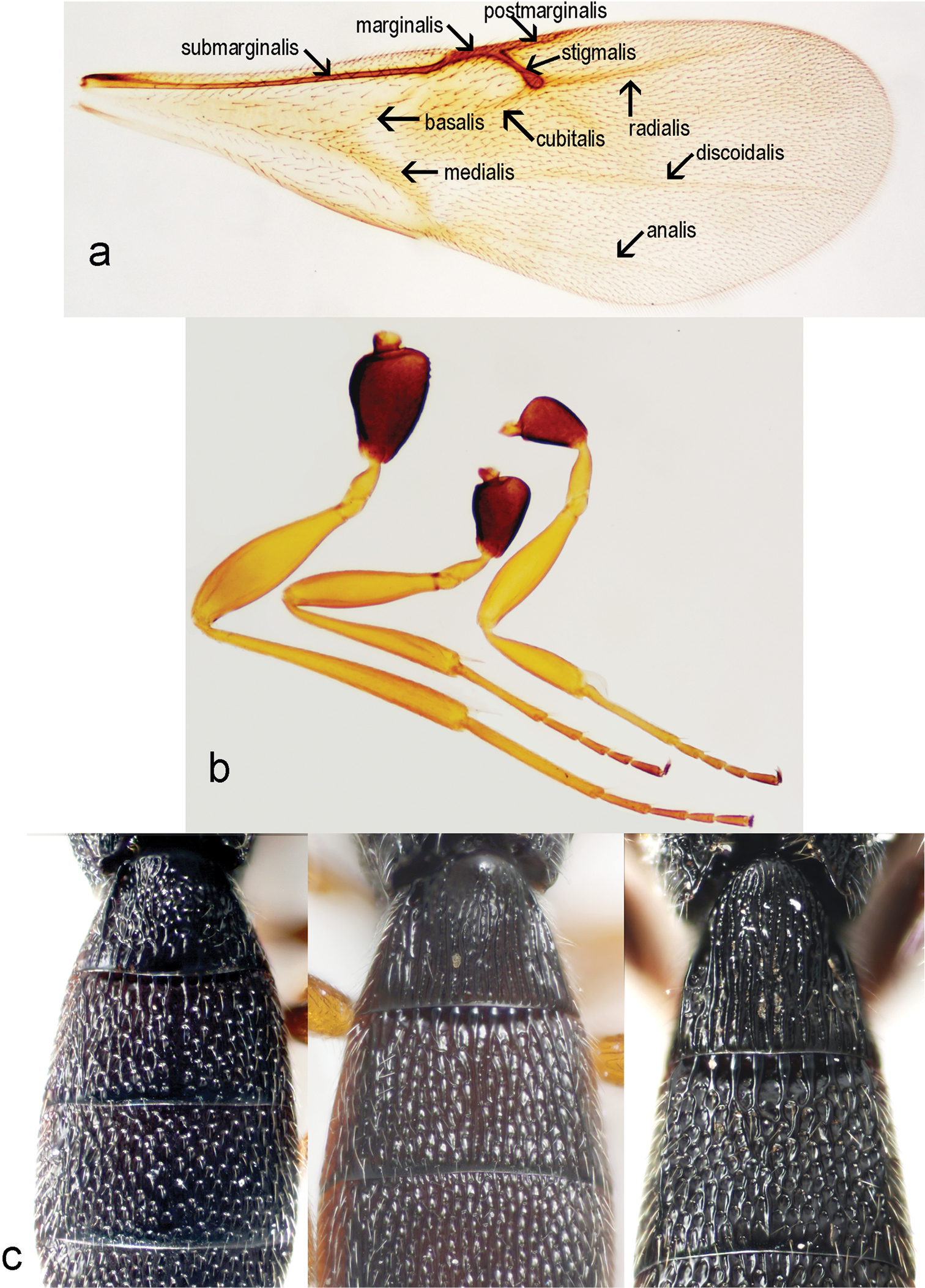
Macropterous, fore wings variable in length, not reaching apex of metasoma. Fore wing (Fig. 4a) covered with dense, short microtrichia. Length of fore wing 2.7–3.3 times width in female (3.0 ± 0.14, n = 60), 2.8–3.1 times width in male (2.9 ± 0.09, n = 22), 1.13– 1.5 times length of hind wing in female (1.3 ± 0.05, n = 60), 1.3–1.4 times length of hind wing in male (1.3 ± 0.04, n = 22), 2.7–3.3 times width of mesosoma in female (3.0 ± 0.11, n = 60), 2.7–3.1 times width of mesosoma in male (2.9 ± 0.1, n = 22). Fore wings with tubular submarginal, marginal, postmarginal and stigmal veins and with nebulous medial, cubital, anal, basal, discoidal and radial veins (Fig. 4a). Length of postmarginal vein 0.91–2.9 times length of marginal vein in female (1.3 ± 0.3, n = 60), 1.0–1.5 times length of marginal vein in male (1.2 ± 0.2, n = 22). Marginal vein length 0.7–1.3 times length of stigmal vein in female (1.03 ± 0.11, n = 60), 0.9–1.3 times length of stigmal vein in male (1.1 ± 0.09, n = 22).
Hind wing 4.0–6.1 times as long as wide in female (4.8 ± 0.4, n = 60), 4.4–5.7 times as long as wide in male (4.8 ± 0.3, n = 22), with three hamuli and complete submarginal vein. Marginal fringe short, width of hind wing 7.6 time length of marginal fringe.
Trochantellus present on all legs, tibial spur formula 1-1-1. The middle leg is the shortest (Fig. 4b).
Metasoma (Figs 1a; 1b) broadly sessile, depressed, in male with seven terga and seven sterna, in female with six terga, six sterna visible externally, homonomously segmented, T2–T4 subequal in length, T3 slightly the longest. Laterotergites well developed, narrow. Length of metasoma 2.0–2.6 (2.2 ± 0.13, n = 60) times length of mesosoma, 2.6–3.9 times width in female (3.2 ± 0.2, n = 60), 2.7–3.5 times width in male (3.0 ± 0.2, n = 22).
T1 with anterior margin carinate (especially visible in male), sublaterally with shallow depressions, with horn in female usually longitudinally costate. The apex of horn can be smooth, almost shining, or with longitudinally costae or with areolate rugulae (Fig. 4c). Length of T1 1.0–1.3 times its minimum width in female (1.1 ± 0.07, n = 60), 0.8–1.1 times its minimum width in male (1.0 ± 0.06, n = 22). Ratio between maximum and minimum width of T1 is 1.3–1.7 in female (1.5 ± 0.08, n = 60) and 1.3–1.5 in male (1.4 ± 0.05, n = 22).
Length of T2, 0.9–1.4 times the length of T1 in female (1.05 ± 0.06, n = 60) and 1.1–1.4 times the length of T1 in male (1.2 ± 0.07, n = 22). Maximum width of T2 1.4–2.0 its length in female (1.7 ± 0.1, n = 60) and 1.3–1.8 its length in male (1.6 ± 0.1, n = 22). Ratio between maximum and minimum width of T2 1.0–1.4 in female (1.3 ± 0.05, n = 60) and 1.2–1.4 in male (1.3 ± 0.04, n = 22). T3 is slightly the longest metasomal tergite, T3 length 1.0–1.3 times length of T2 in female (1.1 ± 0.05, n = 60), 1.0–1.2 times length of T2 in male (1.1 ± 0.04, n = 22) and 1.0–1.25 times length of T4 in female (1.1 ± 0.06), 1.0–1.2 times length of T4 in male (1.1 ± 0.04, n = 22). Maximum width of T3 1.3–1.8 times length in female (1.5 ± 0.11, n = 60), 1.3–1.6 times length in male (1.5 ± 0.1, n = 22). Ratio between maximum and minimum width of T3 is 1.0–1.1 in female (1.0 ± 0.01, n = 60) and 1.0–1.1 in male (1.0 ± 0.02, n = 22). Length of T4 1.2–1.6 times length of T5 in female (1.4 ± 0.07, n = 60), 1.3–1.6 times length of T5 in male (1.5 ± 0.08, n = 22) and length of T5 0.94–1.5 times length of T6 in female (1.2 ± 0.1, n = 60) and 1.7–3.3 times length of T6 in male (2.1 ± 0.4, n = 22). Ratio between maximum and minimum width of T4 is 1.2–1.4 in female (1.3 ± 0.05, n = 60), 1.1–1.3 in male (1.2 ± 0.04, n = 22) and ratio between maximum and minimum width of T5 is 1.2–2.1 in female (1.8 ± 0.13, n = 60) and 1.4–1.7 in male (1.5 ± 0.07, n = 22). Length of T6 0.6–1.2 times its maximum width in female (0.9 ± 0.01, n = 60) and 0.3–0.5 times its maximum width in male (0.4 ± 0.05, n = 22).
Ovipositor Scelio–type (Fig. 5b); the relation between ovipositor assembly length and metasoma length is shown in Fig. 2e.
Ovipositor assembly, very tiny, elongate. Proximal arms slender, short, 0.11 times length of ovipositor assembly; second gonapophyses assembly complex; gonoplacs elongate, 0.62 times ovipositor length; second gonocoxa 0.54 times gonoplac length. Gonoplacs weakly spatulate apically. First gonapophyses apically sharp. We cannot identify the proximal part of ventral membranous plate present in other Triteleia (Fig. 5c).
Lateral apodemes present, incorporated into wall of telescopic tube (Fig. 5e). Telescopic tube membranous with three or four sections. S6 without medial apodeme (Figs 5h; 5j).
Structure of ovipositor in Triteleia dubia, shows this species was misplaced in Apegus by Kieffer, because in Apegus, the ovipositor has a completely different structure, being Ceratobaeus–type (Fig. 5d).
The aedeagus (Fig. 5a) has two parts: the basal ring and aedeago-volsellar shaft. The basal ring is well developed, and represents 0.4 of copulatory organ length and 0.7 of aedeago-volsellar shaft. The aedeago–volsellar shaft has two aedeagal apodemes and two digiti volsellares. Each digitus has a row of five pits, each with a short tooth. The digiti, teeth and aedeagal apodemes are darker, more sclerotized than the rest of the copulatory organ.
Triteleia peyerimhoffi is the third member in a tritrophic system, the other two being the plant-hosts and the orthopteran-host. We examined specimens of Triteleia peyerimhoffi obtained from the following plants: Asphodelus sp. (Asphodelaceae); Ferula sp., Magydaris tomentosa (both Apiaceae), Tilia sp. (Malvaceae) and Quercus sp. (Fagaceae). In most cases, Triteleia peyerimhoffi was obtained from the tettigoniids Uromenus brevicollis insularis or Platycleis albopunctata (Fig. 6). The relationship between Asphodelus ramosum – Uromenus brevicollis insularis – Triteleia peyerimhoffi (under the name Triteleia dubia) was previously noted by
Kieffer did not appreciate the variability of this species since he described the female of this species in 1906 in Caloteleia; males two years later in Apegus; and the male and female again (1910b) as Ceratoteleia lugens. According to
To clarify the taxonomic status of these species (ICZN, article 75.3.1), we here designate neotypes for Parapegus dubius and Ceratoteleia peyerimhoffi. We consider that the types of these species have been lost or destroyed: since we were unable to locate them in BMNH, MNHN, HNHM or in the Naturhistorisches Museum, Vienna. For Parapegus dubius we designate as neotype one female labeled: Hungary, Veröce 47°49.58'N, 19°1, 30'E, 122m, 2–18.ix.2005, leg. Z. Nyiro (Malaise trap, CNCI). For Ceratoteleia peyerimhoffi we only have one male from Algeria. Because we have many specimens from Italy from the same host as the type specimens and because the male from Algeria is very similar to males from Italy, we decided to designate as a neotype a female of Ceratoteleia peyerimhoffi labeled: Italy, Guspini, 3.VIII.1933 (reared from Asphodelus). This neotype will be deposited in BMNH.
Among the Palaearctic species described by Kieffer in Ceratoteleia there is one further species that has an uncertain status: Ceratoteleia mediterranea. Currently it is placed in Calliscelio (
FRANCE: 17 females, Lot Escamps, 5–31.viii.1995, Malaise trap, leg. H. Tussac (CNCI); 1 male, Lot Escamps, 5–31.viii.1995, Malaise trap, leg. H. Tussac (CNCI); 1 female, Dordogne, Couze St. Front, 27.vi–11.vii.1993, Malaise trap, leg. H. Tussac (CNCI); 2 females, Dordogne, Couze St. Front, 1.ix.1994–22.ii.1995, Malaise trap, leg. J. N. Revol (CNCI); 1 female, Gard, St. Félix de Paulliéres, La Hourne Haute, 7–14.vii.1996, Malaise trap, leg. J. F. Vayssiéres (CNCI); 2 females, Bouches-du-Rhône, Fonscolombe, 17.vii.1990, leg. M. de V. Graham (BMNH(E)1995-489); 1 male, Bouches-du-Rhône, nr. Rognes, 16.vii.1979 (BMNH(E)1995-489); 1 female, Bouches-du-Rhône, Fonscolombe, 25.vii.1990, leg. M. de V. Graham (BMNH(E)1995-489); 1 female, Bouches-du-Rhône, Fonscolombe, 4.viii.1986, leg. M. de V. Graham (BMNH(E)1995-489); 1 female, Bouches-du-Rhône, Fonscolombe, 15.viii.1980, ex. Caloteleia coriaria (Fabaceae) gall, leg. M. de V. Graham (BMNH(E)1995-489); 1 female, Bouches-du-Rhône, Fonscolombe, 29.vii.1979, leg. M. de V. Graham (BMNH(E)1995-489); 1 female, Pignans, 4.ix.1965, leg. J. Barbier (MNHN, 7237); 1 female, Esbarres, C. D'OR, 6.viii.1955, leg. J. Barbier (MNHN, I536)
HUNGARY: 3 females, Veröce, 47°49.58'N, 19°1.30'E, 122m, 2–18.ix.2005, Malaise trap, leg. Z. Nyiro (CNCI).
ITALY: 3 females, Bienca, 20.ix–19.x.1985, leg. A. Casale (CNCI); 2 females, Toscana Sesto Fior. ix.1943, leg. L. Ceresa (OPPC); 1 male & 2 females, Sardegna, Macomer, 8.vii.1957, reared from Uromenus brevicollis insularis on Ferula sp. (FBIN); 3 males & 3 females, Sassari, Bunnari, 8.vii.1957, reared from Uromenus brevicollis insularis on Magydaris tomentosa (FBIN); 13 males & 52 females, Guspini, vii.1934, reared from Asphodelus (FBIN); 24 males & 114 females, Guspini, 7.vii.1934, reared from Asphodelus (FBIN); 24 males & 1 female, Guspini, vi–vii.1933, reared from Asphodelus (FBIN); 11 males & 79 females, Guspini, 19.vii.1934, reared from Asphodelus (FBIN); 67 males & 38 females, Sessa Aurunca, 7.vii.1934, reared from Asphodelus (FBIN); 2 females & 4 males, Matera, 1934, reared from Platycleis grisea (FBIN); 1 male & 7 females, ?locality, 1964, reared from Uromenus brevicollis insularis, leg. Crovetti (FBIN); 189 males & 84 females, Guspini, vi.1934, reared from Asphodelus (FBIN); 1 female & 1 male, Toscana Sesto Fior. vii.1943, leg. L. Ceresa (FBIN); 25 males & 13 females, Mandas vii.1933, reared from Asphodelus (OPPC); 5 females, Guspini, vii.1933, reared from Asphodelus (FBIN); 2 males & 1 female, Guspini, 4.viii.1933 (OPPC); 2 females, Guspini, 3.viii.1933, reared from Asphodelus (OPPC); 1 female, Caprioli, 21.vii.1936 (OPPC); 2 males & 1 female, Nuoro, 13.vii.1933, reared from Asphodelus (OPPC); 1 female, Sessa Aurunca 27.vii.1934 (OPPC); 6 males, ?locality, viii.1933, reared from eggs of Orthoptera, leg. Dr. Provasoli (OPPC).
CROATIA: 1 female, Krk Isle 24.viii.2007, swept, leg. M. Mitroiu (OPPC).
ROMANIA: 1 female, Bârnova forest, N46°59'37.0", E27°35'27.1", 8.ix.2004, swept, leg. O. Popovici (OPPC); 1 female, Bârnova forest, N46°59'37.0", E27°35'27.1", 12.viii.2010, Malaise trap, leg. M. Popovici (OPPC); 2 females, Bârnova forest, 27.iii.2006, obtained from dead wood of Tilia sp., leg. L. Fusu & M. Dascălu, (OPPC); 1 female, Mârzeşti forest, 14.ii.2006, from dead wood of Quercus sp., leg. L. Fusu & M. Dascălu (OPPC).
GREECE: 1 female & 1 male, Krousia Mts., N41°11'32, 4", E23°03'59, 5", 18–24.vii.2007, Malaise trap, leg. G. Ramel (OPPC); 1 female, Krousia Mts., N41°11'32, 4", E23°03'59, 5", 8–14.viii.2007, Malaise trap, leg. G. Ramel (OPPC); 1 female, Promohonas site, N41°22'25.32", E23°22'18.84", 11–17.vii.2007, Malaise trap, leg. G. Ramel (OPPC); 2 females, Midway site, N41°18'49.8", E23°16'35, 6", 14–21.vii.2008, Malaise trap, leg. G. Ramel (OPPC); 1 male & 1 female, Midway site, N41°18'49.8", E23°16'35.6", 21– 7.vii.2008, Malaise trap, leg. G. Ramel (OPPC), 1 female, Midway site, N41°18'49.8", E23°16'35.6", 8–14.ix.2008, Malaise trap, leg. G. Ramel (OPPC); 1 male, Midway site, N41°18'49.8", E23°16'35.6", 28.vii–3.viii.2008, Malaise trap, leg. G. Ramel (OPPC); 2 females & 1 male, Thessalia, Kalambaka, 14–20.viii.1979, hillside meadow, leg. M. C. Day, G. R. Else & D. Morgan (BMNH(E)1979-312).
SPAIN: 1 male, Andalucía, Jaén, Santa Elena, 5.vii.1974, leg. Z. Bouček (BMNH(E)1974-321).
PORTUGAL: 1 male, Madeira, pre-1855, leg. Wollaston (BMNH(E)1855-7, ).
ALGERIA: 1 male, Oran, Douar belbaid, reared from Asphodelus, leg. J. Barbier (6565 MNHN).
JORDAN: 1 male, NW corner, c. 16 km WWN Aljun, 21.v.2007,
32°27.074'N, 35°42.404'E, 600m, leg. J. Bezdek (CNCI).
Triteleia peyerimhoffi: a – habitus female, dorsal view b – habitus male, dorsal view c – head, frontal view d – head, frontal view in malformed specimen e – back of head.
Triteleia peyerimhoffi: a – habitus, lateral view b – head and pronotum, lateral view c and d – variability of sculpture in mesopleuron e – metasoma and ovipositor system.
Triteleia peyerimhoffi: a – antenna in female from Italy b – antenna in the smallest specimen female c – antenna in male d – detail with 5 antennal segment, “sex – segment” e – clava in female of Triteleia peyerimhoffi f – clava in female of Apegus sp. g – clava in female of Macroteleia sp.
Triteleia peyerimhoffi: a – fore wing b – legs c – variability of sculpture of first 2 terga.
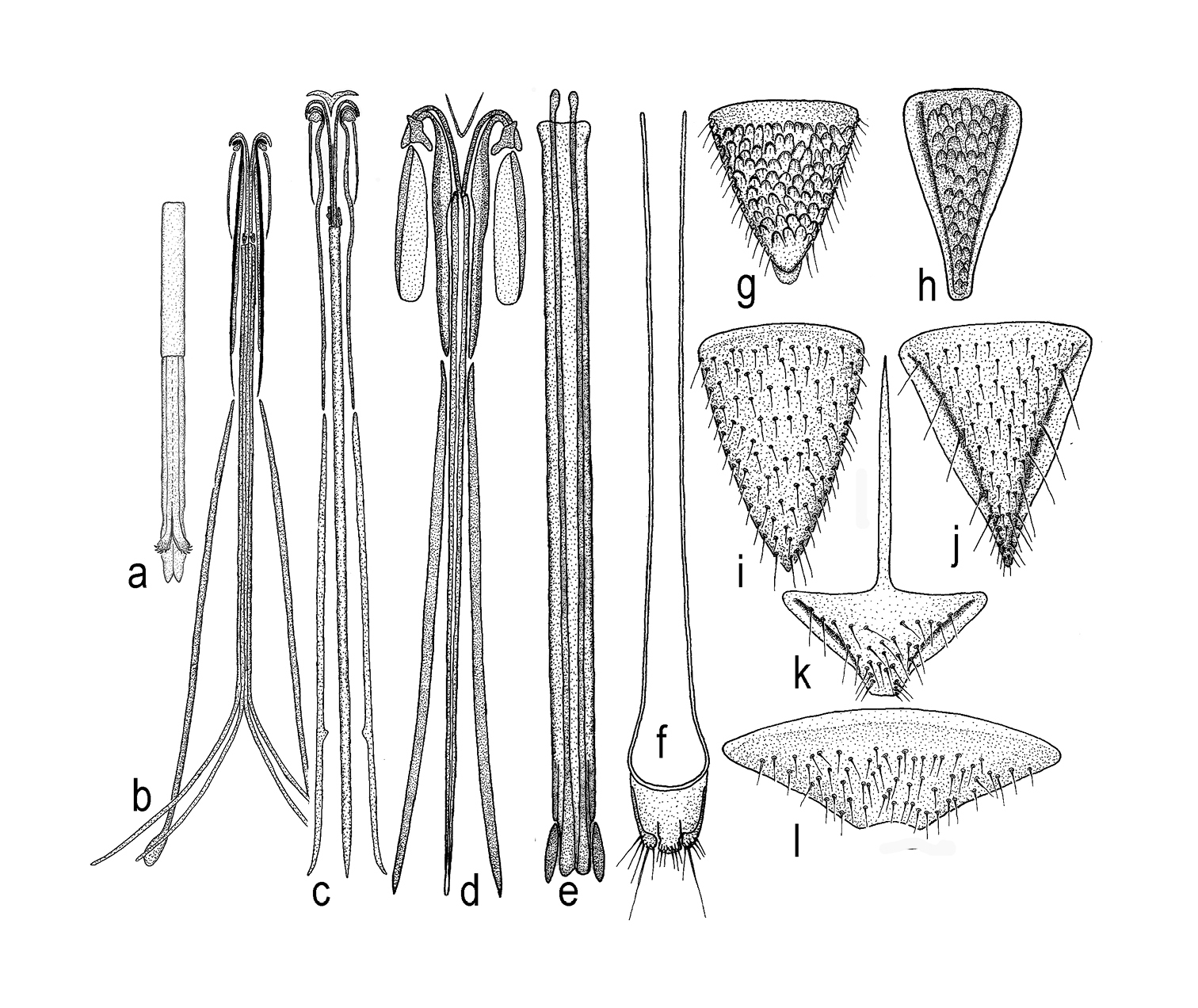
a – aedeagus in Triteleia peyerimhoffi b – ovipositor assembly in Triteleia peyerimhoffi c – ovipositor assembly in Triteleia sp. d – ovipositor assembly in Apegus sp. e – telescopic tube with lateral apodemes, incorporated into wall in Triteleia sp. f – T7, cerci and lateral apodeme in Apegus sp. g – T6 in female of Triteleia peyerimhoffi h – S6 in female of Triteleia peyerimhoffi i – T6 in female of Triteleia sp. j – S6 in female of Triteleia sp. k – S6 in female of Apegus sp. l – T6 in female of Apegus sp.
A female of Triteleia peyerimhoffi and its egg – host belongs to Platycleis albopunctata.
Triteleia peyerimhoffi is a widely distributed species in southern Europe and because of this wide distribution it appears to form local races which appear different. Therefore, it is easy for researchers working with a limited series of specimens from a limited geographic region to misinterpret this intraspecific variability as representing additional species. Indeed, specimens from the extremes of the species’ distribution may look like different species; however, studying a large number of specimens shows an almost continuous morphological gradation between these extremes.
In other parasitic Hymenoptera PCA was successfully used to separate closely related species (
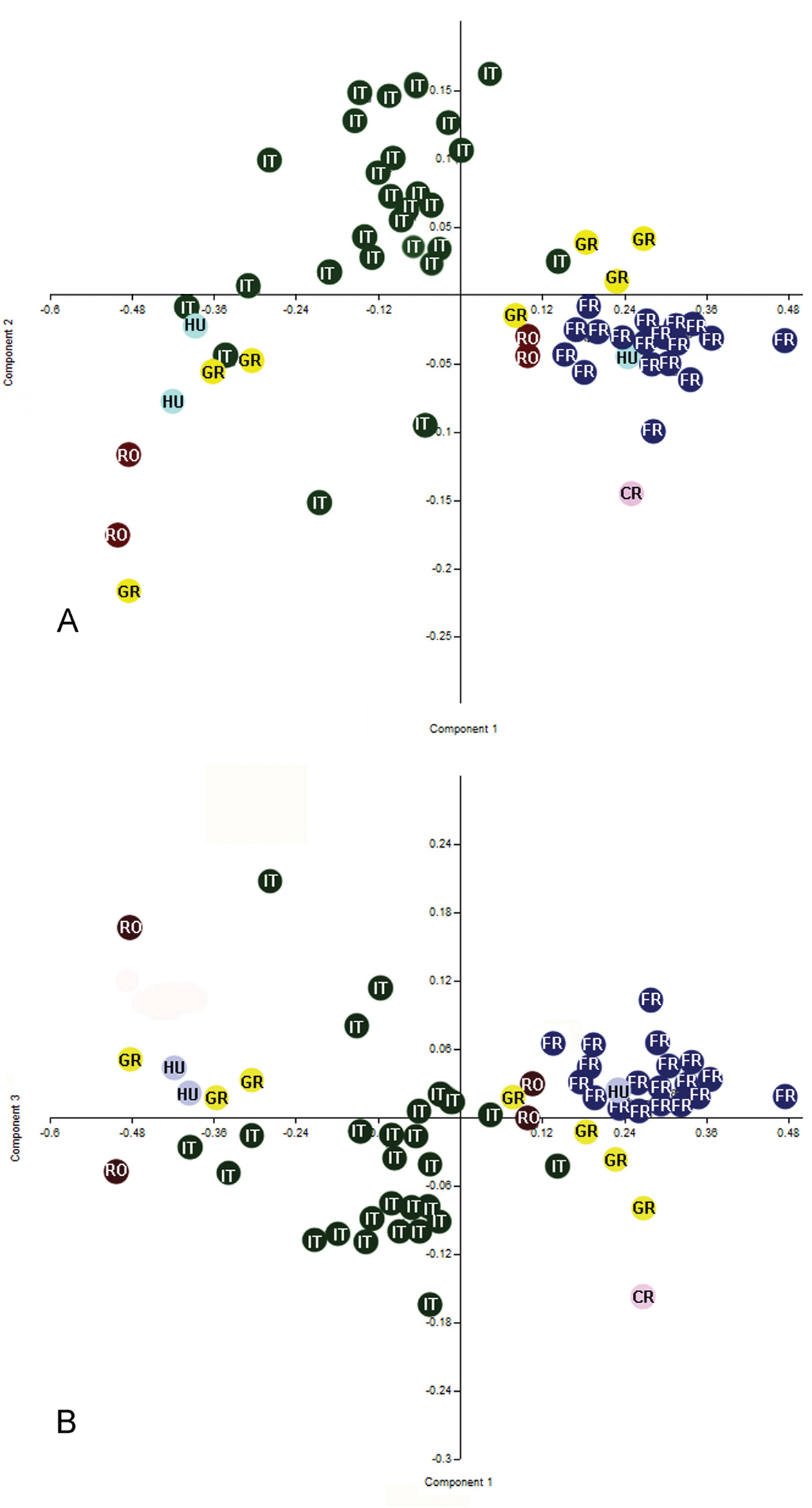
The first six principal components of PCA explain 80.34% of the total variance (one indicated by Jolliffe cut-off value = 0.002; eigenvalues of the first six PCs > 0.002). 95% bootstrapped confidence intervals are given for the eigenvalues (Table 1). The first three principal components of this analysis are plotted in Figures 7a and 7b; they explain 67.35% (PC1), 6.99% (PC2) and 6% (PC3) respectively of the total variance. Both graphs of PC1 on PC2 and PC1 on PC3 displayed a trend of separation between specimens (Figs 7a, 7b). The contributions of variables in this dispersion are showed in the Table 2.
Because in both the graph of PC1 on PC2 and PC1 on PC3, but especially in PC1 on PC2 we obtained a relatively clear separation between the specimens from France and specimens from Italy, we wanted to see if there is a significant difference between these two populations.
One-way ANOVA of all variables indicated significant differences between populations for all variables excepting one (LT6 min: F= 1.865, p= 0.13). The analysis proceeded by pair-wise comparisons (post-hoc) using the Games Howel test after the Levene test revealed unequal variances (p <0.05). The Games Howel test indicated small but significant differences between populations for most variables (p<0.05) (Table 3). The main differences appear between specimens from Italy and specimens from France, in this case almost all characters showed significant differences. Also there were a few significant differences between specimens from France and specimens from Greece (for six characters), between specimens from France and specimens from Hungary (for one character) and between specimens from France and specimens from Romania (for one character). There were no significant differences between specimens from France and the specimen from Croatia. Interestingly there weren’t any significant differences between specimens from Italy and specimens from Greece, Croatia, Romania and Hungary. Also, there were no significant differences between specimens from Greece, Romania, Croatia and Hungary. These results demonstrate that all these specimens from different populations belong to the same species, specimens from France and specimens from Italy being at different extremes with populations from Greece, Croatia, Romania and Hungary, lying between these two populations.
Because characters of the metasoma were very important in the PCA analysis we decided to analyze the size and shape of the metasoma in Triteleia peyerimhoffi to reveal more detail of the variability within this species.
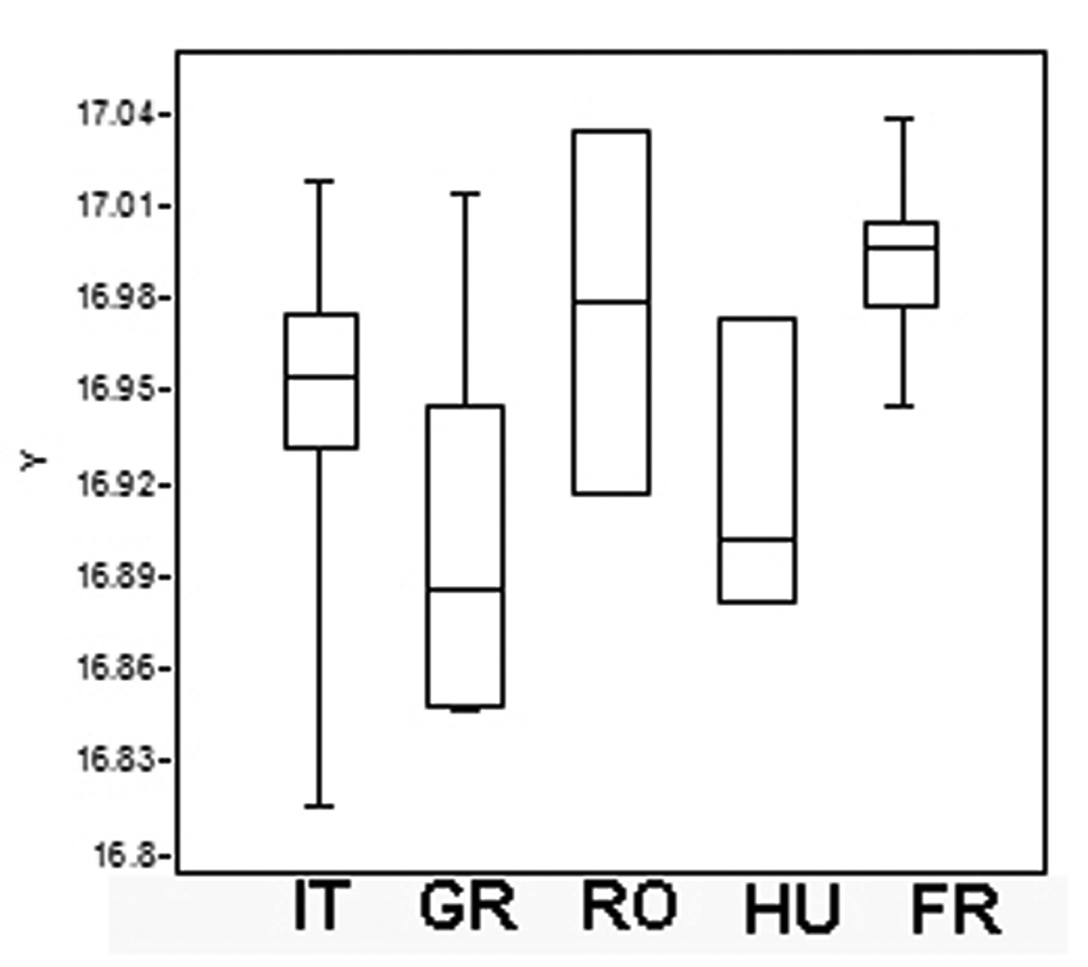
One way ANOVA found significant differences in centroid size between populations (F= 7.17; P = 0.001). The distribution of the log-transformed centroid size is shown in Fig. 8. Levene’s test revealed a normal distribution of all populations (p>0.05) and Tukey’s pairwise mean comparisons showed a significant difference between three of them (Table 4). Again, in the analysis of the metasoma, there were some significant differences between France and Italy, Greece and Romania and between France and Greece. There were no differences however between Hungary and Romania or between Hungary and Greece, no difference between Italy and Romania or between Italy and Greece, no difference between France and Romania, or between specimens from France and Hungary or Croatia (Table 4). We conclude, as before, that although there are some significant differences between some populations, overall the specimens belong to the same species.
The result of analysis of the shape of the metasoma is shown on the plot of principal components. The first two principal components are shown in Figure 9. PC1 accounts for 35.21% of the shape variability and PC2 explains 31.38% of the variability. Thin plate deformation grids show the transformation of shape along the two axes.The MANOVA permutation test found insignificant overall difference between shapes (p>0.05) of metasoma in the six examined populations. The result of this test, confirm our view that all populations belong to the same species. So, in Fig. 9 it is impossible to separate specimens from their provenance, because there is a mixture of specimens belonging to the different populations. Hence we consider this variation in size and shape of the metasoma to be intraspecific variability. Furthermore, we found an almost continuous gradation between the shortest and the longest metasoma (Fig. 10).
Multiple regression of shape on size was performed. The results revealed that only 10.88% of variability of shape is predicted by size (Permutation test against the null hypothesis of independence/ Number of randomization rounds: 10000, P-value: <0.001).
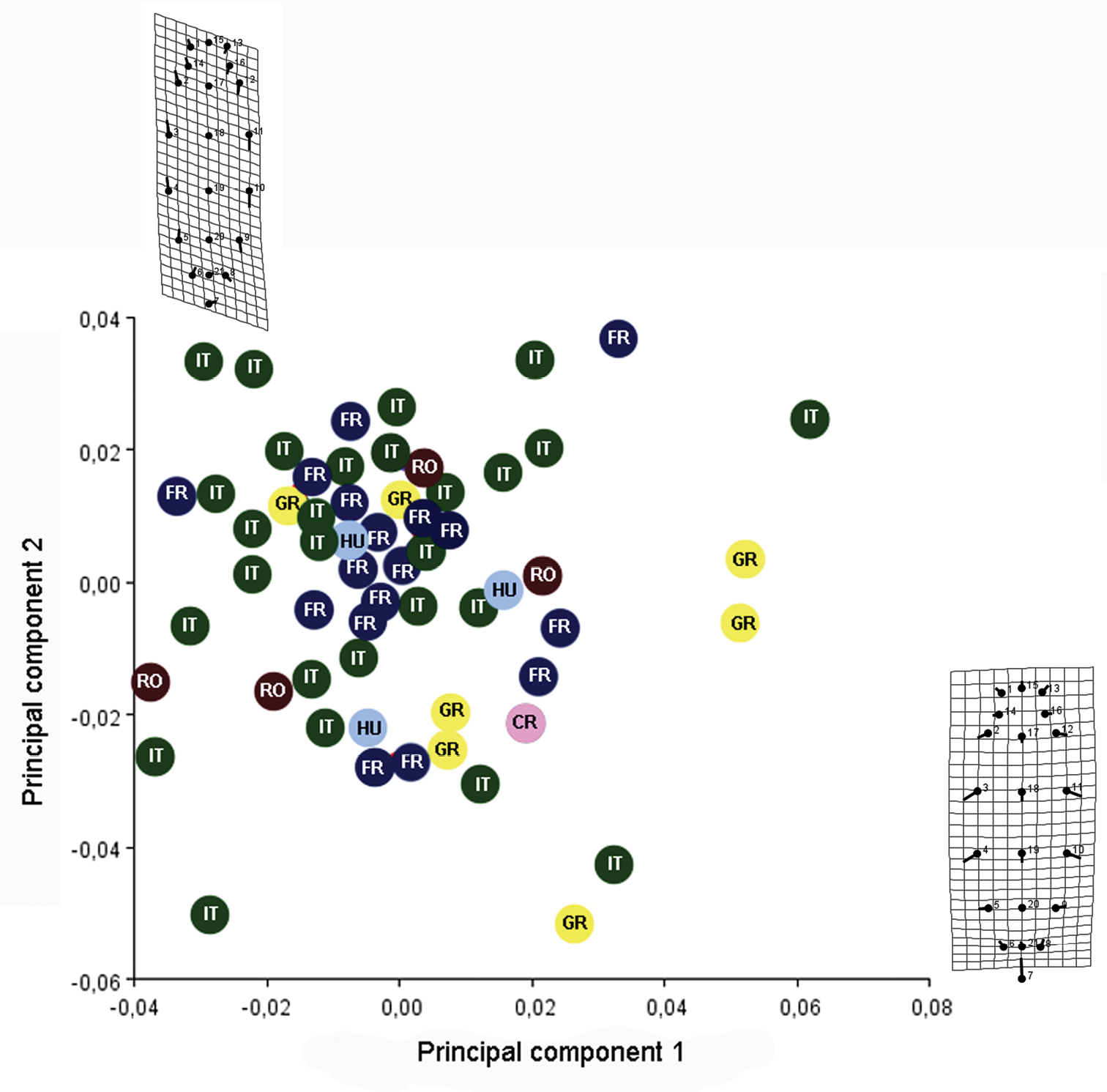
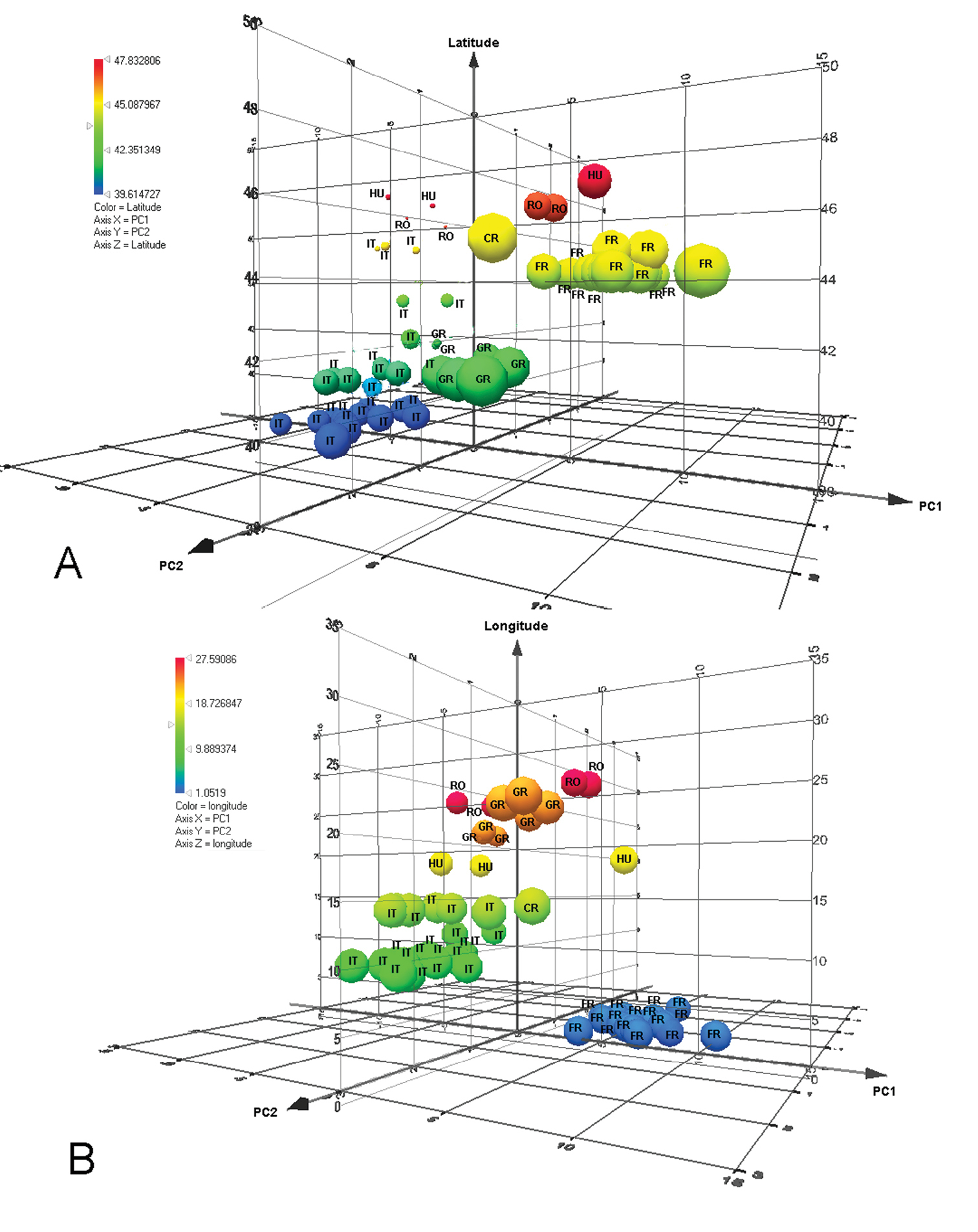
We therefore assert that the great variability of this species to geographical variation. The correlation of geography with these variables was shown by Spearman’s rank coefficient. There was a significant correlation between longitude and PC1 (Spearman’s correlation = - 0.52; p<0.01) and latitude and PC2 (Spearman’s correlation = - 0.62; p<0.01). Therefore, in the case of this species, there is a correlation between longitude and LA3, LT5, width of metascutellum, LT4, LE and LT6 and also, between latitude and minimum width of T6, length of T6, Lt, length of T3, length of T4, length of T2 and length of T5. The relation between latitude and longitude on PC1 and PC2 can be seen in Fig. 11A & B. So, the gap between populations on Fig. 7A and 7B can be explained as a gap between the sites where the specimens were collected. The majority of specimens from Italy were collected from Sardinia and just three from continental Italy. Similarly for the other material: from Greece we analyzed specimens from northern Greece only; from Romania, only specimens from the North-East; from France, only specimens from a limited area. Hence there are many unrepresented areas between the sampled populations. Consequently we think this is the reason for the gap between the populations from France and Italy. Also, we have few specimens from northern Italy, and this population is separated from that in France by the Western Alps.
A large variation in size, shape and sculpture of the metasoma is not something that is unusual within scelionid species, e.g.,
Within the Chalcidoidea,
The influence of hosts on the morphology of parasitoids was demonstrated by
It is very probable that Triteleia peyerimhoffi has not just a single host, but uses a number of similar tettigoniid hosts and differences between the size and shape of the eggs of different host species, and differences between the size and shape of eggs within the same host species under different environmental conditions are a source of intraspecific variability within and between parasitoid populations.
We conclude that Triteleia peyerimhoffi is a species with a wide circum-Mediterranean distribution and is one of the most important eggs parasitoids of Uromenus brevicollis. As an alternative host it uses Platycleis albopunctata which explains its presence in areas (e.g. Romania) where there is no Uromenus, but where Platycleis albopunctata common (Iorgu I., pers. comm.).
Principal Components and the bootstrapped confidence intervals.
| PC | Eigenvalue | % variance | Eig 2.5% | Eig 97.5% |
|---|---|---|---|---|
| 1 | 0.063821 | 67.351 | 61.114 | 74.243 |
| 2 | 0.00663 | 6.997 | 4.5251 | 10.308 |
| 3 | 0.005683 | 5.9973 | 3.2998 | 10.638 |
| 4 | 0.004301 | 4.5388 | 2.7452 | 72.321 |
| 5 | 0.002332 | 2.4606 | 1.1542 | 41.235 |
| 6 | 0.002188 | 2.3087 | 1.2735 | 33.399 |
The contribution of the variables along the first three principal components
| Axis | Loading | Variables |
|---|---|---|
| PC1 | 0.2951 | LA3 |
| 0.2375 | LT5 | |
| 0.2348 | width of metascutellum | |
| 0.209 | LT4 | |
| 0.2032 | LE | |
| 0.2024 | LT6 | |
| PC2 | 0.7473 | minimum width of T6 |
| 0.2352 | length of T6 | |
| 0.2321 | Lt | |
| 0.1575 | length of T3 | |
| 0.1565 | length of T4 | |
| 0.1146 | length of T2 | |
| 0.09395 | length of T5 | |
| PC3 | 0.4948 | minimum width of T6 |
Results of Multiple Comparisons: Games – Howell test (the Table includes only variables and populations with only significant differences)
| Variables | Populations | Mean Difference | Std. Error | p- value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| LH | France | Italy | 0.04 | 0.01 | 0.000 | 0.02 | 0.05 |
| WH | France | Italy | 0.08 | 0.01 | 0.000 | 0.06 | 0.09 |
| WH | France | Greece | 0.06 | 0.02 | 0.045 | 0.00 | 0.12 |
| POL | France | Italy | 0.05 | 0.01 | 0.000 | 0.03 | 0.08 |
| distance between eyes at level of anterior ocellus | France | Italy | 0.06 | 0.01 | 0.000 | 0.05 | 0.08 |
| Hfd | France | Italy | 0.08 | 0.01 | 0.000 | 0.06 | 0.11 |
| distance between compound eye and frontal depression | Romania | France | -0.08 | 0.02 | 0.031 | -0.16 | -0.01 |
| distance between compound eye and frontal depression | France | Italy | 0.06 | 0.02 | 0.027 | 0.01 | 0.12 |
| HE | France | Italy | 0.09 | 0.00 | 0.000 | 0.07 | 0.10 |
| LE | France | Italy | 0.09 | 0.01 | 0.000 | 0.07 | 0.11 |
| gen | France | Italy | 0.08 | 0.01 | 0.000 | 0.04 | 0.11 |
| width of mesosoma | France | Italy | 0.07 | 0.01 | 0.000 | 0.05 | 0.09 |
| length of mesoscutum | France | Italy | 0.07 | 0.01 | 0.000 | 0.05 | 0.08 |
| Wscut | France | Italy | 0.08 | 0.01 | 0.000 | 0.06 | 0.09 |
| width of metascutellum | France | Italy | 0.10 | 0.01 | 0.000 | 0.07 | 0.12 |
| length of T1 | France | Italy | 0.05 | 0.01 | 0.000 | 0.03 | 0.08 |
| minimum width of T1 | France | Italy | 0.08 | 0.01 | 0.000 | 0.06 | 0.10 |
| maximum width of T1 | France | Italy | 0.06 | 0.01 | 0.000 | 0.05 | 0.08 |
| maximum width of T1 | France | Greece | 0.06 | 0.02 | 0.040 | 0.00 | 0.11 |
| length of T2 | France | Italy | 0.05 | 0.01 | 0.000 | 0.03 | 0.07 |
| maximum width of T2 | France | Italy | 0.04 | 0.01 | 0.000 | 0.03 | 0.06 |
| length of T3 | France | Italy | 0.03 | 0.01 | 0.030 | 0.00 | 0.05 |
| maximum width of T3 | France | Italy | 0.06 | 0.01 | 0.000 | 0.04 | 0.07 |
| length of T4 | France | Italy | 0.05 | 0.01 | 0.000 | 0.03 | 0.08 |
| minimum width of T4 | France | Italy | 0.09 | 0.01 | 0.000 | 0.07 | 0.10 |
| minimum width of T1 | France | Greece | 0.08 | 0.02 | 0.010 | 0.02 | 0.14 |
| length of T5 | France | Italy | 0.07 | 0.01 | 0.000 | 0.04 | 0.10 |
| minimum width of T5 | France | Italy | 0.08 | 0.01 | 0.000 | 0.05 | 0.10 |
| minimum width of T5 | France | Greece | 0.11 | 0.02 | 0.002 | 0.05 | 0.16 |
| minimum width of T6 | France | Hungary | 0.00 | 0.00 | 0.003 | 0.00 | 0.00 |
| Lfw | France | Italy | 0.07 | 0.00 | 0.000 | 0.06 | 0.09 |
| Wfw | Greece | France | -0.05 | 0.01 | 0.041 | -0.10 | 0.00 |
| Wfw | France | Italy | 0.06 | 0.00 | 0.000 | 0.05 | 0.07 |
| length of marginal vein | France | Italy | 0.08 | 0.01 | 0.000 | 0.05 | 0.12 |
| Lhw | France | Italy | 0.07 | 0.01 | 0.000 | 0.05 | 0.09 |
| Whw | France | Italy | 0.09 | 0.01 | 0.000 | 0.06 | 0.11 |
| Whw | France | Greece | 0.07 | 0.01 | 0.008 | 0.02 | 0.11 |
Results of Multiple Comparisons: Tukey’s test (the table includes only populations with significant differences)
| Populations | Mean Difference | Std. Error | P value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Italy | France | -0.04 | 0.01 | 0.01 | -0.08 | -0.01 |
| Greece | Romania | -0.08 | 0.03 | 0.03 | -0.16 | -0.01 |
| France | Greece | 0.09 | 0.02 | 0.00 | 0.04 | 0.15 |
Scatter plots of the first three factors from the analysis of the log–transformated data for some population belongs to Triteleia peyerimhoffi (A – PC1 and PC2; B– PC1 and PC3); RO– Romania, IT– Italy, GR– Greece, FR– France, CR– Croatia, HU– Hungary.
Boxplot of metasoma Log– Transformed Centroid Size (Log–CS) screening the metasoma size variation in Triteleia peyerimhoffi populations. (IT– Italy, GR– Greece, RO– Romania, HU– Hungary, FR– France).
PC1 vs PC2 screen plot and thin plate deformation grids show the transformation of shape of metasoma along the two axes. (RO– Romania, IT– Italy, GR– Greece, FR– France, CR– Croatia, HU– Hungary).
Morphological gradation between the shortest metasoma and the longest metasoma in Triteleia peyerimhoffi.
3D–plot of distribution of data according to PC1 and PC 2 and A: latitude; B: longitude. (RO– Romania, IT– Italy, GR– Greece, FR– France, CR– Croatia, HU– Hungary).
The senior author thanks Mr. G. Ramel, a priceless friend, for his huge effort in collecting and sorting samples from Greece; also gratitude to his colleague and friend Dr. L. Fusu for gifts of specimens and for collecting and travels together. Thanks are due to Dr. Svetlana Kononova who allowed Dr. Alex Gumovsky to photograph the holotype of Triteleia striolata; Dr. Alex Gumovsky for his helpfulness; Drs M. Mitroiu and M. Dascălu for the gift of specimens; Drs Sandor Csősz, Claire Villemant, L. Fusu & D. Zimmermann for their help looking for types; Drs I. Iorgu, D. Petit & O. Bardet for information about Uromenus and Platycleis; Drs Norman Johnson & Luciana Musetti for access to The genera of Platygastroidea (Hymenoptera) of the World and Hymenoptera on-line database, valuable resources for any Platygastroidea student and to the anonymous referees for their critical and very useful comments on this paper. This study was financed by the Percy Sladen Memorial Fund, Linnean Society, London, from POSDRU/89/1.5/S/49944 and from the grant SYNTHESYS (GB-TAF-1303).