(C) 2012 Karin Breugelmans. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
In April 2009 two specimens of a terrestrial flatworm were collected from under a rock in an orchard at Ciutadella de Menorca on the easternmost Balearic island of Menorca (Spain). Their external morphology suggested that both specimens belonged to the invasive blue planarian Caenoplana coerulea, a species which is native to eastern Australia. Sequence data of a fragment of the mitochondrial cytochrome c oxidase subunit I (COI) and of the entire 18S ribosomal RNA confirm its identification. This is one of the first records of the species in Europe where it has only been found in one locality in the United Kingdom, France and NE Spain.
Terrestrial flatworm, 18S rDNA, COI, introduction, molecular identification, Balearic Islands, Spain, Europe
Several species of terrestrial planarian are known as invasive, exotic species in soils of the northern hemisphere. For instance, in North America and the British Isles about a dozen species of exotic terrestrial planarians have been introduced (
The impacts of introduced exotic terrestrial flatworms may be especially detrimental in islands and archipelagos that support an endemic invertebrate fauna. This is illustrated by the terrestrial flatworm Platydemus manokwari De Beauchamp, 1962, which has been introduced in many Pacific islands (e.g.
Against this background, we here report for the first time the occurrence of the invasive blue land planarian Caenoplana coerulea Moseley, 1877 in the Balearic Islands (Menorca, Spain). Its identification was confirmed by DNA sequence analysis of the entire nuclear 18S ribosomal RNA (18S rDNA) gene and of a portion of the mitochondrial cytochrome c oxidase subunit 1 (COI) gene.
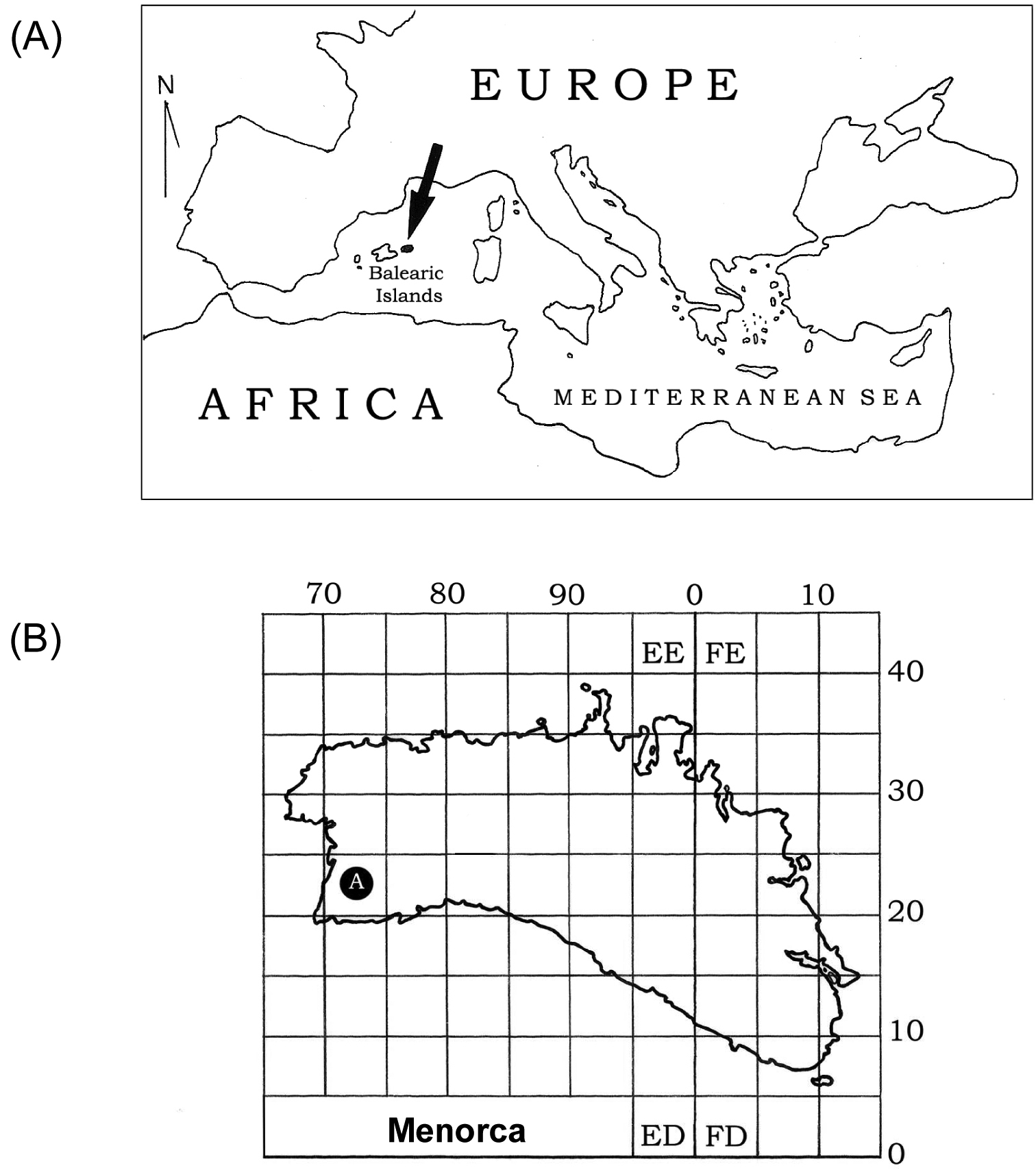
Materials and methodsIn April 2009 two specimens of a terrestrial flatworm were collected by hand under a rock in an orchard at Ciutadella de Menorca on the easternmost Balearic island of Menorca (Spain, 39°57'00"N, 03°51'00"E; Figures 1 and 2). Both specimens (labelled ‘1957’ and ‘1958’) were stored in 100% ethanol.
(A) Location of the Balearic Islands in the Mediterranean Sea. Menorca is in black and indicated by an arrow. (B) Detailed map of Menorca: the locality where Caenoplana coerulea was found is indicated with the letter A.
One of the two specimens of Caenoplana coerulea collected on Menoca.
Genomic DNA was extracted using the NucleoSpin® Tissue Kit (Machery-Nagel, Düren, Germany). A 424 bp fragment of the COI gene was amplified using the primer pair flatCOIL and flatCOIH (modified from
Forward (F) and reverse (R) primers used for amplification and sequencing of the mitochondrial cytochrome c oxidase subunit I (COI) and the nuclear 18S ribosomal RNA (18S rDNA) genes of the two Caenoplana specimens in this study.
| Name | Sequence 5’-3’ | Source |
|---|---|---|
| COI: | ||
| F: flatCOIL | GCAGTTTTTGGTTTTTTGGACATCC | modified from |
| R: flatCOIH | GAGCAACAACATAATAAGTATCATG | modified from |
| 18S rDNA: | ||
| F: 4F18s | CTGGTTGATYCTGCCAGT |
|
| R: 10R18S | TTGGYRAATGCTTTCGC |
|
| F: 9F18S | CGCGGTAATTCCAGCTCCA |
|
| R: 3R18S | GACGGGCGGTGTGTRC |
|
| F: 14F18S | ATAACAGGTCTGTGATGCCC |
|
| R: 16R18S | CYGCAGGTTCACCTACRG |
|
All PCR products were purified using NucleoFast 96 PCR plates (Macherey-Nagel, Düren, Germany) and bidirectionally sequenced using the BigDye Terminator v1.1 chemistry on an ABI 3130xl automated capillary DNA sequencer (Life Technologies). For the sequencing of 18S rDNA several internal primers were used (Table 1). Sequences were visually inspected and aligned in SeqScape v2.5 (Life Technologies). COI and 18S rDNA sequences from other flatworm species of the Continenticola (see e.g.
Two tree reconstruction methods were implemented: Neighbor-Joining (NJ) (
Both specimens have been deposited in the collections of the Royal Belgian Institute of Natural Sciences, Brussels, under catalogue number IG.32062. DNA sequences have been deposited in GenBank under accession numbers JQ639215-JQ639227 (for 18S rDNA) and JQ514564 (for COI).
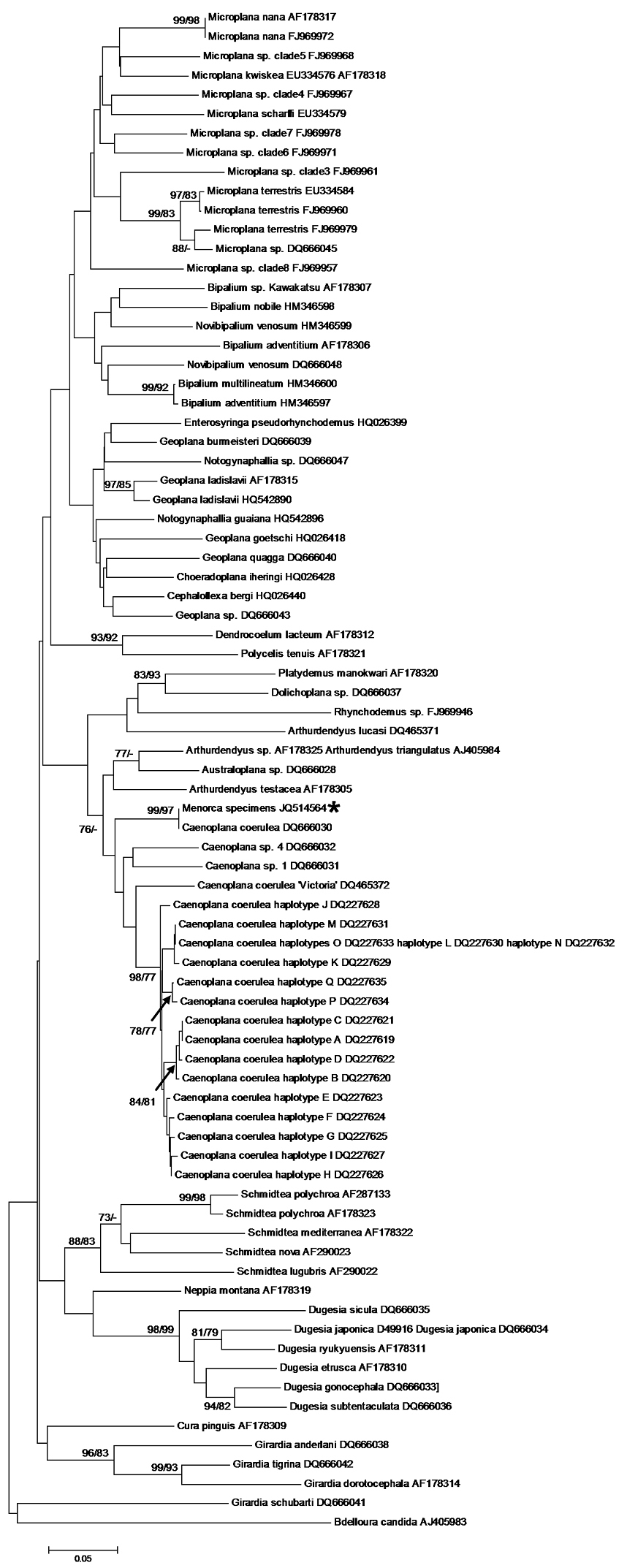
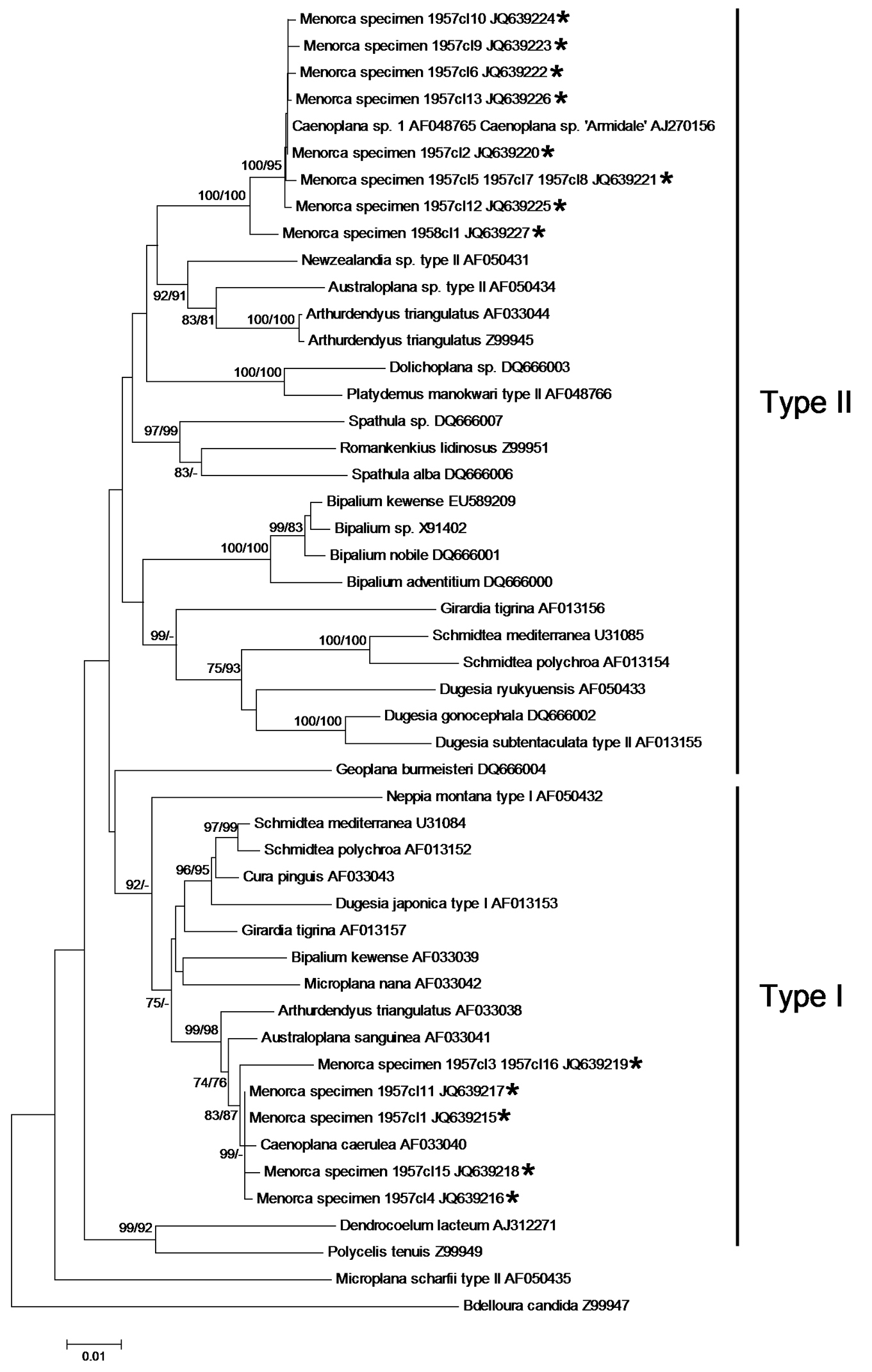
Results and discussionThe dorsal dark blue ground-colour with a thin median dorsal stripe, the intense blue colour of the ventral side, and eyes that are arranged in a single row around the anterior tip and which do not extend dorsally, suggest that the two specimens belong to the species of blue land planarian, Caenoplana coerulea Moseley, 1877 (Geoplanidae). This is corroborated by our phylogenetic analysis of the COI and 18S rDNA genes. Both individuals had the same COI haplotype; as in other triclads, there were two different intra-individual types of 18S rDNA (
Neighbor-Joining and ML tree of the 296 bp dataset of the mitochondrial cytochrome c oxidase subunit I gene (COI). The haplotype of the Menorcan specimens is indicated with an asterisk. Bootstrap values ≥ 70% for the NJ and ML trees are given as NJ/ML and are shown at the nodes. – indicates that the node was not supported by the analysis.
Neighbor-Joining and ML tree of the 1765 bp dataset of the nuclear 18S rDNA gene. The clones (cl) of the Menorcan specimens ‘1957’ and ‘1958’ are indicated with an asterisk. Bootstrap values ≥ 70% for the NJ and ML trees are given as NJ/ML and are shown at the nodes. – indicates that the node was not supported by the analysis. Note that the clades of the type I and type II 18S rRNA variants are not supported.
Caenoplana coerulea is native to eastern Australia but, as a result of human activities, it has been introduced to New Zealand, the United States, the United Kingdom, Norfolk Island (Australia), and France (
In the Iberian Peninsula and Balearic Islands, at present ten autochthonous species of the family Geoplanidae have been reported (
We do not know when exactly this exotic species arrived in the Balearic Islands. The first specimens of Caenoplana coerulea were found in an orchard in April 2009. In 2011 the species had spread to a nearby garden, where it was found at shaded places. As is the case in other land planarians, its spread and distribution in newly colonized areas is probably mainly determined by moisture (
We would like to thank Dr. Leigh Winsor (Condon, Australia) for providing part of the literature and for information on the distribution of Caenoplana coerulea and two anonymous referees for their valuable comments. This work was supported by FWO grant G.0208.08N and the Belgian Network for DNA Barcoding (FWO contract number W0.009.11N).
List of samples used in this study with GenBank accession numbers and sampling locality (if known). The classification follows
| Species | 18S rDNA | COI | Sampling locality | |
|---|---|---|---|---|
| Type I | Type II | |||
| Maricola | ||||
| Family Bdellouridae | ||||
| Subfamily Bdellourinae | ||||
| Bdelloura candida | Z99947 | AJ405983 | ||
| Continenticola | ||||
| Family Planariidae | ||||
| Polycelis tenuis | Z99949 | AF178321 | Spain | |
| Family Dendrocoelidae | ||||
| Dendrocoelum lacteum | AJ312271 | AF178312 | France | |
| Family Dugesiidae | ||||
| Cura pinguis | AF033043 | AF178309 | New Zealand | |
| Dugesia etrusca | AF178310 | Italy | ||
| Dugesia gonocephala | DQ666002 | DQ666033 | The Netherlands | |
| Dugesia japonica | AF013153 | D83382 | DQ666034 | Japan |
| D49916 | ||||
| Dugesia ryukyuensis | AF050433 | AF178311 | Japan | |
| Dugesia sicula | DQ666035 | Spain | ||
| Dugesia subtentaculata | AF013155 | DQ666036 | Spain | |
| Girardia anderlani | DQ666013 | DQ666038 | Brasil | |
| Girardia dorotocephala | AF178314 | USA | ||
| Girardia schubarti | DQ666015 | DQ666041 | Brasil | |
| Girardia tigrina | AF013157 | AF013156 | DQ666042 | France |
| Neppia montana | AF050432 | AF178319 | ||
| Romankenkius libidinosus | Z99951 | |||
| Schmidtea mediterranea | U31084 | U31085 | AF178322 | Spain |
| Schmidtea lugubris | AF290022 | |||
| Schmidtea nova | AF290023 | |||
| Schmidtea polychroa | AF013152 | AF0131154 | AF178323 | Spain |
| AF287133 | ||||
| Spathula alba | DQ666006 | New Zealand | ||
| Spathula sp. | DQ666007 | New Zealand | ||
| Family Geoplanidae | ||||
| Subfamily Bipaliinae | ||||
| Bipalium adventitium | DQ666000 | AF178306 | USA | |
| HM346597 | ||||
| Bipalium kewense | AF033039 | |||
| EU589209 | Japan | |||
| Bipalium multilineatum | HM346600 | Japan / South Korea | ||
| Bipalium nobile | DQ666001 | Japan | ||
| HM346598 | ||||
| Bipalium sp. ‘Kawakatsu’ | X91402 | AF178307 | Japan | |
| Novibipalium venosum | DQ666048 | Japan | ||
| HM346599 | South Korea | |||
| Subfamily Microplaninae | ||||
| Microplana kwiskea | EU334576 | Spain | ||
| AF178318 | ||||
| Microplana nana | AF033042 | AF178317 | Spain | |
| FJ969972 | Spain | |||
| Microplana scharffi | AF050435 | EU334579 | UK | |
| Microplana terrestris | EU334584 | |||
| FJ969960 | Spain | |||
| FJ969979 | Spain | |||
| Microplana sp. | DQ666045 | Spain | ||
| Microplana sp. clade 3 | FJ969961 | Spain | ||
| Micorplana sp. clade 4 | FJ969967 | Spain | ||
| Microplana sp. clade 5 | FJ969968 | Spain | ||
| Microplana sp. clade 6 | FJ969971 | Spain | ||
| Microplana sp. clade 7 | FJ969978 | Spain | ||
| Microplana sp. clade 8 | FJ969957 | Spain | ||
| Subfamily Rhynchodeminae | ||||
| Arthurdendyus lucasi | DQ465371 | |||
| Arthurdendyus testacea | AF178305 | Australia | ||
| Arthurdendyus sp. | AF178325 | Australia | ||
| Arthurdendyus triangulatus | AF033038 | AF033044 | AJ405984 | |
| Z99945 | ||||
| Australoplana sanguinea | AF033041 | Australia | ||
| Australoplana sp. | AF050434 | DQ666028 | Australia | |
| Caenoplana coerulea | AF033040 | DQ666030 | UK | |
| ‘Victoria’ | DQ465372 | Australia | ||
| haplotype A | DQ227619 | Australia | ||
| haplotype B | DQ227620 | Australia | ||
| haplotype C | DQ227621 | Australia | ||
| haplotype D | DQ227622 | |||
| haplotype E | DQ227623 | Australia | ||
| haplotype F | DQ227624 | Australia | ||
| haplotype G | DQ227625 | Australia | ||
| haplotype H | DQ227626 | Australia | ||
| haplotype I | DQ227627 | |||
| haplotype J | DQ227628 | Australia | ||
| haplotype K | DQ227629 | Australia | ||
| haplotype L | DQ227630 | Australia | ||
| haplotype M | DQ227631 | Australia | ||
| haplotype N | DQ227632 | Australia | ||
| haplotype O | DQ227633 | Australia | ||
| haplotype P | DQ227634 | Australia | ||
| haplotype Q | DQ227635 | Australia | ||
| 1957 | JQ514564 | Spain (Menorca) | ||
| 1958 | JQ514564 | Spain (Menorca) | ||
| 1957clone1 | JQ639215 | Spain (Menorca) | ||
| 1957clone4 | JQ639216 | Spain (Menorca) | ||
| 1957clone11 | JQ639217 | Spain (Menorca) | ||
| 1957clone15 | JQ639218 | Spain (Menorca) | ||
| 1957clone3-16 | JQ639219 | Spain (Menorca) | ||
| 1957clone2 | JQ639220 | Spain (Menorca) | ||
| 1957clone5-7-8 | JQ639221 | Spain (Menorca) | ||
| 1957clone6 | JQ639222 | Spain (Menorca) | ||
| 1957clone9 | JQ639223 | Spain (Menorca) | ||
| 1957clone10 | JQ639224 | Spain (Menorca) | ||
| 1957clone12 | JQ639225 | Spain (Menorca) | ||
| 1957clone13 | JQ639226 | Spain (Menorca) | ||
| 1958clone1 | JQ639227 | Spain (Menorca) | ||
| Caenoplana sp.’Armidale’ | AJ270156 | Australia | ||
| Caenoplana sp. 1 | AF048765 | DQ666031 | ||
| Caenoplana sp. 4 | DQ666032 | |||
| Dolichoplana sp. | DQ666003 | DQ666037 | ||
| Newzealandia sp. | AF050431 | |||
| Platydemus manokwari | AF048766 | AF178320 | Australia | |
| Rhynchodemus sp. | FJ969946 | |||
| Subfamily Geoplaninae | ||||
| Cephaloflexa bergi | HQ026440 | |||
| Choeradoplana iheringi | HQ026428 | Brasil | ||
| Enterosyringa pseudorhynchodemus | HQ026399 | |||
| Geoplana burmeisteri | DQ666004 | DQ666039 | Brasil | |
| Geoplana goetschi | HQ026418 | |||
| Geoplana ladislavii | AF178315 | Brasil | ||
| HQ542890 | ||||
| Geoplana quagga | DQ666040 | Brasil | ||
| Geoplana sp. | DQ666043 | Uruguay | ||
| Notogynaphallia guaiana | HQ542896 | |||
| Notogynaphallia sp. | DQ666047 | Brasil | ||