(C) 2011 Angélico Asenjo. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The genus Neolindus Scheerpeltz, 1933, of the tribe Cylindroxystina Bierig, 1943, is recorded from French Guiana for the first time. Two new species, Neolindus irmleri sp. n. and Neolindus hermani sp. n., are described and illustrated. A key to males of Neolindus is provided.
Coleoptera, Staphylinidae, Paederinae, Neolindus, key, French Guiana, new species
The genus NeolindusScheerpeltz, 1933 was revised by
More recently,
In this paper, two new species of Neolindus are described and illustrated and the genus is recorded for the first time from French Guiana, increasing the number of known species to 37. A key to males of the genus is presented based mainly on characters of the genitalia. Neolindus amazonicus
Specimens. Specimens were collected via flight interception traps (FIT or window traps), an especially useful capture method which has resulted in the discovery of many new species of multiple taxa. The specimens studied here were collected by SEAG (Société Entomologique Antilles-Guyane) with FITs hung approximately 1.50 m above ground level (Fig. 1 in
To study morphological characters, dried specimens were macerated in boiling water for five minutes and then cleared in 10% KOH overnight. Dissections were carried out under a Carl Zeiss Stemi SV6 stereoscopic microscope and drawings made with the same equipment. Photographic illustrations were done using IM 50 (Image Manager) software and combined using Auto-Montage Pro (Syncroscopy) software. Measurements were made with an ocular micrometer in the SV6 microscope.
For the type label data, quotation marks “ ” separate different labels and a slash / separates different lines. Text within square brackets [ ] is explanatory and was not included in the original labels.
The following abbreviations are used:
BL body length (from anterior margin of clypeus to posterior margin of tergite IX)
BW body width (maximum width of elytra)
EL elytral length (maximum)
EW elytral width (maximum)
HL head length (from anterior margin of clypeus to posterior margin of head disc)
HW head width (maximum)
PL pronotum length (maximum)
PW pronotum width (maximum)
All measurements are in millimeters and are based on the holotypes. The terminology adopted for the descriptions follows (
All specimens are deposited in the following collections:
DZUP Coleção de Entomologia Pe. J. S. Moure, Departamento de Zoologia, Universidade Federal do Paraná, Curitiba, Brazil (Lucia M. de Almeida).
MNHN Muséum National d’Histoire Naturelle, Paris, France (Thierry Deuve).
MUSM Colección Entomológica del Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Lima, Peru (Gerardo Lamas).
Resultsurn:lsid:zoobank.org:act:DF665432-A4A7-4173-B61B-166F17AADBFD
http://species-id.net/wiki/Neolindus_irmleri
Figs 1–7FRENCH GUIANA: Holotype male, with labels: “GUYANA FRANCESA: / Montagne des chevaux, / 04°43'N, 52°25'W, 90m, / flight intercept trap(glass), / 9.v.2009, S. Brûlé, / P.H. Dalens, E. Poirier” “Holotype / Neolindus / irmleri Asenjo / Desig. Asenjo, 2011” (MNHN).
1 malewith labels: “GUYANA FRANCESA: / Montagne des chevaux, / 04°43'N, 52°25'W, 90m, / flight intercept trap(glass), / 13.vi.2009, S. Brûlé, / P.H. Dalens, E. Poirier” “Paratype / Neolindus / irmleri Asenjo / Desig. Asenjo, 2011” (DZUP).
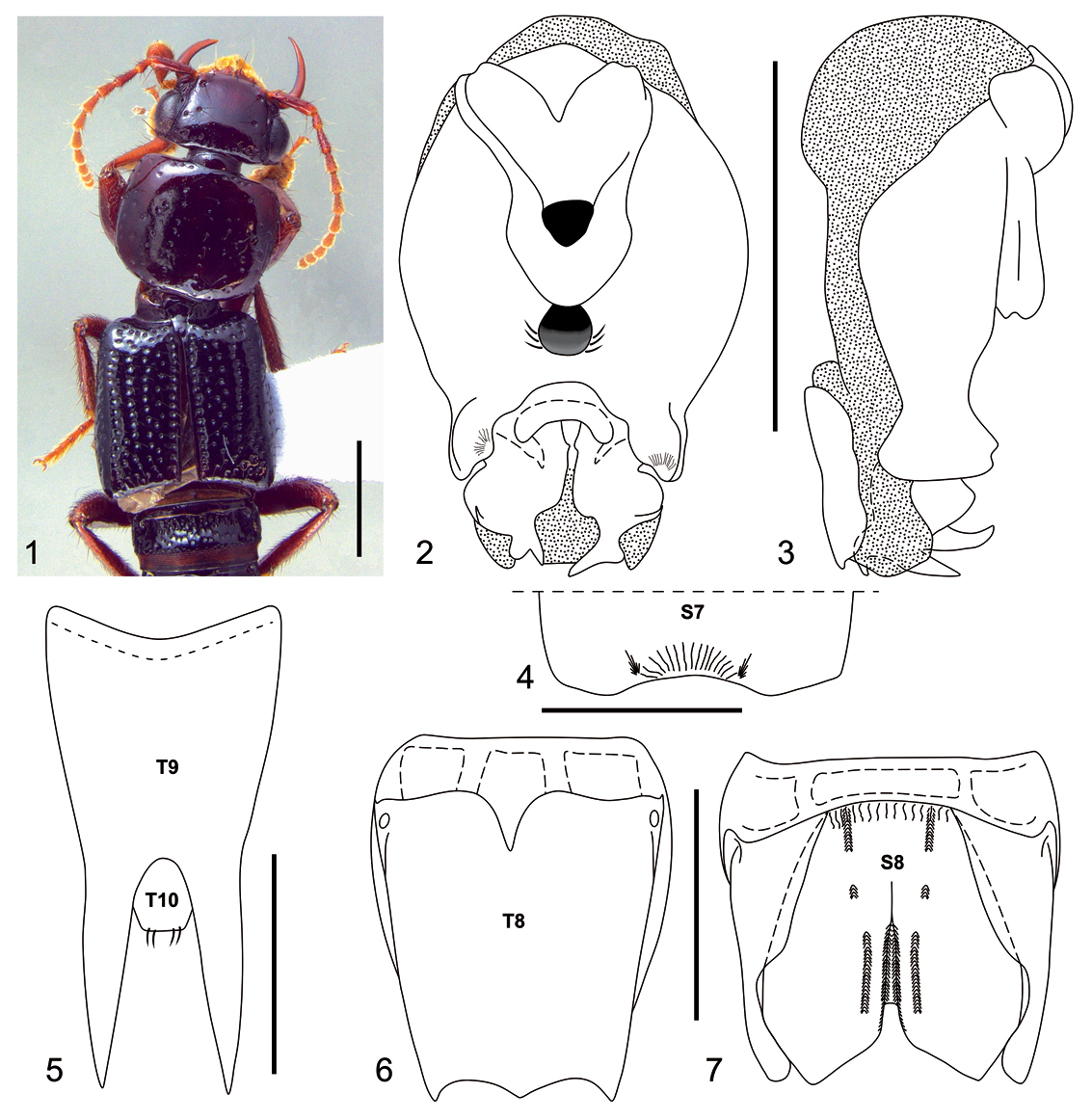
Neolindus irmleri sp. n. can be distinguished from other Neolindus species by the sternum VIII divided into one central and two lateral plates (Fig. 7).
Holotype male, BL: 12.36.
Body dark brown (Fig. 1). Mandibles, femora and tibiae dark reddish brown; antennal segments 1–3 dark reddish brown, segments 4–11 and all tarsi paler.
Head and pronotum moderately flattened dorsoventrally. Head (Fig. 1) wider (HW: 1.61) than long (HL: 1.02), with acute hind angles. Head disk with umbilicate punctures each carrying a black macroseta and one trichobothrium on lateral side of vertex near anterior third of eye, the umbilicate punctures mainly distributed at posterior edge in transversal line. Epicranium shiny without microsculpture and with micropunctures between umbilicate punctures, micropunctures denser anteriorly. Gula with two long setae near anterior margin. Labrum with large, apically rounded lobe near middle of anterior margin and with smaller, apically rounded lobe near lateral edge of anterior margin. Antennae with scape gradually thickened, pedicel (0.24) shorter than 2.6 times the length of scape (0.63), pedicel and segment 3 similar in width (0.12), segment 3 longer (0.33) than pedicel (0.24), segment 4 (length 0.22 : width 0.14) longer than wide, segment 5 (0.24 : 0.14) longer than wide, segments 6 to 8 longer than wide and identical measurements (0.20 : 0.14), segment 9 longer than wide (0.16 : 0.13), segment 10 quadrate (0.14 : 0.14), segment 10 longer than wide (0.22 : 0.12); segments 4–11 densely covered with microsetae; scape to segment 3 with black macrosetae lacking a defined pattern, on segment 4 to 10 arranged in one ring in the apical region, on segment 11 in a ring in the middle region and one tuft in the apical region.
Pronotum (Fig. 1) wider than long (PL: 1.53; PW: 1.86), with anterior margin straight, lateral margin slightly concave and hind angles rounded. Disk polished and shiny without microsculpture, with longitudinal row of 7-9 punctures on each side of midline; several punctures on lateral to paramedial row of punctures; rare micropunctures homogeneously distributed. Elytra (Fig. 1) slightly wider than pronotum (EL: 2.08; EW: 1.96) with epipleural ridge; surface polished and shiny, with irregular rows; with black macrosetae.
Legs uniformly covered with glossy black macrosetae; segments 1–4 of protarsus strongly bilobate and with yellowish pale setae ventrally.
Abdomen polished and shiny, uniformly punctate; the first segments more strongly punctate than the last.
Male with broad and moderately deep, median apical emargination on sternum VII (Fig. 4), posterior margin with small carina on lateral edge of emargination. Segment VIII (Figs 6–7) with four internal canals at base of tergum and sternum. Tergum VIII (Fig. 6) with trilobed posterior margin; basal ridge with short median carina. Sternum VIII (Fig. 7) constituted by two lateral plates and one central plate, fused at the base. Central plate with broad, median emargination; the emargination wide apically and strongly narrowed basally, depression margined laterally by longitudinal carinae; each side of depression with additional lateral carinae; basal ridge with longitudinal small grooves and pair of central carinae; between basal and apical carinae is a small carina. Tergum IX (Fig. 5) fused medially and with long black setae. Aedeagus as in Figs. 2–3; parameres symmetric, fused around basal foramen; with broad, deep median apical emargination; ventral side with median cavity between median depression and basal foramen; median depression with small cluster of setae on lateral margin.
Female not known.
Neolindus irmleri Asenjo sp. n. holotype male. 1 Adult habitus 2 aedeagus, ventral view 3 aedeagus, lateral view 4 apex of sternum VII (S7), setae omitted 5 tergum IX (T9) and tergum X (T10), setae omitted 6 tergum VIII (T8), setae omitted 7 sternum VIII (S8), setae omitted. Scale bars= 1mm.
From window traps in rainforest.
Known from Montagne des Chevaux, French Guiana, 90 m.
This species is named in honor of Dr. Ulrich Irmler of the Institute of Ecosystem Research, Germany.
urn:lsid:zoobank.org:act:2B271F06-AAF9-48EC-B0B7-E904AFA5A527
http://species-id.net/wiki/Neolindus hermani
Figs 8–14FRENCH GUIANA: Holotype male with labels:"GUYANA FRANCESA: / Montagne des chevaux, / 04°43'N, 52°25'W, 90m[altitude in relation to sea level], / flight intercept trap(glass), / 8.iii.2009, S. Brûlé, / P.H. Dalens, E. Poirier" “Holotype / Neolindus / hermani Asenjo / Desig. Asenjo, 2011" (DZUP).
5 males with labels: “GUYANA FRANCESA: / Montagne des chevaux, / 04°43'N, 52°25'W, 90m[altitude in relation to sea level], / flight intercept trap(glass), / 2.v.2009, S. Brûlé, / P.H. Dalens, E. Poirier" [without right elytron] (MNHN); “GUYANA FRANCESA: / Montagne des chevaux, / 04°43'N, 52°25'W, 90m[altitude in relation to sea level], / flight intercept trap(glass), / 10.iii.2009, S. Brûlé, / P.H. Dalens, E. Poirier" [without left elytron] (DZUP); “GUYANA FRANCESA: / Montagne des chevaux, / 04°43'N, 52°25'W, 90m[altitude in relation to sea level], / flight intercept trap(glass), / 8.iii.2009, S. Brûlé, / P.H. Dalens, E. Poirier" [without right elytron] (MUSM); “GUYANA FRANCESA: / Réserve Trésor, [around]~225m[altitude in relation to sea level], 04°36'37.6"N, 52°16'44.5"W, / flight intercept trap (glass), / 1.xi.2009, S. Brûlé, P.H. / Dalens, E. Poirier" (MUSM); “GUYANA FRANCESA:The / Nouragues natural reserve, / Saut Pararé, 04°02'17.1"N, 52°40'22.3"W, 80m[altitude in relation to sea level], flight" “intercept trap (glass), / 20.x.2009, S. Brûlé, P.H. / Dalens, E. Poirier" [without elytra] (MUSM). 2 female with labels: “GUYANA FRANCESA: / Montagne des chevaux, / 04°43'N, 52°25'W, 90m[altitude in relation to sea level], / flight intercept trap(glass), / 20.vi.2009, S. Brûlé, / P.H. Dalens, E. Poirier" (MUSM); “GUYANA FRANCESA:The / Nouragues natural reserve, / Saut Pararé, 04°02'17.1"N, 52°40'22.3"W, 80m[altitude in relation to sea level], " “vii.2009, flight intercept trap / (glass), S. Brûlé, P.H. / Dalens, E. Poirier" (MUSM); all paratypes with label “Paratype / Neolindus / hermani Asenjo / Desig. Asenjo, 2011".
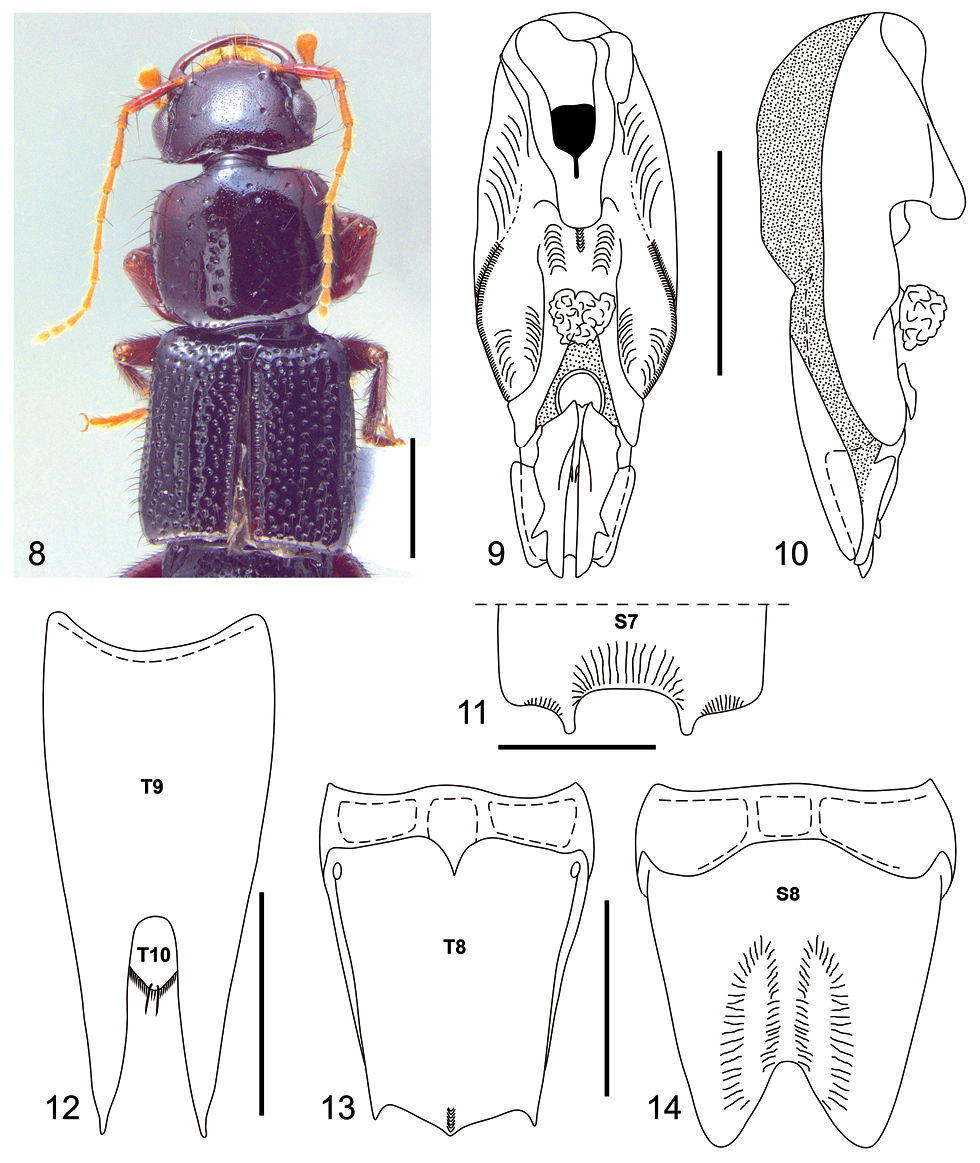
Among Neolindus species, Neolindus hermani sp. n. is similar to Neolindus pastazae, in having the three triangular lobes on the posterior margin of tergum VIII (Fig. 13) and antennal segment 10 shorter than 9. Neolindus hermani sp. n. differs from it by the acute lobe on each side of median apical emargination on sternum VII (Fig. 11) and sternum VIII with a large pair of depressions on each side of the central emargination of the apex (Fig. 14).
Holotype male, BL: 13.75.
Body dark brown (Fig. 8). Mandibles, femora, tibiae and antennal segments 1–2 dark reddish brown; antennal segments 3–11 reddish brown to yellow; all tarsi paler.
Head and pronotum moderately flattened dorsoventrally. Head (Fig. 8) wider (HW: 1.90) than long (HL: 1.22), with acute hind angles. Head disk with umbilicate punctures each carrying a black macroseta and one trichobothrium on lateral side of vertex near anterior third of eye. The umbilicate punctures mainly distributed at posterior edge in transversal line. Epicranium shiny without microsculpture and with micropunctures between umbilicate punctures, micropunctures homogeneously distributed. Gula with transverse cluster of numerous setae near anterior margin. Labrum with large, apically rounded lobe near middle of anterior margin and with smaller, apically rounded lobe near lateral edge of anterior margin. Antennae with scape gradually thickened, pedicel (0.20) shorter than 3.8 times the length of scape (0.76), scape (0.14) wider than pedicel (0.11), segments 3–11 longer than wide and with identical width (0.10), segment 3 (0.47) longer than pedicel (0.20), length segments 4 and 8 (0.31), length segments 5 and 6 (0.35), length segment 7 (0.33), length segment 9 (0.24), length segments 10 and 11 (0.20); segments 3–11 densely covered with microsetae; scape and pedicel with black macrosetae lacking a defined pattern, on segment 3 to 10 arranged in one ring in the apical region, on segment 11 in a ring in the middle region and one tuft in the apical region.
Pronotum (Fig. 8) wider than long (PL: 1.82; PW: 2.12), with anterior margin straight, lateral margin slightly concave and hind angles rounded. Disk polished and shiny without microsculpture; with longitudinal row of 8–11 punctures on each side of midline; several punctures on lateral to paramedial row of punctures; micropunctures homogeneously distributed. Elytra (Fig. 8) slightly wider than pronotum (EL: 2.45; EW: 2.33) with epipleural ridge; surface polished and shiny, with irregular rows; with black macrosetae.
Legs uniformly covered with glossy black macrosetae; segments 1–4 of protarsus strongly bilobate and with yellowish pale setae ventrally.
Abdomen polished and shiny, uniformly punctate; the first segments more strongly punctate than the last. Segments VII and VIII with microsculpture between punctures.
Male with broad and deep median apical emargination on sternum VII (Fig. 11), posterior margin with lobe on lateral edge of emargination; surface adjacent to emargination with shallow median depression. Segment VIII (Figs 13–14) with four internal canals at base of tergum and sternum. Tergum VIII (Fig. 13) with three triangular, apically acute lobes on posterior margin, apex of central lobe longer than lateral lobes; basal ridge with short median carina; surface with slightly midlongitudinal carina on apical portion of median lobe; Sternum VIII (Fig. 14) with large pair of depressions on each side of central emargination of apex. Tergum IX (Fig. 12) fused medially and with long black setae. Aedeagus as in Figs 9–10; parameres symmetric and fused to median lobe; with broad, deep median apical emargination; ventral side with median carina in front of basal foramen; apex of median lobe with many sclerites exposed.
Female with characters of head, pronotum, and elytra as described for male. Abdominal sterna VII and VIII with posterior margin emarginated.
Neolindus hermani Asenjo sp. n. holotype male. 8 Adult habitus 9 aedeagus, ventral view 10 aedeagus, lateral view 11 apex of sternum VII (S7), setae omitted 12 tergum IX (T9) and tergum X (T10), setae omitted 13 tergum VIII (T8), setae omitted 14 sternum VIII (S8), setae omitted. Scale bars= 1mm.
From window traps in rainforest.
Known from Montagne des Chevaux (90m), Nouragues natural reserve-Saut Pararé (80m) and Réserve Trésor (225m) from French Guiana.
This species is named in honor of Dr. Lee Herman of the American Museum of Natural History, USA.
Key to the males of Neolindus speciesThe Peruvian species Neolindus amazonicus, Neolindus hanagarthi and Neolindus peruvianus described for
| 1 | Head with one pair of trichobothria (Fig. 64 in |
2 |
| – | Head with two pairs of trichobothria (Fig. 78 in |
26 |
| 2(1) | Pronotum longer than wide | 3 |
| – | Pronotum wider than long (Fig. 64 in |
10 |
| 3(2) | Tergum VIII with posterior margin rounded or truncate (Fig. 122 & 148 in |
4 |
| – | Tergum VIII with posterior margin emarginate (Fig. 136 in |
7 |
| 4(3) | Antennal segments 3 to 11 with dense pubescence. Peru (Huánuco) | Neolindus verhaaghi |
| – | Antennal segments 4 to 11 with dense pubescence | 5 |
| – | Antennal segments 5 to 11 with dense pubescence | 6 |
| 5(4) | Abdominal tergum VIII (Fig. 122 in |
Neolindus incanalis |
| – | Abdominal tergum VIII ( |
Neolindus cephalochymus |
| 6(4) | Tergum VIII with posterior margin rounded (Fig. 127 in |
Neolindus brewsterae |
| – | Tergum VIII with posterior margin sinuotruncate (Fig. 144 in |
Neolindus agilis |
| 7(3) | Head without midlongitudinal carina at anterior margin | 8 |
| – | Head with midlongitudinal carina at anterior margin | 9 |
| 8(7) | Antennal segment 2 longer than 3, segments 5–11 with dense pubescence. Colombia (Amazonas), Brazil (Pará) | Neolindus densus |
| – | Antennal segment 2 shorter than 3, segments 4–11 with dense pubescence. Peru (Ucayali, Madre de Dios) | Neolindus punctiventris |
| 9(7) | Body length about 9.00 mm; tergum VIII with moderately deeply to shallowly emarginate posterior margin (Fig. 159 in |
Neolindus retusus |
| – | Body length about 6.00 mm; tergum VIII with feebly emarginate posterior margin (Fig. 165 in |
Neolindus procarinatus |
| 10(2) | Tergum VIII with posterior margin rounded or truncate (Fig. 178 & 215 in |
11 |
| – | Tergum VIII with posterior margin emarginate, lobed or trilobed (Figs. 203, 157 & 206 in |
18 |
| 11(10) | Tergum IX with base fused medially (Fig. 180 in |
12 |
| – | Tergum IX with base divided medially (Fig. 171 in |
13 |
| 12(11) | Antennal segment 2 longer than 3. Costa Rica (Puntarenas) | Neolindus cuneatus |
| – | Antennal segment 2 shorter than 3. Ecuador (Napo) | Neolindus milleri |
| 13(11) | Antennal segments 3 to 11 with dense pubescence; median orifice of median lobe of aedeagus with the sclerites hidden (Fig. 170 in |
14 |
| – | Antennal segments 4 to 11 with dense pubescence; median orifice of median lobe of aedeagus with the sclerites exposed (Fig. 214 in |
15 |
| 14(13) | Aedeagus on ventral surface with median apical carina on median lobe (Fig. 170 in |
Neolindus campbelli |
| – | Aedeagus on ventral surface without median apical carina on median lobe (Fig. 174 in |
Neolindus apiculus |
| 15(13) | Tergum VIII with posterior margin rounded (Fig. 183 in |
16 |
| – | Tergum VIII with posterior margin truncate (Fig. 215 in |
Neolindus dichymus |
| 16(15) | Aedeagus without setae on ventral surface (Fig. 186 in |
Neolindus lodhii |
| – | Aedeagus with setae on ventral surface (Fig. 198 in |
17 |
| 17(16) | Sternum VIII with shallow apical emargination; emargination about one-fifteenth of length of sternum (Fig. 199 in |
Neolindus sinuatus |
| – | Sternum VIII with deep apical emargination; emargination about one-fifth of length of sternum (Fig. 195 in |
Neolindus basisinuatus |
| 18(10) | Aedeagus without median hole on ventral surface (Fig. 156 in |
19 |
| – | Aedeagus with median hole on ventral surface (Fig. 210 in |
22 |
| 19(18) | Antennal segment 2 longer than 3; gula with two setae | 20 |
| – | Antennal segment 2 shorter than 3; gula with transverse cluster of setae | 21 |
| 20(19) | Tergum VIII with posterior margin lobed and middle of basal ridge carinated (Fig. 203 in |
Neolindus unilobus |
| – | Tergum VIII with posterior margin emarginated and middle of basal ridge not carinated (Fig. 157 in |
Neolindus bullus |
| 21(19) | Tergum VIII without carina in middle of basal ridge (Fig. 2F in |
Neolindus pastazae |
| – | Tergum VIII with middle of basal ridge pointed (Fig. 206 in |
Neolindus punctogularis |
| 22(18) | Tergum VIII with middle of basal ridge carinate (Fig. 221 in |
23 |
| – | Tergum VIII with middle of basal ridge pointed | 24 |
| 23(22) | Gula with two setae; antennal segments 2 and 3 subequal in length. Ecuador (Pichincha) | Neolindus bidens |
| – | Gula with four setae; antennal segment 2 shorter than 3. Brazil (São Paulo, Rio de Janeiro) | Neolindus schubarti |
| 24(22) | Sternum VIII not divided (Fig. 14) | 25 |
| – | Sternum VIII divided into three plates, one central and two lateral (Fig. 7). French Guiana | Neolindus irmleri sp. n. |
| 25(24) | Gula with two setae; antennal segments 9 and 10 subequal in length and 4 to 11 with dense pubescence; aedeagus with setae on ventral surface (Fig. 224 in |
Neolindus religans |
| – | Gula with transverse cluster of setae; antennal segment 10 shorter than 9 and 3 to 11 with dense pubescence; aedeagus without setae on ventral surface (Fig. 9). French Guiana | Neolindus hermani sp. n. |
| 26(1) | Tergum VIII with posterior margin rounded (Fig. 82 in |
27 |
| – | Tergum VIII with posterior margin emarginate, trilobed (Fig. 109 & 117 in |
31 |
| 27(26) | Aedeagus with apex of median lobe pointed (Fig. 81 in |
28 |
| – | Aedeagus with apex of median lobe emarginate (Fig. 85 in |
30 |
| 28(27) | Sternum VIII with surface of apex of internal canals unmodified. Venezuela (Trujillo) | Neolindus plectrus |
| – | Sternum VIII with depressions in the surface of apex of internal canals (Fig. 92 in |
29 |
| 29(28) | Aedeagus distinctive and without transversal carina or process on ventral surface (Fig. 105 in |
Neolindus rudiculus |
| – | Aedeagus distinctive and with transversal carina or process on ventral surface (Fig. 93 in |
Neolindus pumicosus |
| 30(27) | Antennal segment 2 longer than 3. Ecuador (Napo) | Neolindus parallelus |
| – | Antennal segments 2 and 3 subequal in length. Venezuela (Aragua) | Neolindus brachiatus |
| 31(26) | Pronotum longer than wide; aedeagus with apex of median lobe pointed (Fig. 113 in |
2 |
| – | Pronotum wider than long; aedeagus with apex of median lobe emarginate (Fig. 152 in |
Neolindus hamatus |
| 32(31) | Elytra shorter than pronotum; tergum VIII with posterior margin emarginate (Fig. 109 in |
Neolindus lirellus |
| – | Elytra longer than or as long as pronotum; tergum VIII with posterior margin trilobed (Fig. 117 in |
Neolindus prolatus |
I am grateful to Stéphane Brûlé, Pierre-Henri Dalens, Eddy Poirier, Julien Touroult and Philippe Collet for collecting specimens in French Guiana and allowing me to study them; Dr. Nigel Pitman (Duke University) for suggestions on the manuscript;The Biological Collection Network of Paraná (Taxon-line, UFPR) for the photographs and Brazil’s National Council of Science and Technology Development (CNPq) for scholarships in support of this research.