(C) 2012 Nathalie Yonow. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Seventy species of opisthobranchs are described in this work based on collections from the Persian Gulf, Socotra, Kenya, Zanzibar, Madagascar, La Réunion, Mauritius, the Seychelles, the Maldives, and Sri Lanka. Ten species are newly recorded from the western Indian Ocean and four species are recorded in the scientific literature for the first time since their original descriptions. Two species are described as new: Cyerce bourbonica sp. n. from La Réunion and Doriopsilla nigrocera sp. n. from the Persian Gulf coast of Saudi Arabia. Chromodoris cavae is removed from its synonymy with Chromodoris tennentana and redescribed from specimens from La Réunion, while several new synonyms are proposed for some commonly occurring species. Risbecia bullockii is recorded for the second time from the Indian Ocean and assigned to its correct genus.
Nudibranchs, Cephalaspidea, Anaspidea, Sacoglossa, Nudibranchia, Doridina, biogeography, taxonomy
The year 2010 was designated the International Year of Biodiversity by the United Nations; however, biogeographical and taxonomic works cataloguing collections of opisthobranchs are rare in this age of advanced technology and DNA techniques. Expeditions have been mounted in order to collect and record species of particular groups, but systematic papers on their collections often remain unpublished. For example, in 1990 the Rumphius Biohistorical Expedition revisited the area sampled by the Dutch explorer-naturalist Rumphius to compare the marine invertebrate fauna with his records (see
In this publication, expedition material is supplemented by occasional specimens collected by a number of people from a range of western Indian Ocean localities. Very few of the species were collected in high numbers, but these specimens are none-the-less valuable; if occasional specimens of the uncommon or rare species are never recorded in the scientific literature, our knowledge of the basic ‘data set’ remains hidden, with only a few scientists and museum curators aware of their existence. For example, a description and illustration of an unidentified species from the Maldives based on a single specimen, Thuridilla sp. (Yonow 1994), was recognised as new and described one year later (Thuridilla undula Gosliner, 1995) as having a widespread Indo-West Pacific distribution: the single Maldive specimen provided the only Indian Ocean record.
Four species included in this report have not been recorded in the scientific literature since their original descriptions, and many other species provide records from new localities. Although some of these new locations may not be unexpected, a surprisingly patchy distribution for opisthobranchs within the Indian Ocean is emerging, with several species occurring only in one or two localities. Ten species are recorded from the western Indian Ocean for the first time: some are common western Pacific species, but several have again been rarely recorded in the literature. This work aims to document some of the species occurring in the region and to provide information on their identities and geographical distributions; not all groups are covered, and subsequent papers will deal with the Aeolidina, Dendronotina, Arminina, and Pleurobranchida as well as provide a more thorough discussion on the biogeography of the western Indian Ocean Opisthobranchia.
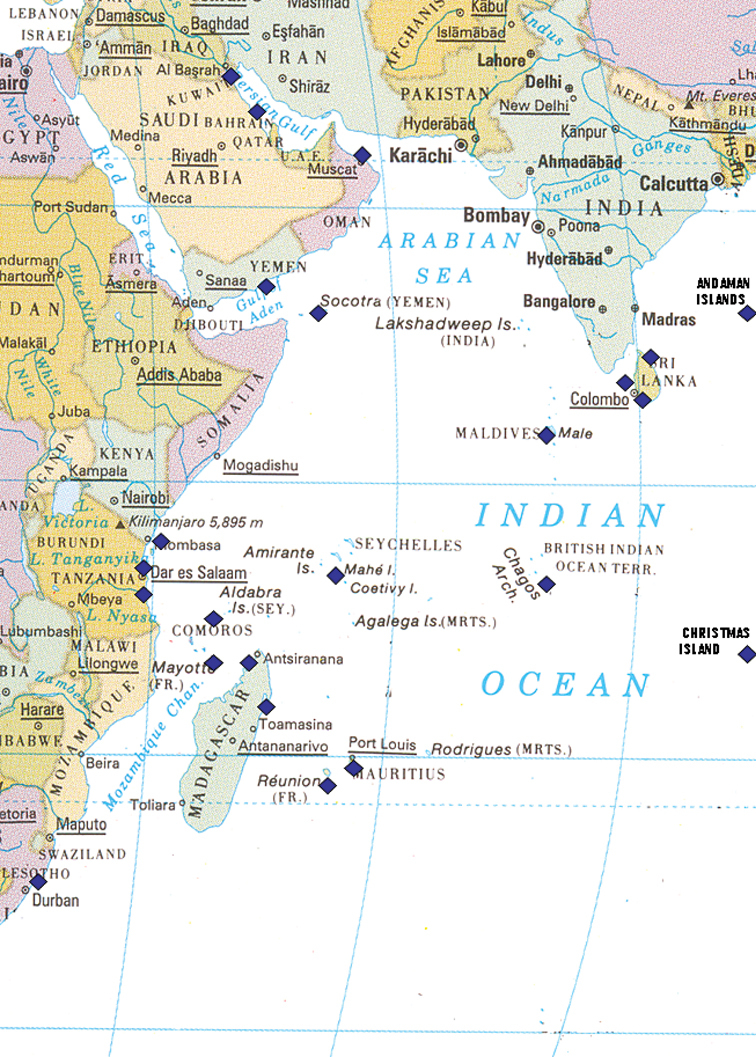
A map of the collection localities of the species included in this work is provided below. Species recorded and discussed by the author in previous publications are listed with a relevant and updated synonymy within the Indian Ocean: new specimens are listed with their collection details and brief descriptions of each individual, with colour photographs to provide a visual reference as well as to enable the readers to make their own assessment. Further information acquired on morphology, behaviour, habitat, and distributions is provided, as are references to any recent research. Species not covered in previous papers by the author are described and illustrated in more detail to verify their identification, especially if they have been rarely recorded or are new records for the western Indian Ocean. “The color (both dorsal and ventral) is critically important in nudibranch taxonomy, not only because it provides potentially good diagnostic characters ... Therefore it is important to evaluate color variation among individuals” (
Map of the western half of the Indian Ocean showing collecting localities listed in text marked by blue squares; note the Andaman Islands and Christmas Island are off the map in reality.
Parts of these collections have already been reported upon by the author; all methods are as described previously (
The literature was carefully reviewed, and the references included in the synonymies are as complete as possible for Indian Ocean records.
The buccal bulb was removed through ventral longitudinal and horizontal cuts in the head and dissolved in a weak solution of sodium hydroxide (NaOH) for at least 48 hours. The radula was rinsed in several washes of water, stained and flattened on a glass slide with lignin pink in lactophenol, and photographed through an Olympus compound microscope. Dissections of phyllidiids was made by cuts in the mantle enabling the removal of the dorsum from the rhinophores to the anal hole. All line drawings were made using a camera lucida attachment to the Olympus for the radulae, a Wild for the anatomy, or traced from micrographs. All drawings are precisely what was seen and labelled for each specimen, and future workers should be able to link unambiguously all illustrations to the specimens and radular preparations.
Systematic accountshttp://species-id.net/wiki/Haminoea_cf_cymbalum
Plate 1Maldives: two specimens approx. 20 mm length (MDV/AB/96/23), Vilufushi lagoon, Thaa Atoll, 3 m depth, 31 March 1993, leg. RC Anderson & SG Buttress. – La Réunion: photographs of numerous individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
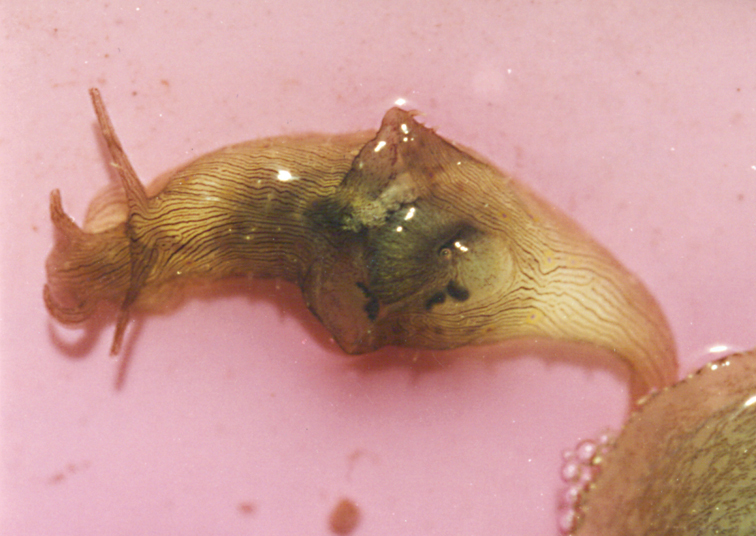
Globose shell thin, transparent, too small to accommodate body completely; shell 15 mm, living animal up to 25 mm. Body pale green with large and small orange spots, part contained within the shell much darker green than both head and tail. Larger orange spots and patches underlying shell outlined with white or very pale green.
Infrequently, huge mating congregations are observed, with numbers of up to 100 individuals per m2 in February 2007 in La Réunion, which lasted approximately one week (P Bidgrain, pers. comm. and http://seaslugs.free.fr/nudibranche/a_intro.htm). These aggregations were not observed again until 2010, but with a much smaller number of animals, only 100 individuals in total (P Bidgrain, pers. comm.).
Haminoea cf. cymbalum has been rarely recorded in the Indian Ocean; it is more common in the western Pacific. Difficulties arise with the name of this well-known species: both Burn (in
http://species-id.net/wiki/Chelidonura_electra
Plate 2Maldives: 29 mm length (10 mm pres., MDV/AB/96/1), Guraidhoo Channel, South Malé Atoll, 16 m depth, 30 April 1996, leg. RC Anderson & SG Buttress. – Zanzibar: two pres. specimens 15 mm × 2 mm and 19 mm × 9 mm, Bawe Island, underside of Porites head with encrusting Tubastrea and sponges, 7 m depth, June 1995, leg. MD Richmond. – Madagascar: 50 mm × 10 mm (PK-C), Ampangorina, Nosy Komba, on algal-encrusted coral, 3 m depth, 30 January 1992, leg. P Kemp. – Mayotte: photographs of two individuals, 15 mm and 25 mm http://seaslugs.free.fr/nudibranche/a_intro.htm.
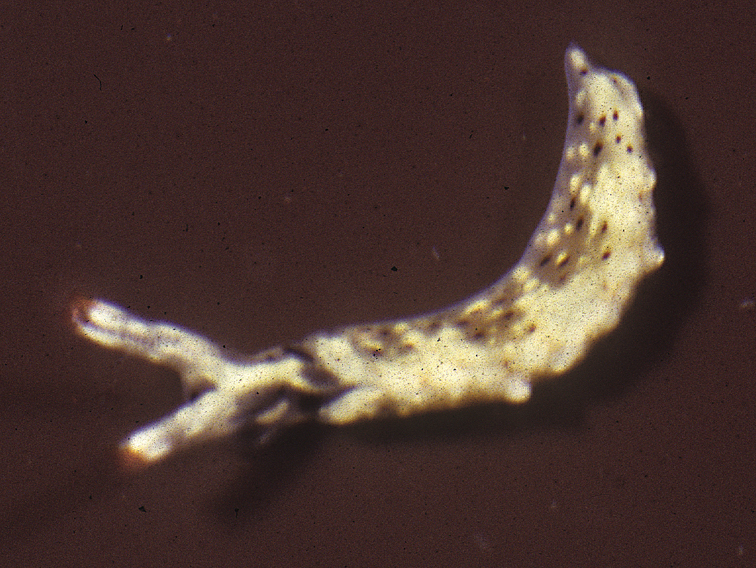
These specimens and photographs are among the first records of Chelidonura electra from the Indian Ocean. Chelidonura electra is distinguished by its white body and yellow edges to the parapodia and tails. There are no similar specimens in the Indian Ocean: Chelidonura pallida Risbec is found in the western Pacific and eastern Indian Ocean only; it has not been recorded from the western Indian Ocean. Also white, it has a black line along the edges, followed by an inner orange-yellow band.
http://species-id.net/wiki/Chelidonura_hirundinina
Plate 3Maldives: three specimens, approx. 5-6 mm length pres., Kuredu, Lhaviyani Atoll, on sandy substrate in shallow water, 10-24 March 1998, leg. J Hinterkircher; several individuals, photos only, 1986-1994, J Hinterkircher. – La Réunion: photographs of numerous individuals, 10 - 40 mm http://seaslugs.free.fr/nudibranche/a_intro.htm.
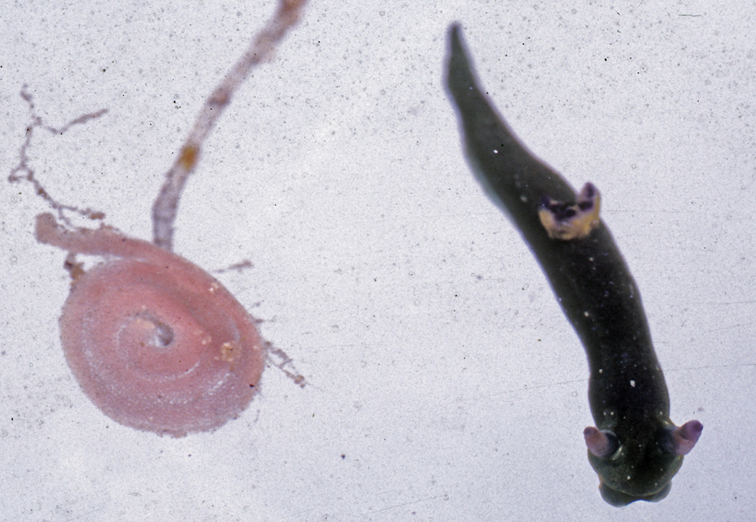
Dark brown to black body with black and orange edging, vivid blue Y- or T-shaped mark on head, small white patch at posterior end of head shield, and large white crescent-shaped mark on posterior shield. The species is rarely recorded in the Indian Ocean, where the pattern is relatively consistent, and somewhat different to those from the Pacific Ocean.
http://species-id.net/wiki/Chelidonura_punctata
Plate 4Maldives: 15 mm (MDV/AB/96/15, “very dark brown, almost black, white edge anteriorly and blue grey edge to parapodia, with orange-red spots”), Fulidhoo Channel, Felidhoo Atoll, 9 m depth on coral rock, 7 May 1996, leg. RC Anderson & SG Buttress. – Mauritius, La Réunion, and Mayotte: five individuals photographed http://seaslugs.free.fr/nudibranche/a_intro.htm.
Chelidonura punctata appears to be restricted to the western Indian Ocean; to date there are no records of the species occurring in the Red Sea, the Gulf of Aden, the Gulf of Oman, or the Persian Gulf. The similar species found in the Gulf of Eilat (recorded erroneously as Chelidonura punctata in
An interesting paper documenting the feeding habits of Chelidonura punctata in Kenya describes the association between it and an acoel flatworm present on the hard coral Platygyra daedalea (
http://species-id.net/wiki/Chelidonura_sandrana
Plates 6 , 7Maldives: 3 mm pres., Maayafushi, Ari Atoll, shallow water in lagoon, February 1995, leg. J Hinterkircher; photos of two individuals, 1986-1994, J Hinterkircher; 7 mm (5 mm pres., “all black”), Maayafushi Channel, Ari Atoll, 7-17 m depth on sandy slope, 12 October 1994, leg. RC Anderson & SG Buttress.
The collecting notes state that there were “plenty of aglajids, all completely black” in Maayafushi lagoon, up to approximately 10 mm in length, and it is very common in the Maldives (Yonow 1994). This species is recorded throughout the western Indian Ocean as well as in the northern Red Sea, and its extensive variability of colouration is now well known; both specimens demonstrate the all-black colouration (Plate 6) while an additional photo (from another locality in the same atoll) depicts an individual which is black with white marbling, but without the orange spots (Plate 7).
http://species-id.net/wiki/Chelidonura_varians
Plate 8Maldives: two specimens, 30 mm and 15 mm pres., Maayafushi Channel, Ari Atoll, 7-17 m depth on sandy slope, 12 October 1994, leg. RC Anderson & SG Buttress.
http://species-id.net/wiki/Philinopsis_speciosa
Plate 9Maldives: two specimens, 18 mm and 12 mm pres., Maayafushi Channel, Ari Atoll, 7-17 m depth on sandy slope, 12 October 1994, leg. RC Anderson & SG Buttress; 24 mm (MDV/AB/96/5), Fulidhoo lagoon, Felidhoo Atoll, 7 m depth, 03 May 1996, leg. RC Anderson & SG Buttress. – La Réunion, photographs of several individuals http://seaslugs.free.fr/nudibranche/a_intro.htm. – Tanzania: photograph of single individual, Mafia Island, shallow water, 31 July 2004, A de Villiers.
The photographs on the
http://species-id.net/wiki/Aplysia_parvula
Plates 10, 11Pale form: Socotra: 10 mm × 5 mm pres. (IT-084, N-171), Rhiy di-Irisal, SE site, 2-8 m depth, 24.II.1999, leg. N Simões. – La Réunion, photographs of numerous individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
Dark form: Maldives: two specimens approx. 14 mm in length, Hulhulé Island, North Malé Atoll, 12 m depth on orange encrusting sponge on outer reef, 30 July 1995, leg. RC Anderson & SG Buttress (“dark brown with numerous tiny white dots, brown tending to orange, greyish white at margins”); two specimens both 14 mm, Old Shark Point, Thilafalhu Reef, North Malé Atoll, 16 m depth, 18 November 1995, leg. RC Anderson & SG Buttress (“dark brown with numerous fine pale dots; edges of [parapodia] and tentacles pale”); 20 mm (MDV/AB/96/13), Fulidhoo Channel, Felidhoo Atoll, 9 m depth, 07 May 1996, leg. RC Anderson & SG Buttress (“no white spots”). – La Réunion, photographs of several individuals http://seaslugs.free.fr/nudibranche/a_intro.htm. – Tanzania: photo of one individual, Mafia Island, shallow water, May 2009, A de Villiers.
The dark colour form is common in the Red Sea and western Indian Ocean (
The radula of the Socotra specimen has the formula 29 (+2) × 4.7.1.7.4. It is comparable in formula and size to those of the dark form previously examined from the Red Sea (26 (+1) × 4.6.1.6.4:
http://species-id.net/wiki/Dolabella_auricularia
Fig. 2, Plate 12Zanzibar: 46 × 28 mm pres., Kizimkazi Dimbani reefs, intertidal, June 1994, leg. Suki/MD Richmond. – La Réunion, Mauritius, and Mayotte: photographs of numerous individuals and two shells http://seaslugs.free.fr/nudibranche/a_intro.htm. – Oman: Muscat, 1-12 April 2009, photo S Kahlbrock. – Seychelles: one individual photographed, Lilôt, NW Mahé, 1988-1989, P Kemp.
Examination of the shell (Fig. 2) identifies the Zanzibar specimen as the tropical Indo-Pacific Dolabella auricularia; the specimen had rounded tubercles in life, and these remain on the preserved animal. There are only two species of Dolabella, but to date no recent specimens of Dolabella gigas (Rang) have been recorded. The two species are said to differ in shell morphology and internal characters (
The radular formulae appear smaller in gigas but it is difficult to make size-for-size comparisons from the literature. This study demonstrates that the shells of three specimens from La Réunion and Zanzibar with rounded tubercles, the external morphology of Dolabella gigas, prove to be identical to Dolabella auricularia (P Bidgrain and M Jay, pers. comm., see http://seaslugs.free.fr/nudibranche/a_intro.htm for shell photos). Looking at the many shells illustrated by Engel (op. cit.) and Eales (op. cit.), there appears to be a clinal variation from one extreme to the other. Smooth and hirsute specimens were recorded together from Mauritius (
Dolabella auricularia, shell of 46 mm specimen (24 mm maximum shell dimension).
The Maldives specimen and shell listed in Yonow (1994) are deposited in The Natural History Museum, London (NHMUK 20110444), and not in the Australian Museum, Sydney, as stated in that paper due to subsequent postal security regulations.
http://species-id.net/wiki/Dolabrifera_dolabrifera
Socotra: 10 mm curled pres. (St-021, MAP-097), rocks with algae, W of Rhiy di-Diblih, Nogid, S coast, 12 March 1999, leg. M Apel. – Maldives: 60 mm approx. (49 mm pres.), in Tridacna culture tank, Marine Research Section, Malé, North Malé Atoll, 30 December 1995, leg. RC Anderson (“mottled olive green; extruded white gelatinous fluid from posterior siphon”); photos only of several individuals, March 1997, March 1998, March 1999, J Hinterkircher. – Mauritius and La Réunion: numerous individuals with various colours and textures http://seaslugs.free.fr/nudibranche/a_intro.htm. – Seychelles: photo of one individual, Lilôt, NW Mahé, 1988-1989, P Kemp.
Preserved specimen from the Maldives are relaxed, with rhinophores and tentacles well extended, and a distinct flange present around margin; Socotra specimen very contracted and curled up. Colours of living specimens are extremely variable, and the villi may or may not be present; Dolabrifera dolabrifera can grow to 50 mm in length, and is common in the western Indian Ocean and Red Sea in shallow inter- and sub-tidal coastal waters; it has a wide Indo-Pacific distribution.
http://species-id.net/wiki/Notarchus_indicus
Socotra: four specimens 15-20 mm preserved lengths (IT-157, RJ-011), 12°18.698'N, 53°48.285'E, 09 April 1999, leg. R Janssen.
Preserved specimens are similar to previously examined material from the Red Sea, severely contracted into spheres with varying amounts of speckling remaining on different specimens. The species is circumtropical, common where it occurs but not often recorded. Records from the western Indian Ocean are summarized by
http://species-id.net/wiki/Stylocheilus_longicauda
Plate 13Maldives: 6 mm in length pres., 2 m depth on algal-covered stone, Kuredu, Lhaviyani Atoll, 10-24 March 1998, leg. J Hinterkircher; three specimens collected of many, 50-70 mm live lengths (28-32 mm preserved), on algae growing on floating grouper cage near surface, Kiadhu Tila, Felidhoo Atoll, 23 July 1994, leg. RC Anderson (striations still visible on preserved specimens). – Zanzibar: numerous specimens 10-50 mm pres. lengths, Mazzini, intertidal and low water forming chains on sand and rocks in sea grass beds, 09 September 1993, leg. MD Richmond (numerous villi, very long rhinophores and oral tentacles). – Mauritius, La Réunion, Mayotte, and Madagascar: photographs of numerous individuals http://seaslugs.free.fr/nudibranche/a_intro.htm. – Oman: Muscat, 01-12 April 2009, photos of several individuals, S Kahlbrock.
Benthic form of this circumglobal species distinguished by fine longitudinal brown-to-black lines usually interrupted by ‘eyespots’ with bright blue centres; tail long, swelling present in middle of body where parapodial lobes enclose large gill and anal siphon; rhinophores and oral tentacles long and slender. Specimens in any given population may have few or numerous villi, and this variation is still visible in the preserved material. It can be locally common in sea grass beds and shallow water, especially during the breeding season, but this does not happen every year (P Bidgrain, pers. comm. and http://seaslugs.free.fr/nudibranche/a_intro.htm).
There is probably only one species of Stylocheilus occurring in two forms, the benthic striated form described and illustrated here, which was known as striatus, and the yellow pelagic form associated with seaweeds currently known as longicauda.
This may be an appropriate location to discuss the problems posed by the aplysiids distributed in the Indo-West Pacific: all species covered in this work are complex and appear to comprise colour forms identified as separate species, as well as having different habitat preferences. Aplysia parvula occurs consistently in at least two forms, the larger pale-coloured shallow water one associated with algae and the tiny black reef form although other colour forms are recorded (http://seaslugs.free.fr/nudibranche/a_intro.htm and http://www.nudipixel.net/species/aplysia_parvula/). It is noted that the radulae and shells were similar, with slight differences. Dolabrifera dolabrifera has been described under many names but has long been recognised as a single species. Many species of Dolabella have been described but were historically resolved to two species, with different external and (possibly) internal morphologies and shells; these are synonymised as one species in this work. The history of Stylocheilus is also complicated, with two very different forms inhabiting very different environments. Clearly the aplysiids are in need of revision, entailing comparison of specimens from different regions and habitats as well as a very thorough review of the literature; it is probable that the numerous species names currently listed will dissolve into fewer, variable species.
urn:lsid:zoobank.org:act:EF8569BD-D10C-4309-A53B-C1A37F1DEB76
http://species-id.net/wiki/Cyerce_bourbonica
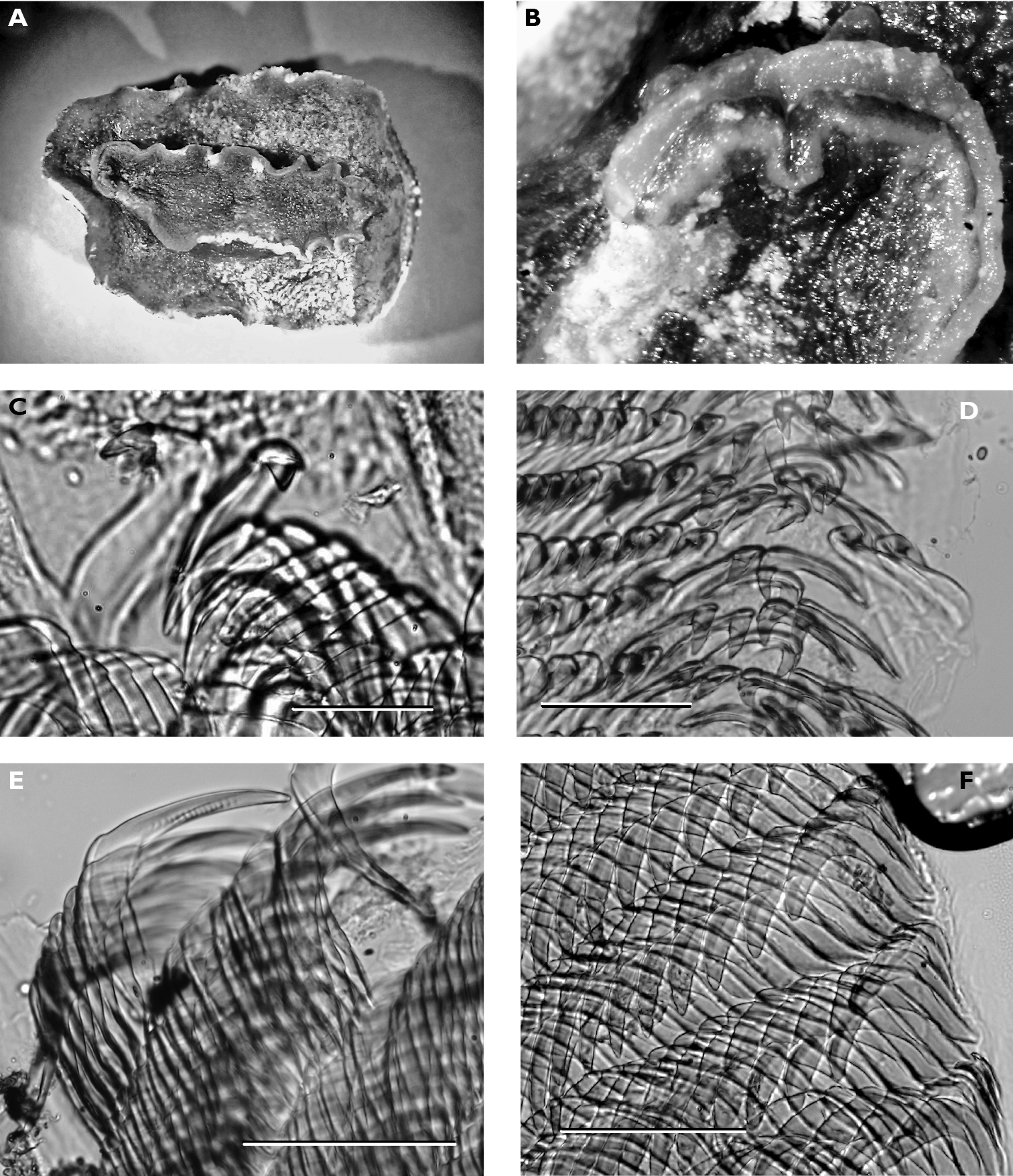
Fig. 3, Plates 14 , 15Holotype: 12 mm alive, 6.5 × 2.5 mm pres. in alcohol, Etang Salé, La Réunion, rocky coast, 1 m depth, 14 December 2009, leg. H. Flodrops, SMF 337104.
Paratypes: approx. 12 mm alive, 4 × 3 mm pres. in formalin (dissected), Etang Salé, La Réunion, rocky coast, 1 m depth, 14 December 2009, leg. H. Flodrops, SMF 337103; 10 mm alive, 4.5 × 2 mm pres. in alcohol, Etang Salé, La Réunion, rocky coast, 1 m depth, 14 December 2009, leg. H. Flodrops, SMF 337205.
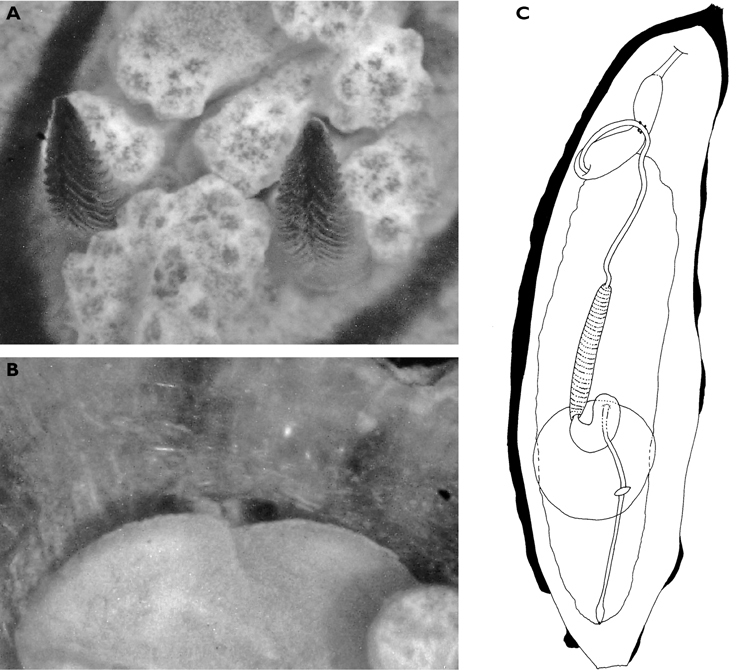
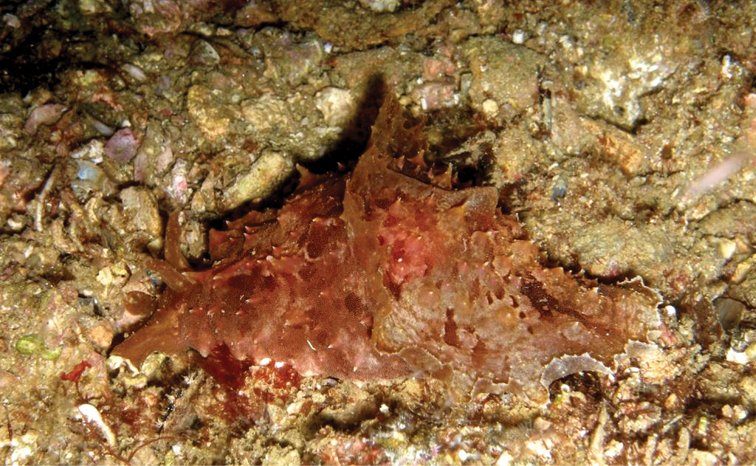
A multi-coloured Cyerce with approximately 30 inflated, pustular, angular cerata whose margins are pale violet-blue with creamy orange patches and black spots. The head has an orange band on either side and black spots on its frontal margin. The body and cerata are marbled light green and white. Ventrally, the propodium and metapodium each bear a row of small black dots, the former anteriorly and the latter marginally and posteriorly. Size up to approximately 15–20 mm.
The description of the colour and pattern is based on a series of photographs of the type specimens: body covered in fewer than 30 slightly inflated and tubercular cerata, loosely arranged in transverse rows along each side. Each ceras can be flat or swollen, angular with rounded corners (Fig. 3B, C), semi-translucent beige to green, margins pale blue-violet ‘beaded’ with opaque pale orange pigment, and sub-marginal black spots. The profile is in one plane along the sides but strongly convex at the distal end. Tubercles opaque white, concentrations of larger black spots in lateral swellings. Very few black spots scattered on cerata but yellow spots, olive-green patches, and light green to light brown marbling present. In some photographs, paired dark brown globular patches visible in alcohol-preserved specimens can be seen as circular red-brown patches at bases of cerata (Plate 14, right-hand cerata at level of pericardium). Anterior portion of body marbled: head and rhinophores semi-translucent; eyes clearly visible at bases of rhinophores (Plate 15). Oral tentacles short and recurved. Frontal margin of head with black spots, and behind eyes and rhinophores, coalesced to form a network. Broad orange band on each side of head, outside the black spots. Pericardium immediately behind first two or three rows of cerata, swollen, ochre-coloured, bare of cerata.
The specimens preserved in alcohol are in excellent condition; the paratype in formaldehyde shed 23 larger cerata. It is cream in colour, while the holotype and second paratype are semi-translucent. Dark patch of pigment anterior to swollen pericardium behind rhinophores and faint pigmented patch beyond. Pericardium a large swelling but dissection of paratype in formaldehyde did not reveal vessels and disintegrated with further prodding. Large swollen anal papillae behind right rhinophore near eye in all preserved specimens. Conical penis visible below right rhinophore in alcohol paratype only, no spine apparent at tip. Ventral views well presented in alcohol (Fig. 3A); rhinophores visible, as are head and oral tentacles; propodium with row of black spots along anterior margin, rounded metapodium with a row of black spots all around its edge except anteriorly. Cerata angular with three rounded corners near distal margin. At the base, doughnut-shaped attachment point and small flap beyond (Fig. 3B). Minute pointed papillae located in a band following the contours on both surfaces of the cerata, white in certain angles of light (Fig. 3C). Edges of some preserved cerata appear serrated (Fig. 3C). Brown triangular patch present on flap at base of each ceras in alcohol-preserved specimens. Two dark brown to black bodies inside cerata, formed of very small globules (Fig. 3B, C), near base above and beside attachment point.
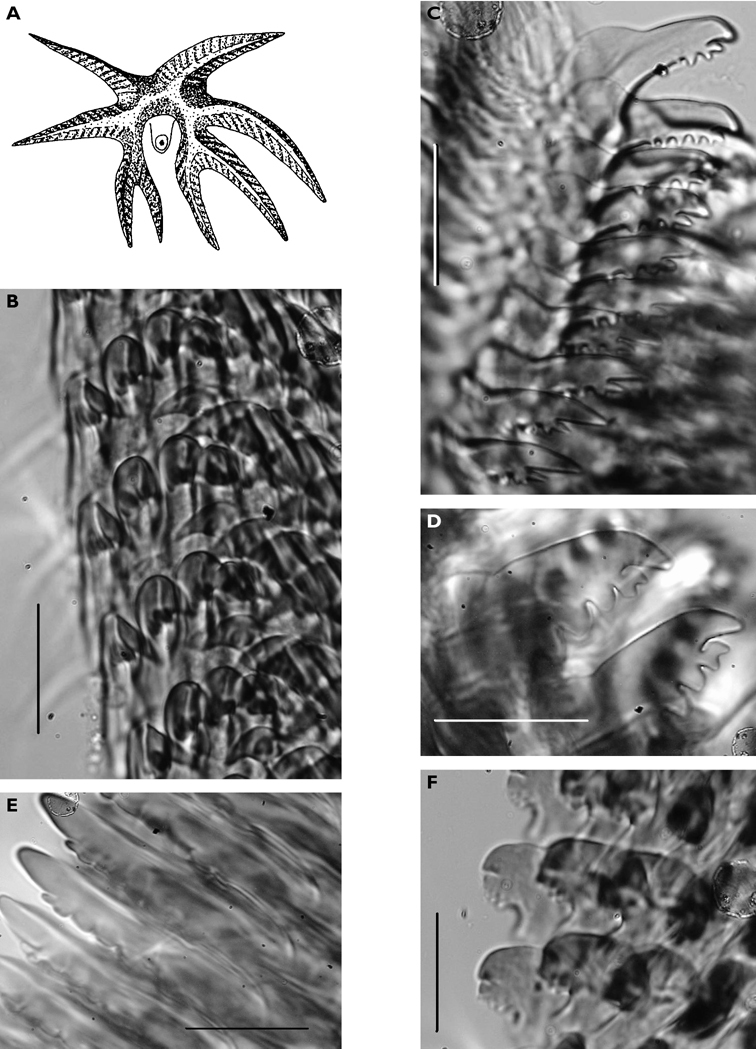
Dissection of the formaldehyde-preserved paratype proved impossible as only the outer skin was preserved, the internal organs decomposed. However, the muscular pharynx remained solid and was removed to extract the radula. Radular formula 16 × 0.1.0, nine teeth in the ascending limb and seven teeth in the descending one (fig. 3D). The teeth are typical in shape for Cyerce (e.g.
Cyerce bourbonicasp. n. A ventral view of holotype B selection of larger cerata of paratype (formaldehyde) showing morphology: globular patches at base near attachment point, the small flap, and some black pigment spots remaining at the distal end C photograph of ceras of paratype (alcohol) from anterior side showing denticulate margin and pointed papillae D whole radula of paratype (formaldehyde) E single tooth magnified to show denticles and shape of shaft. Scale bars 100 µm.
This distinctive species appears to extend throughout the Indo-West Pacific oceans (
The species is named ‘bourbonica’ after the original name of the island of La Réunion, the type locality. The island was renamed La Réunion in 1793 after the fall of the House of Bourbon (Spain and Luxembourg currently have Bourbon monarchs), but the name was changed back and forth several times until the French revolution in 1848.
http://species-id.net/wiki/Elysia_cf_nigropunctata
Fig. 4, Plate 16Seychelles: 20 × 5 mm (PK-HH, “10 mm when parapodia are opened”), Whale Rock, on coral rubble, 26 April 1992, leg. P Kemp; numerous photographs from La Réunion, Mauritius, and Mayotte http://seaslugs.free.fr/nudibranche/a_intro.htm.
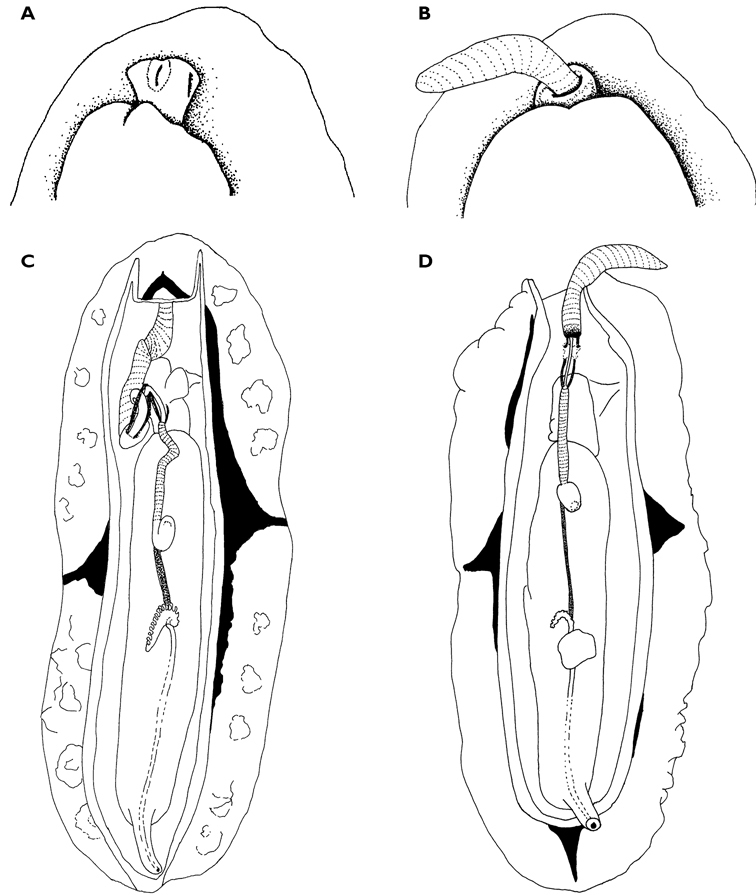
Body pale green with ocellated black spots and orange patches along edges of parapodia where they were thrown into three permanent folds. Rhinophores marbled green with white pigment dorsally, creamy orange submarginal band, and white distal end. Oral tentacles tipped with white.
The single specimen is well preserved, with the parapodia opened completely. Dorsally, there are large black ovate spots (oval along the horizontal axis) on inner surfaces of parapodia and body. Edges of parapodia scalloped in three places, containing opaque white granular pigment spots along the margin (Fig. 4A), presumably the orange marginal pigment in life. The pericardium is elongated, triangular anteriorly and squared posteriorly, with only two un-branched vessels posteriorly. Ventrally, the foot and head are bilobed; there are fewer smaller spots on the foot and more larger spots on the outer surfaces of the parapodia (Fig. 4B).
Elysia cf. nigropunctata, 20 mm A dorsal view of preserved specimen showing irregular parapodial margins and pigmentation B ventral view of same specimen.
This species is recognisable, as evidenced by the internet discussions (http://www.seaslugforum.net/find/elyssp11) and is most probably Pease’s species, described from Tahiti. The many photographs on the internet show slight variations only, and it is remarkable that it has not been recorded before. This is not only the first record for the Indian Ocean, but the first published record since the species was described in 1861. Another specimen measuring 40 mm from Madagascar is lodged in the Natural History Museum, London (NHMUK 20010521, leg. Lindsey Warren) but was not examined by this author.
http://species-id.net/wiki/Plakobranchus_ocellatus
Plate 17Kenya: 25 × 11 mm preserved, Vipingo, 25 miles N of Mombasa, rock pool ELW, 23 September 1984, leg. J Hognerud. – Maldives: 9 mm in length (MDV/AB/96/7, specimen disintegrated, no radula located), Fulidhoo Lagoon, Felidhoo Atoll, 10 m depth on sand, 04 May 1996, leg. RC Anderson & SG Buttress. – Zanzibar: 14 × 8 mm preserved, Matemwe Lagoon, in Xenia sp. and sand near encrusted, partly submerged rock with Didemnum molle and Halimeda sp., 01 March 1995, leg. MD Richmond. – Seychelles: 30 × 10 mm alive (PK-A, one of four individuals preserved), Source d’Argent, La Digue, 1 m depth on broken Acropora sp. behind reef in algal growth, 26 January 1992, leg. P Kemp. – La Réunion and Mayotte: photographs of several individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
Plakobranchus ocellatus has been recorded in excess of 35 mm in the Indian Ocean (
In the Marshall Islands, an interesting commensal association was observed between Plakobranchus ocellatus and the sea cucumber Holothuria atra (
http://species-id.net/wiki/Thuridilla_gracilis
Fig. 5, Plates 18, 19Seychelles: 15 × 5 mm (PK-P, “stripy black/mauve rhinophores and body, scattered blue spots, orange line edging parapodia”), Lilôt, NW Mahé, 7 m depth on underside of rock, 25 March 1992, leg. P Kemp; 25 × 5 mm (PK-W, “striations along parapodia but with white edge with orange line, vivid blue spots, rhinophores white with longitudinal stripes and brown orange at tips, orange under mouth”), Lilôt, NW Mahé, 20 m depth under rock, 09 April 1992, leg. P Kemp; photo of one individual, Lilôt, NW Mahé, 1988-1989, P Kemp. – Maldives: three pres. specimens 6 mm, 7 mm, and 12 mm. (MDV/AB/96/10), Miyaru Kandhu, Felidhoo Atoll, 05 May 1996, leg. RC Anderson & SG Buttress; 15 mm (MDV/AB/96/12; badly preserved, no radula found), Fulidhoo Channel, Felidhoo Atoll, 10 m on algae, 07 May 1996, leg. RC Anderson & SG Buttress; photos of two individuals, 1986-1994, J Hinterkircher.
Several colour forms exist in the Indo-West Pacific under the names of bayeri Er. Marcus (1975) and ratna Er. Marcus (1975).
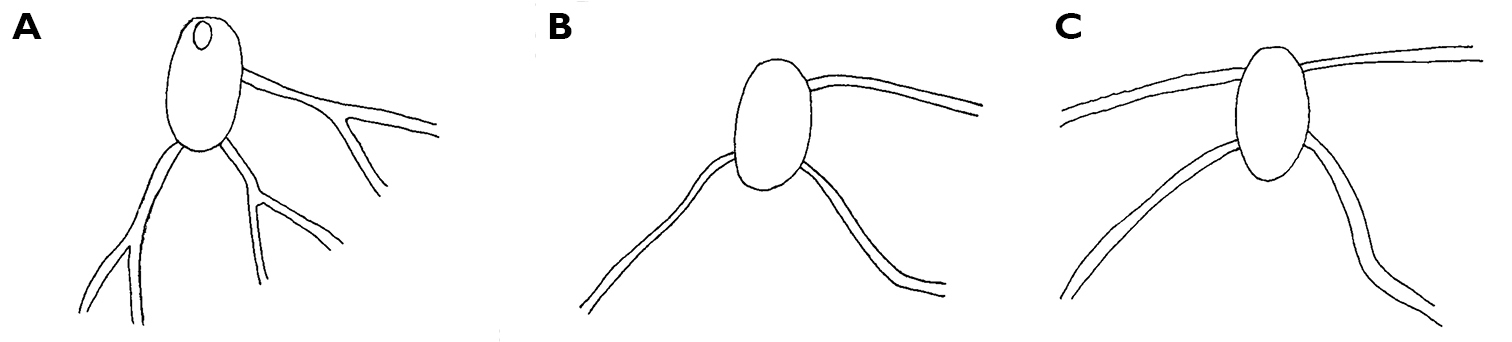
Thuridilla gracilis, drawings of pericardia A Seychelles 15 mm specimen B Maldives 12 mm specimen. C Maldives 6 mm specimen.
Thuridilla gracilis (Risbec) was mentioned in a discussion of a new species as being impossible to re-identify (
http://species-id.net/wiki/Thuridilla_vataae
Fig. 6, Plate 20Maldives: approx. 10 mm alive (3 mm pres.), Coral Garden on filamentous green algae, Vadhoo Reef, South Malé Atoll, 5 m depth, 14 October 1994, leg. RC Anderson & SG Buttress; specimen disintegrated (no radula found), 7 mm alive, Bathala Island reef, Ari Atoll, 8 m depth, 28 July 1995, leg. SG Buttress & RC Anderson (“colour dark with pale streaks, rhinophores appeared white with red tips”). – La Réunion and Mayotte: numerous individuals 10-15 mm in length http://seaslugs.free.fr/nudibranche/a_intro.htm.
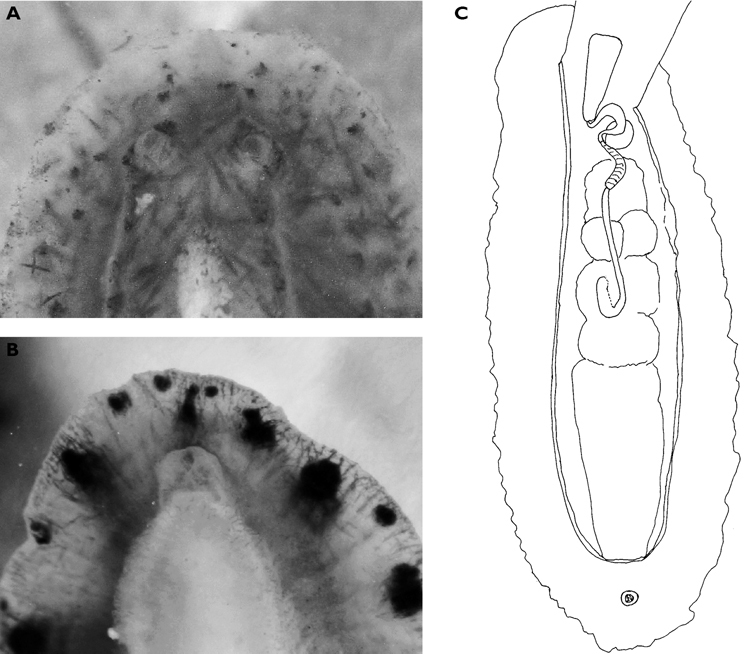
Ground colour grey with hint of purple, covered with yellow spots and pustules, and round black dots. Marginal bands of the parapodia creamy yellow. Head white, forming X-shape into rhinophores anteriorly and body posteriorly; remainder of head and anterior part of body grey-black. Red-orange band at tip of each rhinophore, a distinctive character of the species; in this specimen, the rhinophores were faintly spotted with creamy yellow.
Preserved specimen semi-translucent with no pigment remaining except black spots on body. Parapodia folded over body and frontal margin bilobed (Fig. 6A). Pericardium unusual in Thuridilla vataae in that lateral and posterior vessels are connected. In this specimen, they appear to form a partial ring around the pericardium (Fig. 6A). Ventrally, foot and frontal margin both cleft in preserved specimen (Fig. 6B).
Thuridilla vataae, 10 mm (3 mm pres. specimen) A dorsal view showing bilobed frontal margin and pericardium with connected vessels B ventral view showing bilobed foot and frontal margin.
Thuridilla vataae is a small species recorded sporadically from the Indo-West Pacific.
http://species-id.net/wiki/Nembrotha_guttata
Plate 21Maldives, two specimens, both 35 mm (MDV/AB/96/4), Fulidhoo Channel, Felidhoo Atoll, 9 m depth, 02 May 1996, leg. RC Anderson & SG Buttress; 32 mm (MDV/AB/96/14), Fulidhoo Channel, Felidhoo Atoll, 07 May 1996, leg. RC Anderson & SG Buttress; photo of individual, Maaya Tila, Ari Atoll, 6-8 m depth, March 1994, H Voigtmann.
Easily recognised by velvety black body, large orange pustules edged with green especially along frontal margin, black and orange rhinophores, and green gills. The differences between Nembrotha guttata and Nembrotha cristata Bergh are clearly visible in Plate 21. Nembrotha cristata has been previously recorded from the Indian Ocean, but only in the Maldives (Yonow 1994). A third black and pustulose species known from the region is Nembrotha kubaryana, recorded from Mauritius (
Nembrotha guttata appears to be endemic to the Maldive islands; paler and consistently differently coloured species are recorded from the Philippines (
http://species-id.net/wiki/Roboastra_gracilis
Plate 22Maldives: 8 mm (6 mm pres.), Fulidhoo Channel, Felidhoo Atoll, 12 m depth, 24 July 1994, leg. RC Anderson & SG Buttress; photographs of two individuals 12 mm and 20 mm, March 1999, J Hinterkircher. – Tanzania: photographs of a single individual, M’Nazi Bay, Msimbati, near Mtwara, May 1994, IM Horsfall. – La Réunion: several individuals photographed up to 18 mm in length (http://seaslugs.free.fr/nudibranche/a_intro.htm).
http://species-id.net/wiki/Tambja_amakusana
Plate 23Maldives: 11 mm (6 × 2 mm pres.), Old Shark Point, Thilafalhu reef, south end of North Malé Atoll, 10 m depth, 14 August 1995, leg. RC Anderson & SG Buttress.
Animal dark to medium green with some relatively large white spots. Tail extremely long, almost half body length, and body appears to have been smooth. Five small multi-pinnate gills beige with purple tips; rhinophores beige suffused with purple; margins of rhinophore sheaths pale yellow. Flattened oral tentacles visible when crawling.
The preserved specimen is well relaxed: body light blue-green, gills and rhinophores white, rhinophores with 12 lamellae. Marshall and Willan (1990) state that there is no rhinophoral sheath; none is visible on the specimen but the rim of the right rhinophore is visible in Plate 23 where it catches the light.
Tambja amakusana is recorded for the first time from the Indian Ocean; all literature records to date are listed in the synonymy. The only similarly small species with purple tips to the gills and rhinophores is Tambja limaciformis (Eliot), but this species is usually brick to orange red, with many more white spots, occurring throughout the tropical Indo-West Pacific (e.g.
http://species-id.net/wiki/Tambja_morosa
Mauritius: 22 × 8 × 10 mm, Japan Wreck, 25 m depth, 04 March 1990, leg. H Debelius and (poor) photograph of two mating individuals; photographs of three individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
Preserved specimen translucent but retains blue pigment in creases on tail, gills, and rhinophores. The species was previously recorded from Mauritius by the author, and appears to be an infrequently encountered species in the western Indian Ocean (see
Plate 24
Maldives: 40 mm (35 × 7 mm pres.), Banana Reef, North Malé Atoll, 10 m depth on yellow sponge, night dive, 28 August 1993, leg. RC Anderson & SG Buttress; one individual photographed/described but not preserved, 63 mm (MDV/AB/96/16), Fulidhoo Channel, Felidhoo Atoll, 10 m depth on bare coral rock, 08 May 1996, RC Anderson & SG Buttress; photographs of one individual, 1986-1994, J Hinterkircher.
This dark colour form of Notodoris gardineri appears to be endemic to the Maldives island group (only one photo from more than 50 available on http://www.nudipixel.net/species/aegires_gardineri/ for example). Otherwise, Notodoris gardineri is common in and restricted to the Pacific, with no records from the western Indian Ocean other than form nigerrima from the Maldives.
http://species-id.net/wiki/Notodoris_minor
Plate 25Socotra: 40 × 13 mm preserved (IT-157, RJ-011), 12°18.698'N, 53°48.285'E, 09 April 1999, leg. R Janssen. – Tanzania: photographs of two individuals, Mafia Island, shallow water, May 2009, A de Villiers. – La Réunion, Rodriguez, and Mauritius: several individuals photographed http://seaslugs.free.fr/nudibranche/a_intro.htm. – Seychelles: one photographed individual, Lilôt, NW Mahé, 1988-1989, P Kemp.
http://species-id.net/wiki/Hexabranchus_sanguineus
Plates 26 , 27Socotra: 55 mm pres. length (WPU Rostock, MAR 85, RC-N57), Hadibo, late 1980s, leg. W Wranik. – Yemen: 85 × 60 mm pres., distorted and flattened (WPU Rostock, MAR 85, RC-N16), Al Mukalla, late 1980s, leg. W Wranik. – Kenya: two specimens 60 and 95 mm in length, pres., Vipingo, 25 m N of Mombasa, ELW in rock pools on exposed reef, 23 September 1984, leg. J Hognerud. – Tanzania: photographs of one individual, M’Nazi Bay, Msimbati, near Mtwara, May 1994, IM Horsfall. – Maldives: one juvenile specimen 13 mm (MDV/AB/96/17), Yacht Tila, South Malé Atoll, 25 m depth, 09 May 1996, leg. RC Anderson & SG Buttress; photos of 15 mm juvenile individual, 1986-1994, J Hinterkircher. – La Réunion, Mauritius, Mayotte: photographs of numerous individuals, including juveniles similar to the Maldives specimen examined here (http://seaslugs.free.fr/nudibranche/a_intro.htm) and slides of 27 individuals varying from yellow to mottled pink, M Parmantier. – Seychelles: photos of several individuals including juvenile and sub-adult, Lilôt, NW Mahé, 1988-1989, P Kemp. – Sri Lanka: photographs of one large individual, Unawatuna, S of Galle, on sediment-covered rock, 27 December 2011, S Kahlbrock.
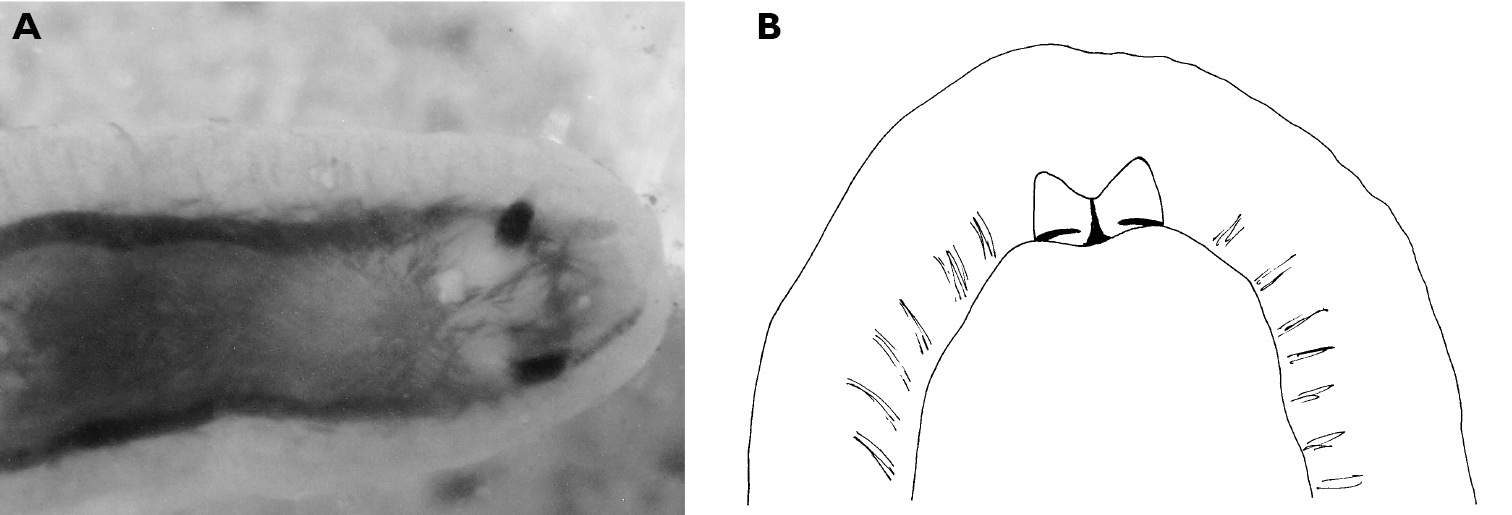
The large specimens and individuals were of the typical Indo-West Pacific colour pattern, blotchy red and cream, especially along the margins. The 13 mm juvenile specimen from the Maldives illustrated here (Plate 26) is well relaxed, and the six gills can be seen to insert into separate openings. The oral tentacles are large rounded lobes, clearly the precursors to the lappets of the adult: these tentacles are huge in comparison to those of a chromodorid (or dorid) of similar size. The rhinophores have 13 lamellae; note that the white edges present in the adults have not yet developed. Another 15 mm juvenile individual was identical in colour pattern, while a slightly larger animal from the Seychelles demonstrated the developing adult colour pattern already had white edges to the 17+ rhinophore lamellae (Plate 27).
The radular formulae of the larger specimens are tabulated below: the sizes listed are of the same dimension of a large lateral tooth – from the tip of the cusp to the flange where the cusp meets the base. There is no relationship between the radular formula and maximum tooth size, nor are they correlated with preserved animal size. A giant Hong Kong specimen is included for comparison, and has the largest teeth but not the largest radula. It had a bubbly texture and was pinkish yellow in life (M Collard pers. comm.; specimen, radula, notes and photographs lodged in the Natural History Museum, London: NHMUK acc. no. 2337 with the “Red Sea Giant”).
Socotra 55 mm pres. 41 × 64.0.64 500 μm
Yemen 85 mm pres. 53 × 93-83.0.83-93 550 μm
Kenya 90 mm pres. 47 × 77.0.77 700 μm
Kenya 95 mm pres. 46 × 79.0.79 450 μm
Hong Kong 190 mm pres. 51 × 67.0.67 800 μm
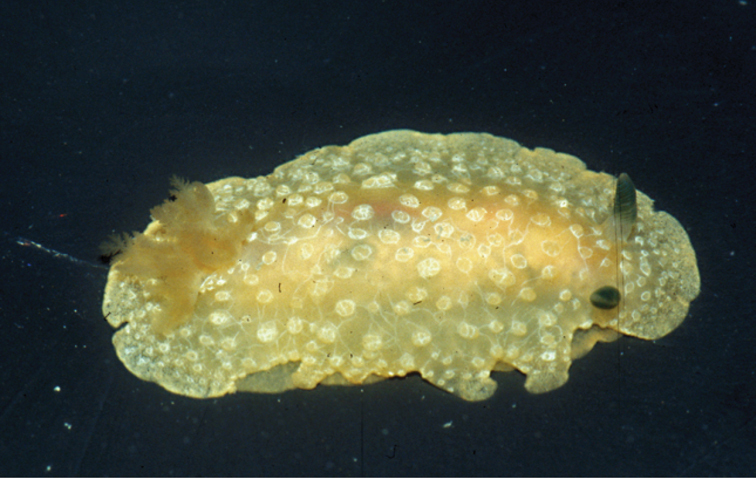
http://species-id.net/wiki/Carminodoris_grandiflora
Fig. 7, Plate 28Zanzibar: 30 mm pres., Matemwe, in sponge, lagoon on east coast, 01 March 1995, leg. MD Richmond. – La Réunion: photographs of one individual, 1990s, M Jay, pers. comm. and http://seaslugs.free.fr/nudibranche/a_intro.htm.
Numerous photographs of living specimen depict an oblong animal slightly broader posteriorly, with pinkish beige gill clump spanning width of animal. Darker central patch extended from just in front of rhinophores to gills, very dark brown where the tubercles were not ringed by white but lighter along the midline where seven large and numerous small tubercles were ringed in white. Many tubercles “frosted” with white pigment, which appears to be a characteristic of living specimens of Hoplodoris grandiflora. Remainder of mantle consisted of paler band of beige tubercles outlined in white; extended white areas formed patches perpendicular to the margin.
Dorsally the preserved specimen still bears distinct hard beige and brown tubercles. The central ones are the largest, and tubercles decrease in size towards the margins. The six gills are multipinnate, and the sheath is scalloped. The rhinophores extend from a raised irregular sheath bearing pinkish beige tubercles. The ventral surfaces of both sole and hyponotum are creamy beige with light brown pigment in the creases, lighter than dorsal tubercles (Fig. 7A). There are small pustules covering the hyponotum but not the sole. The foot is bilaminate for the entire anterior margin extending laterally for a short distance down each side, both laminae are notched, and the oral tentacles are triangular (Fig. 7B).
The radula of the 30 mm preserved specimen has the formula 38-39 × 107.0.107. The first lateral on each side has a long base and a short triangular cusp with a small denticle on each side (Fig. 7C). The remaining teeth have an equally long base with a small flange, but the cusp increases in length (Fig. 7D) and develops serrations along the side (Fig. 7E). The last two or three teeth in each row are very reduced (Fig. 7F).
Carminodoris grandiflora, 30 mm A ventral view of preserved specimen showing small tubercles on hyponotum B view of propodium, head, and tentacles showing notched bilaminate foot and damaged on right side C median pair of radular teeth, new row, scale bar 50 µm D central lateral teeth, scale bar 100 µm. E middle lateral teeth showing denticles on cusp and apical projection, scale bar 150 µm F outer lateral teeth showing rapid reduction in cusp size, scale bar 150 µm.
This species has a widespread distribution in the western Indian Ocean, the Red Sea (
http://species-id.net/wiki/Halgerda_formosa
Plate 29Zanzibar: 10 × 5 mm pres., leg. MD Richmond (California Academy of Sciences, CASIZ 169953). – Kenya: photographs of two individuals, Malindi National Park & Watamu National Park, 5-8 m depth, MD Richmond. – La Réunion, Mauritius, and Mayotte: photographs of numerous individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
Body colour translucent white, yellow to orange-yellow ridges, and several dark spots anteriorly and posteriorly near mantle edge. Foot translucent white with at least one black spot near tip and broad pale yellow margin. Rhinophores long, translucent white with one dark brown spot on stalk; yellow on proximal third, dark brown on distal two-thirds of lamellate portion, white on elongated tip. Six gills white with black band across inner side of each rachis from base to tips of pinnules.
This small species was first recognised by
http://species-id.net/wiki/Halgerda_punctata
Fig. 8, Plate 30Sri Lanka: three pres. specimens 9 × 5 mm, 17 × 10 mm, 20 × 12 mm, and two pres. curled 15 × 10 mm, 15 × 8 mm, Unawatuna, S of Galle, 27-30 December 2010, leg. S Kahlbrock.
The photographs accompanying these five specimens from the type locality are all clearly of the same species: body semi-translucent white with numerous yellow-capped tubercles and few round black spots, tubercles in some specimens arranged in lines approximating ridges of other species of Halgerda. Rhinophores long, tapering; long translucent stalk with black pigment on its posterior surface continuing to lamellate portion, which was black with black knob at tip. First 4-6 lamellae yellow on anterior face and remaining 20-24 lamellae black on all sides. Black spot always present at base of rhinophoral sheath posteriorly in line with black line on stalk, often another black spot laterally or anteriorly. At least one yellow tubercle on rhinophoral pocket rim. The gills distinctive in this species with only four gill branches. Anterior two gills larger than posterior two, inner rachides marked with brown-black line. The anal papilla protruded in some photographs of living specimens, and was identical to Farran’s description of “rather long and tubular, white with a black crenulated margin.”
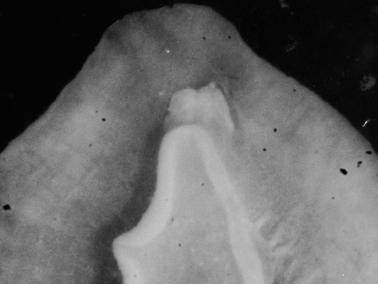
The preserved specimens are firm, grey-white with pale yellow spots on low tubercles in all but one specimen, which has pale orange spots. All specimens have round black spots. The rhinophoral pockets have a posterior black spot on the rim extending to the mantle on all specimens and an anterior black spot in four specimens. There are black spots on the top of the metapodium, which also has an orange spot at the tip fading into a line along the middle. The branchial pocket is faintly tuberculate and there is always a large round or oval black spot placed anteriorly on the mantle in each specimen, also visible in Plate 30. The gills, retain their black and white markings. The hyponotum is narrow with a few small spots. There are larger spots on the sides of the foot but the sole is bare. The head is reduced, and the mouth is visible with very small rounded tentacles (Fig. 8).
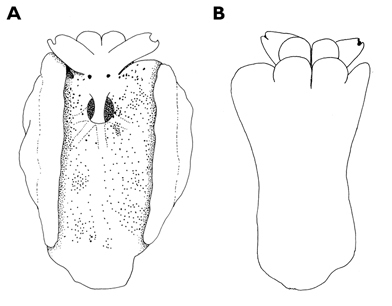
Halgerda punctata, 15 mm pres. specimen, ventral view showing head and very reduced oral tentacles.
This appears to be the first record in the scientific literature of this species since its description. It was originally described from Sri Lanka, and most photographic records are from the type locality, with a few from Thailand and Burma to the east (
http://species-id.net/wiki/Halgerda_tessellata
Kenya: two specimens 15 × 15 mm and 9 × 5 mm, Vipingo, 25 miles N of Mombasa, rock pools at ELW, 23 September 1984, leg. J Hognerud (California Academy of Sciences CASIZ 169945). – Tanzania: photograph of single individual, Mafia Island, shallow water, May 2009, A de Villiers. – Maldives: two specimens 15 mm and 25 mm (MDV/AB/96/11), Foteo Channel, Felidhoo Atoll, 15 m depth, 05 May 1996, leg. RC Anderson & SG Buttress (California Academy of Sciences CASIZ 169996); photos of two individuals, 1986-1994, J Hinterkircher. – Mayotte: photographs of two individuals http://seaslugs.free.fr/nudibranche/a_intro.htm. – Seychelles: photos of two individuals, Lilôt, NW Mahé, 1988-1989, P Kemp.
Easily recognised by pattern of orange ridges enclosing brown depressions speckled with white. Both specimens from Kenya were examined when they were received in 1984: the 15 mm specimen was still yellow-orange, as was the foot; the brown reticulated pattern also remained. Very few spots were present on hyponotum but between the hyponotum and foot, there was a band of brown dots/patches. The smaller specimen retained an orange edge to the mantle and the foot: the dorsum was yellow-orange with the brown pattern remaining; there were more spots on the hyponotum than in the larger animal, and there were spots and patches present in a band between the hyponotum and the foot. The preserved Maldives specimens were also clearly recognizable several years after collection, with the brown reticulated pattern remaining between the ridges as well as the brown spots scattered on the hyponotum.
Halgerda tessellata has a wide Indo-Pacific distribution although, like some species with such a large range, it has a consistently different colour form in the Pacific: a band of white dots is present around the margin and there is dense white pigmentation on the gills (
http://species-id.net/wiki/Cadlinella_ornatissima
Fig. 9, Plate 31Sri Lanka: 13 × 6 mm pres., Unawatuna, S of Galle, SW coast, 28 December 2010, leg. S Kahlbrock. – Gulf of Oman: photos of two individuals, Muscat, Oman, 01-12 April 2009, S Kahlbrock. – La Réunion and Mayotte http://seaslugs.free.fr/nudibranche/a_intro.htm.
The collected specimen was deep yellow in life with a white foot, long white rhinophores, and five white pinnate gills. The tubercles were elongated, and some were rounded whilst others were somewhat pointed; they were yellow basally and pink-red distally.
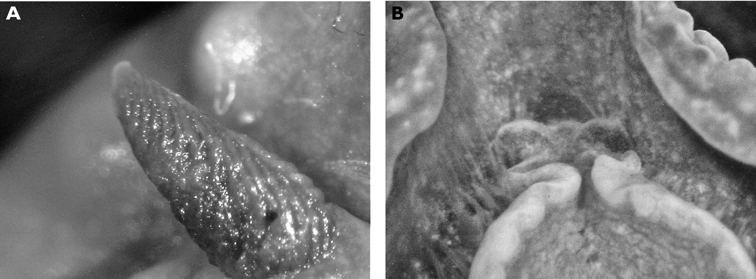
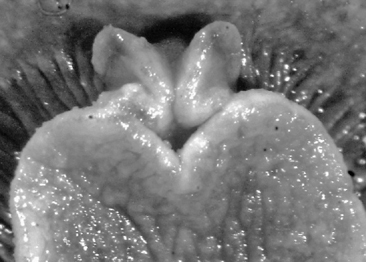
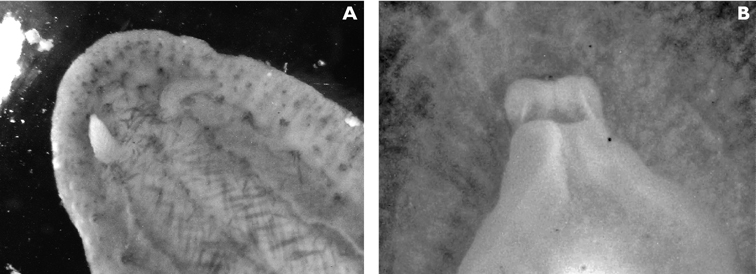
The specimen is well preserved, with some yellow remaining on the dorsum several months after collection. There is a tubercle in front of and behind each rhinophore, and three tubercles located just in front of the gill pocket. The spicules on the dorsum and in the tubercles are visible, as are the yellow tubercle bases; the distal parts are now white. Ventrally the hyponotum has an orange submarginal band and the submarginal bands of mantle glands are visible. The foot is deeply divided anteriorly, and the oral tentacles are so contracted as to be non-existent (Fig. 9).
Cadlinella ornatissima, 13 mm pres. specimen, ventral view of propodium showing divided foot margin.
This appears to be only the third record of Cadlinella ornatissima in the scientific literature from the Indian Ocean, in addition to photographic records from the Gulf of Eilat (
http://species-id.net/wiki/Ceratosoma_miamirana
Plate 32Zanzibar: 36 × 34 mm pres., in Sargassum off reef crest, Matemwe Lagoon, 01 March 1995, leg. MD Richmond (examined 1995: dorsum cream with rusty/orange-brown patches and pigment in creases, knobbly; rhinophores very close together, located far anteriorly; gills located far posteriorly, pocket with six lappets; ventrally with bright rusty ‘rings’ of pigment on hyponotum) (examined 1998: no pigment left except faint marks in creases and tucked-in gills; rings still bright on hyponotum). – Tanzania: photograph of two individuals, Mafia Island, shallow water, 31 December 2003 and 16 October 2005, A de Villiers. – Maldives: 65 mm (47 × 44 mm preserved, MDV/AB/96/19), 13 m depth, Yacht Tila, South Malé Atoll, 09 May 1996 (examined 1998: very well extended, very soft, completely bleached: no marks or colour left; morphology of foot, oral tentacles, and dorsum as above), leg. RC Anderson & SG Buttress. – La Réunion: photographs of several individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
Recognised by its oval shape with scalloped margin, and blurred green, beige, and blue colour pattern. Ventrally, green reticulations and ocelli located in junction between foot and mantle are distinctive. Widespread if infrequently recorded in the tropical Indo-West Pacific.
http://species-id.net/wiki/Chromodoris_africana
Plate 33Kenya: 25 mm in length, preserved, Vipingo, 25 m N of Mombasa, ELW in rock pools on exposed reef, 23 September 1984, leg. J Hognerud (Australian Museum, Sydney, C431128). – Madagascar: 50 × 20 mm (PK-F), Ampangorina, Nosy Komba, 3 m depth in live Acropora, 02 March 1992, leg. and photos P Kemp; photographs of two individuals, Nosy Bé, October 2007, J Hinterkircher. – Tanzania: photographs of two individuals 1994 and Pegasus Wreck, 10 m depth, March 1995, MD Richmond; photographs of two individuals, M’Nazi Bay, Msimbati, near Mtwara, May 1994 and May 1995, IM Horsfall.
A large species, growing to 80 mm in length. Ground colour black, two thick or thin white lines running length of dorsum and joined behind gills forming a loop, not extending to pale orange mantle border anteriorly or posteriorly in any of these specimens but may do so anteriorly in the species. Broad, somewhat crumpled, pale orange tending-to-yellow mantle skirt, separated from the black dorsum by a broad white line. No white line at the margin, visible on the preserved specimen from Madagascar which retains its black, white, and orange bands fifteen years after collection. Gills and rhinophores darker orange than border, arising from pockets with raised orange rims. This species has been recorded from the Red Sea and south along the East African coast only (
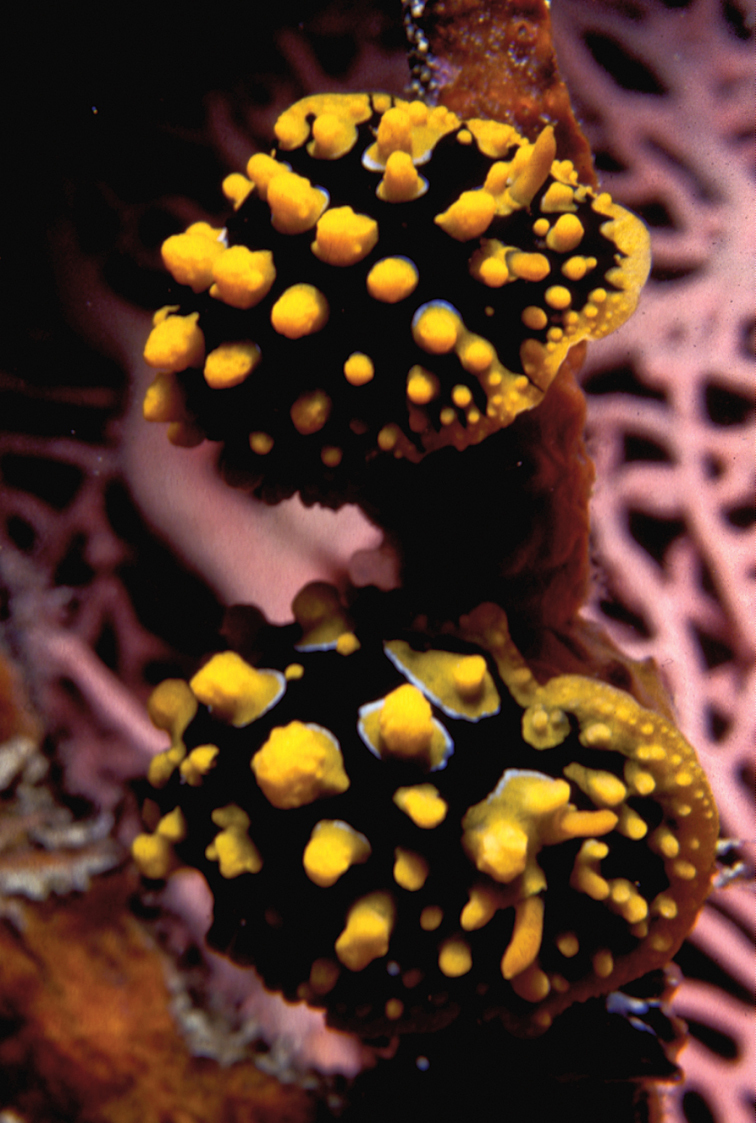
http://species-id.net/wiki/Chromodoris_annulata
Plates 34 , 35Persian Gulf: photograph of one individual, Dahwat ad Daffi, Jubail, Saudi Arabia, April 1992, F Krupp; photographs of numerous individuals, 2000-2011, GT Smith; photos of several individuals, Qaruh Island, Kuwait, 2008-2011, M Nithyandanan. – Gulf of Oman: photos of two individuals, Muscat, Oman, 01-12 April 2009, S Kahlbrock; photographs of one individual less than 10 mm, Inchcape 2 (a ship wreck), 20 m depth, 08 January 2010, GT Smith; two individuals, approx. 15 mm, Sharm Rock, 09 January 2010, GT Smith. – Mayotte and La Réunion: photographs of numerous individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
Easily recognised, not to be confused with any other species found in the western Indian Ocean and Red Sea. Large and fleshy with raised, medium-sized bright orange pustules, deep purple rings around rhinophores and gills joined by broken line in the Dahwat ad Daffi individual and the majority of photographs of individuals from the Persian Gulf (Plate 34). In the Gulf, the purple rings are not perfect as in the Red Sea, presenting with gaps, dots, dashes, and extra markings (Plate 35). Mantle margin is of the same purple pigment, foot without purple margin but does have few orange spots on its upper surface; rhinophores and gills purple.
Chromodoris annulata is known from the Red Sea (
http://species-id.net/wiki/Chromodoris_boucheti
Plate 36Maldives: 30 mm, Bathala Island, Ari Atoll, 28 July 1995, 10 m depth, leg. RC Anderson & SG Buttress (“colour duller and darker than usual”); photographs of several individuals incl. one 5 mm juvenile, March 1997, March 1998, March 1999, J Hinterkircher. – Tanzania: photo of one individual, Mafia Island, shallow water, May 2009, A de Villiers.
Pale blue body with three black lines, black sub-marginal line forming a ring, paler blue to white margin; white and orange rhinophores and six gills with distinctive black marks at base on both sides. There may be a general orange wash dorsally. Recent published records of Chromodoris boucheti are only from the Maldive Islands and Mayotte: Chromodoris boucheti was not found in the Chagos archipelago despite similar sampling techniques, nor has it been recorded from Mauritius, and there is only one record from La Réunion (http://seaslugs.free.fr/nudibranche/a_intro.htm). However, photographs on websites show individuals from Kenya, Tanzania, and South Africa, and this extended distribution is supported by a photograph from Mafia Island, Tanzania (A de Villiers, pers. comm.).
http://species-id.net/wiki/Chromodoris_cavae
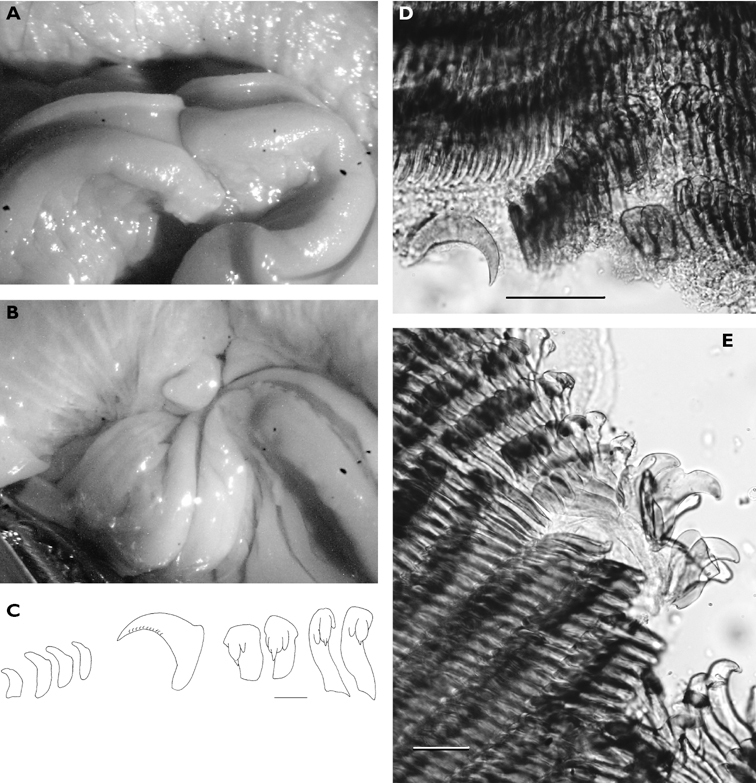
Fig. 10, Plates 37 , 38La Réunion: 60 mm (25 × 15 mm pres., #1), Bassin des Hirondelles, St. Gilles les Bains, 05 November 2006, 1-2 m depth, leg. P Bidgrain; 76 mm (37 × 20 mm pres., #2), Bassin des Hirondelles, St. Gilles les Bains, 17 September 2009, 1-2 m depth, leg. P Bidgrain; 75 mm (34 × 17 mm pres., #3), Bassin des Hirondelles, St. Gilles les Bains, 17 September 2009, 1-2 m depth, leg. P Bidgrain [all in permanent shallow pool on granite coast]; numerous photographs as Chromodoris cf. geminus on http://seaslugs.free.fr/nudibranche/a_intro.htm. – Sri Lanka: photographs of two individuals, Unawatuna, S of Galle and Negombo, N of Colombo, 30 December 2010 and 12 January 2011, S Kahlbrock.
The three specimens are well preserved, accompanied by 10-20 photographs each. They were all similar in life, with an ochre dorsum extending towards a white band around the edge of the mantle; the purple margin normally present in the species was lacking in all three. The first specimen (60 mm) was rusty orange, deeper along central dorsum and fading toward edge; creamy white band along margin but no purple edge. Some photographs are of the hyponotum as the animal flapped its margin, and there was no purple crescent or line ventrally. Six large wine-red patches with white annulus outside and white speckles inside. Around edge of rust-coloured area was a ring of small round wine red spots, each also with white ring. Rhinophores white, distal part of frontal surfaces and tips purple: 35 lamellae on right rhinophore, very faint purple wash at tips remaining on preserved specimen. 25 pinnate gills arranged with two ends of an arc spiralling inwards: each is triangular, flat surface white and translucent pinnae on other two surfaces. Both branchial and rhinophoral pockets slightly raised, orange in life. Foot and digitate oral tentacles white, but upper lamina of foot very faintly purple; no spots ventrally.
Specimen 2 (76 mm) was deeper orange in life, with more brown centrally and more yellow-orange marginally than specimen 1; faint submarginal band of yellow-orange before white margin. 13 large brown-red spots encircled with white, few smaller ones arranged irregularly around central patch. Additionally, this specimen had some white patches outside and amongst the small spots. 22 gills all had purple tips; rhinophores had more extensive purple pigment than those of specimen 1. Foot yellow-orange posteriorly followed by white band around margin. Ventrally foot bilaminate for its entire anterior margin (Fig. 10A). This specimen was dissected to remove the radula: body wall of foot thick and rose-red internally, digestive gland dark brown-pink while buccal mass white with pink tint. Muscles were very strong, glistening pink; reproductive system opaque white. The radula is large, but the teeth are minute: the largest laterals measure 100 μm (Fig. 10C). The formula is 58 (+2) × 56.1.56. There is a much reduced median thickening in some of the older rows (Fig. 10D). The first lateral bears a small rounded denticle on each side of a sharp cusp. The next 12 laterals have 10-12 weak denticles along the cusp (Fig. 10D). The remaining teeth are simply hook-shaped, blunt and rounded at the old end of the radula (Fig. 10E) but with extremely long sharp cusps at the newer end. Most of the jaw elements are simple unicuspid structures, with a single slightly curved cusp; very few are bicuspid.
Specimen 3 (75 mm) was dark like specimen 2, with 19 gills tipped in purple; rhinophores dark violet, with many closely spaced lamellae (Plate 37). Orange raised rhinophoral pocket rims can be seen in the plate. Ventrally, top of foot white with dark spots, violet margin on upper lamina of foot, white oral tentacles; orange-yellow marking posteriorly but no purple margin; faint orange line in crease between hyponotum and foot. Conical oral tentacles of preserved specimen visible in Fig. 10B, although left one is difficult to see. Margin of anterior foot distinctive, with the two laminae quite separated and extending across entire margin.
Chromodoris cavae A ventral view of 76 mm specimen showing bilaminate propodium B ventral view of 75 mm specimen showing conical oral tentacles. Radular teeth of 76 mm specimen C first two lateral teeth on each side of median, lateral 16, and last 4 teeth (from new rows), scale bar 40 µm D central lateral teeth of first row and one displaced early middle lateral tooth showing denticles on cusp, scale bar 100µm E outer lateral teeth from an old row showing reduction in cusp, scale bar 100 µm.
Many photographs of additional individuals from La Réunion as well as those from Sri Lanka (Plate 38) clearly belong to the same species. Variations range from large to small spots with solid to diffuse pigmentation, wine-red to almost black-red in colour, all with white ocelli; the presence or absence of smaller spots with a very broad to ‘normal’ white marginal band; a violet margin is very rarely present in the La Réunion individuals but present on the Sri Lanka individuals. Ventrally, there is also some variability, from pure white to having a (faint or dark) rusty orange line along the crease between the foot and the hyponotum, from no spots to few spots on the foot below this line, and there may or may not be an orange patch on the tail. The Sri Lanka individuals had a very faint purple line on the foot margin.
The specimens are all distinctive in preservative, either alcohol or formaldehyde: they are violet-purple to plum red-violet, and all have a thick undulating mantle skirt (including that from Chagos, see
Comparison of the radulae of tennentana, vicina, leopardus, cf. leopardus, and one La Réunion specimen show only slight variations in morphology but large differences in tooth size; the sizes listed are of the same dimension of a lateral tooth – from the tip of the cusp to the flange where the cusp meets the base.
20 mm alive (Rudman, tennentana) 40(+2) × 37.0.37 40 µm
36 mm alive (Edmunds, vicina = tennentana) 52 × 49.1.49 60 µm
59 mm alive (Rudman, leopardus) 74(+2) × 61.0.61 90 µm
34 mm alive (Yonow et al., cf. leopardus = cavae) 52 × 62.1.62 25 µm
80 mm alive (this paper, cavae) 58(+2) × 56.1.56 100µm
The three specimens examined from La Réunion belong to a form of Chromodoris cavae in which the violet mantle margin is lacking; of the additional photographs of a further 25 individuals from La Réunion and Mauritius (P Bidgrain, pers. comm., H. Flodrops, pers. comm., and http://seaslugs.free.fr/nudibranche/a_intro.htm) all but one individual lack the violet margin on both the mantle and the foot. The Chagos specimens both had a pale purple margin to the mantle (
http://species-id.net/wiki/Chromodoris_conchyliata
Plates 39, 40Seychelles: 20 × 7 mm (PK-Z), 5 m depth under rock, off Ade’s house, Lilôt, NW Mahé, 10 April 1992, leg. P Kemp (specimen retains orange gills and rhinophores, examined 2009) and photos of one larger individual, P Kemp. – La Réunion and Mayotte: photographs of numerous individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
It is exciting to be recording this species once more; there is one other published record from South Africa as Chromodoris geometrica (
http://species-id.net/wiki/Chromodoris_decora
Plates 41 , 42Maldives: 10 mm (8 × 3 mm pres.), 12 m depth, Banana Reef, North Malé Atoll, 19 November 1995, leg. SG Buttress & RC Anderson (“dorsal surface creamy green with white dots, spots, and faint lines; margin orange with purple spots and white dots; larger purple spots inside margin; rhinophores and gills cream”). – Oman: photographs of one individual, Muscat, 01-12 April 2009, S Kahlbrock. – Sri Lanka: photographs of one individual, Unawatuna, S of Galle, 28 December 2010, S Kahlbrock.
The single specimen is accompanied by a photograph reproduced here (Plate 41) and is not of the typical colour pattern. The white lines were represented by a series of dots and dashes, forming an irregular scalloped line around the central dorsal hump, contiguous to an orange band containing larger purple spots and smaller white dots. Purple spots coalesced to form small patches. Some white dots and dashes within this central region, but only vaguely indicate a median. The five simply pinnate gills, rhinophores, and top of the tail all bore opaque white markings: the gills and the rhinophores as a core, and the tail as a white band centrally tapering on the long metapodium. Two individuals photographed in Oman and Sri Lanka have an identical pattern of distinct marginal bands of orange and lilac, the lilac bearing rounded purple spots (Plate 42). There is one white line dorsally splitting around the branchial pocket. One photograph shows the red-violet spots located between the foot and the hyponotum, characteristic of this species.
The preserved specimen is well extended but badly preserved. It is translucent white, and the black digestive gland is visible through the thin epidermis, as is the white intestine loop. The translucent white metapodium is long and pointed.
This is the first literature record of the species from the western Indian Ocean. There have been photographs made available on
http://species-id.net/wiki/Chromodoris_geometrica
Plate 43Maldives: 15 mm, Banana Reef, North Malé Atoll, 12 m depth, 19 November 1995, leg. SG Buttress & RC Anderson, and photos of another individual, RCA. – Mayotte: photographs of numerous individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
The small specimen illustrated in Plate 43 was very dark brown, with a few large pustules distributed centrally and marginally, and speckled greenish-yellow gills and rhinophores: Maldives specimens are usually darker brown than individuals from other localities. It is noteworthy that the only Indian Ocean specimens available are once again from the Maldive Islands. Currently, the only photographic records from anywhere else in the Indian Ocean are from Mayotte (http://seaslugs.free.fr/nudibranche/a_intro.htm), although there are other internet photos of this Indo-West Pacific species from Tanzania and South Africa (
http://species-id.net/wiki/Chromodoris_gleniei
Plate 44Maldives: 25 mm, Fulidhoo Channel, Felidhoo Atoll, 10 m depth, 24 April 1994, leg. RC Anderson & SG Buttress; photographs of one individual 40 mm, March 1999, J Hinterkircher. – Sri Lanka: photographs of mating pair, Negombo, N of Colombo, 16 January 2011, S Kahlbrock.
http://species-id.net/wiki/Chromodoris_hamiltoni
Plate 45Kenya: six specimens 15-20 mm pres. lengths, Vipingo, 25 miles N of Mombasa, ELW in rock pools on exposed reef, 23 September 1984, leg. J Hognerud (Australian Museum, Sydney, C431127). – Tanzania: photographs of two individuals, 1994, and reef flat, Matemwe, August 1993, MD Richmond; photo of one individual, Mafia Island, shallow water, May 2009, A de Villiers. – La Réunion: photograph of single individual, P Bidgrain, pers. comm.
The preserved specimens were blue in life with a broad pale orange mantle skirt and a distinctive orange patch in the centre of the dorsum, bisected by a longitudinal black line. All published records are listed above, and indicate that this species is known only from eastern Africa: Chromodoris hamiltoni has not been recorded from any of the island groups except La Réunion. It is similar only to Chromodoris quadricolor (see below), but usually lacks the distinctive white line between the black outermost line and the orange margin, the orange margin is paler and slightly more pink, and usually it has additional patches of orange and/or black dorsally. It is a much more variable species than Chromodoris quadricolor.
http://species-id.net/wiki/Chromodoris_quadricolor
Socotra: 20 × 9 mm pres. (St-190, F-59), Abd al-Kuri, Khaisat en Naum, western tip, 10 April 1999, leg. U Zajonz.
There are no photographs accompanying the Socotra specimen, but the preserved specimen is semi-relaxed (examined 2009), with dark orange rhinophores and gills. The mantle is black with two white stripes fading to cream with a faint orange tinge on the dorsum. There is a relatively broad white band between the black dorsum and the orange marginal band (cf. Chromodoris hamiltoni above, which normally does not have this white separation, and Chromodoris elisabethina Bergh, which has a fine white line). The white line at the very edge of the margin can still be seen (cf. Chromodoris africana p. 34 which does not have this white line). There are two black stripes on the sides of the foot, and the foot retains an orange margin. The preserved specimen is identical to specimens collected from the Red Sea in 1990: Chromodoris quadricolor appears to hold its colour extremely well in formaldehyde.
Originally described from the Red Sea, its presence in Socotra is not unexpected:
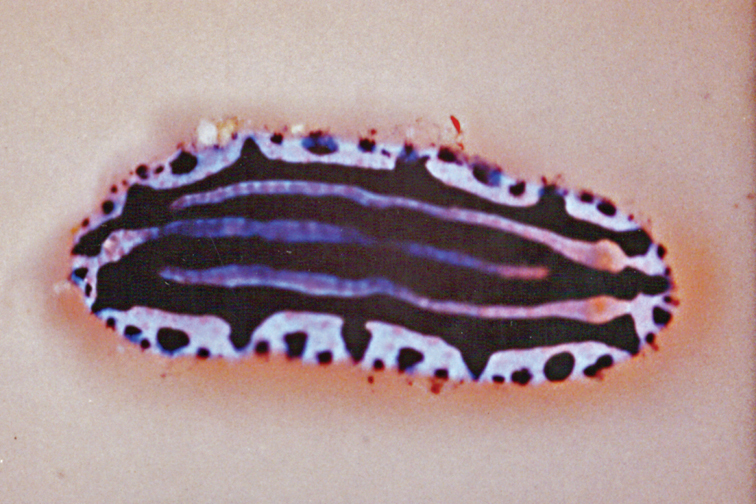
http://species-id.net/wiki/Chromodoris_tennentana
Fig. 11, Plate 46Seychelles: 8 × 5 mm pres. (PK–BB), Lilôt, NW Mahé, 17 m depth on algal-encrusted rock, 26 April 1992, leg. P Kemp (mantle glands obvious on preserved specimen). – Madagascar: photos of one juvenile, 10 mm in length, Nosy Bé, October 2007, J Hinterkircher.
The Seychelles specimen has the typical colour pattern: a central patchy ochre-brown dorsum with ocellated violet spots, a creamy sub-marginal band with orange and purple spots, a blue-violet margin, and ochre rhinophores and gills. The specimen preserved in formaldehyde is translucent, with a rosy-orange and black gut. The flat translucent mantle skirt is extended, white mantle glands visible in a band along the edge: most are irregular in shape, some are stellate (Fig. 11A). Gills translucent, flat, and very simply pinnate; oral tentacles digitiform (Fig. 11B). The similarities and differences between this species and Chromodoris cavae, removed from synonymy of Chromodoris tennentana, are discussed under that species (see p. 36).
Chromodoris tennentana, 8 mm pres. specimen A drawing showing mantle glands located in a band around the mantle skirt B photograph of bilaminate propodium and oral tentacles.
http://species-id.net/wiki/Glossodoris_cincta
Plate 47Seychelles: 30 × 15 mm (PK-N), Horseshoe Rock, Lilôt, NW Mahé, 15 m depth on algal-encrusted rock, 24 March 1992, leg. P Kemp (“pink with dark blue edging”; the preserved specimen is brick red with a green margin). – Maldives: 15 mm (7 mm pres.), 5 m depth on coral encrusted with algae and sponge, Maayafushi, South Malé Atoll, March 1997, leg. J Hinterkircher. – Persian Gulf: photograph of one individual, Dahwat ad Daffi, Jubail, Saudi Arabia, April 1992, F Krupp. – Kenya: 30 mm long pres., Vipingo, 25 m N of Mombasa, ELW in rock pools on exposed reef, 23 September 1984, leg. J Hognerud (preserved animal more orange and purple than red and blue-green) (Australian Museum, Sydney, lost?). – Tanzania: photographs of two individuals, Mafia Island, shallow water, May 2009, A de Villiers. – Sri Lanka: photographs of one individual, Unawatuna, south of Galle, 01 January 2011, S Kahlbrock.
http://species-id.net/wiki/Glossodoris_pallida
Plate 48Maldives: approx. 20 mm alive (11 × 5 mm pres.), Bathala Island, Ari Atoll, 11 m depth, night dive, 27 July 1995, leg. SG Buttress & RC Anderson. – Persian Gulf: photographs of two individuals, Dahwat ad Daffi, April 1992, and N of Abu Ali, 26 May 1992, Jubail, Saudi Arabia, F Krupp; photo of three individuals, Muscat, Oman, 01-12 April 2009, S Kahlbrock. – La Réunion: photos of several individuals 6-20 mm http://seaslugs.free.fr/nudibranche/a_intro.htm. – Seychelles: photographs of one individual, Lilôt, NW Mahé, 1988-1989, P Kemp. – Sri Lanka: photographs of two individuals, Unawatuna, S of Galle, 03 January 2011, S Kahlbrock.
The preserved specimen is well relaxed: it is opaque white, and no markings remain; the digestive system is not visible through the skin. Twelve gills are extended: the rachis is flat on the outside and lamellae face inwards on the two other sides. In life, the lamellae can be seen to extend laterally. One photograph from Oman (S Kahlbrock, pers. comm.) depicts the individual beginning to lay an egg mass, a flat white ribbon laid on its edge.
This species appears to be present but rarely recorded in the western Indian Ocean:
http://species-id.net/wiki/Glossodoris_undaurum
Plate 49Sri Lanka: 13 × 5 mm pres., Unawatuna, S of Galle, 27 December 2010, leg. and photos S Kahlbrock.
This description of the live animal is based on many photographs of the specimen: dorsum broad with narrow mantle overlap; skirt thick with single pair of permanent undulations. Dorsum grey to off-white with irregularly shaped white, slightly raised pustules. Mantle skirt whiter than dorsum with only a few small white pustules; margin lemon-yellow. Rhinophores very small: distal half translucent lemon-yellow, proximal half paler translucent yellow, posteriorly an opaque line brighter yellow towards the tip. Simply pinnate gills translucent white with opaque white line on each side of rachis, red tips, and short red line running down from tips.
The preserved specimen is semi-translucent grey with irregular opaque white spots, slight crumpling of mantle around each. Mantle skirt with thickened margin, no coloured or thickened margin on skirt or foot. Long rhinophores translucent grey with white line and white tip, lamellae indistinct. Gills retracted but the red ends visible though translucent skin and open pocket.
This is the first record in the scientific literature of Glossodoris undaurum from the western Indian Ocean. There are book and internet records from South Africa, Kenya, Oman, and La Réunion. There are several named and un-named species occurring in the Indian Ocean, but these differ externally in the combination of margin, rhinophore, and gill colouration: collection of specimens will enable comparisons. It may be that some are colour variations but this cannot be established at present.
http://species-id.net/wiki/Hypselodoris_maculosa
Plate 50Seychelles: 15 × 5 mm (PK-DD), Lilôt, NW Mahé, 17 m depth on granite wall, 26 April 1992, leg. P Kemp. – Maldives: 15 mm length, Maayafushi, Ari Atoll, 3m depth, on coral rubble encrusted with algae and sponges, March 1997, leg. J Hinterkircher. – La Réunion, Mauritius, and Mayotte: numerous photographs http://seaslugs.free.fr/nudibranche/a_intro.htm. – Sri Lanka: photographs of one individual, Unawatuna, S of Galle, 27 December 2010, S Kahlbrock.
Elongated body with spatulate head. Translucent body with opaque white markings comprising several longitudinal white lines on dorsum and small white dots on orange-red margin. Magenta spots and patches between dorsal white lines. Foot translucent with magenta spots and patches, and small white dots. Rhinophores distinctive with white core bearing two red bands. Gills small, held upright, red proximally and white distally.
Small specimens are similar to several species of Thorunna, such as Thorunna australis (Risbec), recorded only from South Africa in the western Indian Ocean: it also has orange bands on the gills and rhinophores but the white markings are limited to one or two lines on the dorsum. Thorunna florens (Baba) also has similar gills and rhinophores but has purple spots around the margin as well as an orange border to the frontal margin, and it is limited to the western Pacific. Several un-named species are illustrated in Gosliner et al. (2008) but at present they are only known from few localities in the western Pacific.
http://species-id.net/wiki/Risbecia_bullockii
Fig. 12, Plates 51, 52, 53La Réunion: 30 mm (24 × 10 mm pres.) on wall of cave, Etang Salé les Bains, 38 m depth, 21 December 2010, leg. H Flodrops; 35 mm (23 × 10 mm pres.), Sec Jaune, Saint-Leu, 12 m depth (night dive), 30 December 2010, leg. H Flodrops. – Mauritius: photographs only of three individuals, 1990’s, M. Parmantier pers. comm. and http://seaslugs.free.fr/nudibranche/a_intro.htm. – Sri Lanka: three specimens 11 × 5 mm, 13 × 4 mm, and 16 × 7 mm all pres., Unawatuna, S of Galle, 29 December 2010, and Negombo, N of Colombo, 16 January 2011, leg. S Kahlbrock; photo of one individual, Pigeon Island, Trincomalee, 16 m depth, April 1995, RC Anderson.
All five specimens are of the original colour form as described and illustrated by Collingwood (1881) and reproduced on Plate 51: dorsum mauve with central orange tinge and distinct narrow white margin; gills and rhinophores bright orange with magenta bases, gills raised high on a peduncle. La Réunion specimens pale violet with white margin, bicoloured gills and rhinophores (Plate 52). Smaller Sri Lanka specimens with rosier orange central dorsum (Plate 53). Seven gills in La Réunion specimens and nine in Sri Lanka specimens. In some photographs, the peculiar arrangement described and illustrated by Collingwood (Plate 51, "fig. 17") is visible, in which the gills are connected: the last two or three gills on each side are joined at the base and there are three single central ones (Fig. 12A). Anal papilla protruded as a tube in most images showing open gills, same colour as mantle. Gills bright orange but deep magenta where connected basally. Pockets of both rhinophores and gills uniformly coloured with mantle; raised rim translucent. White marginal line present on both surfaces as well as edge of mantle.
The specimens are well preserved, solid with a high profile, the metapodium extending beyond the posterior mantle margin. All five specimens retain the white line around the margin but no mantle glands are evident. The gills are extended in the La Réunion specimens, and each gill is almost round in section. Those of the 35 mm specimen are still pink, especially at the base (April 2011). The head is rounded with small oral tentacles identical to that figured for Risbecia apolegma Yonow (2002, fig. 11). The rosy hue remains on the dorsum, rhinophores, and gills of the Sri Lanka material several months after collection. The radula of the 16 mm Sri Lankan specimen examined had a formula of 72 × 100.0.100. All teeth are denticulate: there is no median thickening; the first 10 laterals in each row are recurved: the main cusp has a strong “brow ridge” with 1 or 2 very small denticles on each side (Fig. 12B). The majority of the laterals rapidly become straighter with a large cusp bearing 5 or 6 small denticles along its edge; the largest cusp measures at least 100 μm. In the last 5-7 teeth of this group, the first denticle next to the main cusp is doubled (Fig. 12C, D), even in the newest row (Fig. 12E). The final 10 teeth of each row rapidly reduce in size of cusp and denticles to become almost rounded; however, the double first denticle persists (Fig. 12F). The jaw rodlets of Risbecia bullockii have a slightly curved point with a swollen base.
Risbecia bullockii A view of gills showing their arrangement (compare with Plate 35 “fig. 17”). Radular teeth of 16 mm pres. Sri Lankan specimen B first lateral teeth from right half of radula C view of one row of lateral teeth showing divided first denticle on each cusp D higher magnification of two lateral teeth E several laterals from the newest row, also with divided first denticles F outermost laterals showing very reduced cusps but still with divided first denticles. Scale bars 50 µm.
Much controversy surrounds the identity of this species, here recorded from the Indian Ocean for the second time: photographs from La Réunion and Mauritius are also amethystine violet, intensifying anteriorly and posteriorly, a thin, bright white line is present around the mantle but not around the foot, and the gills and rhinophores are orange with magenta at the base (http://seaslugs.free.fr/nudibranche/a_intro.htm); these are identical to Collingwood’s illustration. Controversy also surrounded the description of Risbecia apolegma Yonow, 2002: Rudman stated on Sea Slug Forum that he could “find no anatomical grounds to place this ‘species’ in the genus Risbecia” while also querying its distinction from Hypselodoris “bullocki” (sic). However, in the numerous pages showing variations in colour pattern he stated that “unpublished studies of the anatomy show that it should probably be placed in the genus Risbecia.” Examination of the radula of the La Réunion specimen, re-examination of all micrographs of the radulae of Risbecia apolegma and of Risbecia pulchella (see below) show that they are all very similar and bear the diagnostic features as described by
Coincidentally, a recent paper by
http://species-id.net/wiki/Risbecia_pulchella
Fig. 13, Plate 54Seychelles: 50 × 25 mm, one of two animals on algal-encrusted rock, Bug Rock, Brissare, 05 April 1992, leg. P Kemp; photographs of several individuals, Lilôt, NW Mahé, 1988-1989, P Kemp. – Gulf of Oman: photos of two individuals trailing, Muscat, 01-12 April 2009, S Kahlbrock; photographs of pairs, Musandam, 13 May 2009, trailing pair, Siri Island, 15 m depth, 13 May 2009, trailing pair, Anemone Garden, just off Khorfakkan, 8 Jan 2012, GT Smith. – La Réunion, Mauritius, Mayotte: photographs of several individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
The radular formula is 83 (+3) × 90-95.0.95-90: there is no median tooth. The first 7-8 laterals are duck bill shaped with a large denticle on the outside (Fig. 13A). The central laterals increase rapidly in size to a cusp length of approximately 75 μm bearing 4-5 denticles, of which the first is divided (Fig. 13B). The last 3-4 laterals in each row are very reduced with a rounded main cusp and multiple denticles (Fig. 13C).
Risbecia pulchella, 50 mm A first 3–5 laterals on both sides of the midline, no central thickening or rhachidian B mid-lateral teeth showing single main cusp and divided first denticle C outermost laterals in newish row with reduction in cusp. Scale bars 50 µm.
http://species-id.net/wiki/Thorunna_cf_horologia
Fig. 14, Plate 55Sri Lanka: 5 × 4 mm pres., Pigeon Island, Trincomalee, 16 m depth, April 1995, leg. SG Buttress & RC Anderson.
The single specimen appears to have been stocky with little mantle overlap in life. Margin permanently convoluted with three indentations: one behind rhinophores, largest in centre of body, one just beyond gills. Colour opaque white with violet pigment concentrated around edges and on head around and between rhinophores. Thin sub-marginal white line around the violet, followed by translucent margin with orange in patches and in permanent folds, most especially the central one in which there was orange pigment overlying the submarginal white line. Rhinophores very large with translucent stalk and white core covered in 13 orange-red lamellae; rhinophoral pockets with low violet rim. Eight gills simply pinnate, arranged in circle around anal papilla; white ‘core’ and orange lamellae; orange line along both inner and outer edges of each gill. Foot pale violet, darker concentration of violet in band around metapodium.
The preserved specimen is translucent cream, with a thin mantle skirt. The rhinophores are retracted but some of the gills are extended: they are simply pinnate. The foot is convoluted laterally but anteriorly it is relaxed. The bilaminate margin spans the entire width and the upper lamina is notched. The oral tentacles are plump rounded structures (Fig. 14).
Thorunna cf. horologia, 5 mm pres. specimen, ventral view of propodium showing bilaminate edge and oral tentacles.
This specimen differs from Thorunna horologia, also found in the western Indian Ocean: horologia is opaque white with red marginal and orange-yellow sub-marginal lines, a single deep indentation which is red, and a white foot with a violet band on the margin (
http://species-id.net/wiki/Dendrodoris_fumata
Plate 56Socotra: 17 × 10 mm pres. (IT-177, RJ-032), 12°41.326'N, 54°05.175'E, 14 April 1999, leg. R Janssen. – Persian Gulf: four pres. specimens 17 × 10 mm, 15 × 12 mm, 16 × 10 mm, and 9 × 5 mm, Rescue Centre, Jubail, Saudi Arabia, 09 December 1991, leg. D Fiege; photographs of one individual, Dahwat al Musallamiya, Jubail, Saudi Arabia, 25 November 1991, F Krupp. – La Réunion: photographs of four individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
Both Dendrodoris nigra and Dendrodoris fumata are found in similar habitats in the western Indian Ocean, and both species are present in these collections. Two colour forms occur in the Indo-West Pacific, a grey form and an orange/red form, the latter being much more common in the Indian Ocean (pers. obs.,
A third species of Dendrodoris is present in the Persian Gulf material: the preserved animals are very different from both Dendrodoris fumata and Dendrodoris nigra, but there are no notes or photographs of the living material for adequate comparison or identification (Abu Ali, 28 March 1992). In preservative, these two specimens measure 10 × 8 mm and 15 × 9 mm. They are both translucent beige-yellow and may have been pustular (? possibly Dendrodoris coronata Kay & Young). Both have very prominent raised rhinophoral and branchial sheaths; the smaller specimen has 9 extended simply pinnate gills and the rhinophoral pockets are crumpled in both specimens. Ventrally, the foot is broad and squared anteriorly with two laminae, and the ridge-like oral tentacles meet medially as in all dendrodorids.
http://species-id.net/wiki/Dendrodoris_nigra
Plates 57 , 58Socotra: two pres. specimens 18 × 13 mm and 16 × 9 mm (IT-177, RJ-032; the smaller has a white submarginal band), 12°41.326'N, 54°05.175'E, 14 April 1999, leg. R Janssen. – Maldives: two specimens 16 × 4 mm and 19 × 5 mm (MDV/AB/96/6), found separately under rocks, Fulidhoo Lagoon, Felidhoo Atoll, 04 May 1996, leg. RC Anderson & SG Buttress; photo of one red individual, 1986-1994, J Hinterkircher. – Zanzibar: 30 × 15 mm pres. (“black, frilly skirt, domed dorsum, no white specks”), in coral rock, 1994, leg. MD Richmond. – Gulf of Oman: photo of three individuals, Muscat, 01-12 April 2009, S Kahlbrock. – Seychelles, one individual photographed, Lilôt, NW Mahé, 1988-1989, P Kemp. – La Réunion, Mauritius, and Mayotte: photographs of several individuals http://seaslugs.free.fr/nudibranche/a_intro.htm.
The preserved specimens of Dendrodoris nigra are all black, a little longer and thinner, and much firmer than Dendrodoris fumata, which has a thinner but more extensive mantle skirt. In the two Socotra specimens of Dendrodoris nigra, black or dark grey in preservative ten years later, the large white mantle glands are still clearly visible; additionally, in the smaller specimen, there is a substantial and distinct white submarginal band, presumably red in life. The two Maldives specimens of Dendrodoris nigra are relaxed and also retain their small white spots (examined 15 years later). The gills and rhinophores are extended and the gill clump is relatively small. The photograph of the only non-black Dendrodoris nigra from the Maldives is clearly a juvenile: it had a narrow mantle skirt and was pale translucent red with small white spots and flecks as well as a black marginal band (Plate 58). The gill clump was small (but translucent red), the tri-coloured rhinophores were translucent red basally, black distally, with a large white knob at the tip; these are characters typical of Dendrodoris nigra, reviewed by
urn:lsid:zoobank.org:act:5ADE579C-AC59-4EBB-8456-0AEE2F204176
http://species-id.net/wiki/Doriopsilla_nigrocera
Fig. 15, Plate 59Holotype: Persian Gulf (Saudi Arabia): 25 × 17 mm pres., Jubail, Dahwat ad Daffi, 03 April 1992, leg. F Krupp, SMF 337105.
Creamy yellow elongate oval body with ochre gills and charcoal black rhinophores. Variously sized spiculose tubercles are covered in opaque white lines, with some fainter ones extending from the tubercles to the mantle.
The photograph depicts an elongate oval animal with a wrinkled and damaged margin in life. Dorsum covered in tubercles, themselves covered and sometimes faintly linked to each other with fine white lines. Body was creamy yellow, plumose gills ochre in colour, darker than dorsum, but the rhinophores were black: the stalk was translucent cream and the approximately 15 fine lamellae were charcoal black. The midline on each side and the tip were dusty white. The gut was visible through the skin, opaque creamy orange with black patches.
The preserved specimen has a pink cast and is semi-translucent. Surprisingly, the retracted rhinophores are no longer black, and not visible by translucence through the pockets or the body wall. The tubercles are full of spicules. Tubercles are largest centrally and become progressively smaller toward the margin (Plate 59); in preservative, many are mushroom-shaped. The margin itself is rather thick (and damaged on the right side). Ventrally, the anterior margin of the foot is simple, not bilaminate, and there is a fold under the mouth (Fig. 15).
Doriopsilla nigrocerasp. n., holotype, ventral view of propodium, head, and oral tentacles.
The single specimen is unlike any species of Doriopsilla published to date, and different from the photographs available in books and on the internet. The most similar species is Doriopsilla miniata (Alder & Hancock), described from India and occurring throughout the Indo-West Pacific in a wide range of colours from nearly white to bright orange. Usually, there are tracings of white lines crossing the dorsum transversely but these may be absent. More critically, the gills and rhinophores of Doriopsilla miniata are the same colour as the dorsum or a little darker and this, amongst other characteristics, distinguishes this new species. An un-named species of Doriopsilla similar to Doriopsilla nigrocera sp. n. is illustrated in Gosliner et al. (2008),
The specific epithet refers to the very distinctive black rhinophores, unique to this species of Doriopsilla.
http://species-id.net/wiki/Phyllidia_marindica
Plate 60Maldives: 35 mm in length (MDV/AB/96/20), 14 m depth, Banana Reef, North Malé Atoll, 10 May 1996, leg. RC Anderson & SG Buttress; photos of several individuals 30-40 mm, March 1999, J Hinterkircher. – La Réunion and Mauritius: numerous photographs of many individuals http://seaslugs.free.fr/nudibranche/a_intro.htm. – Tanzania: photographs of three individuals, Mafia Island, shallow water, March 2004 and May 2009, A de Villiers. – Seychelles: photo of one individual, Lilôt, NW Mahé, 1988-1989, P Kemp.
The single specimen is not of the typical western Indian Ocean colour pattern, but more like the animals found in the eastern Indian Ocean and is therefore illustrated here. All other photos are of the typical colour form (as described by Yonow 1994), and range in size from 22 mm to 60 mm in length. The longitudinal pattern of black lines is lacking in this specimen and only transverse rays to the margin are present, connected to each other and crossing the dorsum. There are usually no tubercles on the black lines but if present, these are only small pustules. Ground colour granular blue-white, with more blue pigment present around margin; larger tubercles orange-yellow and arranged in three lines of partially contiguous tubercles along centre of dorsum; in the marginal area, row of single orange-yellow tubercles on each side followed by smaller ones.
http://species-id.net/wiki/Phyllidia_picta
Fig. 16Socotra: two specimens, both 27 × 12 mm pres. (St. 018, GR-22), Qualansiyah Bay 12°41.026'N, 53°28.309'E, 10 March 1999, leg. G Reinicke.
This is the first record of Phyllidia (Fryeria) picta in the western Indian Ocean, identified by colour pattern and ventral anus. The colour pattern does not appear to differ from that found in the western and central Pacific: the white-in-life areas around the tubercles are now grey-white, and the orange-in-life tubercles are a dirty yellow colour. There are three rows of individual tubercles which are not contiguous (as in marindica, see above) although the white bases of two or three tubercles may merge along the central line (Fig. 16A). Ventrally, the anterior foot margin is divided and the oral tentacles are triangular (Fig. 16B). The two specimens differ from preserved specimens of marindica (above) and Phyllidia (Fryeria) rueppelii Bergh, from the Red Sea: the black pigment covers much more of the dorsum in Phyllidia (Fryeria) picta, extending to the margin in rays. The black areas in Phyllidia (Fryeria) picta are smooth, while they are tuberculate in Phyllidia (Fryeria) rueppelii and small pustules may be present in the black areas of Phyllidia (Fryeria) marindica. All three species, however, are similar in having three central lines of orange tubercles, orange rhinophores, and a ventral anus.
Fryeria picta, 27 mm pres. specimen A dorsal view B ventral view of propodium, head, and tentacles.
http://species-id.net/wiki/Phyllidia_alyta
Fig. 17, Plate 61Maldives: 25 mm in length, Dhigu Tila, near Gulhi, South Malé Atoll, 12 m depth, 14 October 1994, leg. SG Buttress & RC Anderson; 28 × 12 mm, Bathala Island, Ari Atoll, 6 m depth, 28 July 1995, leg. SG Buttress & RC Anderson; 15 × 7 mm pres. (“light grey with black lines, rhinophores and last tubercle yellow”), Bathala Island, Ari Atoll, 10 m depth, 27 July 1995, leg. SG Buttress & RC Anderson; photographs of many individuals, 25-40 mm, March 1997, March 1998, March 1999, J Hinterkircher. – Sri Lanka: photos of two individuals, Negombo, N of Colombo, April 1995, RC Anderson; photos of one individual, Unawatuna, S of Galle, 27 December 2010, S Kahlbrock.
The preserved 15 mm juvenile is in perfect condition and easily recognised as Phyllidia alyta. Broken black line present on sole of foot despite its small size (Fig. 17A), but only two of the four longitudinal dorsal black lines are complete (Plate 61). Three rows of tubercles on midline between start of two lines: central row of tubercles joined by low ridge and only the last tubercle orange. Black crossbar between rhinophores lacking. Two larger specimens typical, with four dorsal black lines, black bar between rhinophores, and ventral broken black line. Foot thickened and concave anteriorly, and triangular tentacles bear groove along each outer side (Fig. 17B). Hyponotum hatched with spicules, dorsal black pigment showing through.
Phyllidia alyta, 15 mm pres. specimen A ventral view showing line on sole of foot B view of propodium, head, and oral tentacles.
It is probable that the species described by Eliot (1906) is Phyllidia alyta: he distinguishes it from Phyllidia varicosa (he had specimens of both), and Phyllidia alyta is the only other species in the Indian Ocean with linearly-arranged orange tubercles and black lines dorsally in combination with a black line on the sole of the foot. This species appears to be restricted to the western Indian Ocean; there are only two internet records from south western Thailand on NudiPixel.
http://species-id.net/wiki/Phyllidia_coelestis
Plate 62Madagascar: 65 × 25 mm (PK-C), Ampangorina, Nosy Komba, 1 m depth on Acropora, 30 January 1992, leg. P Kemp. – Tanzania: photographs of two individuals, Mafia Island, shallow water, 15 January 2005 and 04 July 2005, A de Villiers. – Seychelles: 32 × 12 mm (PK-FF), Lilôt, NW Mahé, 15 m on encrusted coral, 26 April 1992, leg. P Kemp; 32 × 15 mm preserved (NHMUK acc. no. 2222), slightly curled, east side of East Channel, Aldabra, 1 m depth in coral, 25 September 1967, leg. JD Taylor; 49 × 15 mm preserved (NHMUK acc. no. 2222), Passe Femme, Aldabra, in shallow water beneath coral, 29 November 1967, leg. JD Taylor. – Maldives: 23 × 11 mm preserved (NHMUK ref. M/02/B/42), Gan, 04 September 1964, PSD Maldive Islands Expedition. – La Réunion and Mayotte: numerous individuals photographed, 20-45 mm in length http://seaslugs.free.fr/nudibranche/a_intro.htm.
Ground colour granular blue-white with three black lines: median line containing orange-tipped tubercles, two smooth lateral lines. Lateral lines normally meet anteriorly, and extend to margin usually as U-shape, but remain separated posteriorly. Outside the black lines, mantle bears tubercles, black flecks, and smaller scattered pustules toward edge; larger tubercles may have orange tips. Rhinophores bright orange, up to 16 lamellae; row of orange tubercles originates behind each rhinophore. Ventrally, propodium notched or deeply concave; all specimens have long tapering oral tentacles, grooved laterally and bearing black pigment on dorsal surfaces extending ventrally onto bases.
These specimens and photographed individuals belong to the typical form of Phyllidia coelestis, a commonly recorded species in the western Indian Ocean and occurring as far south as South Africa. Another group of specimens also identified as Phyllidia coelestis (
http://species-id.net/wiki/Phyllidia_exquisita
Fig. 18 , Plate 63Maldives: single specimen 15 mm (11 × 6 mm pres.), Dhigu Tila, near Gulhi, South Malé Atoll, 12 m depth, 14 October 1994, leg. SG Buttress & RC Anderson. – Madagascar: photo of one individual, Nosy Bé, 12-14 m depth, 25 July 2010, S Bachel (http://seaslugs.free.fr/nudibranche/a_intro.htm). – Mayotte: photo of one individual, Ilot Blanc de Mtsamboro, 4 m depth, 30 Dec 2010, M Deuss (http://seaslugs.free.fr/nudibranche/a_intro.htm).
Elongated oval animal with granular white ground colour and three black lines connected anteriorly around rhinophores but not posteriorly; central one shortest and widest. Tubercles present in black areas. Three main rows of orange-tipped tubercles in life, middle ones located along central black line, lateral ones between black lines. Several isolated orange-tipped tubercles outside black lines within marginal band of granular white, which also contains non-orange tubercles. Ring of rounded black spots at edge of granular white and bordering the orange margin. Rhinophores same orange colour as tubercles and margin; anal papilla faintly orange. When the colour images of the living specimen are magnified, a completely separate band is visible around the margin, which is translucent when not pigmented with orange (Plate 63).
The preserved specimen does not retain any orange pigmentation. The tubercles located on the black lines are clearly spiculose, while those located on white areas are covered by granular pigment (Fig. 18A). The anus is very difficult to see, located far posteriorly and not on a tubercle. Ventrally, the anterior margin of the foot appears to be concave; the oral tentacles are conical (Fig. 18B).
Phyllidia exquisita, 11 mm pres. specimen A dorsal view showing granular white pigmentation and spiculose black areas B ventral view of propodium, head, and oral tentacles (left oral tentacle folded over).
http://species-id.net/wiki/Phyllidia_koehleri
Fig. 19 , Plates 64 , 65Maldives: 11 × 6 mm pres. (NY-142, orange with black triangles), Fulidhoo House Reef, Felidhoo Atoll, 20 m depth, 12 January 1991, leg. N Yonow; 14 × 7 mm pres. (SH-65, “bright yellow” with black ring), Maaya Tila, Ari Atoll, 16 m depth, August 1991, leg. S Harwood; 17 × 7 mm pres. (“yellow with black” ring), Hans’ Place, North Malé Atoll, 23 m depth, 3 November 1994, leg. SG Buttress & RC Anderson; photographs only of two individuals together, 10 mm approx. lengths (yellow with black ring), 12 m depth, July 1992, J Hinterkircher.
Phyllidia koehleri is a small species (the largest recorded size is 17 mm preserved length, above), bright yellow or orange, with a central longitudinal black line and a black ring, solid or broken, encompassing the anus and rhinophores. Body oval with single raised ridge behind each rhinophore, either side of central black line. Apart from two strong central ridges and tiny single tubercles covering skirt, the mantle is smooth. Broad central black band extending from behind the rhinophores to the anus, black ring then encloses central area (Plate 64); colour in life of these specimens yellow. Sometimes, as in one specimen, the black ring is broken into dashes which form bases of black triangular rays extending to margin (Plate 65); colour of this specimen orange but both patterns occur in both colours in images on the internet. There may be a few black spots in the marginal band and the orange form appears more tuberculate than the yellow ringed form. Rhinophores same colour as body, yellow or orange depending on body colour, extremely long and thin, bearing little more than 10 diagonal lamellae. Anal papilla translucent, extending from raised rim at junction of two ridges very near the posterior border, surrounded by some small tubercles. Ventrally, foot white in life, but hyponotum and gills yellow or orange in life. Anterior margin of foot notched, head white, and large oral tentacles triangular, grooved on outer margins, tipped with orange in life (Fig. 19A-C).
Internally, all specimens are similar, and illustrated here (Fig. 19D-F). The pharyngeal bulb is conical and slightly asymmetrical. Its posterior surface is glandular, the ventral projections a little longer than the dorsal ones. The oesophagus is very long, exiting the bulb posteriorly and bending anteriorly to pass through the nerve ring sitting on the bulb.
Phyllidia koehleri A ventral view of propodium, head, and oral tentacles of 11 mm pres. specimen. B ventral view of propodium, head, and oral tentacles of 14 mm pres. specimen C ventral view of propodium, head, and oral tentacles of 17 mm pres. specimen D digestive anatomy of 11 mm pres. specimen E digestive anatomy of 14 mm pres. specimen F digestive anatomy of 17 mm pres. specimen.
This small species appears to be endemic to the Maldives; a dozen photographs on various websites are recorded only from the Maldive archipelago. Colour variation appears standard: either animals have a black ring or they have black triangles, and both patterns occur in yellow and orange. Although they appear rather different, they are consistent in external (two strong ridges, scattered marginal tubercles, rhinophores with few diagonal lamellae) and internal (asymmetrical buccal bulb, a long oesophagus bending anteriorly before passing through the nerve ring) morphologies.
http://species-id.net/wiki/Phyllidia_multituberculata
Fig. 20 , Plate 66Typical form: Socotra: 30 × 16 mm pres. (St. 064, SAM1), Quatub Bay, 18 March 1999, leg. S Al-Moghrabi.
Black form: Maldives: single specimen 14 × 8 mm crawling (NY-167), Kudhiboli Tila, Felidhoo Atoll, 21 m depth, 15 January 1991, leg. N Yonow; two specimens together 28 × 20 mm and 30 × 21 mm both preserved, Maayafushi House Reef, Ari Atoll, 34 m depth, 18 December 1991, leg. H Voigtmann; 14 mm (MDV/AB/96/2), Cocoa House reef, South Malé Atoll, 16 m depth, 01 May 1996, leg. RC Anderson & SG Buttress; photos of one individual, 1986-1994, J Hinterkircher. – Sri Lanka: two pres. specimens 21 × 15 mm and 25 × 18 mm and photographs of a juvenile, Unawatuna, S of Galle, 26-28 December 2010, S Kahlbrock.
The Socotra specimen is of the typical form described and illustrated by Boettger, with five black patches on each side. This is the commonly occurring species in the Indian Ocean; see
The Maldives and Sri Lankan specimens differ in colour pattern from the Socotra specimen but are identical to each other, completely black with numerous orange mushroom-shaped tubercles: some had a white ring at the base when alive (Plate 66), and still visible in the preserved specimens. Tubercles vaguely organized in longitudinal rows over dorsum, tubercles in central row largest. Rhino-tubercles present as well as single very large tubercle behind and between them. Margin orange, somewhat scalloped along inner margin bordering black, with single small tubercles aligned along edge. In Sri Lankan specimens, white submarginal line. Rhinophores deeper shade of orange than tubercles and margin, raised pocket rims also orange. Ventrally, foot of black and orange specimens deeply divided (convoluted for its entire margin in the largest specimen), head bipartite, separated oral tentacles digitiform (Fig. 20B), most similar to those of Phyllidia multituberculata form undula (fig. 5B in
Phyllidia multituberculata A ventral view showing propodium, head, and oral tentacles, 30 mm pres. Socotra specimen B ventral view showing head and oral tentacles, 28 mm pres. Maldives specimen C anatomy of 28 mm pres. Maldives specimen.
Dissection of the 28 mm Maldive animal shows that the digestive anatomy is typical for the genus (Fig. 20C). Significantly, it has a segmented asymmetrical pharyngeal bulb as described and illustrated for Phyllidia multituberculata and Phyllidia multituberculata form undula. This feature, in addition to the colour patterns, separates the Indian Ocean Phyllidia multituberculata from the Pacific Phyllidia ocellata Cuvier, which has a symmetrical pharyngeal bulb (e.g.
http://species-id.net/wiki/Phyllidia_varicosa
Socotra: two pres. specimens 65 × 31 mm and 42 × 12 mm curled, (St. 064, SAM1), Quatub Bay, 18 March 1999, leg. S Al-Moghrabi; single pres. specimen 48 × 18 mm (St. 063, GR-57), 12°38.945'N, 53°56.028'E, off Quatub fishing village, in front of airport, 18 March 1999, leg. G Reinicke; 33 × 15 mm pres. (St. 068, MAP-138), 12°41.062'N, 54°04.508'E, Hawlaf, off jetty, 19 March 1999, leg. M Apel. – South Africa: three specimens (Natal Museum refs 51894, 5908). – Kenya: 35 × 15 mm and 15 × 8 mm both preserved (completely bleached dorsally and ventrally, notch on foot anteriorly), Turtle Bay, subtidal on rock, July 1969, leg. K Brander (nudibranch XII, TE Thompson collection, NHMUK acc. no. 2364). – Tanzania: photographs of two individuals, 1994, MD Richmond; photographs of four individuals, M’Nazi Bay, Msimbati, near Mtwara, May 1994, IM Horsfall; photographs of several individuals, Mafia Island, shallow water, 12 February 2004 and 06 January 2008, A de Villiers. – Zanzibar: photo of one individual, MD Richmond. – Mauritius: 65 × 32 mm pres. (dissected), MNHN, Paris (no label other than ‘Mauritius’). – La Réunion, Mauritius, Rodrigues, and Mayotte Islands: http://seaslugs.free.fr/nudibranche/a_intro.htm. – Seychelles: 53 × 25 mm pres., Victoria Harbour, 12 May 2005, leg. MD Richmond; photo of one individual, Lilôt, NW Mahé, 1988-1989, P Kemp. – Maldives: numerous individuals documented and/or photographed but not preserved, 8-80 mm, Maldives Expedition, N Yonow (see Yonow 1994, 1996) and 1997-1999 by J Hinterkircher. – Sri Lanka: photos of two individuals (1 adult & 1 very small juvenile), Negombo, N of Colombo, April 1995, RC Anderson; photos of two large individuals, Negombo, N of Colombo, 16 January 2011, S Kahlbrock.
http://species-id.net/wiki/Phyllidiella_meandrina
Fig. 21 , Plate 67Socotra: 40 × 21 mm curled, pres. (St. 018, GR-23), Qualansiyah Bay 12°41.026'N, 53°28.309'E, 10 March 1999, leg. G Reinicke. – Kenya: three pres. specimens 26 × 15 mm (bent and curled), 17 × 11 mm, and 17 × 8 mm (slightly curled), Vipingo, 25 miles N of Mombasa, rock pool ELW, 23 September 1984, leg. J Hognerud; four pres. specimens curled and faded, approx. 15 mm, Kibirijini, intertidal pool, 27 August 1969/ nudibranch IXX, Turtle Bay, subtidal rock, 12 July 1969/nudibranch XXIII, Mida 30’ rock, 25 July 1969/nudibranch XX, Turtle Bay, intertidal rock, July 1969, all leg. K Brander (TE Thompson coll. NHMUK acc. no. 2364). – Tanzania: photographs of two individuals, Mafia Island, shallow water, May 2009, A de Villiers; photographs of two individuals, M’Nazi Bay, Msimbati, near Mtwara, May 1994 and May 1995, IM Horsfall. – Zanzibar: photo of few individuals, MD Richmond and A de Villiers. – South Africa: two specimens (Natal Museum specimen ref. nos. S1773 and E4898 + photo slide ref. 0061). – Madagascar: 40 × 15 mm (PK-D2), Ampangorina, Nosy Komba, 1 m depth (“cruising over sand”), 30 January 1992, leg. P Kemp; 50 × 15 mm (PK-E), Ampangorina, Nosy Komba, 1 m depth (“on algal-encrusted stag horn coral”), 20 February 1992, leg. P Kemp. – Sri Lanka: 47 × 17 mm pres. (curled), via Tropical Marine Centre, London (fish importers), 14 August 1990. – Mauritius: photos of several individuals, M Parmantier, and La Réunion http://seaslugs.free.fr/nudibranche/a_intro.htm.
Phyllidiella meandrina A left rhinophore of 40 mm pres. Socotra specimen B ventral view of 50 mm Madagascar specimen showing propodium and rounded black oral tentacles.
The Socotra specimen is an almost perfect replica of the type specimen, with two incomplete median circles of small tubercles. First lateral encircling band of tubercles composed of small tubercles followed by row of single isolated tubercles and narrow black line before pink margin; long black rhinophores (Fig. 21A). Large fused oral tentacles tinged with black in life; pigment still remains on Madagascar specimens 20 years later (Fig. 21B).
Although easily recognized, Phyllidiella meandrina is not a common species in the western Indian Ocean; it has not been recorded from the Maldives (contrary to my statement in
http://species-id.net/wiki/Phyllidiella_pustulosa
Plate 68Socotra: 28 × 9 mm curled pres. (IT-157, RJ-011), 12°18.698'N, 53°48.285'E, 09 April 1999, leg. R Janssen. – Little Aden: 40 × 17 mm preserved (NHMUK), curled at edges, BP Refinery, PO Box 3003, 05 February 1968, leg. Miss Gobnait Murphy. – Seychelles: preserved (NHMUK acc. no. 2222), Passe Femme, Aldabra, shallow water beneath coral, 29 November 1967, leg. JD Taylor. – Sri Lanka: 17 × 12 mm pres., near Pigeon Island, Nilaveli, Trincomalee, 11 March 1995, leg. SG Buttress & RC Anderson; photo of 1 individual, Negombo, N of Colombo, April 1995, RC Anderson; photos of one individual, Negombo, N of Colombo, 11 January 2010, S Kahlbrock. – Tanzania: photo of one individual, 1994, MD Richmond. – Andaman Islands: one pres. specimen (NHMUK 1960.1334, acc. no. 1838), Winckworth coll. – Christmas Island: 25 × 11 mm pres. (NHMUK 1899.5.26.12), Flying Fish Cove, leg. Sir J Murray (Andrews).
http://species-id.net/wiki/Phyllidiella_rosans
Fig. 22, Plate 69Maldives: two pres. specimens 52 × 16 mm and 27 × 16 mm (with 3 median lines and 2 rings), Gangehi Island lagoon, Ari Atoll, 1-5 m depth, February 1988, leg. G Corriero; 16 × 11 mm (3 median lines and 2 rings), Gangehi Island lagoon, Ari Atoll, < 5 m depth, August 1990, leg. Cabona; 43 × 14 mm (NY-4 “green ridges, tubercles in 3 median lines + 3 rings, long black anal papilla, long black rhinophores”), Bodhuhithi Channel, North Malé Atoll, 21.5 m depth, 11 May 1990, leg N Yonow; 21 × 14 mm pres., curled (NY-19 “3 median line of tubercles + 3 rings”), Rasfaree outer reef, North Malé Atoll, 27 m depth, 13 May 1990, leg. N Yonow; 34 × 14 mm (NY- 24 “pink edge, smelly”), Kuramathi, Rasdhu Atoll, 20 m depth, 13 May 1990, leg. N Yonow; 33 × 17 mm (NY- 62) and 21 × 17 mm (NY- 63), Kandholudhu Tila, Ari Atoll, 10 m depth, 15 May 1990, leg. N Yonow; 21 × 8 mm (NY-103 “green with 3 rings”), Maaya Tila, Ari Atoll, 11 m depth, 18 May 1990, leg. N Yonow; 48 × 15 mm (NY-119 “crests in 3 lines + 2 rings, produced slimy mucus”), Tin Tila, Ari Atoll, 27 m depth, 21 May 1990, leg. N Yonow; 22 × 11 mm (NY-126 “median ridge + 3 rings”), Maayafushi Tila, Ari Atoll, 46 m depth, 22 May 1990, leg. N Yonow; 45 × 18 mm (NY-145 “green, separated grey oral tentacles, sole of foot mottled black concentrated centrally, smelly, exuded white mucus”) and 40 × 16 mm (NY-146), Fulidhoo house reef, Felidhoo Atoll, 20 m depth, 12 January 1991, leg. N Yonow; four specimens 33 × 16 mm (NY-159), 30 × 11 mm (NY-160), 18 × 15 mm (NY-161) (all “green and black ringed”), 25 × 13 mm (NY-162, “pink & black, very ridged, black rhinophores”), Kunarvashi Tila, Felidhoo Atoll, 22 m depth, 14 January 1991, leg. N Yonow; six specimens 30 × 15 mm pres. curled (“3 median + 3 rings”), 42 × 18 mm pres. (“3 medians + 3 rings”), 28 × 12 mm pres. (“3 medians slightly broken”), 26 × 12 mm pres. (“3 medians + 2 rings”), 24 × 11 mm pres., 22 × 10 mm pres. (all as NY dive 42), Maaya Tila, Ari Atoll, 33 m depth, 19 January 1991, leg. N Yonow; 21 × 5 mm (NY- 208), Manta Point, North Malé Atoll, 17 m depth, 21 January 1991, leg. N Yonow; numerous individuals photographed & measured but not collected, 21-52 mm, Maldives Expedition, N Yonow (see above and Yonow 1994, 1996); photographs only of many individuals, 1986-1994, J Hinterkircher. – Tanzania: photographs of numerous individuals, M’Nazi Bay, Msimbati, near Mtwara, May 1994, May 1995, IM Horsfall. – Mauritius and La Réunion: numerous photographs, 18-35 mm, http://seaslugs.free.fr/nudibranche/a_intro.htm.
Phyllidiella rosans, 42 mm (NY dive 42), view of propodium, head, and black pigment on oral tentacles.
http://species-id.net/wiki/Phyllidiella_rudmani
Fig. 23, Plate 70Maldives: 10 × 3 mm alive (NY-11a), Nakatcha Tila, North Malé Atoll, 20 m depth, 12 May 1990, leg. N Yonow.
This juvenile specimen has identical marking to adult specimens, white with two black lines; dorsum completely smooth, tubercles not yet developed and spicules clearly visible in skin (Fig. 23A). Ventrally, minute tentacles same shape as in adults (
Phyllidiella rudmani, juvenile 10 mm specimen A dorsal view showing black markings, lack of tubercles, and spicule arrangement B ventral view showing propodium, head, and oral tentacles.
http://species-id.net/wiki/Phyllidiella_striata
Fig. 24 , Plate 71Maldives: 22 × 11 mm pres., Gan, Maldives Island Expedition, 20 m depth, 28 July 1964, leg. PSD (NHMUK ref. no. M/01/0); 45 × 11 mm (NY-2, “tuberculate ridges, grey oral tentacles with grooves”), Blue Cave, North Malé Atoll, 25 m depth, 11 May 1990, leg. N Yonow; 47 × 9 mm (NY-9), Nakatcha Tila, North Malé Atoll, 27 m depth, 12 May 1990, leg. N Yonow; 26 × 10 mm (NY-17), Nakatcha Kura Tila, North Malé Atoll, 12 m depth, 12 May 1990, leg. N Yonow; three specimens 20 × 7 mm, 21 × 7 mm, and 22 × 8 mm (NY-20, 21, 22, “sole grey cf. rosans black”), Rasfaree Outer Reef, North Malé Atoll, 27 m depth, 13 May 1990, leg. N Yonow; four specimens 34 × 10 mm (NY-25, “broken crests, 2 rings, ring around anus, grey oral tentacles”), 34 × 13 mm (NY-26, “similar to NY-25 but crests more continuous, distinctly elongated”), 21 × 6 mm (NY-28, “median line + laterals + ring, metapodium with black median line, oral tentacles grey with black tip, ”), 16 × 4 mm (NY-29, “black with broken med + laterals + 1 ring tubercles, oral tentacles grey pink”), Kuramathi, Rasdhu Atoll, 20 m depth, 13 May 1990, leg. N Yonow; six specimens 30 × 10 mm (NY- 48), 24 × 7 mm (NY- 50), 20 × 5 mm (NY- 56), 23 × 6 mm (NY- 57), 33 × 10 mm (NY- 58), 11 × 5 mm (NY- 66, “black with median + 2 lateral + 1 ring, pink tubercular crests”), Kandholudhu Tila, Ari Atoll, 10 m depth, 15 May 1990, leg. N Yonow; three specimens all pres. 16 × 10 mm (bent), 22 × 10 mm, 25 × 8 mm (NY dive 11, “pink & black crested”), Kandholudhu fringing reef, Ari Atoll, max 25 m depth, 15 May 1990, leg. N Yonow; three specimens pres. 22 × 8 mm bent, 22 × 10 mm curled, 25 × 10 mm (NY dive 12), Maayafushi fringing reef, Ari Atoll, max 30 m depth, 16 May 1990, leg. N Yonow; two specimens 26 × 10 mm (NY-104, “pink crested”) and 12 × 6 mm (NY-105), Maaya Tila, Ari Atoll, 11 m depth, 18 May 1990, leg. N Yonow; 15 × 7 mm pres. (NY-108), Toroca, Ari Atoll, 10 m depth, 19 May 1990, leg. N Yonow; 24 × 10 mm (NY-127), Maayafushi House Reef, Ari Atoll, 46 m depth, 22 May 1990, leg. N Yonow; 24 × 10 mm (NY-141), Small Banana Reef, North Malé Atoll, 25 m depth, 10 January 1991, leg. N Yonow; 20 × 7 mm (NY-147), Felidhoo House Reef, Fulidhoo Atoll, 20 m depth, 12 January 1991, leg. N Yonow; two specimens 24 × 18 mm (NY-149) and 24 × 7 mm (NY-150, “pink crested, 2 rings, smelly & exuded slime”), Fulidhoo House Reef, Felidhoo Atoll, 20 m depth, 12 January 1991, leg. N Yonow; 25 × 10 mm (NY-179, “pink crested”), Dhageti Tila, Ari Atoll, less than 17 m depth, 16 Jan 1991, leg. N Yonow; four specimens 40 × 13 mm (NY-199), 40 × 13 mm (NY-200), 25 × 10 mm (NY-201), 35 × 14 mm (NY-202), Bathala Faro, Ari Atoll, 15 m depth, 17 January 1991, leg. N Yonow; two specimens pres. 17 × 7 mm and 18 × 8 mm (NY dive 42, “pink broken crested”), Maaya Tila, Ari Atoll, 33 m depth, 18 January 1991, leg. N Yonow; 45 × 17 mm (NY-203, “pink broken crested”), Maayafushi lagoon, Ari Atoll, 22 m depth, 18 Jan 1991, leg. N Yonow; 13 × 5 mm (NY-205, “pink crested”), Bodhu Fahlu, Ari Atoll, 15 m depth, 19 Jan 1991, leg. N Yonow; 30 mm length (SH-6, “3 broken median lines, edge of mantle white”), Huravalhi Reef, Lhaviyani Atoll, 20 m depth, 1 March 1991, leg. S Harwood; 18 × 8 mm pres. (SH-55), Foteo West Reef, Felidhoo Atoll, 20 m depth, 25 April 1991, leg. S Harwood; 12 × 7 mm pres., Naifaro House Reef, Lhaviyani Atoll, 29 m depth, 3 November 1991, leg. H Debelius; 16 mm, Bathala Island, Ari Atoll, 10 m depth, 27 July 1995, leg. SG Buttress & RC Anderson; numerous individuals photographed & measured but not preserved, 13-35 mm, Maldives Expedition, N Yonow (see above and Yonow 1994, 1996) and 1986-1999, J Hinterkircher. – Christmas Island: 15 × 8 mm preserved (NHMUK 1899.5.26.13), Flying Fish Cove, leg. Sir J Murray (Andrews).
The recognition of Bergh’s species by Yonow et al. (2002) is further supported by these 45 specimens from the Maldives and Christmas Island. The Maldives appears to be a real hot spot for phyllidiid nudibranchs, supporting at least one endemic species (Phyllidia koehleri, p. 61) and vast numbers of Phyllidiella striata and Phyllidiella rosans (see above). Phyllidiella striata is most similar to Phyllidiella rosans, but the (usually) broken dorsal ridges which are angled behind the rhinophores, which form a circle in front of the rhinophores, and which form a circle around the anus are characteristic (Plate 71). Additionally, the two species differ ventrally, with Phyllidiella striata lacking the black pigmentation on the foot and oral tentacles (compare Fig. 22 and Fig. 24). Phyllidiella striata ranges from 13-35 mm while Phyllidiella rosans is usually larger, measuring up to 52 mm (p. 67) and is much darker ventrally.
Phyllidiella striata, 35 mm (NY-202), view of propodium, head, and oral tentacles.
http://species-id.net/wiki/Phyllidiella_zeylanica
Plates 72 , 73Seychelles: 30 × 15 mm (PK-Y), off Ade’s house, Lilôt, NW Mahé, 10 April 1992, leg. P Kemp; photos of three individuals, Lilôt, NW Mahé, 1988–1989, P Kemp. – Maldives: photos of several individuals, 20–25 mm, March 1997, March 1998, March 1999, J Hinterkircher. – Sri Lanka: photos of one individual, Unawatuna, S of Galle, 26 December 2010, S Kahlbrock.
http://species-id.net/wiki/Phyllidiopsis_gemmata
Fig. 25 , Plate 74Maldives: 33 × 15 mm pres. (NY-35), Maayafushi Tila, Ari Atoll, 23 m depth, 14 May 1990, leg. N Yonow (“white with 4 black lines, sole pale grey/white, foot extends beyond margin, has black line dorsally”); photographs of one individual, Maayafushi, South Malé Atoll, March 1997, J Hinterkircher.
Living specimen very white and grey with two longitudinal black lines on each side of centre; each pair meets in front of its respective rhinophore. Outer black lines additionally meet posteriorly beyond anus and extend to mantle margin. Tubercles small and simple, although they may occur in clusters along tuberculate lines. Anus located on a tubercle, and was extended in life. Sole pale grey to white but top of foot with black line.
In preservative, specimen is distinctive: relaxed, with a very broad but thin mantle skirt. Central tubercles bead-like, arranged in three longitudinal lines; median one several tubercles wide. Skirt has scattered tubercles arranged neither in groups nor in lines. Anterior margin of foot narrow with thickened edge. Head distinctive, forming a “funnel” below broad fused oral tentacles, difficult to see in Fig. 25 (but similar to Phyllidiella zeylanica); gills and head white in preserved material.
Phyllidiopsis gemmata, 33 mm (NY-35), view of propodium, head, and oral tentacles.
There appear to be very few records of this species in the literature: the species identified as Phyllidia cf. gemmata by
http://species-id.net/wiki/Phyllidiopsis_krempfi
Fig. 26 , Plate 75Maldives: 35 mm (SH-49), Fulidhoo Reef, Felidhoo Atoll, 21 April 1991, leg. S Harwood. – Sri Lanka: 40 × 20 mm pres., near Pigeon Island, Nilaveli, Trincomalee, 11 March 1995, leg. SG Buttress & RC Anderson.
Sri Lankan and Maldives specimens bear large compound tubercles centrally, as well as on extended mantle skirt. Pair of black lines meets in V-shape in front of rhinophores (Fig. 26A) and extends to margin; posteriorly, they normally do not meet but bend outwards near the level of the anus and extend to the mantle margin. Traces of black in between central tubercles, as well as spots and streaks on skirt. Anus protrudes in preserved specimens, located on edge of last tubercular cluster. Gills grey, fused oral tentacles with black tips (Fig. 26B). Digestive anatomy typical of genus (Fig. 26C).
Phyllidiopsis krempfi, 40 mm pres. specimen A close-up view of rhinophores and rhino-tubercles B ventral view of propodium, head, and rounded oral tentacles with black tips C digestive anatomy.
These records from the Maldives and Sri Lanka are the first records of this species for the western Indian Ocean; it is better known in the western Pacific, where it is larger and more complex: the tubercles become so compound that the longitudinal black lines are almost lost. In the Indian Ocean, it is one of the larger pink species with numerous compound tubercles bearing black lines which always meet in a V-shape in front of the rhinophores.
http://species-id.net/wiki/Phyllidiopsis_shireenae
Fig. 27 , Plate 76Maldives: three specimens 60 × 24 mm (NY-100), 75 × 25 mm (NY-98), and 95 × 33 × 20 mm (NY-99), Maaya Tila, Ari Atoll, 8–30 m depth, 17 May 1990, leg. N Yonow, photos H Voigtmann; 80 mm length (NY dive 42), Maaya Tila, Ari Atoll, 33 m depth, 18 January 1991, leg. N Yonow; individual 40 mm length (photographed but not preserved), Vihafushi Tila, Baa Atoll, 18 m depth, 7 March 1991, S Harwood; individual 30 mm length (photographed but not preserved), Vadhoo Reef, South Malé Atoll, 16 m depth, 3 April 1991, S Harwood; photographs of several individuals, Ari Atoll, approx. 20 m depth, late 1980s, H Voigtmann.
Body elongate oval, flexible and rubbery, with high profile. Ground colour semi-translucent pink with black line surrounding central region; four smaller perpendicular lines extending to margin in opposing pairs. Central region bears high median crest and lower lateral one either side. Crests composed of irregular granular compound tubercles, opaque in comparison with body. Rhinophores and anal papilla located within black oval. Rhinophores translucent pink, 12 to 19 oblique lamellae; anus conical, located on last tubercle of central crest, visible on larger specimen Plate 76; both extend through raised rims. Outside the black ellipse, a row of large tubercles is followed by a row of smaller ones, also compound and opaque pink-white in life. Metapodium projected beyond the mantle in life. Black band in crevice where hyponotum meets foot behind gills; this black line extends posteriorly along top of metapodium in one specimen. Gills black, genital opening a white swelling on right side. Propodium nearly divided in smaller specimens and notched in the largest. White head and fused oral tentacles, very short with groove on outer sides (figs. 27A).
Internally, three specimens from the Maldives proved to be identical (the fourth was badly preserved). One specimen (95 mm) everted its mouthparts completely during preservation (Fig. 27B, D). The mouth leads to a short oral tube which, in one specimen, is narrow and contracted like a concertina (Fig. 27C). Where it thickens into the pharyngeal bulb, there is a band of pigment, incomplete dorsally. The pharyngeal bulb is extensible, as evidenced by the specimen illustrated in Fig. 27D. A sharp demarcation exists where the pharynx joins the bulb; the bulb turns anteriorly and loops posteriorly, passing through the nerve ring. The posteriorly-directed portion is of equal length to the anteriorly-directed segment, and inserts into the oesophagus at a constriction. The oesophagus is a thicker banded tube leading into a sac-like structure with which it is continuous (the muscular oesophageal segment), and opens into the digestive gland. The pathway from the oesophagus to the digestive gland follows a Z-shaped route, located in the anterior quarter of the digestive gland. Almost in a continuous line with the oesophagus, a long muscle attaches and runs under the intestine and pericardium to emerge on the other side. The intestine then runs along the midline to the anus.
Phyllidiopsis shireenae A ventral view of 80 mm specimen B ventral view of 95 mm specimen with mouthparts extruded C digestive anatomy of 75 mm specimen D digestive anatomy of 95 mm specimen.
These records greatly extend the range of the species from the tropical western Pacific to the central Indian Ocean. It was previously described from eastern Australia, the Solomon Islands (
http://species-id.net/wiki/Phyllidiopsis_sphingis
Fig. 28 , Plates 77 , 78Maldives: 17 mm (SH-7, “yellow rhinophores”), Fehigili, Lhaviyani Atoll, 30 m depth, 05 March 1991, leg. S Harwood; 19 × 10 mm (MDV/AB/96/18, “pale blue, no tubercles, rhinophores cream/pale yellow”), Yacht Tila, near Medufinolhu, South Malé Atoll, 10 m depth, 09 May 1996, leg. RC Anderson & SG Buttress.
Both specimens well relaxed, virtually identical to each other with black linear pattern composed of four lines: median pair merges between rhinophores and extends to margin anteriorly, posteriorly they remain separate to anus or meet outer lines at level of anus. Outer lines straight medially but scalloped laterally in extensions to margin; scallops not symmetrical on each side, with five on left and six on right on both specimens. Collectors’ sketches identical to illustration in Brunckhorst, 1993; although only one collector comments on blue colour, both comment on ochre-yellow rhinophores, visible in photographs of both specimens. Anal papilla ochre, very near posterior margin. Photograph of 19 mm specimen (Plate 77) shows central area between black lines to have been blue, outer scalloped areas were granular white with blue spots along margin. Faint orange tinge behind rhinophores (and generally) is a photographic artefact.
The specimens, both preserved in formaldehyde, are very different: one is perfectly marked as if alive, with extended rhinophores and black markings (17 mm, Plate 78). The second appears ‘bleached’ and translucent, to the point where the black spicules aligned in the central region above the yellowish gut are perfectly clear (Fig. 28A). Ventrally, the head is typical of species of Phyllidiopsis, a large fused unit with the grooves of the tentacles just visible in the pigmented specimen; spicules visible along foot margin and on hyponotum (Fig. 28B). The hyponotum, foot sole, and gills are cream coloured.
Internally, the digestive anatomy is typical of the genus: the solid pharyngeal bulb has a flat end anteriorly and tapers posteriorly. The tubular pharynx is narrow and turns on itself to pass through the nerve ring before joining the muscular oesophagus, which is banded and brown-black. There is a long narrow cream oesophagus which enters the digestive gland at approximately the one third mark (fig. 28C). The anal papilla is slightly swollen.
Phyllidiopsis sphingis A dorsal view of rhinophores and spicule alignment of 19 mm specimen B ventral view of 17 mm specimen showing the large fused oral tentacles C digestive anatomy of 19 mm specimen.
This is the first record of Phyllidiopsis sphingis for the Indian Ocean. Both specimens are small, but within the range reported for West Pacific specimens (4–23 mm). They differ from the Pacific records which have blue pigment only on the mantle skirt. The species in the Maldives is similar to Phyllidia koehleri in its small size, a ridged dorsum rather than a tuberculate one, and with the anus located very far posteriorly.
http://species-id.net/wiki/Phyllidiopsis_xishaensis
Fig. 29 , Plate 79Maldives: 21 × 8 mm (NY-34, “translucent white, tiny oral tentacles ochre like rhinophores”), Maaya Tila, Ari Atoll, 15 m depth, 14 May 1990, leg. N Yonow; 15 mm (SH-12, “yellow rhinophores make him different”), Boamandhifurihurai, Lhaviyani Atoll, 6 m depth, 05 March 1991, leg. S Harwood; 12 × 5 mm pres. (“with dots around edge”), Hans Place, south end of North Malé Atoll, 9 m depth, 11 October 1994, leg. SG Buttress & RC Anderson; photographs of five individuals, one measuring 15 mm, March 1997, March 1998, March 1999, J Hinterkircher. – Mauritius, La Réunion, and Mayotte: numerous individuals photographed, 6–19 mm alive (http://seaslugs.free.fr/nudibranche/a_intro.htm). – Seychelles: photos of two individuals, Lilôt, NW Mahé, 1988–1989, P Kemp.
The resurrection of this species and the rationale behind this were described in Yonow et al. (2002). These specimens from the western Indian Ocean clearly conform to the original (and subsequent) illustrations of the species (erroneously identified as Phyllidiopsis striata in the literature and on many websites) and further illustrations are provided here of the anterior dorsum (Fig. 29A) and head/oral tentacles (Fig. 29B). It has an isolated distribution in the western Indian Ocean, from the Laccadive and Maldive islands south to Chagos and west to Mauritius, La Réunion, and Mayotte; there are no specimen records from East or South Africa.
Phyllidiopsis xishaensis A dorsal view of anterior end of 15 mm specimen B ventral view of propodium of 21 mm specimen.
Preliminary evaluation of this particular assemblage of species shows no real patterns at present. Without doubt there are a number of species which seem to be limited to the western Indian Ocean, especially within the chromodorids and phyllidiids. At present, only the species covered in this paper are tabulated for locality, with a total for each location (Table 1). La Réunion and Mauritius are ranked as the most diverse, with a total of 48 species, closely followed by the Maldives with 45 species (except Kenya, see below). A number of species appear to be limited to the Maldives, despite similar collection methodologies in the other regions studied: Nembrotha guttata and Notodoris gardineri form nigerrima only occur here, as does Phyllidia koehleri. Of the ten new records for the Indian Ocean, seven are recorded from the Maldives, four of which are recorded exclusively from that locality. Three somewhat rare West Pacific phyllidiids are also recorded only from the Maldives, or also at one other locality: Phyllidia exquisita, Phyllidiella rudmani, and Phyllidiopsis gemmata. It is a little surprising that Mauritius, with its much longer history of research, does not rank as highly as the Maldives for this clutch of species, despite the establishment of the Mauritius/
The highest number of species recorded for the countries listed in Table 1 was for Kenya; this is an ‘experimental’ result produced by using the locations tab on the
Dr. RC and SG Anderson (Maldives and U.K.) have been very helpful in collecting specimens during their travels, and I am grateful for Susan’s excellent notes and drawings, and Charles’ photographs. I would like to thank Dr F Krupp and Dr R Janssen (Senckenberg Museum) for specimens and photographs from the Socotra Project and Saudi Arabia. Dr M Richmond (Tanzania) provided a number of specimens and photographs while editing his book on East Africa, and Ms P Kemp (U.K.) has also spent time collecting and photographing opisthobranchs, scanning many slides, and typing the first draft, for all of which I am grateful. Mr J Hinterkircher (Germany), Mr S Kahlbrock (Germany and Egypt), Mr P Bidgrain (formerly La Réunion, currently Mayotte), and Mr H Flodrops (La Réunion) have been generous in providing photographs over the years, and more recently by collecting specimens, and I would like to express my gratitude to them. Mr GT Smith (U.K. and Oman) and Mrs A de Villiers (Tanzania) have been kind enough to send photographs for identification over the years, and given permission to use them. Mrs A Ratcliffe (Swansea) spent many hours cleaning and scanning old slides, and for her cheerful assistance, I am ever grateful. My sincere gratitude also goes to Dr. SJ Wainwright (Swansea) for construction of my microscope camera. Finally, I would like to thank Dr W Harris (Swansea) for her professional enhancement of the images, Dr RC Willan (Darwin, Australia) for discussions and comments on the manuscript, and Dr M Nithyandanan (Sea City, Kuwait) for Indian literature. Two referees provided helpful and valuable comments, and for their time and patience, I am very grateful.
Haminoea cf. cymbalum, 20 mm, Maldives, photo: RC Anderson.
Chelidonura electra, 20–25 mm, Mayotte, photo: P Bidgrain.
Chelidonura hirundinina, 5–6 mm pres., Maldives, photo: J Hinterkircher.
Chelidonura punctata, 18–20 mm, La Réunion, photo: P Bidgrain.
Chelidonura cf. punctata, Seychelles, photo: P Kemp (no spcm).
Chelidonura sandrana, 7 mm, Maldives, photo: J Hinterkircher.
Chelidonura sandrana, Maldives, photo: J Hinterkircher (no spcm).
Chelidonura varians, 30 mm, Maldives, photo: RC Anderson.
Philinopsis speciosa, 18 mm pres., Maldives, photo: RC Anderson.
Aplysia parvula, 20 mm, La Réunion, photo: P Bidgrain (no spcm).
Aplysia parvula, 14 mm, Maldives, photo: RC Anderson.
Dolabella auricularia, Oman, photo: S Kahlbrock (no spcm).
Stylocheilus longicauda form striata, 10–50 mm pres., Zanzibar, photo: MD Richmond.
Cyerce bourbonica sp. n., 12 mm holotype, La Réunion, photo: H Flodrops.
Cyerce bourbonica sp. n., 12 mm paratype, La Réunion, photo: H Flodrops.
Elysia cf. nigropunctata, 35 mm, La Réunion, photo: H Flodrops (no spcm).
Plakobranchus ocellatus, 14 mm pres., Zanzibar, photo: MD Richmond.
Thuridilla gracilis form bayeri, Maldives, photo: J Hinterkircher (no spcm).
Thuridilla gracilis form ratna, 25 mm, Seychelles, photo: P Kemp.
Thuridilla vataae, 10 mm, Maldives, photo: RC Anderson.
Nembrotha guttata, Maldives, photo: H Voigtmann (with N. cristata, no spcms).
Roboastra gracilis, 20 mm, Maldives, photo: J Hinterkircher (no spcm).
Tambja amakusana, 11 mm with egg mass, Maldives, photo: RC Anderson.
Notodoris gardineri form nigerrima, 40 mm, Maldives, photo: RC Anderson.
Notodoris minor, Pemba Island, photo: A de Villiers (no spcm).
Hexabranchus sanguineus, juvenile 13 mm, Maldives, photo: RC Anderson.
Hexabranchus sanguineus, sub-adult, Seychelles, photo: P Kemp (no spcm).
Carminodoris grandiflora, 30 mm pres., Zanzibar, photo: MD Richmond.
Halgerda formosa, Kenya, photo: MD Richmond (no spcm).
Halgerda punctata, 17–20 mm pres., Sri Lanka, photo: S Kahlbrock.
Cadlinella ornatissima, 13 mm pres., Sri Lanka, photo: S Kahlbrock.
Ceratosoma miamirana, 36 mm pres., Zanzibar, photo: MD Richmond.
Chromodoris africana, Madagascar, photo: J Hinterkircher (no spcm).
Chromodoris annulata, Persian Gulf, photo: G Smith (no spcm).
Chromodoris annulata, Persian Gulf, photo: S Kahlbrock (no spcm).
Chromodoris boucheti, Maldives, photo: J Hinterkircher (no spcm).
Chromodoris cavae, 75 mm, La Réunion, photo: P Bidgrain.
Chromodoris cavae, Sri Lanka, photo: S Kahlbrock (no spcm).
Chromodoris conchyliata, juvenile 20 mm, Seychelles, photo: P Kemp.
Chromodoris conchyliata, Seychelles, photo: P Kemp (no spcm).
Chromodoris decora, 10 mm, Maldives, photo: RC Anderson.
Chromodoris decora, Oman, photo: S Kahlbrock (no spcm).
Chromodoris geometrica, 15 mm, Maldives, RC Anderson.
Chromodoris gleniei, Sri Lanka, photo: S Kahlbrock (no spcm).
Chromodoris hamiltoni, Tanzania, photo: A de Villiers (no spcm).
Chromodoris tennentana, 10 mm, Madagascar, photo: J Hinterkircher (no spcm).
Glossodoris cincta, 15 mm, Maldives, on the bryozoan Steginoporella, photo: J Hinterkircher.
Glossodoris pallida, 20 mm, Maldives, photo: RC Anderson.
Glossodoris undaurum, 13 mm pres., Sri Lanka, S Kahlbrock.
Hypselodoris maculosa, 15 mm, Maldives, photo: J Hinterkircher.
Chromodoris bullockii Collingwood, 1881: figs 15, 16, 17.
Risbecia bullockii, 35 mm, La Réunion, H Flodrops.
Risbecia bullockii, 11 and 16 mm pres., Sri Lanka, S Kahlbrock.
Risbecia pulchella, Oman, photo: S Kahlbrock (no spcm).
Thorunna cf. horologia, 5 mm pres., Sri Lanka, RC Anderson.
Dendrodoris fumata, Saudi Arabia, photo: F Krupp (no spcm).
Dendrodoris nigra, Oman, photo: S Kahlbrock (no spcm).
Dendrodoris nigra, red juvenile, Maldives, photo: J Hinterkircher (no spcm).
Doriopsilla nigrocera sp. n., 25 mm pres., Saudi Arabia, photo: F Krupp.
Fryeria marindica with Pacific pattern, 35 mm, Maldives, photo: RC Anderson.
Phyllidia alyta, 15 mm, Maldives, photo: RC Anderson.
Phyllidia coelestis, Tanzania, photo: A de Villiers (no spcm).
Phyllidia exquisita, 15 mm, Maldives, photo: RC Anderson.
Phyllidia koehleri, 10 mm, Maldives, photo: J Hinterkircher.
Phyllidia koehleri, 11 mm pres., Maldives, photo: N Yonow.
Phyllidia multituberculata black form, 28 and 30 mm pres., Maldives, H Voigtmann.
Phyllidiella meandrina, Tanzania, photo: A de Villiers (no spcm).
Phyllidiella pustulosa, Sri Lanka, photo: RC Anderson (no spcm).
Phyllidiella rosans, 30 mm, Maldives, photo: N Yonow (note defensive exudates).
Phyllidiella rudmani, 35 mm, Maldives, photo: N Yonow (spcm in Yonow 1996).
Phyllidiella striata, 20 mm, Maldives, photo: N Yonow.
Phyllidiella zeylanica, Maldives, photo: J Hinterkircher (no spcm).
Phyllidiella zeylanica, 38 mm, Maldives, photo: H Debelius (spcm in Yonow 1996).
Phyllidiopsis gemmata, Maldives, photo: J Hinterkircher (no spcm).
Phyllidiopsis krempfi, 40 mm pres., Sri Lanka, photo: RC Anderson.
Phyllidiopsis shireenae, 60 & 95 mm, Maldives, photo: H Voigtmann.
Phyllidiopsis sphingis, 19 mm, Maldives, photo: RC Anderson.
Phyllidiopsis sphingis, 17 mm, Maldives, photo: S Harwood.
Phyllidiopsis xishaensis, 10 mm, Maldives, photo: RC Anderson.
List of species recorded in this paper with locality data for each. (doi: 10.3897/zookeys.197.1728.app). File format: Excel spreadsheet (xls).
Explanation note: List of species recorded in this paper with locality data for each; the total number of species for each locality is listed along the last row. References of previous records are listed and X denotes species recorded in this work. All references are included in the synonymy and reference list.